ABSTRACT
The use of azole fungicides in agriculture is believed to be one of the main reasons for the emergence of azole resistance in Aspergillus fumigatus. Though widely used in agriculture, imidazole fungicides have not been linked to resistance in A. fumigatus. This study showed that elevated MIC values of imidazole drugs were observed against A. fumigatus isolates with TR34/L98H/S297T/F495I mutation, but not among isolates with TR34/L98H mutation. Short-tandem-repeat (STR) typing analysis of 580 A. fumigatus isolates from 20 countries suggested that the majority of TR34/L98H/S297T/F495I strains from China were genetically different from the predominant major clade comprising most of the azole-resistant strains and the strains with the same mutation from the Netherlands and Denmark. Alignments of sterol 14α-demethylase sequences suggested that F495I in A. fumigatus was orthologous to F506I in Penicillium digitatum and F489L in Pyrenophora teres, which have been reported to be associated with imidazole resistance. In vitro antifungal susceptibility testing of different recombinants with cyp51A mutations further confirmed the association of the F495I mutation with imidazole resistance. In conclusion, this study suggested that environmental use of imidazole fungicides might confer selection pressure for the emergence of azole resistance in A. fumigatus.
KEYWORDS: Aspergillus fumigatus, imidazole drugs, drug resistance, Cyp51A, evolution
INTRODUCTION
Aspergillus fumigatus is an opportunistic fungal pathogen. In recent years, with the increase in the number of susceptible patients, such as those with malignant tumors and hematopoietic stem cell transplantation, the incidence of invasive pulmonary aspergillosis (IPA) has increased, and A. fumigatus has now become one of the main infectious pathogens associated with the death of hospitalized patients (1, 2). Azoles are the main drug class used in the management of Aspergillus diseases. However, since the emergence of itraconazole (ITC)-resistant A. fumigatus in 1997, reports of azole-resistant A. fumigatus strains from clinical and environmental sources have been increasing (3). The prevalence of azole resistance in A. fumigatus isolates from some clinical care centers was as high as 10%, posing a great challenge for clinical treatment of Aspergillus disease (4). It has been believed that there are two distinct routes of resistance development (5–11). One is long-term azole therapy for patients, which leads to point mutations in the sterol 14α-demethylase gene cyp51A, including substitutions at G54, P216, M220, G138, and G448. The other is the application of azole fungicides in the environment, which leads to mutations in the cyp51A gene in combination with a tandem repeat (TR) in the promoter region of the gene, including the TR34/L98H and TR46/Y121F/T289A mutations.
The emergence of A. fumigatus strains with different cyp51A mutations might be associated with selection pressure from different azole drugs and the ability of A. fumigatus to adapt to the human host and the natural environment. Therefore, it has been suggested that starting, switching, and stopping azole therapy involves a risk of selecting for highly resistant strains with wild-type fitness (12). TR34/L98H was the predominant type of mutation for azole resistance in A. fumigatus strains from the Netherlands, Germany, France, India, and many other countries (13–16). However, our previous study and other studies from China (7, 17, 18) showed that the prevalence of the TR34/L98H/S297T/F495I mutation was comparable to, or even higher than, that of the TR34/L98H mutation among azole-resistant A. fumigatus isolates in China, while the former mutation was less frequently identified in other countries. The genotypic and phenotypic characterizations of A. fumigatus strains with different cyp51A mutation types and their association with the use of agricultural azole compounds merit further studies.
This study aims to demonstrate the in vitro susceptibility of A. fumigatus to major azole fungicides and its genetic relationship with different cyp51A mutations, as well as to verify the association of elevated MIC values with imidazole fungicides and TR34/L98H/S297T/F495I mutation by site-directed mutagenesis.
RESULTS
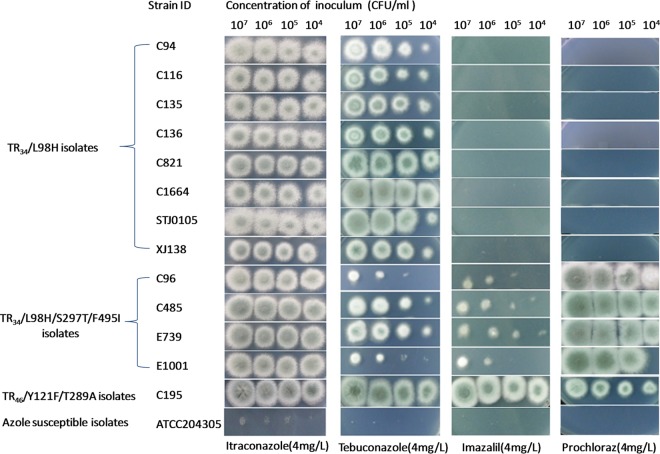
The results of in vitro susceptibility testing showed that five triazole fungicides—epoxiconazole, bromuconazole, tebuconazole, difenoconazole, and propiconazole—exhibited elevated MIC values (MICs, ≥8 mg/liter) for all the A. fumigatus isolates harboring the TR34/L98H, TR34/L98H/S297T/F495I, or TR46/Y121F/T289A mutation. Prochloraz and imazalil had higher MIC values for all four TR34/L98H/S297T/F495I isolates than for the eight TR34/L98H isolates. The MIC values of the seven azole fungicides tested against the two azole-resistant A. fumigatus isolates with G54R or G54V mutation were similar to those for the azole-susceptible isolates (Table 1). The growth phenotypes of these isolates on drug-containing minimal medium (MM) plates are shown in Fig. 1. All the TR34/L98H/S297T/F495I isolates could grow on MM plates containing 4 mg/liter of prochloraz and imazalil, while the eight TR34/L98H isolates could not.
