ABSTRACT
The clinical pathogen Klebsiella pneumoniae is a relevant cause of nosocomial infections, and resistance to current treatment with carbapenem antibiotics is becoming a significant problem. Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) used for controlling plasma cholesterol levels. There is clinical evidence showing other effects of statins, including decrease of lung inflammation. In the current study, we show that pretreatment with atorvastatin markedly attenuated lung injury, which was correlated with a reduction in the cellular influx into the alveolar space and lungs and downmodulation of the production of proinflammatory mediators in the initial phase of infection in C57BL/6 mice with K. pneumoniae. However, atorvastatin did not alter the number of bacteria in the lungs and blood of infected mice, despite decreasing local inflammatory response. Interestingly, mice that received combined treatment with atorvastatin and imipenem displayed better survival than mice treated with vehicle, atorvastatin, or imipenem alone. These findings suggest that atorvastatin could be an adjuvant in host-directed therapies for multidrug-resistant K. pneumoniae, based on its powerful pleiotropic immunomodulatory effects. Together with antimicrobial approaches, combination therapy with anti-inflammatory compounds could improve the efficiency of therapy during acute lung infections.
KEYWORDS: Klebsiella pneumoniae, lung inflammation, atorvastatin, imipenem, combination therapy
INTRODUCTION
Community-acquired pneumonia (CAP) is among the most typical infectious diseases and is a serious health problem leading to significant morbidity and mortality, especially in immunocompromised patients and elderly people (1–4). Klebsiella pneumoniae is one of the major opportunistic pathogens responsible for severe community-acquired pneumonia and is often associated with nosocomial infections, including sepsis, meningitis, liver abscess, and urinary dysfunctions (5, 6). Treatment of nosocomial infections caused by K. pneumoniae can be complicated by the severity of disease, the presence of a limited number of drugs available, and the rising occurrence of resistant bacteria. Carbapenems are widely regarded as drugs of choice for the treatment of severe infections caused by resistant K. pneumoniae (7, 8). In this context, the discovery of new agents to control pulmonary inflammation during antibiotic treatment of Gram-negative pneumonia could lead to reduced tissue damage during infection.
Infection with K. pneumoniae triggers inflammatory cell influx to the lung parenchyma and is accompanied by the release of proinflammatory cytokines and chemokines (9–12). Host defense against acute pulmonary bacterial infection requires effective responses that involve recruitment and activation of neutrophils and monocytes to the lungs (13, 14). The protective immune response against K. pneumoniae infection is dependent on a dynamic balance between production of essential lipid mediators such as leukotrienes, reactive oxygen species (ROS) generation, and release of cytokines and chemokines, which are able to efficiently control replication of invading microorganisms (15–18).
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors that interfere with cholesterol biosynthesis, and they are some of the most-prescribed drugs in clinical practice for the treatment of hypercholesterolemia, showing beneficial effects on cardiovascular disease (19). For years, statins have been ascribed additional beneficial pleiotropic effects on inflammation and immunity, which include antioxidative (20), anti-inflammatory (21), anticarcinogenic (22), and immunomodulatory (23) properties. A number of studies suggest that statin treatment is associated with a positive outcome during inflammatory diseases of the respiratory tract (24–29). Interestingly, epidemiological data have shown that patients administered statins may have decreased mortality rates during viral and bacterial respiratory infections (21, 30, 31). However, due to the heterogeneity of these studies and the lack of well-designed prospective studies, the use of statins in critically ill patients/clinical trials remains inconclusive.
In the present study, we have investigated the effects of treatment with atorvastatin in a mouse model of pneumonia caused by the pathogen K. pneumoniae. In addition, we examined the efficacy of combination therapy with statin and antibiotic (i.e., atorvastatin plus imipenem) in mouse survival and inflammatory responses following pulmonary bacterial infection. Our findings showed that atorvastatin exhibits important immunomodulatory effects that regulate lung injury and that may provide additional benefits in the prophylaxis therapy against K. pneumoniae infection. Our study is the first to provide evidence that combined therapy with atorvastatin and imipenem improves survival of pneumonia triggered by K. pneumoniae infection. The beneficial outcome of the drug combination may be attributed to bacterial clearance due to the antibiotic and pleiotropic anti-inflammatory and immunomodulatory effects of atorvastatin.
RESULTS
Atorvastatin treatment did not alter bacterial load in mice infected with K. pneumoniae.
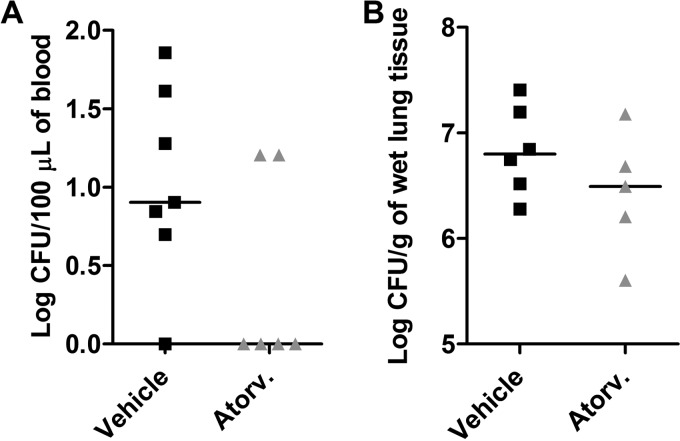
To determine whether atorvastatin administered prior to bacterial inoculation (pretreatment) could interfere with total live bacterial count, one of the hallmarks of acute lung injury due to K. pneumoniae-induced pneumonia, groups of C57BL/6 mice were treated with a single dose of the drug 24 h before inoculation with K. pneumoniae (Fig. 1). The atorvastatin treatment alone did not alter bacteremia or bacterial load recovered from the lungs of infected mice in comparison to vehicle-treated mice (Fig. 1A and B). The results were similar when mice were inoculated with a different bacterial strain (K. pneumoniae ATCC 13883) (Table 1). In addition, the numbers of bacteria recovered from the lung and blood of infected mice were the same as in vehicle- and atorvastatin-treated mice, regardless of the time point assessed (12 or 48 h) (data not shown).
FIG 1.
Atorvastatin treatment did not interfere with bacterial load after K. pneumoniae infection. C57BL/6 mice were pretreated with atorvastatin (10 mg/kg/day) or vehicle and challenged with 3 × 106 bacteria via intranasal inoculation. Bacterial burden was recovered in the blood (A) and lung tissue (B) of vehicle-treated (Vehicle) and atorvastatin-treated (Atorv.) mice after 24 h of infection with K. pneumoniae. The horizontal line represents the median log CFU per group with each symbol representing one mouse. Results representative of two independent experiments.
TABLE 1.
Bacterial counts in the lungs and total and differential cell counts in the BAL fluid of C57BL/6 mice infected with K. pneumoniae strain ATCC 13883
| Group | CFU/ga | No. of cells (106/ml)b |
||
|---|---|---|---|---|
| Total leukocytes | Neutrophils | Macrophages | ||
| Uninfected | NDd | 0.25 ± 0.13 | ND | 0.25 ± 0.13 |
| Vehicle | 509.5 | 13.5 ± 6.7* | 12.13 ± 5.82* | 1.35 ± 0.91* |
| Atorvastatinc | 481.8 | 2.25 ± 0.98# | 2.13 ± 0.78# | 0.34 ± 0.14# |
Bacterial burden was recovered in the lung tissue of vehicle-treated and atorvastatin-treated mice after 24 h of infection with K. pneumoniae. The results are expressed as median log CFU per gram of lung tissue (n = 6/group).
Total numbers of leukocytes infiltrated in cells and numbers of neutrophils and macrophages recovered in the BAL fluid were quantified after 24 h of infection. Results are expressed as mean ± SEM (n = 6/group). *, P < 0.05 compared to uninfected control mice; #, P < 0.05 compared to vehicle-treated mice.
