ABSTRACT
The rapid spread of strains of malaria parasites that are resistant to several drugs has threatened global malaria control. Hence, the aim of this study was to predict the antimalarial activity of chemical compounds that possess anti-hemozoin-formation activity as a new means of antimalarial drug discovery. After the initial in vitro anti-hemozoin-formation high-throughput screening (HTS) of 9,600 compounds, a total of 224 hit compounds were identified as hemozoin inhibitors. These 224 compounds were tested for in vitro erythrocytic antimalarial activity at 10 μM by using chloroquine-mefloquine-sensitive Plasmodium falciparum strain 3D7A. Two independent experiments were conducted. The physicochemical properties of the active compounds were extracted from the ChemSpider and SciFinder databases. We analyzed the extracted data by using Bayesian model averaging (BMA). Our findings revealed that lower numbers of S atoms; lower distribution coefficient (log D) values at pH 3, 4, and 5; and higher predicted distribution coefficient (ACD log D) values at pH 7.4 had significant associations with antimalarial activity among compounds that possess anti-hemozoin-formation activity. The BMA model revealed an accuracy of 91.23%. We report new prediction models containing physicochemical properties that shed light on effective chemical groups for synthetic antimalarial compounds and help with in silico screening for novel antimalarial drugs.
KEYWORDS: malaria, antimalarial, hemozoin, prediction, drug
INTRODUCTION
Despite recent advances in antimalarial drug discovery and development, malaria remains one of the most serious medical burdens worldwide, resulting in 212 million new cases and 429,000 deaths in 2015 (1). According to the different stages of the parasite life cycle, several therapies can affect these stages and exert their therapeutic roles. Quinine, amodiaquine, chloroquine, and mefloquine, which contain a quinoline scaffold, are fast-acting and highly effective blood schizontocidal therapies against malaria parasites, including Plasmodium falciparum (2).
Indeed, the resistance of malaria to chloroquine and other 4-aminoquinoline-based therapies, in addition to the antifolate combination sulfadoxine-pyrimethamine, has turned the spotlight on artemisinin-based combinations to achieve higher response rates (3, 4). However, rapidly spreading resistance of P. falciparum to artemisinin-based combinations has been reported, posing a global challenge for malaria control (5, 6). Thus, it is important to discover new antimalarial drugs, especially for countries where malaria is endemic.
Recently, several new classes of antimalarials have entered clinical studies with patients with malaria, such as the fast-acting agents KAF156 (7), cipargamin (8), and artefenomel (9), whereas ferroquine remains the only long-acting novel antimalarial in clinical development (10, 11). However, these drugs have not yet been approved, and no vaccine to help in the prevention, control, elimination, and eradication of malaria has been approved yet. Only one vaccine candidate, RTS,S/AS01, reached phase III clinical trials, with relatively low efficacy (13, 14). Therefore, there is an urgent need for the discovery and development of novel antimalarial chemotherapies for which there are no preexisting resistance mechanisms.
At present, one of the most promising and ideal targets is interference with the parasite's heme detoxification pathway, which is the target for some current antimalarial drugs, such as quinine, which is still efficacious against chloroquine-resistant P. falciparum (15–19). Recently, inhibition of the heme detoxification pathway of the parasite has been highlighted as a target in several antimalarial screening projects (20–22). This target is based on the inhibition of hemozoin, which is a crystalline pigment produced by the malaria parasite as a result of the hemoglobin degradation process to protect it against the toxic heme produced as an end product of hemoglobin catabolism (23, 24).
Hemozoin formation is a protective physiochemical process that needs parasite protein (25–27) and/or food vacuole lipids or membranes (28, 29) for synthesis. Therefore, lipophilic detergents that mimic intraparasite conditions, like Nonidet P-40 and Tween 20, can be used as surrogate substances for high-throughput screening (HTS) of novel antimalarials because they have the ability to promote the crystallization of heme (20, 30). This makes hemozoin inhibition suitable for research using HTS assays to build prediction models for novel antimalarial drugs.
Recently, several studies used HTS and predicted models for β-hematin, synthetic hemozoin, inhibitors. Sandlin et al. screened 144,330 and produced 530 hits, 171 of which were active against parasites: 73 hits had parasite 50% inhibitory concentrations (IC50s) of <5 μM, and 25 hits had IC50s of <1 μM (31). In addition, using physiochemical properties (22), we recently developed an in silico model to predict drug-like compounds that possess antihemozoin activity.
As previously suggested, prediction models possess advantages for antimalarial design because other approaches, such as analog development based on existing agents or natural products, mainly detect new antimalarials by chemical modifications of previously known compounds (32); however, new antimalarial compounds can be discovered by the prediction equation based on a well-known metabolic target. Thus, prediction models aid in the discovery of new chemical scaffolds. Moreover, specialized labware and expensive equipment are not required for these models, so millions of library compounds can be screened in silico by using the prediction models. Also, the relationship between the compound's properties and antihemozoin activity is interpreted from the prediction models. Therefore, we continued previous work by developing new prediction models for novel antimalarial activities of hemozoin inhibitors using the physiochemical properties of these small chemical compounds.
RESULTS
In vitro antimalarial assay.