TABLE 1.
In vitro testing of the antifungal susceptibilities of 24 Aspergillus fumigatus isolates to ten azole compoundsa according to the EUCAST method
| Strain ID | Source | Azole resistant | cyp51A mutation(s) | EUCAST MIC (mg/liter) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITC | VRC | POS | EPO | BRO | TEB | DIF | PRO | IMA | PRC | ||||
| C94 | Clinical | Yes | TR34/L98H | ≥16 | 2–4 | 1 | ≥32 | 8 | 8 | 8 | ≥32 | 2 | 0.5–1 |
| C116 | Clinical | Yes | TR34/L98H | ≥16 | 4 | 0.5 | ≥32 | 8 | 16 | 8 | ≥32 | 4 | 0.5–1 |
| C135 | Clinical | Yes | TR34/L98H | ≥16 | 2 | 1 | ≥32 | 16 | 8 | 16 | ≥32 | 2 | 0.5–1 |
| C136 | Clinical | Yes | TR34/L98H | ≥16 | 2 | 0.5 | ≥32 | 8 | 8 | 8 | ≥32 | 2 | 0.5–1 |
| C821 | Clinical | Yes | TR34/L98H | ≥16 | 4 | 1 | ≥32 | 16 | 16 | ≥32 | ≥32 | 2 | 1 |
| C1664 | Clinical | Yes | TR34/L98H | ≥16 | 8 | 1 | ≥32 | 16 | ≥32 | ≥32 | ≥32 | 2 | 2 |
| STJ0105 | Clinical | Yes | TR34/L98H | ≥16 | 8 | 2 | ≥32 | 8 | 16 | ≥32 | ≥32 | 2 | 1 |
| XJ138 | Clinical | Yes | TR34/L98H | ≥16 | 2–4 | 0.5 | ≥32 | 8 | 16 | ≥32 | ≥32 | 1 | 0.5 |
| C96 | Clinical | Yes | TR34/L98H/S297T/F495I | ≥16 | 1 | 0.5 | ≥32 | ≥32 | 16 | ≥32 | ≥32 | 8 | ≥32 |
| C485 | Clinical | Yes | TR34/L98H/S297T/F495I | ≥16 | 2 | 1 | ≥32 | ≥32 | 16 | ≥32 | ≥32 | 8 | ≥32 |
| E739 | Environmental | Yes | TR34/L98H/S297T/F495I | ≥16 | 2 | 0.5 | ≥32 | ≥32 | 16 | ≥32 | ≥32 | 8 | ≥32 |
| E1001 | Environmental | Yes | TR34/L98H/S297T/F495I | ≥16 | 1 | 0.5 | ≥32 | ≥32 | 16 | 16 | ≥32 | 8 | ≥32 |
| C195 | Clinical | Yes | TR46/Y121F/T289A | 1 | ≥16 | 0.5–1 | ≥32 | ≥32 | 8–32 | ≥32 | ≥32 | ≥32 | 16–32 |
| C02810 | Clinical | Yes | G54R | ≥16 | 0.5 | ≥8 | 1 | 0.25–0.5 | 1–2 | 0.25 | 1–2 | 0.06 | 0.25 |
| STJ0119 | Clinical | Yes | G54V | ≥16 | 0.25 | 1 | 0.5–1 | 0.5–1 | 1 | 0.5 | 1–2 | 0.06–0.125 | 0.25 |
| C79 | Clinical | No | D262Y | 0.25 | 0.5 | 0.06 | 2 | 1 | 2 | 2 | 4 | 0.125 | 0.125 |
| C98 | Clinical | No | F46Y/M172V/N248T/D255E/E427K | 1 | 1 | 0.25 | 4 | 4 | 2 | 2 | 8–16 | 0.25 | 0.25 |
| C490 | Clinical | No | N248K | 1 | 0.25–0.5 | 0.125 | 1 | 0.5 | 0.5 | 0.5 | 2 | 0.125 | 0.125 |
| C68 | Clinical | No | None | 0.5 | 0.5–1 | 0.25 | 4 | 4 | 4 | 2–4 | 8 | 0.25 | 1 |
| C239 | Clinical | No | None | 0.5 | 0.5 | 0.125 | 4 | 2–4 | 2 | 2 | 8 | 0.25 | 0.5 |
| E509 | Environmental | No | N248K | 0.5–1 | 0.25 | 0.06–0.125 | 2 | 0.5–1 | 1 | 1 | 1–2 | 0.125 | 0.125 |
| E631 | Environmental | No | None | 0.25 | 0.25–0.5 | 0.125 | 2 | 1 | 2 | 1–2 | 1–2 | 0.125 | 0.25 |
| E1069 | Environmental | No | A9T | 0.25–0.5 | 0.5 | 0.125 | 2 | 2 | 2 | 2 | 8 | 0.25 | 0.25 |
| E1109 | Environmental | No | None | 0.25 | 0.5 | 0.06 | 2 | 1–2 | 2 | 2 | 4 | 0.125 | 0.25 |
ITC, itraconazole; VRC, voriconazole; POS, posaconazole; EPO, epoxiconazole; BRO, bromuconazole; TEB, tebuconazole; DIF, difenoconazole; PRO, propiconazole; IMA, imazalil; PRC, prochloraz.
FIG 1.
Growth of 14 Aspergillus fumigatus isolates on minimal medium plates containing four different azole drugs (35°C, 72 h).