Atorvastatin (10 mg/kg/day) was administered 24 h prior to infection with 3 × 106 bacteria of K. pneumoniae strain ATCC 13883 via intranasal inoculation.
ND, not detected.
To evaluate any potential direct effect of atorvastatin on the viability of K. pneumoniae, the MIC of the statin was evaluated by the microdilution method. It has been reported that the bacterium Staphylococcus aureus is killed in the presence of atorvastatin in vitro (32). As a positive control, we observed 100% inhibition of viable S. aureus with atorvastatin at the concentration of 250 μg/ml. In contrast, various concentrations (0.975 to 500 μg/ml) of atorvastatin had no inhibitory effect on the growth of K. pneumoniae in vitro. The concentrations of dimethyl sulfoxide (DMSO) (2.5%, vol/vol) used in all tests did not interfere with bacterial growth. The interaction of atorvastatin with imipenem was also evaluated by the checkerboard microdilution method. The imipenem MIC for the K. pneumoniae strain was 0.5 μg/ml. Atorvastatin exhibited an indifferent effect on the K. pneumoniae strain when combined with imipenem (data not shown).
Pretreatment with atorvastatin decreased cell recruitment and tissue damage after K. pneumoniae infection in mice.
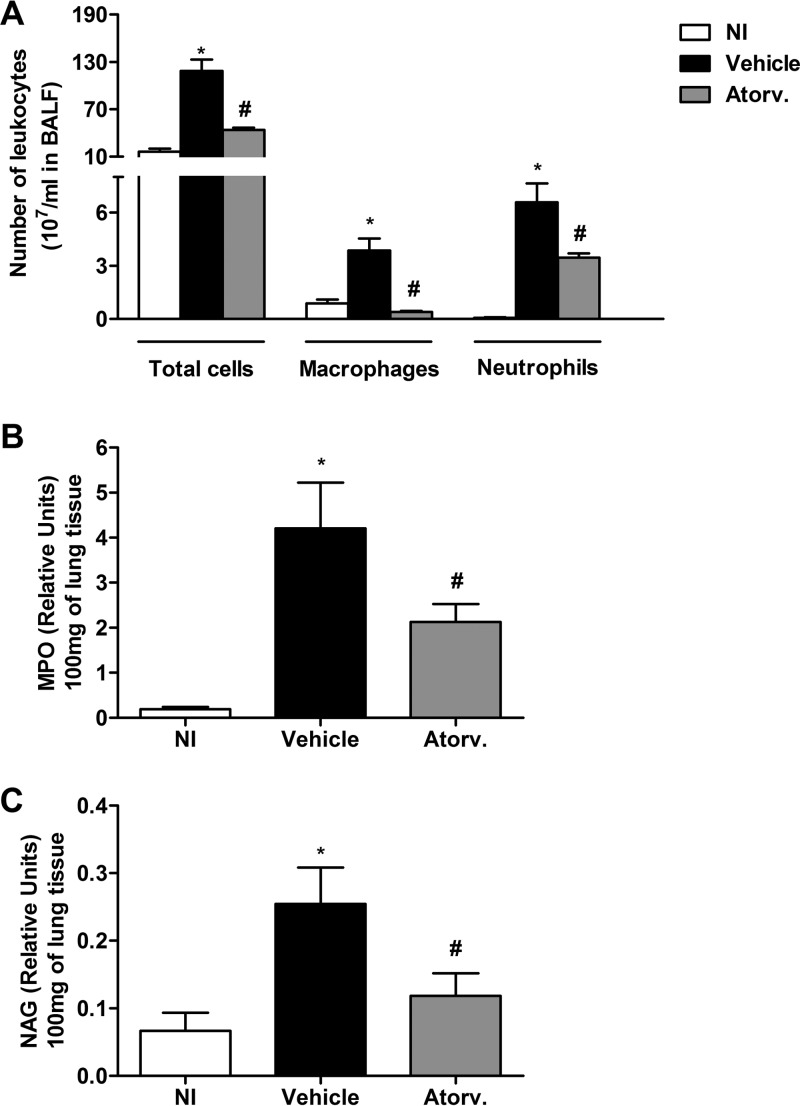
As illustrated in Fig. 2A, infection with K. pneumoniae induced an increased influx of leukocytes in the airway spaces of untreated mice 24 h after infection. The inflammatory infiltrate found in the bronchoalveolar space of untreated infected mice was composed mostly of neutrophils and macrophages. The infection with a different K. pneumoniae strain (ATCC 13883) induced a similar phenotype of inflammatory immune response (Table 1). In addition, there was significant infiltration of neutrophils and macrophages in lung parenchyma of vehicle-treated mice after infection, as assessed by myeloperoxidase (MPO) (Fig. 2B) and N-acetyl-β-d-glucosaminidase (NAG) (Fig. 2C) activity, respectively. Treatment with atorvastatin decreased the number of neutrophils and macrophages in airway spaces of mice infected with both strains of K. pneumoniae to the number of cells similarly found in control mice (Fig. 2A and Table 1). There was also significant reduction in MPO (Fig. 2B) and NAG (Fig. 2C) activity in lung parenchyma of infected mice treated with atorvastatin.
FIG 2.
Treatment of mice with atorvastatin decreased the influx of inflammatory cells in infection with K. pneumoniae. Animals were pretreated with atorvastatin (10 mg/kg/day) or vehicle and inoculated with 3 × 106 bacteria via intranasal inoculation. (A) Total numbers of leukocytes infiltrated and neutrophils and macrophages recovered in the BAL fluid (BALF) were quantified after 24 h of infection. (B and C) Myeloperoxidase (MPO) (B) and N-acetyl-β-d-glucosaminidase (NAG) (C) activity in the lungs was used as an index of neutrophil and macrophage recruitment, respectively, in vehicle-treated (Vehicle) and atorvastatin-treated (Atorv.) mice after 24 h of infection. Results representative of two independent experiments are expressed as mean ± SEM (n = 7/group). *, P < 0.05 compared to uninfected control mice; #, P < 0.05 compared to vehicle-treated mice. NI, uninfected control mice.
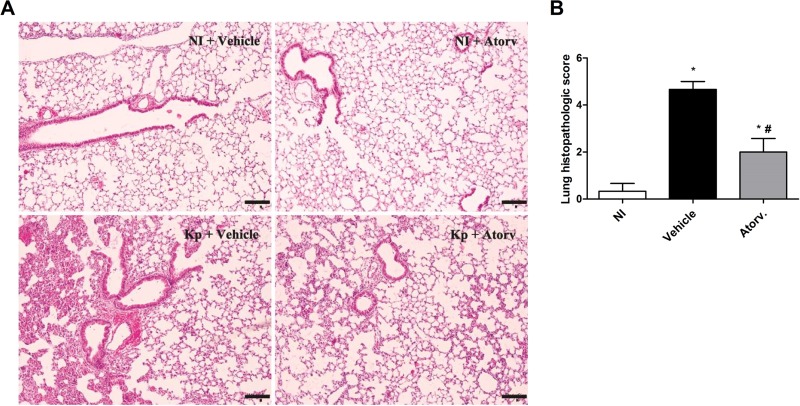
To examine inflammation and tissue injury induced by infection with K. pneumoniae, lung sections stained with hematoxylin and eosin (H&E) were analyzed and graded to estimate leukocyte infiltration into the lung tissue (Fig. 3A). Infected C57BL/6 mice showed extensive areas of inflammation with intense inflammatory infiltrates in the alveolar parenchyma and perivascular area, with thickening of alveolar walls and edema. Interestingly, pretreatment with atorvastatin reduced lung inflammation, as seen by the normal thickness of alveoli and fewer parenchymal inflammatory infiltrates than in untreated infected mice (Fig. 3A). Grading scores confirmed the histological evaluation and showed that inflammatory infiltrates were present in smaller areas of the parenchyma in treated infected mice. Indeed, whereas 25% of tissue was scored as injured in atorvastatin-treated animals, 75% of tissue was damaged in untreated infected mice (Fig. 3B). Therefore, data demonstrated that atorvastatin controlled the lung inflammatory response in the initial phase of infection.