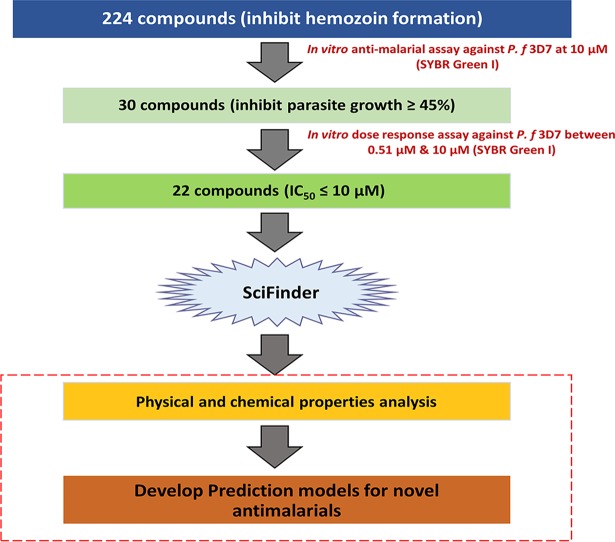
A total of 224 compounds with hemozoin inhibitory activity (22) were selected for in vitro antimalarial assays. Among them, 30 compounds with ≥45% growth-inhibitory activity at a concentration of 10 μM were further subjected to a dose-response assay to remove false-positive compounds from the initial screening (Fig. 1), resulting in only 22 compounds with a clear sigmoid dose-response curve to determine the IC50 (Fig. 2 and Table 1).
FIG 1.
Workflow of this study. First, 224 compounds that showed antihemozoin activity in our previous study (22) underwent in vitro antimalarial assays at 10 μM. Second, 30 compounds with ≥45% parasite-inhibitory efficacy underwent an in vitro dose-response assay at concentrations of between 0.5 nM and 10 μM. Next, 22 compounds with IC50s of ≤10 μM were identified. Subsequently, the main structures and physicochemical properties of these compounds were searched by using SciFinder. Finally, the prediction models were generated by the traditional approach versus the Bayesian approach, using their physical and chemical properties.
FIG 2.
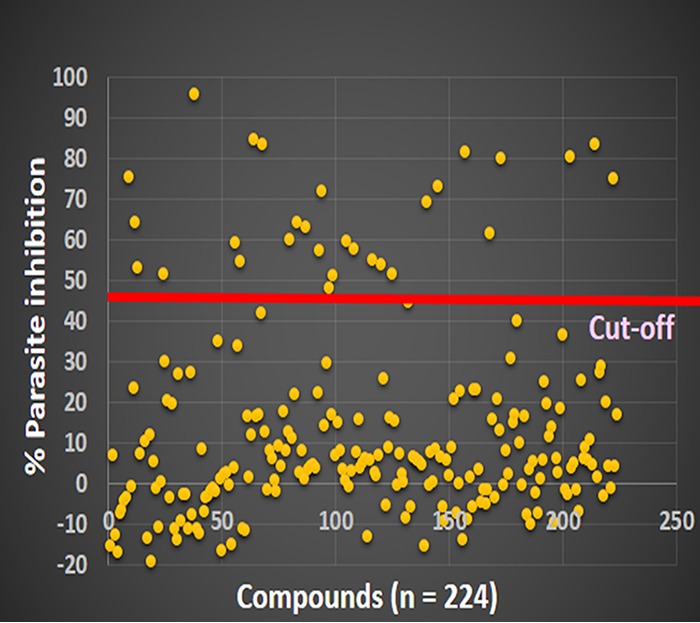
Initial in vitro antimalarial screening of 224 compounds using the chloroquine-mefloquine-sensitive P. falciparum 3D7A strain. The circle dots represent the percentages of parasite inhibition of 224 compounds, including 30 compounds with parasite inhibition values of ≥45%.
TABLE 1.
Antimalarial and antihemozoin activities of 22 positive compounds
| Compound | Compound identification | Antimalarial activity (3D7A) IC50 (μM) ± SD | Antihemozoin activity IC50 (μM) |
|---|---|---|---|
| 1 | UTDIF3A11 | 0.66 ± 0.10 | 78.1 |
| 2 | UTDIF4A14 | 8.15 ± 2.60 | 34.67 |
| 3 | UTDIF5A15 | 9.00 ± 1.40 | 14.01 |
| 4 | UTDIF6C12 | 8 | 4.58 |
| 5 | UTDIF7D16 | 10.50 ± 2.10 | 87.76 |
| 6 | UTDIF8E4 | 10 | 38.54 |
| 7 | UTDIF9E10 | 1.54 ± 0.07 | 53.44 |
| 8 | UTDIF10E14 | 10.00 ± 1.40 | 41.18 |
| 9 | UTDIF11F12 | 9 | 36.16 |
| 10 | UTDIF12F15 | 8.95 ± 1.30 | 103.5 |
| 11 | UTDIF13G5 | 9.26 ± 1.80 | 30.69 |
| 12 | UTDIF14I6 | 7.00 ± 1.40 | 43.98 |
| 13 | UTDIF15I15 | 1.01 ± 0.50 | 25.96 |
| 14 | UTDIF16J16 | 9.28 ± 2.40 | 28.5 |
| 15 | UTDIF17K7 | 4.80 ± 1.70 | 198.1 |
| 16 | UTDIF18L5 | 3.06 ± 1.30 | 110 |
| 17 | UTDIF19L16 | 6.80 ± 4.40 | 29.04 |
| 18 | UTDIF20M7 | 8.00 ± 2.80 | 156 |
| 19 | UTDIF21O9 | 0.56 ± 0.27 | 18.3 |
| 20 | UTDIF22P6 | 0.06 | 42.98 |
| 21 | UTDIF23P14 | 9.50 ± 0.70 | 24.72 |
| 22 | UTDIF24G15 | 9 | 160 |
The interference of 22 compounds with SYBR Green l in the presence of malaria parasites was measured by culturing Plasmodium falciparum 3D7A for 48 h and then adding 22 compounds followed by addition of SYBR Green l with 1 h incubation in the dark. Subsequently, the fluorescence was measured by using a multiplate reader (ARVO1430; PerkinElmer, MA) in the fluorescence detection mode (excitation [Ex] at 485 nm and emission [Em] at 515 nm) for a 0.1-s exposure. The results showed that all fluorescence variations were <10%, indicating that there was almost no interference of the compounds, artemisinin, and chloroquine with SYBR green I fluorescence (see Fig. S1 in the supplemental material). Furthermore, our results demonstrated that all 22 compounds possessed antihemozoin activities, with IC50s ranging from 4.58 to 198.1 μM (Table 1).