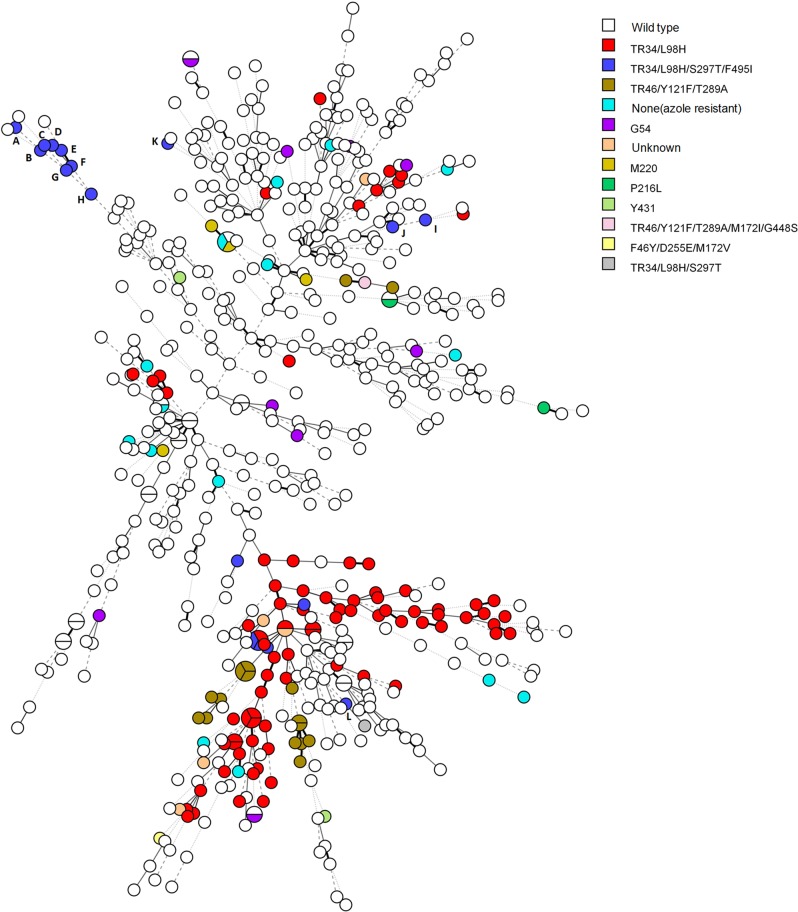
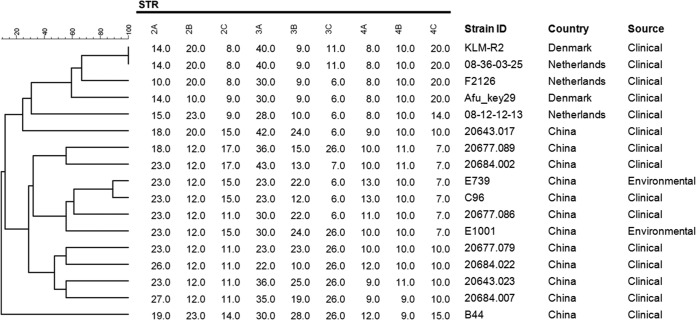
The population structure of 580 A. fumigatus isolates based on short-tandem-repeat (STR) typing analysis is shown in Fig. 2. Most of the TR34/L98H strains were distributed in the lower right clade of the minimum spanning tree, which represents the major clone complex of the azole-resistant A. fumigatus strains disseminating all around the world. Eight of 12 TR34/L98H/S297T/F495I strains originated from China were distributed in the upper left clade of the tree. This distribution suggested that these strains might have different evolutionary sources than the major TR34/L98H clone complex. The genotypic relationships of 17 A. fumigatus isolates with TR34/L98H/S297T/F495I mutation are shown in Fig. 3, which indicates that the TR34/L98H/S297T/F495I strains from China are genetically unrelated to the TR34/L98H/S297T/F495I strains from the Netherlands and Denmark.
FIG 2.
Minimum spanning tree of 580 Aspergillus fumigatus isolates based on all nine microsatellite markers of STR typing. Each circle represents one unique genotype. Different colors indicate different cyp51A mutations as shown in the key. Circles labeled A to L correspond to the 12 Chinese isolates with the TR34/L98H/S297T/F495I mutation (A, 20684.007; B, C96; C, 20684.022; D, 20677.086; E, 20643.023; F, E739; G, 20677.079; H, E1001; I, 20677.089; J, 20684.002; K, B44; L, 20643.017).
FIG 3.
Genotypic relationships among 17 Aspergillus fumigatus isolates with TR34/L98H/S297T/F495I mutation from China, the Netherlands, and Denmark. The dendrogram is based on a categorical analysis of nine microsatellite markers in combination with UPGMA clustering. The bar indicates the percentage of identity.
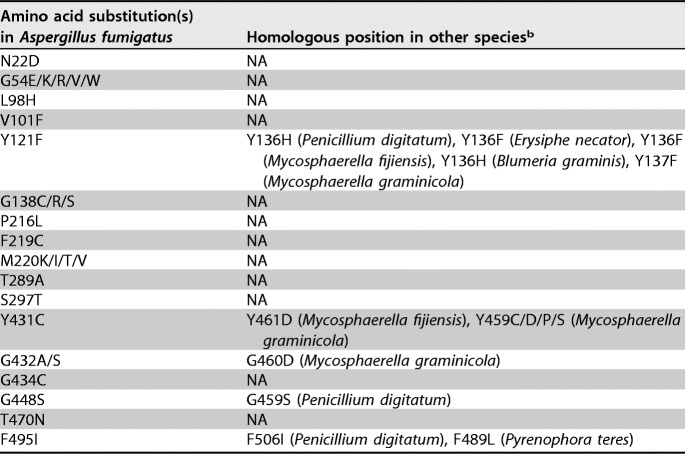
An amino acid sequence alignment based on the Cyp51A sequence from A. fumigatus and orthologous protein sequences from 10 other fungal pathogens is shown in Fig. S1 in the supplemental material. The alignments have been summarized, and fungicide resistance-associated mutations are given in Table 2. The F495I mutation in A. fumigatus is demonstrated to be orthologous to F506I in Penicillium digitatum and F489L in Pyrenophora teres, while no orthologous mutation was found for the L98H or S297T mutation in A. fumigatus. The Y121F mutation in A. fumigatus is also demonstrated to be orthologous to numerous mutations in agricultural fungal pathogens, including Y136H in P. digitatum, Y136F in Erysiphe necator, Mycosphaerella fijiensis, and Mycosphaerella graminicola, and Y136H in Blumeria graminis.