FIG 3.
Protective effect of atorvastatin treatment on lung inflammation induced by K. pneumoniae (Kp) infection. Animals were pretreated with atorvastatin (10 mg/kg/day) or vehicle and inoculated with 3 × 106 bacteria via intranasal inoculation. (A) Representative photographs of H&E-stained sections of lung from uninfected control mice treated with vehicle (NI + Vehicle), uninfected mice treated with atorvastatin (NI + Atorv), infected mice treated with vehicle (Kp + Vehicle), and infected mice treated with atorvastatin (Kp + Atorv). Pictures were taken under ×100 magnification. Bars, 25 mm. (B) Histopathological score (maximum of 5) of mice treated or not with atorvastatin after 24 h of infection. *, P < 0.05 compared to uninfected control mice; #, P < 0.05 compared to vehicle-treated mice. NI, uninfected control mice.
Atorvastatin treatment enhanced phagocytic activity of neutrophils and macrophages but did not alter ROS production.
Considering that treatment with atorvastatin diminished recruitment of neutrophils and macrophages to the lungs but did not modify the bacterial load, the next step was to evaluate the effect of atorvastatin treatment in the effector function of leukocytes during infection with K. pneumoniae. A minimum of 200 neutrophils was observed under a microscope, and the number of phagocytic neutrophils (neutrophils containing at least 2 bacteria in the cytoplasm) was estimated in the bronchoalveolar lavage (BAL) fluid of vehicle- and atorvastatin-treated mice after 24 h of infection with K. pneumoniae. The results demonstrated that approximately 28% (56 ± 38, mean ± standard deviation) of neutrophils from untreated mice contained bacteria in the cytoplasm, whereas almost 55% (110 ± 55) of neutrophils recovered from atorvastatin-treated mice presented internalized bacteria (P < 0.05 versus vehicle-treated mice) (Table 2). Treatment with atorvastatin also changed the ability of macrophages to phagocytize bacteria in vitro. Macrophages were pretreated with vehicle (dimethyl sulfoxide [DMSO]) or atorvastatin (10 μM) and then infected with K. pneumoniae. Coculture experiments revealed that, 2 h after incubation, macrophages pretreated with atorvastatin showed a higher phagocytic index than vehicle-treated macrophages (Table 2). We also analyzed the production of reactive oxygen species (ROS) and nitric oxide (NO) in macrophages treated or not with atorvastatin after incubation with K. pneumoniae. The results showed that pretreatment of macrophages with atorvastatin did not affect the levels of reactive species produced by infected macrophages compared with vehicle-treated cells (Table 2). There was no significant detection of nitric oxide in the supernatant of macrophages in the experimental groups evaluated (data not shown).
TABLE 2.
Effect of treatment with atorvastatin on phagocytosis and production of ROS by peritoneal macrophages after infection with K. pneumoniae
| Treatmentc | No. of neutrophils in BAL containing internalized bacteria (mean ± SD)d | Peritoneal macrophages | |
|---|---|---|---|
| Phagocytic indexa | ROS production (MFI)b | ||
| Mock | ND | ND | 36.1 ± 5.3 |
| Vehicle | 56 ± 38 | 33.3 ± 14.3 | 194.6 ± 14.5* |
| Atorvastatin | 110 ± 55* | 62.5 ± 28.4# | 205.4 ± 36.8* |
Phagocytic index was calculated from the percentage of phagocytic macrophages multiplied by the number of bacteria per cell and expressed as mean ± standard deviation from 2 independent experiments, each performed in triplicate. #, P < 0.05 compared to vehicle-treated macrophages. ND, not detected.
Intracellular ROS production by macrophages after infection with bacteria was measured by using an intracellular fluorescent probe. Data are expressed as mean fluorescence intensity (MFI) ± standard deviation and represent two independent experiments performed in triplicate. *, P < 0.05 compared to uninfected macrophages (Mock).
Mice were treated with vehicle or atorvastatin (10 mg/kg, orally) starting 24 h prior to the infection of mice with K. pneumoniae. Murine peritoneal macrophages (3 × 105 cells/well) were treated for 30 min with atorvastatin (10 μM) prior to infection with K. pneumoniae in a 1:30 cell/bacterium ratio and incubated at 37°C for 2 h.
A minimum of 200 neutrophils were observed under a microscope and the number of phagocytic neutrophils (neutrophils containing at least 2 bacteria in the cytoplasm) was estimated in the bronchoalveolar lavage fluid (BAL) of vehicle- and atorvastatin-treated mice after 24 h of infection with K. pneumoniae. Data represent the number of phagocytic neutrophils and are expressed as the mean ± standard deviation from two independent experiments, each performed in triplicate. *, P < 0.05 when compared to vehicle-treated mice.
Pretreatment with atorvastatin reduced cytokine and chemokine levels in bronchoalveolar fluid and lungs of mice infected with K. pneumoniae.
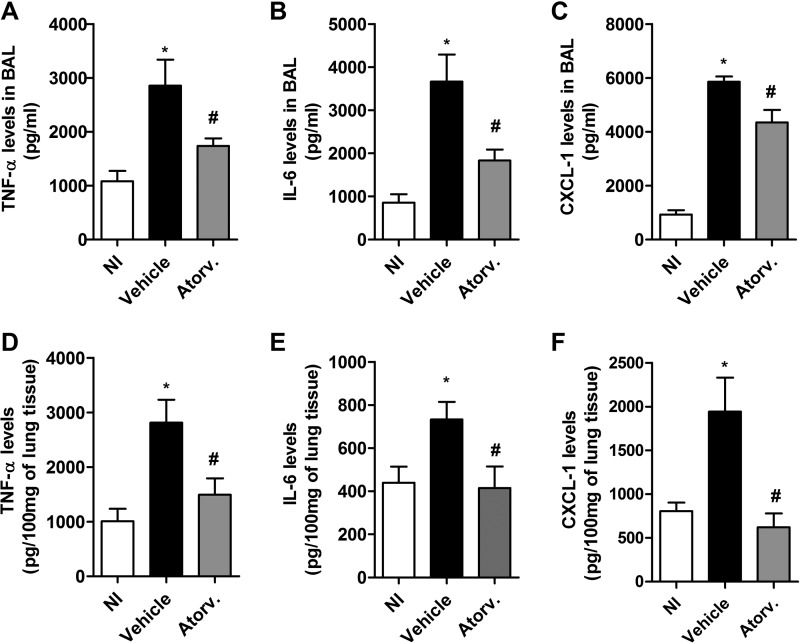
Infection with K. pneumoniae induced the production of the cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and the chemokine CXCL-1 in bronchoalveolar lavage fluid and lung homogenates of vehicle-treated mice compared to uninfected mice. Nonetheless, pretreatment with atorvastatin reduced the levels of TNF-α, IL-6, and CXCL-1 in both compartments evaluated, compared to vehicle-treated mice (Fig. 4A to F). There was no difference in the concentration of the cytokines IL-1β and IL-10 between vehicle- and atorvastatin-treated mice after 24 h of infection (data not shown). Therefore, our data demonstrate an important modulatory effect of atorvastatin on production of proinflammatory mediators during the initial phase of infection with K. pneumoniae.
FIG 4.
Downmodulation of cytokine/chemokine production by atorvastatin treatment after infection with K. pneumoniae. Animals were pretreated with atorvastatin (10 mg/kg/day) or vehicle and inoculated with 3 × 106 bacteria via intranasal inoculation. The concentrations of TNF-α, IL-6, and CXCL-1 were measured by ELISA in BAL fluid (A to C) and lung homogenates (D to F) after 24 h of infection. Results representative of two independent experiments are expressed as mean ± SEM (n = 5 to 7/group). *, P < 0.05 compared to uninfected control mice; #, P < 0.05 compared to vehicle-treated mice. NI, uninfected control mice.