Development of prediction models for antimalarial activities.
Numbers of C and S atoms and H acceptors; the H donor-acceptor sum; log D at pH 1, 2, 3, 4, and 5; mass solubility at pH 1 and 3; and polarizability had P values of <0.1 in the univariable logistic regression (LR), and partition coefficient (log P); organic carbon-water partition coefficient (KOC) at pH 5.5 and 7.4; log D at pH 5.5 and 7.4; density; refractive index; rule-of-five violations; and numbers of freely rotatable bonds, H bond donors, and H and O atoms were significant according to the previous study (22), while the number of N atoms and surface tension were significant according to the present analysis and the previous study, respectively (22) (Table 2).
TABLE 2.
Univariable logistic regression statistics of the physicochemical properties of the compoundsa
| Predictor | Univariable analysis |
|
|---|---|---|
| OR (95% CI) | P | |
| No. of C atoms | 1.11 (0.98–1.25) | 0.092 |
| No. of H atomsb | 1.07 (0.98–1.17) | 0.111 |
| No. of N atoms | 1.5 (1.15–1.95) | 0.003 |
| No. of O atomsb | 1.02 (0.77–1.35) | 0.912 |
| No. of S atoms | 0.45 (0.19–1.06) | 0.067 |
| No. of F atoms | 0 (0–Inf) | 0.992 |
| No. of Cl atoms | 1.45 (0.67–3.16) | 0.348 |
| No. of Br atoms | 0 (0–infinity) | 0.991 |
| Avg mass of atoms | 1.01 (0.997–1.01) | 0.214 |
| No. of monoisotopic atoms | 1.01 (0.998–1.01) | 0.192 |
| Densityb | 0.89 (0.07–11.81) | 0.929 |
| No. of rotatable bondsb | 1.03 (0.85–1.26) | 0.753 |
| No. of H acceptors | 1.38 (1.08–1.77) | 0.01 |
| No. of H donorsb | 1.11 (0.76–1.61) | 0.605 |
| H donor-acceptor sum | 1.25 (1.03–1.52) | 0.022 |
| KOC at pH 1 | 1 (0.9999–1.0001) | 0.647 |
| KOC at pH 2 | 1 (0.9999–1.0001) | 0.568 |
| KOC at pH 3 | 1 (0.9998–1.0001) | 0.499 |
| KOC at pH 4 | 1 (0.9998–1.0001) | 0.457 |
| KOC at pH 5 | 1 (0.9998–1.0001) | 0.469 |
| KOC at pH 6 | 1 (0.9999–1.0001) | 0.562 |
| KOC at pH 7 | 1 (0.9999–1.0001) | 0.538 |
| KOC at pH 8 | 1 (0.9999–1.0001) | 0.65 |
| KOC pH 9 | 1 (0.9999–1.0001) | 0.603 |
| KOC at pH 10 | 1 (0.9998–1.0001) | 0.526 |
| Log D at pH 1b | 0.73 (0.58–0.92) | 0.007 |
| Log D at pH 2 | 0.74 (0.59–0.93) | 0.009 |
| Log D at pH 3 | 0.73 (0.58–0.92) | 0.008 |
| Log D at pH 4 | 0.72 (0.56–0.92) | 0.008 |
| Log D at pH 5 | 0.76 (0.59–0.97) | 0.027 |
| Log D at pH 6 | 0.85 (0.66–1.09) | 0.199 |
| Log D at pH 7 | 0.94 (0.74–1.2) | 0.643 |
| Log D at pH 8 | 0.9995 (0.79–1.27) | 0.997 |
| Log D at pH 9 | 1.04 (0.82–1.32) | 0.756 |
| Log D at pH 10 | 1.09 (0.86–1.38) | 0.481 |
| Log Pb | 0.85 (0.62–1.17) | 0.319 |
| Intrinsic mass | 0.97 (0.79–1.19) | 0.762 |
| Mass solubility at pH 1 | 1.002 (0.9999–1.003) | 0.067 |
| Mass solubility at pH 2 | 1.001 (0.9997–1.003) | 0.106 |
| Mass solubility at pH 3 | 1.002 (0.9998–1.003) | 0.078 |
| Mass solubility at pH 4 | 1.001 (0.9991–1.004) | 0.24 |
| Mass solubility at pH 5 | 1.002 (0.9993–1.005) | 0.136 |
| Mass solubility at pH 6 | 1.001 (0.998–1.004) | 0.535 |
| Mass solubility at pH 7 | 0.997 (0.99–1.01) | 0.616 |
| Mass solubility at pH 8 | 0.97 (0.89–1.06) | 0.525 |
| Mass solubility at pH 9 | 0.84 (0.57–1.24) | 0.376 |
| Mass solubility at pH 10 | 0.97 (0.91–1.03) | 0.312 |
| Mass solubility | 0.47 (0.076–2.86) | 0.409 |
| Molar vol | 1.01 (0.997–1.01) | 0.218 |
| Mol wt | 1 (0.997–1.01) | 0.283 |
| pKa1 | 1.05 (0.93–1.17) | 0.444 |
| Polar surface | 1 (0.99–1.02) | 0.733 |
| Total score | 1.22 (0.82–1.82) | 0.334 |
| Polarizability | 1.07 (0.996–1.14) | 0.066 |
| Surface tension | 1.02 (0.99–1.05) | 0.256 |
| Refractive indexc | 1.36 (0.72–2.57) | 0.348 |
| Rule-of-five violations | 2 (0.67–5.9) | 0.212 |
| ACD log D at pH 5.5b | 0.87 (0.68–1.11) | 0.256 |
| ACD log D at pH 7.4b | 1.07 (0.83–1.36) | 0.617 |
| ACD BCF at pH 5.5 | 1 (1–1) | 0.771 |
| ACD BCF at pH 7.4 | 1 (0.9998–1.0001) | 0.737 |
| ACD KOC at pH 5.5b | 1 (1–1) | 0.77 |
| ACD KOC at pH 7.4b | 1 (0.9999–1.0001) | 0.766 |
Abbreviations: OR, odds ratio; CI, confidence interval; BCF; bioconcentration factor. Significant predictors are underlined.