TABLE 2.
Corresponding mutation sites in agricultural pathogens based on alignment to the reference sequence from Aspergillus fumigatus cyp51Aa
GenBank accession number AF338659.
NA, not available.
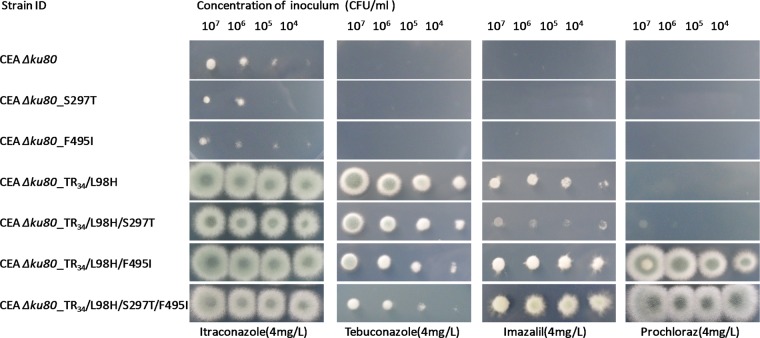
Successful construction of recombined A. fumigatus strains containing S297T, F495I, TR34/L98H, TR34/L98H/S297T, TR34/L98H/F495I, and TR34/L98H/S297T/F495I was confirmed by diagnostic PCR testing and sequencing of the cyp51A genes of the recombinants. The MICs for recombinant A. fumigatus strains with different cyp51A mutations are shown in Table 3. The recombinants with an S297T or F495I mutation produced MICs similar to those for the wild-type A. fumigatus CEA Δku80 recipient strain. The MICs of all three antifungal drugs and the seven fungicides for TR34/L98H mutants showed increases over those for CEA Δku80. When S297T was introduced into the TR34/L98H strain, the MICs of the two imidazoles remained similar. When F495I alone or both F495I and S297T were introduced in addition to the TR34/L98H mutation, a higher prochloraz MIC value (≥32 mg/liter) was observed. The MIC values of itraconazole, posaconazole, and the seven fungicides for the recombinants with TR34/L98H/S297T/F495I mutation were at least 2-fold higher than those for CEA Δku80. The differences in growth ability on azole-containing MM plates between CEA Δku80 and the six constructed strains are clearly shown in Fig. 4 and are consistent with the results of in vitro susceptibility testing.
TABLE 3.
MICs for recombinant Aspergillus fumigatus strains with different Cyp51A amino acid substitutions according to the EUCAST method
| Isolate | Cyp51A substitution(s) |
EUCAST MIC (mg/liter)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRb | Coding gene | ITC | VRC | POS | EPO | BRO | TEB | DIF | PRO | IMA | PRC | |
| CEA Δku80 recipient strain | − | − | 0.5 | 0.5 | 0.125 | 2–4 | 2 | 2 | 1–2 | 4 | 0.125 | 0.25 |
| CEA Δku80_S297T | − | S297T | 0.5–1 | 1 | 0.25 | 4 | 4 | 2 | 2 | 4 | 0.25 | 0.5 |
| CEA Δku80_F495I | − | F495I | 0.5 | 0.5–2 | 0.03–0.125 | 2–4 | 1 | 1–2 | 2 | 8 | 0.5 | 0.5–1 |
| CEA Δku80_TR34/L98H | + | L98H | ≥16 | 4 | 1 | ≥32 | 16 | 16–32 | 16 | ≥32 | 4–8 | 2 |
| CEA Δku80_TR34/L98H/S297T | + | L98H/S297T | 8–16 | 2 | 0.5–1 | ≥32 | 32 | 16 | 16 | ≥32 | 2–4 | 4 |
| CEA Δku80_TR34/L98H/F495I | + | L98H/F495I | ≥16 | 2 | 0.5 | ≥32 | 8 | 16–32 | ≥32 | ≥32 | 8 | >32 |
| CEA Δku80_TR34/L98H/S297T/F495I | + | L98H/S297T/F495I | ≥16 | 1–2 | 1 | ≥32 | ≥32 | 16 | ≥32 | ≥32 | 16 | ≥32 |
ITC, itraconazole; VRC, voriconazole; POS, posaconazole; EPO, epoxiconazole; BRO, bromuconazole; TEB, tebuconazole; DIF, difenoconazole; PRO, propiconazole; IMA, imazalil; PRC, prochloraz.
TR, 34-bp tandem repeat in the promoter region. −, absent; +, present.
FIG 4.
Growth of Aspergillus fumigatus CEA Δku80 and six constructed strains on minimal medium plates containing four different azole drugs (35°C, 72 h).