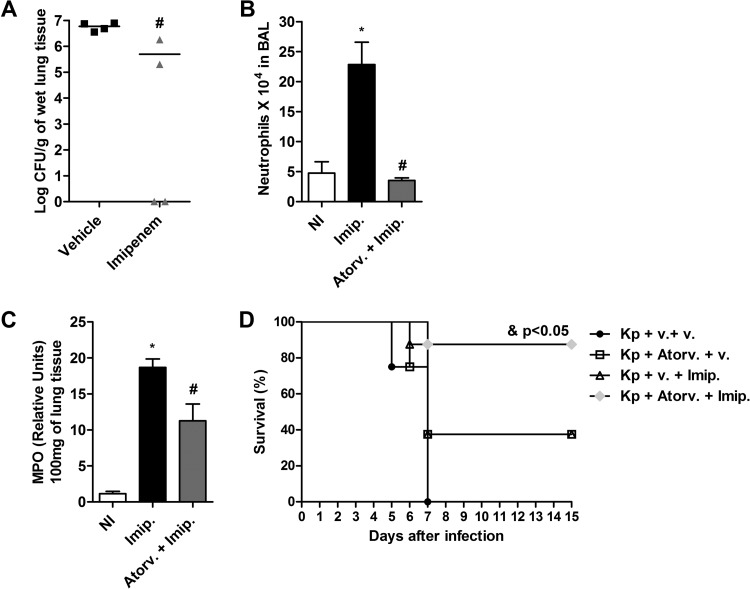
Combined therapy with atorvastatin and imipenem improved resistance to K. pneumoniae infection.
Next, we evaluated the effect of treatment with imipenem during infection of mice with K. pneumoniae. Imipenem is a carbapenem antibiotic frequently used in clinical severe nosocomial infections, and it has a wide spectrum of action. This treatment approach resulted in decreased numbers of bacteria recovered in lungs of infected mice compared to vehicle-treated mice (Fig. 5A). The effect of combination therapy with atorvastatin and imipenem on the inflammatory response was analyzed in mice after infection with K. pneumoniae. The results showed that mice infected and treated with imipenem alone presented significant recruitment of neutrophils into alveolar space and lung parenchyma compared to uninfected control mice (Fig. 5B and C). However, combination therapy promoted a significant reduction in neutrophil influx within bronchoalveolar lavage (BAL) fluid and lung tissue after infection with K. pneumoniae compared to imipenem treatment alone (Fig. 5B and C). In addition, treatment with atorvastatin or imipenem alone resulted in survival of 38% of mice after 15 days of infection, while 100% of the untreated infected mice succumbed by the 7th day of infection. Interestingly, combination therapy with atorvastatin plus imipenem resulted in a significant survival improvement in mice infected with of K. pneumoniae (88%) compared to untreated infected mice or mice treated with atorvastatin or imipenem alone (Fig. 5D). Thus, pretreatment with atorvastatin and administration of imipenem could modulate the host immune response and control bacterial growth, contributing positively to clinical outcomes during infection with K. pneumoniae.
FIG 5.
Effect of combined treatment with atorvastatin and imipenem on inflammatory response and survival during K. pneumoniae infection. Atorvastatin (10 mg/kg/day) was administered 24 h prior to infection with K. pneumoniae (3 × 106 bacteria via intranasal inoculation), and imipenem (40 mg/kg/day) was administered intravenously every 12 h from the day after infection. (A) Bacterial burden recovered from lungs of C57BL/6 mice treated with vehicle or imipenem after 24 h of infection. The horizontal line represents the median log CFU per group with each symbol representing one mouse. (B and C) Number of neutrophils in BAL fluid (B) and MPO activity in the lungs of uninfected (NI), imipenem-treated (Imip.), and atorvastatin-plus-imipenem-treated (Atorv. + Imip.) mice (C) after 24 h of infection. (D) Survival curves for mice inoculated with K. pneumoniae following treatment with vehicle (Kp + v. + v.), atorvastatin (Kp + Atorv. + v.), imipenem (Kp + v. + Imip.), and an atorvastatin-imipenem combination (Kp + Atorv. + Imip.) (n = 8/group). Results representative of two independent experiments are expressed as mean ± SEM. *, P < 0.05 compared to uninfected control mice; #, P < 0.05 compared to vehicle-treated mice. &, P < 0.05 compared to groups of mice treated with atorvastatin (Kp + Atorv. + v.) and imipenem (Kp + v. + Imip.) alone. NI, uninfected control mice.
DISCUSSION
As one of the major causes of community- and hospital-acquired respiratory infection, the pathogenic bacterium K. pneumoniae leads to intense lung injury and mortality worldwide. Pneumonia triggered by K. pneumoniae infection is marked by an exacerbated immune response, associated with excessive recruitment of neutrophils and macrophages, production of large amounts of proinflammatory cytokines, and extensive lung damage, which can often progress to bacteremia and sepsis (33, 34). The initial host defense against pulmonary bacterial infection is driven through the stimulation of a local inflammatory response that mainly comprises recruitment and activation of neutrophils. Although local inflammation is favorable after infection by inhibiting pathogen proliferation and dissemination, neutrophil recruitment needs to be restrained to reduce tissue damage (35). This hyperinflammation is commonly observed in pneumonia in which continuous neutrophilic inflammation is accompanied by collateral damage to pulmonary tissue leading to acute lung injury (12).
Emerging antimicrobial drug resistance, including carbapenem-resistant K. pneumoniae, is now recognized as a major health threat and a serious treatment challenge to clinicians, due to limited antibiotic options available (7, 36). In addition, the absence of applicable management of the harmful inflammatory response remains a significant limitation in the medical care of bacterial pneumonia. Strategies to control lung inflammation could provide new therapeutic opportunities against pneumonia induced by K. pneumoniae infection. In this context, repositioning existing drugs for new indications has emerged as an excellent approach for the establishment of new therapeutic perspectives, since discovery and production of new compounds are a laborious, lengthy, and costly process that involves many safety and clinical concerns (32).
Since epidemiological data point to reduced risk of nosocomial bacterial infections in patients previously treated with statins and atorvastatin is related to pleiotropic anti-inflammatory effects (37, 38), experiments with K. pneumoniae infection were performed in mice treated with atorvastatin. In this study, we have investigated the effects of atorvastatin on the survival, bacterial load, and immune response in a mouse model of pulmonary infection caused by Gram-negative bacterium K. pneumoniae. Previous studies developed in our laboratory showed that after intratracheal (i.t.) inoculation with K. pneumoniae, mice developed pneumonia with signs and features resembling the human disease (11, 13, 39–41). According to our data, pretreatment of mice with atorvastatin is accompanied by a better prognosis in severe pneumonia induced by K. pneumoniae infection. These data are consistent with the beneficial effect of therapy with statins in other models, including septic shock induced by lipopolysaccharide (LPS) challenge or bacterial or fungal infection and sepsis triggered by cecal ligation and puncture (CLP) (42–47). In addition, clinical trials have indicated the potential of atorvastatin in therapeutic improvement of patients with pneumonia. In population-based cohort studies, current exposure to statins was associated with a decreased risk of community-acquired pneumonia and reduced mortality of patients after hospital admission (48, 49). Therefore, the results show that prophylactic use of statins may be associated with beneficial effects in the prevention of serious infectious diseases, including pneumonia induced by K. pneumoniae.
Initial experiments evaluated the effects of pre- and posttreatment of mice with statins. The results show that posttreatment with atorvastatin had no effect on survival rates of mice infected with K. pneumoniae compared to infected mice only (data not shown). Therefore, pretreatment with atorvastatin is required for the positive effect in our model of Gram-negative pneumonia induced by K. pneumoniae in mice. This is surely a limitation of this strategy. Furthermore, our conclusion is limited to specific strains of K. pneumoniae. Here, we tested two different strains of the bacterium, ATCC 27736 and ATCC 13883. Although results were similar for the two strains, other studies with other Klebsiella strains and other microorganisms are needed in order to confirm the benefits of the prophylactic effect of statins in pneumonia.