Variables with prior significance in our previous study (22).
The refractive index was multiplied by 10 to obtain a lower OR.
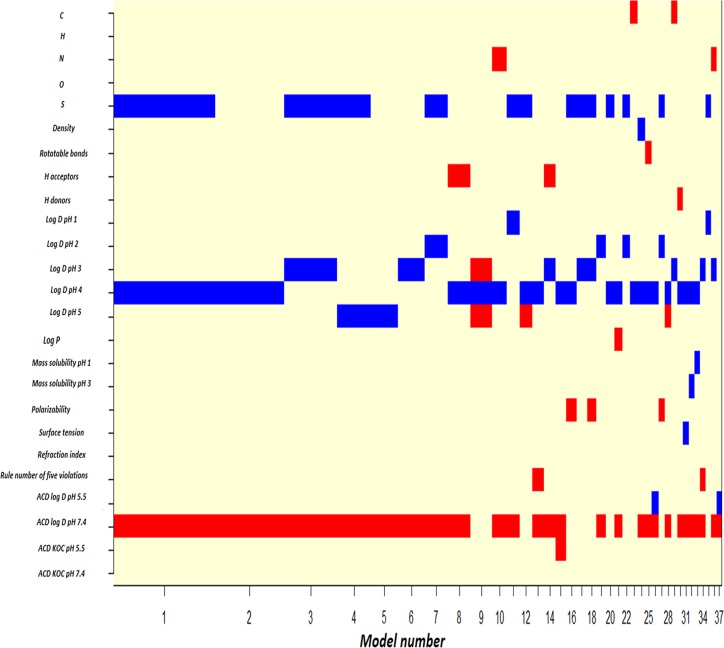
The Bayesian model averaging (BMA) analysis selected 37 models, from which the best 5 models had 5 variables, as the best predictors of the chemical compound activity, including the number of S atoms; log D at pH 3, 4, and 5; and ACD log D at pH 7.4 (Fig. 3 and Tables 2 and 3). This reflected that the antimalarial activity was significantly associated with a lower number of S atoms; lower log D at pH 3, 4, and 5; and higher ACD log D at pH 7.4.
FIG 3.
Models selected by Bayesian model averaging. Blue and red represent positive and negative variable estimates, respectively, while uncolored variables were not included in the model. On the x axis, models were listed in the order of the declines in the posterior probability.
TABLE 3.
The five best parsimonious models selected by BMAa
| Model | Predictor | Coefficient of predictor | BIC of model | PP of model |
|---|---|---|---|---|
| 1 | Intercept | −2.21 | −648.24 | 0.17 |
| No. of S atoms | −1.26 | |||
| Log D at pH 4 | −0.86 | |||
| ACD log D at pH 7.4 | 0.78 | |||
| 2 | Intercept | −2.76 | −647.46 | 0.11 |
| Log D at pH 4 | −0.84 | |||
| ACD log D at pH 7.4 | 0.81 | |||
| 3 | Intercept | −2.22 | −646.94 | 0.09 |
| No. of S atoms | −1.3 | |||
| Log D at pH 3 | −0.78 | |||
| ACD log D at pH 7.4 | 0.68 | |||
| 4 | Intercept | −2.05 | −646.02 | 0.06 |
| No. of S atoms | −1.22 | |||
| Log D at pH 5 | −0.92 | |||
| ACD log D at pH 7.4 | 0.85 | |||
| 5 | Intercept | −2.57 | −645.61 | 0.05 |
| Log D at pH 5 | −0.92 | |||
| ACD log D at pH 7.4 | 0.88 |
Abbreviations: BIC, Bayesian information criterion; PP, posterior probability. The cumulative posterior probability was 0.468.
Validation of the prediction model.
The BMA model, with a cutoff of 0.09, revealed a sensitivity, specificity, and maximum accuracy of 60%, 69.23%, and 91.23%, respectively.
DISCUSSION
Our BMA prediction model identified several physicochemical properties from which we can design and develop novel drugs. We found that a lower number of S atoms; lower log D values at pH 3, 4, and 5; and a higher ACD log D value at pH 7.4 could serve as good predictors for the development of novel antimalarial drugs.
Kumar et al. found that the replacement of the ring oxygen by sulfur to produce 4,5-dihydroxythioxanthone results in decreased inhibitory activity on hemozoin formation and parasite growth compared to the corresponding xanthone homolog (33).