DISCUSSION
Azoles are an important class of drugs widely used in human and animal health, as well as in agriculture and horticulture. Over the past few years, evidence for an environmental route of resistance development in A. fumigatus due to applications of azole fungicides in agriculture has been accumulating (9); however, this link has not been proven (19). One of the main arguments has arisen from the lack of evidence of an association between two imidazoles, imazalil and prochloraz, and resistance in patients, although these imidazoles have been widely used since the 1970s (20). Our study clearly demonstrates an association of elevated MIC values of imidazole fungicides with TR34/L98H/S297T/F495I mutation in A. fumigatus. Although MICs have been used to evaluate the in vitro activities of fungicides against A. fumigatus (9, 21), there is currently no agreement on breakpoints to define resistance in the fungicides tested. Therefore, the significance of a 1- or 2-fold difference in the MICs of azole fungicides could not be determined (e.g., it is not known whether the clinical isolates C116 and XJ138 harbor different adaptive abilities in a natural soil environment sprayed with imazalil fungicides). This is one major limitation of this study. More studies are needed in the future to determine the significance of minor differences in MIC values of azole fungicides for A. fumigatus.
The results of STR typing analysis (Fig. 2) showed that 8 of the 12 Chinese TR34/L98H/S297T/F495I strains clustered in the upper left clade seem to belong to the same clone complex, while the other 4 strains were dispersed in the tree and possibly have different evolutionary origins. Both Fig. 2 and Fig. 3 reveal that the Chinese TR34/L98H/S297T/F495I strains are genetically different from the strains with the same mutation from the Netherlands and Denmark. These findings suggested that most of the A. fumigatus strains with TR34/L98H/S297T/F495I mutation from China possibly evolved from an extremely adaptive recombinant event under the selection pressure of imidazole fungicides within China, in a manner similar to the emergence of clonal TR34/L98H strains in India (16). The recent report of agricultural TR34/L98H/S297T/F495I isolates in China further supports our hypothesis (8).
Another piece of evidence comes from alignments of Cyp51 sequences from A. fumigatus and agricultural pathogens. These showed that the F495I mutation in A. fumigatus corresponds to F506I in P. digitatum and F489L in P. teres. It has been suggested that the F506I mutation plays an important role in the structure and function of Cyp51 and contributes to prochloraz resistance in P. digitatum strains from China (22). This mutation was found in combination with G459S mutation (22). F489L mutation in P. teres has also been shown to be associated with a high level of prochloraz resistance, and further structural in silico modeling analysis showed that interaction of F489L with the heme cavity produced a localized constriction of the region adjacent to the docking site that is predicted to result in lower binding affinities (22). In P. teres, the F495L mutation was the sole polymorphism of Cyp51A to be found only in resistant and not in sensitive isolates (23). The insertion of TR in the promoter region also represents an important mechanism of fungicide resistance and could contribute to overexpression of the cyp51 gene in many agricultural pathogens (11, 24, 25). More importantly, an investigation of 25 prochloraz-resistant P. digitatum isolates from China showed that F506I mutations in the cyp51 gene arose in combination with an insertion of 199 bp in the promoter region (22), suggesting that azole-resistant agricultural and clinical fungal pathogens harbor similar resistance mechanisms and probably have originated from the same evolutionary routes.
Repeated in vitro susceptibility testing showed that there were one or two 2-fold MIC differences for some strains when the same isolate was tested several times, suggesting that the results of susceptibility testing were generally stable and reliable. The susceptibility testing of different recombinants with cyp51A mutations further confirms the association of the F495I mutation with imidazole resistance. Although the single F495I mutation causes only a slight increase in the MIC values of imidazole fungicides, it could lead to high levels of prochloraz and imazalil resistance together with the introduction of the 34-bp tandem repeat and the L98H mutation. It has been shown that the 34-bp TR in the cyp51A gene of A. fumigatus is by itself not sufficient for the multi-azole resistance phenotype, and the substitution at codon 98 is a key alteration in this resistance mechanism (26). Our study suggested that the introduction of the F495I mutation was important for the survival of TR34/L98H/S297T/F495I strains under selection pressure from imidazole fungicides. The exact role of the S297T mutation is unknown. One hypothesis is that it is required to compensate for the harmful effect of F945I on normal protein function, just as T289A does in TR46/Y121F/T289A strains (27). The emergence of these special combinations of mutations possibly represent evolutionary consequences of the genotype–fitness maps (28). TR34/L98H/S297T/F495I and TR46/Y121F/T289A strains could be regarded as two of the multiple fitness peaks in the fitness landscape of A. fumigatus. Imazalil showed lower MIC values for clinical isolates than for the laboratory mutants in our study. This difference was possibly due to the absence of selection pressure from imidazole drugs on clinical isolates, which might have evolved to compensate for the adverse effect of cyp51A mutations.
Our findings raise many questions for future studies. First, could the TR34/L98H/S297T/F495I mutation be induced under selection pressure from imidazole fungicides under experimental conditions? Second, why do the TR34/L98H/S297T/F495I isolates from different countries or regions have different evolutionary sources? Third, is there any dose-effect relationship between amounts of imidazole consumption and the prevalence of imidazole resistance in agriculture or other environments? Could the problem of TR34/L98H/S297T/F495I dissemination be contained by reducing the application of imidazole fungicides in the environment?
In conclusion, our study is the first to demonstrate the association of elevated MIC values of imidazole fungicides with TR34/L98H/S297T/F495I mutation in A. fumigatus, which deepens our understanding of environmental routes of azole resistance development. Additional epidemiological and experimental evidence is needed for guiding the rational use of environmental fungicides and containing the problem of increasing antifungal resistance.
MATERIALS AND METHODS
In vitro antifungal susceptibility testing.