In agreement with the limitations above, therapeutic use of statins after onset of bacterial infections remains inconclusive in the clinical trials. Most of these previous studies have been conducted on one specific type of infection and/or did not specify which types of statins were prescribed. Even less clear is the association between statin therapy timing and duration and subsequent effects on mortality, including the influence of statins as adjunctive therapy to antibiotics. In our study, pretreated mice under continued statin therapy experienced decreased rates of mortality after pulmonary infection. Thus, considering this particular condition, atorvastatin relevance in the clinic must be recognized in patients already receiving lipid-lowering therapy at the time of clinical admission with bacterial lung infection. We agree that new initiation of statins as adjunctive therapy to antibiotics still demands further analysis as a promising system to optimize positive clinical outcomes and should comprise clinical observational research and efficient trials to provide greater application of the findings.
The improved survival observed after atorvastatin administration was correlated with a reduced infiltration of inflammatory cells into the BAL fluid and lung during infection with K. pneumoniae. Our data are consistent with those of other researchers who showed that cellular influx is markedly attenuated by treatment with atorvastatin (50, 51). Kobashigawa et al. (52) suggest that statins can interfere with the ability of neutrophils to express adhesion molecules and subsequently adhere to endothelial cells (52). In addition, it was demonstrated that statins attenuate neutrophil migration through inhibition of the activity of Ras homolog gene family member A (RhoA), a small GTPase protein involved in the control of distinct cellular functions such as cytoskeleton rearrangement, migration, and ROS generation (53). Of note, the inhibition of cell recruitment to the lungs is not associated with a decrease in bone marrow-derived leukocytes or circulating cells, as no difference was observed in bone marrow and peripheral blood mononuclear cell (PBMC) counts between groups of animals treated or not with atorvastatin (data not shown). Likewise, there was no difference in the numbers of lymphocytes between the groups of mice evaluated (noninfected mice, infected mice treated with vehicle, and infected mice treated with atorvastatin) in the BAL fluid after 24 h of infection (data not shown). Thus, the protective effect of atorvastatin may be secondary to the modulation of cellular recruitment into the lungs, an important contributing step in the pathogenesis of pneumonia caused by K. pneumoniae. Importantly, decrease of neutrophil and macrophage influx into the airways was followed by lower pulmonary damage and may account for the beneficial effects of treatment with atorvastatin during K. pneumoniae infection.
Furthermore, treatment of mice with atorvastatin reduced concentrations of proinflammatory cytokines TNF-α and IL-6 and the chemokine CXCL-1 in airway fluid and lung homogenates. Our data are in accordance with previous experimental studies that have shown the effect of statins on the regulation of cytokine production (54–56). These results suggest that statins, especially atorvastatin, exhibit anti-inflammatory properties by decreasing the release of proinflammatory cytokines, which might also provide insights into the protective benefits of statins during infection with K. pneumoniae.
Despite the significant anti-inflammatory impact, treatment with atorvastatin alone had no effect on the bacterial load in mice infected with K. pneumoniae. These data could indicate that atorvastatin does not have antibacterial properties at this early stage of infection. In accordance, atorvastatin in different concentrations did not present any inhibitory activity on growth or viability of K. pneumoniae in vitro. However, some studies have demonstrated a growth-inhibitory potential for statins against pathogenic bacteria (33, 57). A recent study evaluated the antimicrobial activity of atorvastatin, pravastatin, and simvastatin against a range of clinically important pathogens. No MIC of atorvastatin and pravastatin was found at the concentrations tested, and simvastatin showed 100% inhibition only against S. aureus (58). The divergent phenotypes in antimicrobial activity of some statins seem to be related to differences in their chemical characteristics and the microbial strains evaluated (59, 60). In addition, researchers have mentioned that the concentrations of statins found to have antimicrobial properties in vitro are much higher than what can be achieved in humans. Thus, a direct bactericidal effect of atorvastatin in vivo is probably not the mechanism behind the observed beneficial effect against infection with K. pneumoniae.
The results of the current study showed an increased phagocytosis of bacteria in either neutrophils from BAL fluid of infected mice or peritoneal macrophages infected in vitro with K. pneumoniae. In this context, Djaldetti et al. (61) have reported that administration of pravastatin, simvastatin, or atorvastatin to mice caused a marked increase of both the percentage of phagocytizing peritoneal macrophages and their individual engulfing capacity. It is well known that the enhancement of the membrane cholesterol content has a marked impact on lipid bilayer fluidity. Thus, any modification in membrane bilayer lipid architecture and particularly its cholesterol content may affect phagocytic cell function either by changes in membrane rigidity or by redistribution of the cholesterol in the cell membrane (62). Therefore, it is believed that the enhanced phagocytic capacity of neutrophils and macrophages following treatment with atorvastatin in the current study was a consequence of increased membrane fluidity due to a reduction of membrane and possible cellular cholesterol content. However, pathogens express multiple factors to subvert the fast-acting inflammatory response and survive inside phagocytic cells during the early stages of infection. Of note, macrophages treated with atorvastatin showed release of ROS and NO elicited by coincubation with K. pneumoniae comparable with that of vehicle-treated macrophages, which could account for the lack of difference in bacterial load observed in mice treated or not with atorvastatin after infection with K. pneumoniae.
The protective phenotype of atorvastatin appears to be related to the immunomodulatory effect in the innate immune response of the host during infection with K. pneumoniae. Despite its anti-inflammatory effects, atorvastatin treatment did not reduce or increase the bacterial load in blood and lung tissue. In spite of antibacterial therapy, infections caused by resistant K. pneumoniae remain a major cause of morbidity and mortality in health care settings. Current treatment options for nosocomial infections caused by multiresistant K. pneumoniae include the use of the carbapenem antibiotic imipenem (8). Thus, we speculated in this study that combination therapy using pretreatment with atorvastatin and administration of imipenem could exert beneficial effects during infection by acting in the control of exacerbated inflammation and initial bacterial growth, respectively. Our results showed that combined treatment with atorvastatin and imipenem significantly inhibited mortality in mice infected with K. pneumoniae and improved survival compared to treatment with drugs alone. Consistent with our data, Choudhury et al. reported that combined treatment with atorvastatin and imipenem dampened sepsis-induced acute lung injury (63). The results observed in this study clarify that combined therapy with atorvastatin and imipenem improves the survival of the host by a mechanism associated with the anti-inflammatory effect of the statin on neutrophil accumulation and modulation of cytokine production and the reduction of bacterial load by administration of the antibiotic. In addition, our study also showed that pretreatment with a statin upregulated the phagocytosis of neutrophils and macrophages, an important effector mechanism of immune cells to protect the host during infections. We should also mention that our analysis of combined therapy was conducted in experiments using a single strain of K. pneumoniae, and studies utilizing other strains, including multiresistant ones, are required before we broaden our conclusion that the association between statins and antibiotics provides benefit to patients experiencing K. pneumoniae pneumonia. Nevertheless, these findings can contribute to the understanding of the positive role of statins as a promising potential prophylactic in bacterium-induced lung injury.
Taken together, the results obtained in this study show for the first time the beneficial possibility of using combination therapy with antimicrobial and anti-inflammatory drugs for improving patient treatment efficacy during acute lung infections caused by K. pneumoniae. In conclusion, our findings point to the potential usefulness of atorvastatin as an adjunct to host-directed therapies for multidrug-resistant K. pneumoniae, based on its powerful pleiotropic immunomodulatory activity.
MATERIALS AND METHODS
Animals.
Wild-type female C57BL/6 (8- to 12-week-old) mice were obtained from the Animal Care Facilities of the Federal University of Minas Gerais (UFMG). Mice were bred and housed at the Institute of Biological Sciences, UFMG, Belo Horizonte, Brazil. The animals were hold under pathogen-free conditions and had free access to commercial food and water. All experimental procedures were developed in accordance with ethical principles in animal research adopted by the Brazilian Society for Laboratory Animal Science (Federal Law 11,794) and were preapproved by the Ethics Committee of UFMG (protocol number 224/11).