Low log D values and low lipophilicity at pH 3, 4, and 5 through high inhibitory activities against heme crystallization (hemozoin formation) were significantly associated with better antimalarial activity. It is well known that antimalarial drugs must enter the parasite food vacuole, where the pH is around 4 to 5.5. It is noteworthy that log P is an indicator of lipophilicity and is a component of Lipinski's rule of five, a rule of thumb to predict drug-like properties. However, log D is a better indicator for specific pH environments. Because hemozoin formation occurs inside the acidic food vacuole, such compounds are expected to decrease its lipophilicity after uptake into the parasite food vacuole and to be accumulated within it.
Log P is widely used; however, it fails to take into account the variation in the lipophilicity of a drug regarding the ionic states present at key biological pH values. Given that the majority of commercial pharmaceuticals contain an ionizable moiety, Bhal et al. reported that log D is a better descriptor for lipophilicity in the context of the rule of five. It gives more physiologically relevant results, subsequently decreasing the number of potential false-negative compounds incorrectly eliminated during screening. Those authors also showed that the adapted rule of five using log D instead of log P gives a notable improvement in the pass rate for compounds that have the desired lipophilicity at a relevant physiological pH (34).
In 11 p-aminopyridine antimalarials, a quadratic association between the log lipid accumulation ratio (LAR), antilog log D at pH 7.4, and the log resistance index was confirmed, while the log(LAR/vacuolar accumulation ratio [VAR]) versus log resistance index for 12 antimalarials was linear. Both predictors might be able to predict the utility of structural adjustments (35).
This confirms for chloroquine-sensitive parasites that while the charged drug concentration in the food vacuole increases as the vacuolar pH decreases, the concentration of uncharged drug (base) in the vacuolar membrane and other lipid sites in the digestive vacuole has a constant value for each individual drug, because the proportion of drug partitioned into lipid decreases as log D (the log of [drug]lipid/[drug]water at equilibrium) decreases with reduced pH.
Log D can predict gastrointestinal absorption and lipophilic properties because it is a pH-dependent function. Calculated values demonstrated high gastrointestinal tract absorption (pH 3 to 7) and lipophilic properties. A good correlation between the calculated distribution coefficients at pH 7.4 and pH 5.2 (log D at pH 5.2 and 7.4) and the measured inhibition percentages for the tested compounds was observed (36).
In addition, the finding that antimalarial activity increases with increasing ACD log D values at pH 7.4 is most probably because this favors the entry of the compound into red blood cells (RBCs), the parasite, and, ultimately, the digestive vacuole. On the other hand, a low ACD log D value at a low pH (pH 3 to 5) disfavors the egress of the compound out of the digestive vacuole. The consequence would be greater accumulation in the digestive vacuole and, hence, higher concentrations at the site of action. Indeed, antilog[ACD log D (pH 7.4) − ACD log D (pH 5)] is an experimental measure of vacuolar pH trapping. It would make sense that this would apply only in the case of hemozoin inhibitors because they are the compounds that act in the digestive vacuole. Since researchers prefer to use [ACD log D (pH 7.4) − ACD log D (pH 5)] for each compound, this might weaken the correlation and undermine this argument. Hence, we built a new model with that variable, [ACD log D (pH 7.4) − ACD log D (pH 5)], to investigate its effect; however, we found that it was not significant.
It is noteworthy that we have overcome our previous limitation, in which we could not find any heavy atoms playing an important role in the refractive index used to investigate the characteristics of the material (22). The presence and even the quantity of some heavy atoms and functional groups with a high refractive index, such as sulfur (37), have an important role in increasing molar refraction. It is noteworthy that out of the 224 included compounds, there were 87 compounds that had at least 1 S atom, with a mean of 1 atom, and 131 compounds that had no S atoms. Moreover, among negative and positive compounds, there were 83 compounds and 4 compounds that had at least one atom, respectively. Additionally, among our positive antimalarial compounds, we found four compounds with quinolines that had no S atoms; hence, this suggests a negative association between S atoms and antimalarial activity. However, our results did not show an association between the refractive index and high antimalarial activities, suggesting that the refractive index is related only to antihemozoin but not antimalarial activity. Although our models have not been validated with external samples, it can be the first clue for understanding the mechanism of action of antimalarials (22). Moreover, future studies need to validate our results with a larger number of compounds, especially the positive ones.
Conclusion.
Using these physicochemical properties of compounds, we report new prediction models that can predict antimalarial activity for chemical compounds that possess antihemozoin activities. Our findings revealed that lower numbers of S atoms; lower log D values at pH 3, 4, and 5; and higher ACD log D values at pH 7.4 were independent predictors of higher antimalarial activity. This information can be used for in silico screening and modifying chemical structures to develop antimalarials.
MATERIALS AND METHODS
Ethics statement.
Experiments that required human materials, RBCs and serum, were approved by the institutional ethical review board of the Institute of Tropical Medicine (NEKKEN), Nagasaki University.
Materials.