A collection of 13 azole-resistant A. fumigatus clinical isolates, 5 azole-susceptible clinical isolates, 2 azole-resistant environmental isolates, and 4 azole-susceptible environmental isolates was selected for investigation of the in vitro activities of seven azole fungicides and three clinical azole drugs. Among the 24 isolates tested, 5 azole-resistant clinical isolates (C1664, STJ0105, XJ138, C02810, and STJ0119) were newly collected through a surveillance program, while the remaining 19 isolates were from a previous study (7). All data for the 5 newly collected isolates, as well as in vitro activity data of the seven fungicides with the 19 previously collected isolates, were generated in this study.
In vitro testing of the susceptibilities of 24 A. fumigatus isolates to seven azole fungicides was conducted according to the EUCAST broth microdilution E.DEF 9.3 reference method (29). The seven fungicides were epoxiconazole, bromuconazole, tebuconazole, difenoconazole, propiconazole, imazalil, and prochloraz. The concentrations tested for all seven fungicides ranged from 0.06 to 32 mg/liter. Identification of strain species and cyp51A mutations, as well as in vitro testing of the susceptibilities of the five newly collected isolates to ITC (0.03 to 16 mg/liter), voriconazole (VRC) (0.03 to 16 mg/liter), and posaconazole (POS) (0.0156 to 8 mg/liter), were conducted as reported previously (7). A. fumigatus ATCC 204305 was included as a reference for quality control. The MICs for each strain were determined through at least three repeats of testing. The ranges of MICs are provided for those strains with different results after repeated testing. The growth of 14 A. fumigatus isolates on minimal medium (MM) plates containing 4 mg/liter of ITC, tebuconazole, imazalil, or prochloraz was also assessed. A series of freshly harvested spores were spotted onto the antifungal-drug-containing medium, and then the inoculated plate was cultured at 35°C for 72 h for observation.
STR typing analysis.
A search of published papers giving the results of STR typing for A. fumigatus was conducted. Among isolates harboring the same cyp51A mutation and STR type from each country, only the first isolate was included for analysis. As a result, STR typing data of 424 isolates from 20 countries (16, 30–54) were obtained. A total of 151 isolates with distinct STR types from our previous study (7) and 5 newly collected isolates in this study were also included for final analysis. The nine microsatellite markers (STRAf 2A, 2B, 2C, 3A, 3B, 3C, 4A, 4B, and 4C) for the five newly collected isolates were determined as described previously (41). The STR typing data were analyzed by Bionumerics 7.5 and are presented as a minimum spanning tree for categorical data with default settings. The genetic relationships of 19 A. fumigatus isolates with TR34/L98H/S297T/F495I mutation were analyzed by a categorical analysis of nine microsatellite markers using UPGMA (unweighted pair group method with arithmetic means) clustering.
Alignments of sterol 14α-demethylase sequences.
Amino acid sequences of sterol 14α-demethylase (Cyp51) from A. fumigatus, Aspergillus flavus, and nine agricultural fungal pathogens (Blumeria graminis, Monilinia fructicola, Mycosphaerella fijiensis, Penicillium digitatum, Oculimacula yallundae, Pyrenophora teres, Zymoseptoria tritici, Erysiphe necator, and Venturia inaequalis) were downloaded from NCBI GenBank. Reported amino acid substitutions associated with azole resistance were annotated. Alignments of sequences were generated using ClustalW (http://www.genome.jp/tools/clustalw/). The alignments are available as .pdf files in the supplemental material.
cyp51A site-directed mutagenesis.
In order to explore the potential roles of S297T and F495I mutations in fungicide resistance, amino acid substitutions were introduced into the cyp51A gene of an A. fumigatus strain through fusion PCR methods. The 5′ flank and 3′ flank fragments of the cyp51A gene were amplified from A. fumigatus CEA Δku80 genomic DNA using the primers listed in Table S1 in the supplemental material, and the pyrithiamine or hygromycin resistance gene was also amplified from plasmids constructed in our laboratory. The four PCR products were then fused with the nested primer pair cyp51A-S297T-up1S/cyp51A-S297T-dwA or cyp51A-F495I-up1S/cyp51A-F495I-dwA (Table S1). The fusion PCR product was transformed into the recipient strain A1160 to generate the S297T or F495I mutation. For the construction of A. fumigatus with a TR34/L98H, TR34/L98H/S297T, TR34/L98H/F495I, or TR34/L98H/S297T/F495I mutation, the genomic DNA of a previously identified TR34/L98H or TR34/L98H/S297T/F495I isolate was used as the template for the amplification of corresponding cyp51A fragments. The transformants were confirmed by sequencing of the cyp51A coding region (7) as well as by diagnostic PCR with two pairs of primers (cyp51A-T960A-yS/cyp51A-T960A-yA and cyp51A-T1554A-yS/cyp51A-T1554A-yA [Table S1]) targeting cyp51A and the adjacent regions of the constructed strains. In vitro susceptibility testing of constructed strains and the growth experiment with these strains on drug-containing MM plates were conducted using the same methods as those for the 24 clinical and environmental A. fumigatus isolates.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by grants from Beijing Natural Science Foundation (grant 7172157), the National Key R&D Program of China (grant 2016yfc1200100), and the Beijing Nova Program.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01549-17.