Bacterial strain.
The bacterium K. pneumoniae ATCC 27736 has been kept at the Laboratory of Microorganism/Host Interaction, Department of Microbiology (UFMG), and its pathogenicity was stimulated by 10 passages in C57BL/6 mice. Bacteria were frozen in the logarithmic phase of growth and kept at −80°C at a concentration of 1 × 109 CFU/ml in tryptic soy broth (Difco, Detroit, MI, USA) containing 10% (vol/vol) glycerol until use. The strain K. pneumoniae ATCC 13883, kindly provided by Lirlândia Pires de Sousa, has been kept under the same conditions described above in the Department of Clinical and Toxicological Analyses, Faculty of Pharmacy, UFMG, Brazil, and was used in some experiments.
Bacterial growth conditions and pulmonary infection.
K. pneumoniae was cultured in tryptic soy broth for 18 h at 37°C prior to infection. The concentration of bacteria in broth was routinely determined by serial 1:10 dilutions. A total of 100 μl of each dilution was plated onto MacConkey agar and incubated for 24 h at 37°C, and then the colonies were counted. Each animal was anesthetized intraperitoneally with 0.2 ml of a solution containing xylazine (0.02 mg/ml), ketamine (50 mg/ml), and saline in a proportion of 1:0.5:3, respectively. Mice were challenged intranasally with 3 × 106 CFU of K. pneumoniae in a volume of 30 μl of bacterial suspension.
Blood and lung bacterial burden.
At the time of euthanasia, the right ventricle was perfused with 3 ml of sterile saline and the lungs were harvested. Tissue homogenates were placed on ice, and serial dilutions (1:10) in sterile saline were prepared. A total of 100 μl of each dilution was plated onto MacConkey agar and incubated for 24 h at 37°C for determining the number of CFU. Blood samples were plated without any dilution method. The detection limit of the assay was 100 bacteria per ml or 100 bacteria per 100 mg of tissue.
Atorvastatin and imipenem treatment in vivo.
Atorvastatin was suspended in 0.5% carboxymethyl cellulose (CMC) and administered by gavage. Each mouse in the atorvastatin group received a daily dose of 10 mg/kg of body weight/day atorvastatin (Pfizer) starting 24 h before the infection. During the survival experiments, atorvastatin (10 mg/kg, orally) was administered 24 h prior to the infection of mice with K. pneumoniae and once daily during the experimental period (15 days of infection). Imipenem (Tiepem) was administered intravenously (40 mg/kg/day) every 12 h from the day after infection (total of 5 days).
BAL.
The evaluation of leukocytes in the alveolar spaces was performed in bronchoalveolar lavage (BAL) fluid. The trachea was exposed, and a 1.7-mm-outside-diameter polyethylene catheter was inserted. BAL was performed by instilling 1-ml aliquots of phosphate-buffered saline (PBS) (three times), and approximately 2 ml of fluid was retrieved per mouse. The number of total leukocytes was determined by counting them in a modified Neubauer chamber after staining with Turk's solution. Differential counts were obtained from cytospin preparations (Shandon III) by evaluating the percentage of each leukocyte on a slide stained with May-Grünwald-Giemsa stain and examined by light microscopy. Phagocytosis was assessed by determining the number of neutrophils in the BAL fluid that contained at least 2 bacteria in the cytoplasm. In total, 200 neutrophils were counted under each condition.
Determination of MPO activity.
The extent of neutrophil accumulation in the lung tissue was measured by assaying myeloperoxidase (MPO) activity as previously described. Under the conditions described below, the methodology is very selective for the determination of neutrophils over macrophages (64). Briefly, a portion of the left lungs of animals was removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (0.1 g of tissue per 1.9 ml of buffer) was homogenized in buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M sodium EDTA; pH 4.7) and centrifuged at 3,000 × g for 10 min, and the pellet was subjected to hypotonic lysis (1.5 ml of 0.2% NaCl solution followed 30 s later by the addition of an equal volume of a solution containing 1.6% NaCl and 5% glucose). After a further centrifugation, the pellet was homogenized in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB). Overall, 1-ml aliquots of the suspension were transferred into 1.5-ml tubes followed by three freeze-thaw cycles using liquid nitrogen. The aliquots were then centrifuged for 15 min at 3,000 × g, and supernatants were diluted (1:20) prior to assay. MPO activity was assayed by measuring the change in optical density (OD) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). The results were expressed as myeloperoxidase index and were calculated by comparing the OD of tissue supernatant with the OD of mouse peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of mice by injecting 3 ml of 5% casein. A standard curve of neutrophil (95% purity) numbers versus OD was obtained by processing purified neutrophils as described above and assaying for MPO activity.
NAG assay.
The infiltration of macrophages into the lungs was quantified by measuring the levels of the lysosomal enzyme N-acetyl-β-d-glucosaminidase (NAG) present in high levels in activated macrophages (65, 66). Lung samples were homogenized in NaCl solution (0.9%, wt/vol) containing 0.1% (vol/vol) Triton X-100 (Promega, Madison, WI, USA) and centrifuged (3,000 × g, 10 min at 4°C). Samples (100 μl) of the supernatant were incubated at 37°C for 10 min with 100 μl of p-nitrophenyl-N-acetyl-beta-d-glucosaminide (Sigma, St. Louis, MO, USA) prepared in citrate-phosphate buffer (0.1 M citric acid, 0.1 M Na2HPO4; pH 4.5) to yield a final concentration of 2.24 mM. The reaction was stopped by the addition of 100 μl of 0.2 M glycine buffer (pH 10.6). Results were determined by measuring the absorption at 400 nm and expressed as NAG activity compared with 3% thioglycolate-elicited murine peritoneal macrophages processed in the same manner.
Measurement of cytokines and chemokine concentrations in BAL fluid and lungs.
The concentrations of cytokines (TNF-α and IL-6) and chemokine CXCL-1 were measured in BAL fluid and lungs, using enzyme-linked immunosorbent assay (ELISA) techniques with commercially available antibodies and according to the instructions supplied by the manufacturer (R&D Systems). Overall, 100 mg of the lung tissue was homogenized in 1 ml of phosphate-buffered saline (PBS) (0.4 mM NaCl and 10 mM NaPO4) containing antiproteases (0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1 mM benzethonium chloride, 10 mM EDTA, and 20 Ki aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3,000 × g, and the supernatant was immediately used for ELISAs at a 1:5 dilution in the assay dilution buffer. The detection limit of the ELISAs was 16 pg/ml.
Macrophage phagocytosis of K. pneumoniae in vitro.
Mice were injected intraperitoneally with 2 ml of 3% sterile thioglycolate broth, and the resulting peritoneal exudate was harvested by lavage of the peritoneal cavity of female C57BL/6 mice with serum-free, sterile RPMI 1640 medium 4 to 5 days later. Resident macrophages (2 × 105 cells) were seeded on glass coverslips in 24-well plates. After 2 h at 37°C in a humidified incubator containing a 5% CO2 atmosphere, cells were washed and cultured overnight in RPMI 1640 medium containing 10% fetal bovine serum. Before the infection with K. pneumoniae (multiplicity of infection [MOI], 30:1), macrophages were pretreated for 30 min with 10 μM atorvastatin (Pfizer) and appropriate concentrations of vehicle controls. After the incubation period, cell culture supernatants were collected and stored for further nitric oxide (NO) assay. Slides were washed three times with PBS, stained with Grünwald-Giemsa stain, and examined by light microscopy (magnification, ×1,000) to enumerate 200 cells. The phagocytic index was determined by multiplying the percentage of cells containing at least one bacterium by the number of bacteria per positive cell.
Assay of NO and ROS production.