Chloroquine (Sigma-Aldrich Chemical Company, UK) was used to monitor the assay system, stored in a 4°C freezer, and mixed well before use. Dimethyl sulfoxide (DMSO; Wako Pure Chemicals Ltd., Osaka, Japan) was used as a negative control. SYBR green I (10,000× stock concentration) was obtained from Lonza (Rockland, ME, USA) and stored at −30°C. alamarBlue was purchased from Funakoshi (Tokyo, Japan). Lysis buffer containing Tris (20 mM; pH 7.5), EDTA (10 mM), saponin (0.01%, wt/vol), and Triton X-100 (0.1%, vol/vol) was prepared in advance and kept at 4°C. Human O+ RBCs and serum were obtained from the Japanese Red Cross Society (reception number 28J0060). Compounds that were positively identified from our previous study (22) were obtained from the Open Innovation Center for Drug Discovery (OCDD), University of Tokyo (Tokyo, Japan). All compounds were received from the University of Tokyo at a concentration of 10 μM; however, the stock solutions were prepared in DMSO at a concentration of 2 mM. The final DMSO concentration used in the experiment was ≤0.5%, which had no inhibitory effect on parasite culture.
Parasite culture.
For this study, chloroquine-mefloquine-sensitive P. falciparum strain 3D7A was used. P. falciparum strain 3D7A was cultured and maintained as previously described (38), with slight modifications (39). In brief, parasites were maintained in RPMI 1640 medium (Thermo Fisher Scientific, MA, USA) supplemented with 5% AB+ human serum, 0.25% AlbuMAX I (Thermo Fisher Scientific), 12.5 μg/ml gentamicin (Sigma-Aldrich), and human RBCs (O+) at 2% hematocrit levels with 0.2 to 2% parasitemia in culture flasks at 37°C under an atmosphere of 5% CO2, 5% O2, and 90% N2.
Asexual antimalarial assay: in vitro assay using chloroquine-mefloquine-sensitive P. falciparum strain 3D7A.
The erythrocytic antimalarial activity of the compounds was measured by growth inhibition (percent) of the parasites in the presence of compounds, using a SYBR green I assay. In brief, 50 μl of P. falciparum strains was seeded into a 96-well clear-bottom black plate (Thermo Fisher Scientific) with 0.75% parasitemia and 2% hematocrit, followed by the addition of 50 μl of the compound at a final concentration of 10 μM. Wells in the absence of parasites were considered positive controls, and parasites in DMSO (≥5%) without any treatment were considered negative controls. Plates were then incubated for 48 h at 37°C with 5% CO2, 5% O2, and 90% N2 by using a closed jar. After 48 h of incubation under standard culture conditions, 100 μl of lysis buffer containing SYBR green I (1× final concentration) was directly added to the wells. Plates were then placed on a shaker with gentle mixing for incubation for 1 h at room temperature in the dark. The fluorescence of each well then measured by using a multiplate reader (ARVO1430; PerkinElmer, MA, USA) in the fluorescence detection mode (excitation [Ex] at 485 nm and emission [Em] at 515 nm) for a 0.1-s exposure. Two independent experiments were performed under similar conditions. Subsequently, an in vitro dose-response assay was performed.
In vitro antimalarial dose-response assay.
Active compounds identified by the above-described antimalarial assay were eventually subjected to an in vitro erythrocytic dose-response assay at 10 different dilutions ranging between 0.5 nM and 10 μM to exclude any false-positive compounds and measure the half-maximal inhibitory concentrations (IC50s). Each experiment was conducted twice by using a 96-well clear-bottom black plate, and fluorescence was measured as described above. The data obtained were analyzed to calculate the IC50 of each compound based on a typical sigmoid dose-response curve by using GraphPad Prism 6 software.
Antihemozoin dose-response assay.
The IC50s of potential hit compounds against hemozoin formation were further determined, as previously described (22). Briefly, hemin solution (10 mM heme in DMSO and 100 mM acetate buffer) was incubated with the detergent NP-40 (as hemozoin formation inducer) and compounds (0 μM to 208 μM) for 250 min at 37°C, followed by the addition of pyridine solution with 10 min of shaking. Finally, the absorbance of each compound was measured at 405/705 nm. The IC50s of each compound were calculated by using GraphPad Prism 6 software.
Physicochemical properties of 224 hemozoin inhibitors.
Physicochemical properties of chemical compounds were obtained from the SciFinder Scholar Database (American Chemical Society, Washington, DC) or ChemSpider (http://www.chemspider.com/), as previously described (22).
Statistical analysis.
We conducted blind experiments without knowledge of compound names or structures. The code was opened only after the antimalarial and antihemozoin results were sent to the Open Innovation Center for Drug Discovery, University of Tokyo. Moreover, the statistician who performed the analysis is not in the chemistry field and was not involved in the experiments. Variables of physical properties and compounds with many missing data, including pKa2 (negative log value of acid dissociation constant), vapor pressure (torr), flash point (degrees Celsius), enthalpy of vaporization (kilojoules per mole), and boiling point (degrees Celsius), were excluded. Finally, 206 compounds were included in our analysis.
The outcome variable was the antimalarial activity of the chemical compounds (coded as 1 for active and 0 for inactive). The predictor variables were the physicochemical properties of our chemical compounds. We performed univariable LR to examine the association between physicochemical properties and the above-mentioned outcomes. Subsequently, variables with P values of <0.1 and/or with prior significance in our previous study (22) were included in the multivariable analyses using BMA to find the independent predictors of our outcome. We multiplied the index of the refraction variable by 10 to have a lower odds ratio (OR).
Development and validation of the prediction models.
We divided the original data randomly into training and testing sets at a ratio of 70:30 (nearly 157 compounds [including 17 positive compounds] and 67 compounds [including 5 positive compounds], respectively). The training data set was constructed to develop prediction models using the model. Results are shown as the posterior probability (PP) and Bayesian information criterion (BIC) of each model (20, 40) (Tables 2 and 3). The PP, P (B ≠ 0), is the probability that a predictor variable has an effect on compound activity and that the coefficient is nonzero. The best models were then illustrated visually by depicting the variables included in them (Fig. 1).