REFERENCES
- 1.Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, Sendino J, Hernando FL, Ponton J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27:155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Drgona L, Khachatryan A, Stephens J, Charbonneau C, Kantecki M, Haider S, Barnes R. 2014. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis 33:7–21. doi: 10.1007/s10096-013-1944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lestrade PP, Meis JF, Arends JP, van der Beek MT, de Brauwer E, van Dijk K, de Greeff SC, Haas PJ, Hodiamont CJ, Kuijper EJ, Leenstra T, Muller AE, Oude Lashof AM, Rijnders BJ, Roelofsen E, Rozemeijer W, Tersmette M, Terveer EM, Verduin CM, Wolfhagen MJ, Melchers WJ, Verweij PE. 2016. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses 59:101–107. doi: 10.1111/myc.12440. [DOI] [PubMed] [Google Scholar]
- 5.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Lu Z, Zhao J, Zou Z, Gong Y, Qu F, Bao Z, Qiu G, Song M, Zhang Q, Liu L, Hu M, Han X, Tian S, Zhao J, Chen F, Zhang C, Sun Y, Verweij PE, Huang L, Han L. 2016. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother 60:5878–5884. doi: 10.1128/AAC.01005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu Y. 2016. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater 326:54–60. doi: 10.1016/j.jhazmat.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, van der Lee HA, Klaassen CH, Melchers WJ, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 56:10–16. doi: 10.1128/AAC.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 12.Verweij PE, Zhang J, Debets AJM, Meis JF, van de Veerdonk FL, Schoustra SE, Zwaan BJ, Melchers WJG. 2016. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect Dis 16:e251–e260. doi: 10.1016/S1473-3099(16)30138-4. [DOI] [PubMed] [Google Scholar]
- 13.Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob Agents Chemother 54:2425–2430. doi: 10.1128/AAC.01599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morio F, Aubin GG, Danner-Boucher I, Haloun A, Sacchetto E, Garcia-Hermoso D, Bretagne S, Miegeville M, Le Pape P. 2012. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR/L98H, in a French cohort of patients with cystic fibrosis. J Antimicrob Chemother 67:1870–1873. doi: 10.1093/jac/dks160. [DOI] [PubMed] [Google Scholar]
- 15.Bader O, Tunnermann J, Dudakova A, Tangwattanachuleeporn M, Weig M, Gross U. 2015. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother 59:4356–4359. doi: 10.1128/AAC.00100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS One 7:e52871. doi: 10.1371/journal.pone.0052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Zeng R, Zhang L, Li D, Lv G, Shen Y, Zheng H, Zhang Q, Zhao J, Zheng N, Liu W. 2015. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob Agents Chemother 59:4321–4325. doi: 10.1128/AAC.00003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Centre for Disease Prevention and Control. 2013. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. ECDC, Stockholm, Sweden. [Google Scholar]
- 20.Hollomon D. 2017. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest Manag Sci 73:1987–1993. doi: 10.1002/ps.4607. [DOI] [PubMed] [Google Scholar]
- 21.Kano R, Sobukawa H, Murayama SY, Hirose D, Tanaka Y, Kosuge Y, Hasegawa A, Kamata H. 2016. In vitro resistance of Aspergillus fumigatus to azole farm fungicide. J Infect Chemother 22:133–136. doi: 10.1016/j.jiac.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yu J, Liu J, Yuan Y, Li N, He M, Qi T, Hui G, Xiong L, Liu D. 2014. Novel mutations in CYP51B from Penicillium digitatum involved in prochloraz resistance. J Microbiol 52:762–770. doi: 10.1007/s12275-014-4112-2. [DOI] [PubMed] [Google Scholar]
- 23.Mair WJ, Deng W, Mullins JG, West S, Wang P, Besharat N, Ellwood SR, Oliver RP, Lopez-Ruiz FJ. 2016. Demethylase inhibitor fungicide resistance in Pyrenophora teres f. sp. teres associated with target site modification and inducible overexpression of Cyp51. Front Microbiol 7:1279. doi: 10.3389/fmicb.2016.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Wang J, Feng D, Ma Z, Li H. 2011. PdCYP51B, a new putative sterol 14α-demethylase gene of Penicillium digitatum involved in resistance to imazalil and other fungicides inhibiting ergosterol synthesis. Appl Microbiol Biotechnol 91:1107–1119. doi: 10.1007/s00253-011-3355-7. [DOI] [PubMed] [Google Scholar]
- 25.Luo CX, Schnabel G. 2008. The cytochrome P450 lanosterol 14α-demethylase gene is a demethylation inhibitor fungicide resistance determinant in Monilinia fructicola field isolates from Georgia. Appl Environ Microbiol 74:359–366. doi: 10.1128/AEM.02159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelders E, Karawajczyk A, Verhoeven RJ, Venselaar H, Schaftenaar G, Verweij PE, Melchers WJ. 2011. The structure-function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: the mechanism of L98H azole resistance. Fungal Genet Biol 48:1062–1070. doi: 10.1016/j.fgb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Snelders E, Camps SM, Karawajczyk A, Rijs AJ, Zoll J, Verweij PE, Melchers WJ. 2015. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet Biol 82:129–135. doi: 10.1016/j.fgb.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 28.de Visser JA, Krug J. 2014. Empirical fitness landscapes and the predictability of evolution. Nat Rev Genet 15:480–490. doi: 10.1038/nrg3744. [DOI] [PubMed] [Google Scholar]
- 29.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ; Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 22 December 2015. EUCAST Definitive Document EDEF 9.3. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_Mould_testing_definitive.pdf.