The macrophages were isolated from the peritoneal cavity and cultured as described above. In some experiments, culture supernatants were harvested after K. pneumoniae infection. The nitric oxide levels in the culture supernatants were determined by Griess assay. Briefly, 50 μl culture supernatants was mixed with an equal volume of Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylethylenediamide hydrochloride in 2.5% acetic acid). After 30 min of incubation at room temperature, the mixture was measured spectrophotometrically at 550 nm. The nitric oxide concentration was determined from a standard curve prepared with sodium nitrite (NaNO2). To analyze ROS production, cells were then loaded for 30 min with the ROS-specific fluorescent probe H2DCFDA (2′,7′-dichlorofluorescein diacetate; 50 μM final concentration [Sigma-Aldrich, St. Louis, MO, USA]). Fluorescence was assessed after 30 min with a spectrofluorometer (Synergy 2; BioTek) with a fluorescein isothiocyanate filter (excitation, 485 nm; emission, 538 nm).
Antimicrobial susceptibility test.
The antimicrobial activity of atorvastatin was evaluated against Klebsiella pneumoniae ATCC 27736 and Staphylococcus aureus ATCC 6538. Bacterial strains were routinely cultured on tryptic soy agar (TSA) plates, under aerobic conditions, at 35°C. MIC values were determined using a broth microdilution method in 96-well flat-bottomed microtiter plates by measuring the OD of the microbial cultures. In all experiments, Mueller-Hinton broth (MHB; Difco Co.) was used for MIC tests. For all of the following tests, the bacterial inoculum was prepared in 0.9% NaCl at an optical density of 0.1 at 660 nm, which was equivalent to 1 × 108 to 2 × 108 CFU/ml. In each test, the amount of the initial bacterial load was 5 × 105 CFU/ml. The statin (atorvastatin calcium salt trihydrate) was dissolved in 100% DMSO to a stock concentration of 5 mg/ml. Series of 2-fold dilutions ranging from 500 to 0.975 μg/ml were prepared in culture medium and were mixed with equal amounts (100 μl) of microbial suspensions in the microtiter plates. The final concentration of DMSO in each well was 2.5%. The plates were incubated for 24 h at 35°C, and the lowest concentration without any visible bacterial growth was taken as the MIC. The fractional inhibitory concentration index (FICI) was calculated using formulae previously published (67): FICI = (Ac/Aa) + (Bc/Ba), where A and B are the 2 drugs being tested, Aa and Ba are the MICs obtained when each drug was tested alone, and Ac and Bc are the concentrations of each compound at the lowest effective combination. Synergy was defined as a FICI of ≤0.5, no interaction was defined as a FICI of >0.5 to 4.0, and antagonism was defined as a FICI of >4.0. In addition, microbial growth was assessed by optical density measurement at 660 nm with a microtiter plate reader. After the indicated times, 100 μl from each well was diluted in PBS and plated on agar plates. After incubation, numbers of viable CFU were counted.
Histopathology.
For histopathological analysis, mice were euthanized and lungs were fixed for at least 24 h with 10% neutral buffered formalin. After embedding in paraffin, 5-μm lung sections were obtained and stained with hematoxylin and eosin to allow assessment of inflammation in the lung tissue. Lungs were scored according to the relative degree of inflammatory infiltration as previously described (68). Inflammation was scored as follows: 0, no inflammation; 1, perivascular cuff of inflammatory cells; 2, mild inflammation, extending throughout 25% of the lung; 3, moderate inflammation covering 25 to 50% of the lung.
Statistical analysis.
Results were presented as means ± standard errors of the means (SEM). Normalized data sets were compared by using analysis of variance (ANOVA) followed by the Student-Newman-Keuls post hoc analysis. Survival curves were compared using Fisher's exact test. The results were considered significant when P was <0.05.
ACKNOWLEDGMENTS
We thank Frankcinéia Assis, Gilvânia Ferreira da Silva Santos, and Ilma Marçal (Instituto de Ciências Biologicas/Universidade Federal de Minas Gerais) for technical assistance.
This work was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG; Brazil), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq; Brazil), Instituto Nacional de Ciências e Tecnologia em Dengue (INCT Dengue; Brazil), and Pró-reitoria de Pesquisa—Universidade Federal de Minas Gerais (PRPq-UFMG; Brazil).
All authors declare that they have no conflicts of interest.
REFERENCES
- 1.Tudose C, Moisoiu A, Bogdan M. 2010. Mortality risk and etiologic spectrum of community-acquired pneumonia in hospitalized adult patients. Maedica 5:258–264. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel G, Hupricar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 3.Isturiz RE, Luna CM, Ramirez J. 2010. Clinical and economic burden of pneumonia among adults in Latin America. Int J Infect Dis 14:e852–e856. doi: 10.1016/j.ijid.2010.02.2262. [DOI] [PubMed] [Google Scholar]
- 4.Lai CC, Lee PL, Tan CK, Huang YT, Kao CL, Wang JT, Hsueh PR. 2012. Pneumonia due to pandemic (H1N1) 2009 influenza virus and Klebsiella pneumonia capsular serotype K16 in a patient with nasopharyngeal cancer. J Microbiol Immunol Infect 45:382–384. doi: 10.1016/j.jmii.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. 2007. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 6.Fung CP, Lin YT, Lin JC, Chen TL, Yeh KM, Chang FY, Chuang HC, Wu HS, Tseng CP, Siu LK. 2012. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis 18:1322–1325. doi: 10.3201/eid1808.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordman P, Cason C, Naas T. 2009. The real threat of Klebsiella pneumonia carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 8.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. 2010. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol 185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Windt GJ, Florquin S, De Vos AF, Van't Veer C, Queiroz KC, Liang J, Jiang D, Noble PW, Van der Poll T. 2010. CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation during Gram-negative pneumonia. Am J Pathol 177:2483–2494. doi: 10.2353/ajpath.2010.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagundes CT, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. 2012. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol 188:1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 12.Sordi R, Menezes-de-Lima O, Della-Justina AM, Rezende E, Assreuy J. 2013. Pneumonia-induced sepsis in mice: temporal study of inflammatory and cardiovascular parameters. Int J Exp Pathol 94:144–155. doi: 10.1111/iep.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares AC, Souza DG, Pinho V, Vieira AT, Nicoli JR, Cunha FQ, Mantovani A, Reis LF, Dias AA, Teixeira MM. 2006. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect 8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Batra S, Cai S, Balamayooran G, Jeyaseelan S. 2012. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol 188:3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. 1996. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumoniae in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 157:5221–5247. [PubMed] [Google Scholar]
- 16.Broug-Holub E, Toews GB, Van Iwaarden JF, Strieter RM, Kunkel SL, Paine R III, Standiford TJ. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Iwakurai Y, Yamaguchi K. 2001. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-γ through activation of phagocytic cells and stimulation of production of other cytokines. J Med Microbiol 50:959–964. doi: 10.1099/0022-1317-50-11-959. [DOI] [PubMed] [Google Scholar]
- 18.Hickman-Davis JM, O'Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. 2002. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 282:L944–L956. doi: 10.1152/ajplung.00216.2001. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E, HOPE-3 Investigators . 2016. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 20.Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. 2003. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 108:426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 21.Jain MK, Ridker PM. 2005. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 22.Karp I, Behlouli H, Lelorier J, Pilote L. 2008. Statins and cancer risk. Am J Med 121:302–309. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Broady R, Levings MK. 2008. Graft-versus-host disease: suppression by statins. Nat Med 14:1155–1156. doi: 10.1038/nm1108-1155. [DOI] [PubMed] [Google Scholar]
- 24.Hothersall E, McSharry C, Thomson NC. 2006. Potential therapeutic role for statins in respiratory disease. Thorax 61:729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao HW, Mao LG, Zhu JP. 2006. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol 33:793–797. doi: 10.1111/j.1440-1681.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers JD, Singanayagam A, Murray MP, Hill AT. 2008. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med 121:1002–1007. doi: 10.1016/j.amjmed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Zeki AA, Franzi L, Last J, Kenyon NJ. 2009. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med 180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim KH, Kim YK. 2010. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol 37:1055–1063. doi: 10.1111/j.1440-1681.2010.05431.x. [DOI] [PubMed] [Google Scholar]