Comparison of the best models.
The sensitivity and specificity of the best models were calculated. The discriminatory powers of the best prediction models obtained from BMA were compared according to the area under the curve (AUC) from the receiver operating characteristic (ROC) and their accuracies (Fig. 2). All analyses were performed by using RStudio software version 1.0.44 (https://www.rstudio.com/). A P value of <0.05 was considered statistically significant in all analyses. The data and R script can be obtained from M.G.K. or N.T.H.
Supplementary Material
ACKNOWLEDGMENTS
This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Moreover, we are grateful for all Online Research Club native speakers who revised the English language of the manuscript, especially Vincent Hou from McMaster University in Canada.
Farhana Mosaddeque, Awet Alem Teklemichael, and Michiko Fukuda did the experiments under the supervision of Shusaku Mizukami, Osamu Kaneko, Nguyen Tien Huy, and Kenji Hirayama. Farhana Mosaddeque, Mohamed Gomaa Kamel, Truong Van Dat, Dinh Van Toan, Satoshi Mizuta, Yoshimasa Tanaka, Ali Mahmoud Ahmed, Hoang Thi Nam Giang, Tran Ngoc Dang, Lam K. Huynh, and Mohamed Tamer Elhady extracted the data from ChemSpider and SciFinder. Mohamed Gomaa Kamel and Nguyen Lam Vuong performed the statistical analysis under the supervision of Nguyen Tien Huy and Kenji Hirayama. Farhana Mosaddeque, Mohamed Gomaa Kamel, Truong Van Dat, Ali Mahmoud Ahmed, Osamu Kaneko, Timothy J. Egan, and Nguyen Tien Huy interpreted data from the analysis and wrote the manuscript. All authors revised and approved the final version.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02424-17.
REFERENCES
- 1.World Health Organization. 2016. World malaria report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Parhizgar A, Tahghighi A. 2017. Introducing new antimalarial analogues of chloroquine and amodiaquine: a narrative review. Iran J Med Sci 42:115–128. [PMC free article] [PubMed] [Google Scholar]
- 3.Eastman R, Fidock D. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plowe C. 2009. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg 103(Suppl 1):S11–S14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp A, Ringwald P. 2013. Artemisinin resistance is a clear and present danger. Trends Parasitol 29:359–360. doi: 10.1016/j.pt.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Dondorp A, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. 2010. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 7.White NJ, Duong TT, Uthaisin C, Nosten F, Phyo AP, Hanboonkunupakarn B, Pukrittayakamee S, Jittamala P, Chuthasmit K, Cheung MS, Feng Y, Li R, Magnusson B, Sultan M, Wieser D, Xun X, Zhao R, Diagana TT, Pertel P, Leong FJ. 2016. Antimalarial activity of KAF156 in falciparum and vivax malaria. N Engl J Med 375:1152–1160. doi: 10.1056/NEJMoa1602250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White NJ, Pukrittayakamee S, Phyo AP, Rueangweerayut R, Nosten F, Jittamala P, Jeeyapant A, Jain JP, Lefèvre G, Li R, Magnusson B, Diagana TT, Leong FJ. 2014. Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med 371:403–410. doi: 10.1056/NEJMoa1315860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phyo A, Jittamala P, Nosten F, Pukrittayakamee S, Imwong M, White NJ, Duparc S, Macintyre F, Baker M, Mohrle JJ. 2016. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax. Lancet Infect Dis 16:61–69. doi: 10.1016/S1473-3099(15)00320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held J, Supan C, Salazar C, Tinto H, Bonkian LN, Nahum A, Moulero B, Sie A, Coulibaly B, Sirima SB, Siribie M, Otsyula N, Otieno L, Abdallah AM, Kimutai R, Bouyou-Akotet M, Kombila M, Koiwai K, Cantalloube C, Din-Bell C, Djeriou E, Waitumbi J, Mordmuller B, Ter-Minassian D, Lell B, Kremsner PG. 2015. Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study. Lancet Infect Dis 15:1409–1419. doi: 10.1016/S1473-3099(15)00079-1. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy J, Rückle T, Djeriou E, Cantalloube C, Ter-Minassian D, Baker M, O'Rourke P, Griffin P, Marquart L, Hooft van Huijsduijnen R, Mohrle JJ. 2016. A phase II pilot trial to evaluate safety and efficacy of ferroquine against early Plasmodium falciparum in an induced blood-stage malaria infection study. Malar J 15:469. doi: 10.1186/s12936-016-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.RTS, S Clinical Trials Partnership, Agnandji S, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, et al. . 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. New Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targett GA, Moorthy VS, Brown GV. 2013. Malaria vaccine research and development: the role of the WHO MALVAC committee. Malar J 12:362. doi: 10.1186/1475-2875-12-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams PA, Berman PA, Egan TJ, Marsh PJ, Silver J. 1996. The iron environment in heme and heme-antimalarial complexes of pharmacological interest. J Inorg Biochem 63:69–77. doi: 10.1016/0162-0134(95)00212-X. [DOI] [PubMed] [Google Scholar]