- 30.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 32.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad S, Joseph L, Hagen F, Meis JF, Khan Z. 2015. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J Antimicrob Chemother 70:412–415. doi: 10.1093/jac/dku410. [DOI] [PubMed] [Google Scholar]
- 34.Tashiro M, Izumikawa K, Hirano K, Ide S, Mihara T, Hosogaya N, Takazono T, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, Miyazaki T, Nishino T, Tsukamoto M, Kakeya H, Yamamoto Y, Yanagihara K, Yasuoka A, Tashiro T, Kohno S. 2012. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob Agents Chemother 56:4870–4875. doi: 10.1128/AAC.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S, Khan Z, Hagen F, Meis JF. 2014. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ Res 133:20–26. doi: 10.1016/j.envres.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Badali H, Vaezi A, Haghani I, Yazdanparast SA, Hedayati MT, Mousavi B, Ansari S, Hagen F, Meis JF, Chowdhary A. 2013. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses 56:659–663. doi: 10.1111/myc.12089. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen KL, Jensen RH, Johansen HK, Skov M, Pressler T, Howard SJ, Leatherbarrow H, Mellado E, Arendrup MC. 2011. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol 49:2243–2251. doi: 10.1128/JCM.00213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhren J, Voskuil WS, Boel CH, Haas PJ, Hagen F, Meis JF, Kusters JG. 2015. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother 70:2894–2898. doi: 10.1093/jac/dkv177. [DOI] [PubMed] [Google Scholar]
- 39.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 40.Balajee SA, de Valk HA, Lasker BA, Meis JF, Klaassen CH. 2008. Utility of a microsatellite assay for identifying clonally related outbreak isolates of Aspergillus fumigatus. J Microbiol Methods 73:252–256. doi: 10.1016/j.mimet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 41.de Valk HA, Meis JF, Curfs IM, Muehlethaler K, Mouton JW, Klaassen CH. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43:4112–4120. doi: 10.1128/JCM.43.8.4112-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escribano P, Recio S, Pelaez T, Bouza E, Guinea J. 2011. Aspergillus fumigatus strains with mutations in the cyp51A gene do not always show phenotypic resistance to itraconazole, voriconazole, or posaconazole. Antimicrob Agents Chemother 55:2460–2462. doi: 10.1128/AAC.01358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CJ, Wang HC, Lee JC, Lo HJ, Dai CT, Chou PH, Ko WC, Chen YC. 2015. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses 58:544–549. doi: 10.1111/myc.12354. [DOI] [PubMed] [Google Scholar]
- 44.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 6:428. doi: 10.3389/fmicb.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nabili M, Shokohi T, Moazeni M, Khodavaisy S, Aliyali M, Badiee P, Zarrinfar H, Hagen F, Badali H. 2016. High prevalence of clinical and environmental triazole resistant Aspergillus fumigatus in Iran: is it a challenging issue? J Med Microbiol 65:468–475. doi: 10.1099/jmm.0.000255. [DOI] [PubMed] [Google Scholar]
- 46.Jensen RH, Hagen F, Astvad KM, Tyron A, Meis JF, Arendrup MC. 2016. Azole-resistant Aspergillus fumigatus in Denmark: a laboratory-based study on resistance mechanisms and genotypes. Clin Microbiol Infect 22:570.e1–570.e9. doi: 10.1016/j.cmi.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Van Waeyenberghe L, Pasmans F, Beernaert LA, Haesebrouck F, Vercammen F, Verstappen F, Dorrestein GM, Klaassen CH, Martel A. 2011. Microsatellite typing of avian clinical and environmental isolates of Aspergillus fumigatus. Avian Pathol 40:73–77. doi: 10.1080/03079457.2010.540229. [DOI] [PubMed] [Google Scholar]
- 48.Guinea J, Garcia de Viedma D, Pelaez T, Escribano P, Munoz P, Meis JF, Klaassen CH, Bouza E. 2011. Molecular epidemiology of Aspergillus fumigatus: an in-depth genotypic analysis of isolates involved in an outbreak of invasive aspergillosis. J Clin Microbiol 49:3498–3503. doi: 10.1128/JCM.01159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang H, Ashu E, Sharma C, Kathuria S, Chowdhary A, Xu J. 2016. Diversity and origins of Indian multi-triazole resistant strains of Aspergillus fumigatus. Mycoses 59:450–466. doi: 10.1111/myc.12494. [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara D, Takahashi H, Fujimoto M, Sugahara M, Misawa Y, Gonoi T, Itoyama S, Watanabe A, Kamei K. 2016. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother 22:577–579. doi: 10.1016/j.jiac.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 51.de Valk HA, Meis JF, de Pauw BE, Donnelly PJ, Klaassen CH. 2007. Comparison of two highly discriminatory molecular fingerprinting assays for analysis of multiple Aspergillus fumigatus isolates from patients with invasive aspergillosis. J Clin Microbiol 45:1415–1419. doi: 10.1128/JCM.02423-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidd SE, Ling LM, Meyer W, Orla Morrissey C, Chen SC, Slavin MA. 2009. Molecular epidemiology of invasive aspergillosis: lessons learned from an outbreak investigation in an Australian hematology unit. Infect Control Hosp Epidemiol 30:1223–1226. doi: 10.1086/648452. [DOI] [PubMed] [Google Scholar]
- 53.Olias P, Gruber AD, Hafez HM, Lierz M, Slesiona S, Brock M, Jacobsen ID. 2011. Molecular epidemiology and virulence assessment of Aspergillus fumigatus isolates from white stork chicks and their environment. Vet Microbiol 148:348–355. doi: 10.1016/j.vetmic.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 54.Hagiwara D, Takahashi H, Watanabe A, Takahashi-Nakaguchi A, Kawamoto S, Kamei K, Gonoi T. 2014. Whole-genome comparison of Aspergillus fumigatus strains serially isolated from patients with aspergillosis. J Clin Microbiol 52:4202–4209. doi: 10.1128/JCM.01105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.