- 29.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. 2005. A role for hydroxy-methylglutaryl coenzyme A reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med 171:606–615. doi: 10.1164/rccm.200406-729OC. [DOI] [PubMed] [Google Scholar]
- 30.Björkhem-Bergman L, Bergman P, Andersson J, Lindh JD. 2010. Statin treatment and mortality in bacterial infections—a systematic review and meta-analysis. PLoS One 5:e10702. doi: 10.1371/journal.pone.0010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T. 2009. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med 169:1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 32.Ashburn TT, Thor KB. 2004. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 33.Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, Alnasser Z. 2012. Antibacterial activity of statins: a comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrob 11:13. doi: 10.1186/1476-0711-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizgerd JP. 2006. Lung infection—a public health priority. PLoS Med 3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 36.Arnold RS, Thom KA, Sharma S, Phillips M, Johnson JK, Morgan DJ. 2011. Emergence of Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria. South Med J 104:40–45. doi: 10.1097/SMJ.0b013e3181fd7d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motzkus-Feagans CA, Pakyz A, Polk R, Gambassi G, Lapane KL. 2012. Statin use and the risk of Clostridium difficile in academic medical centres. Gut 61:1538–1542. doi: 10.1136/gutjnl-2011-301378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou SY, Chu H, Chao PW, Ou SM, Lee YJ, Kuo SC, Li SY, Shih CJ, Chen YT. 2014. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Med 40:1509–1517. doi: 10.1007/s00134-014-3418-1. [DOI] [PubMed] [Google Scholar]
- 39.Soares AC, Pinho VS, Souza DG, Shimizu T, Ishii S, Nicoli JR, Teixeira MM. 2002. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria. Br J Pharmacol 137:621–628. doi: 10.1038/sj.bjp.0704918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soares AC, Souza DG, Pinho V, Vieira A, Barsante MM, Nicoli JR, Teixeira MM. 2003. Impaired host defense to Klebsiella pneumoniae infection in mice treated with the PDE4 inhibitor rolipram. Br J Pharmacol 140:855–862. doi: 10.1038/sj.bjp.0705517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira AT, Rocha VM, Tavares L, Garcia CC, Teixeira MM, Oliveira SC, Cassali GD, Gamba C, Martins FS, Nicoli JR. 2016. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 5(1A). Microbes Infect 18:180–189. doi: 10.1016/j.micinf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Merx MW, Liehn EA, Graf J, Van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C. 2005. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. 2006. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinozaki S, Inoue Y, Yang W, Fukaya M, Carter EA, Yu YM, Fischman A, Tompkins R, Kaneki M. 2010. Farnesyltransferase inhibitor improved survival following endotoxin challenge in mice. Biochem Biophys Res Commun 391:1459–1464. doi: 10.1016/j.bbrc.2009.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, Orihuela CJ, Tuomanen EI. 2010. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest 120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tashiro M, Kimura S, Tateda K, Saga T, Ohno A, Ishii Y, Izumikawa K, Tashiro T, Kohno S, Yamaguchi K. 2012. Pravastatin inhibits farnesol production in Candida albicans and improves survival in a mouse model of systemic candidiasis. Med Mycol 50:353–360. doi: 10.3109/13693786.2011.610037. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro NQ, Costa MC, Magalhães TFF, Carneiro HCS, Oliveira LV, Fontes ACL, Santos JRA, Ferreira GF, Araujo GRS, Alves V, Frases S, Paixão TA, de Resende Stoianoff MA, Santos DA. 2017. Atorvastatin as a promising anticryptococcal agent. Int J Antimicrob Agents 49:695–702. doi: 10.1016/j.ijantimicag.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. 2008. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med 168:2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 49.Vinogradova Y, Coupland C, Hippisley-Cox J. 2011. Risk of pneumonia in patients taking statins: population-based nested case-control study. Br J Gen Pract 61:e742–e748. doi: 10.3399/bjgp11X606654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barsante MM, Roffê E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, Castro MS, Teixeira MM. 2005. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol 516:282–289. doi: 10.1016/j.ejphar.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Chello M, Patti G, Candura D, Mastrobuoni S, Di Sciasco G, Agro F, Carassiti M, Covino E. 2006. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med 34:660–667. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 52.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, Trosian K, Hamilton MA, Moriguchi JD, Kawata N, Hage A, Drinkwater DC, Stevenson LW. 1995. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 53.Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RW. 2009. Statins alter neutrophil migration by modulating cellular Rho activity—a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol 85:186–193. doi: 10.1189/jlb.0608382. [DOI] [PubMed] [Google Scholar]
- 54.Rosenson RS, Tangney CC, Casey LC. 1999. Inhibition of proinflammatory cytokine production by pravastatin. Lancet 353:983–984. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 55.Grip O, Janciauskiene S, Lindgren S. 2002. Atorvastatin activates PPAR-gamma and attenuates the inflammatory response in human monocytes. Inflamm Res 51:58–62. doi: 10.1007/BF02684000. [DOI] [PubMed] [Google Scholar]
- 56.Wang HR, Li JJ, Huang CX, Jiang H. 2005. Fluvastatin inhibits the expression of tumor necrosis factor-alpha and activation of nuclear factor-kappaB in human endothelial cells stimulated by C-reactive protein. Clin Chim Acta 353:53–60. doi: 10.1016/j.cccn.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, Amichay D, Almog Y. 2009. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 35:1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 58.Graziano TS, Cuzzullin MC, Franco GC, Schwartz-Filho HO, de Andrade ED, Groppo FC, Cogo-Müller K. 2015. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One 10:e0128098. doi: 10.1371/journal.pone.0128098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergman P, Linde C, Putsep K, Pohanka A, Normark S, Henriques-Normark B, Andersson J, Björkhem-Bergman L. 2011. Studies on the antibacterial effects of statins—in vitro and in vivo. PLoS One 6:e24394. doi: 10.1371/journal.pone.0024394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nyilasi I, Kocsubé S, Krizsán K, Galgóczy L, Pesti M, Papp T, Vágvölgyi C. 2010. In vitro synergistic interactions of the effects of various statins and azoles against some clinically important fungi. FEMS Microbiol Lett 307:175–184. doi: 10.1111/j.1574-6968.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 61.Djaldetti M, Salman H, Bergman M, Bessler H. 2006. Effect of pravastatin, simvastatin and atorvastatin on the phagocytic activity of mouse peritoneal macrophages. Exp Mol Pathol 80:160–164. doi: 10.1016/j.yexmp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Morita I, Sato I, Ma L, Murota S. 1997. Enhancement of membrane fluidity in cholesterol poor endothelial cells pretreated with simvastatin. Endothelium 5:107–113. doi: 10.3109/10623329709079868. [DOI] [PubMed] [Google Scholar]
- 63.Choudhury S, Kannan K, Pule Addison M, Darzi SA, Singh V, Singh TU, Thangamalai R, Dash JR, Parida S, Debroy B, Paul A, Mishra SK. 2015. Combined treatment with atorvastatin and imipenem improves survival and vascular functions in mouse model of sepsis. Vascul Pharmacol 71:139–150. doi: 10.1016/j.vph.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 64.De Matos IM, Souza DG, Seabra DG, Freire-Maia L, Teixeira MM. 2009. Effects of tachykinin NK1 or PAF receptor blockade on the lung injury induced by scorpion venom in rats. Eur J Pharmacol 376:293–300. doi: 10.1016/S0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- 65.Bailey PJ. 1988. Sponge implants as models. Methods Enzymol 162:327–334. [DOI] [PubMed] [Google Scholar]
- 66.Ferreira MAND, Barcelos LS, Campos PP, Vasconcelos AC, Teixeira MM, Andrade SP. 2004. Sponge-induced angiogenesis and inflammation in PAF receptor-deficient mice (PAFR-KO). Br J Pharmacol 141:1185–1192. doi: 10.1038/sj.bjp.0705731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 68.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. 2009. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]