- 16.Dinio T, Gorka AP, McGinniss A, Roepe PD, Morgan JB. 2012. Investigating the activity of quinine analogues versus chloroquine resistant Plasmodium falciparum. Bioorg Med Chem 20:3292–3297. doi: 10.1016/j.bmc.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan TJ, Hunter R, Kaschula CH, Marques HM, Misplon A, Walden J. 2000. Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of β-hematin formation, and antiplasmodial activity. J Med Chem 43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- 18.Ridley RG, Dorn A, Vippagunta SR, Vennerstrom JL. 1997. Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann Trop Med Parasitol 91:559–566. doi: 10.1080/00034983.1997.11813174. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan D. 2002. Theories on malarial pigment formation and quinoline action. Int J Parasitol 32:1645–1653. doi: 10.1016/S0020-7519(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 20.Sandlin RD, Carter MD, Lee PJ, Auschwitz JM, Leed SE, Johnson JD, Wright DW. 2011. Use of the NP-40 detergent-mediated assay in discovery of inhibitors of β-hematin crystallization. Antimicrob Agents Chemother 55:3363–3369. doi: 10.1128/AAC.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wicht KJ, Combrinck JM, Smith PJ, Egan TJ. 2015. Bayesian models trained with HTS data for predicting β-haematin inhibition and in vitro antimalarial activity. Bioorg Med Chem 23:5210–5217. doi: 10.1016/j.bmc.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huy NT, Chi PL, Nagai J, Dang TN, Mbanefo EC, Ahmed AM, Long NP, Thoa LT, Hung LP, Titouna A, Kamei K, Ueda H, Hirayama K. 2017. High-throughput screening and prediction model building for novel hemozoin inhibitors using physicochemical properties. Antimicrob Agents Chemother 61:e01607-16. doi: 10.1128/AAC.01607-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihekwereme CP, Esimone CO, Nwanegbo EC. 2014. Hemozoin inhibition and control of clinical malaria. Adv Pharmacol Sci 2014:984150. doi: 10.1155/2014/984150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hänscheid T, Egan TJ, Grobusch MP. 2007. Haemozoin: from melatonin pigment to drug target, diagnostic tool, and immune modulator. Lancet Infect Dis 7:675–685. doi: 10.1016/S1473-3099(07)70238-4. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani K, Ishikawa H, Aono S, Mizutani Y. 2014. Identification of essential histidine residues involved in heme binding and hemozoin formation in heme detoxification protein from Plasmodium falciparum. Sci Rep 4:6137. doi: 10.1038/srep06137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan DJ Jr, Gluzman IY, Goldberg DE. 1996. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271:219–222. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 27.Jani D, Nagarkatti R, Beatty W, Angel R, Slebodnick C, Andersen J, Kumar S, Rathore D. 2008. HDP—a novel heme detoxification protein from the malaria parasite. PLoS Pathog 4:e1000053. doi: 10.1371/journal.ppat.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. 1995. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi AK, Gupta A, Garg SK, Tekwani BL. 2001. In vitro β-hematin formation assays with plasma of mice infected with Plasmodium yoelii and other parasite preparations: comparative inhibition with quinoline and endoperoxide antimalarials. Life Sci 69:2725–2733. doi: 10.1016/S0024-3205(01)01349-2. [DOI] [PubMed] [Google Scholar]
- 30.Huy NT, Uyen DT, Maeda A, Trang DT, Oida T, Harada S, Kamei K. 2007. Simple colorimetric inhibition assay of heme crystallization for high-throughput screening of antimalarial compounds. Antimicrob Agents Chemother 51:350–353. doi: 10.1128/AAC.00985-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandlin RD, Fong KY, Wicht KJ, Carrell HM, Egan TJ, Wright DW. 2014. Identification of β-hematin inhibitors in a high-throughput screening effort reveals scaffolds with in vitro antimalarial activity. Int J Parasitol Drugs Drug Resist 4:316–325. doi: 10.1016/j.ijpddr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenthal PJ. 2003. Antimalarial drug discovery: old and new approaches. J Exp Biol 206:3735–3744. doi: 10.1242/jeb.00589. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Guha M, Choubey V, Maity P, Bandyopadhyay U. 2007. Antimalarial drugs inhibiting hemozoin (β-hematin) formation: a mechanistic update. Life Sci 80:813–828. doi: 10.1016/j.lfs.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Bhal SK, Kassam K, Peirson IG, Pearl GM. 2007. The rule of five revisited: applying log D in place of log P in drug-likeness filters. Mol Pharm 4:556–560. doi: 10.1021/mp0700209. [DOI] [PubMed] [Google Scholar]
- 35.Warhurst DC, Craig JC, Raheem KS. 2016. Influence of LAR and VAR on para-aminopyridine antimalarials targetting haematin in chloroquine-resistance. PLoS One 11:e0160091. doi: 10.1371/journal.pone.0160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas Ruiz FA, García-Sánchez RN, Estupiñan SV, Gómez-Barrio A, Torres Amado DF, Pérez-Solórzano BM, Nogal-Ruiz JJ, Martínez-Fernández AR, Kouznetsov VV. 2011. Synthesis and antimalarial activity of new heterocyclic hybrids based on chloroquine and thiazolidinone scaffolds. Bioorg Med Chem 19:4562–4573. doi: 10.1016/j.bmc.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Nakamura Y, Shibasaki Y, Ando S. 2007. High refractive index polyimides derived from 2,7-bis(4-aminophenylenesulfanyl)thianthrene and aromatic dianhydrides. Macromolecules 40:4614–4624. doi: 10.1021/ma070706e. [DOI] [Google Scholar]
- 38.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 91:484–486. [DOI] [PubMed] [Google Scholar]
- 39.Alexandre JS, Yahata K, Kawai S, Torii M, Kaneko O. 2011. PEXEL-independent trafficking of Plasmodium falciparum SURFIN4.2 to the parasite-infected red blood cell and Maurer's clefts. Parasitol Int 60:313–320. doi: 10.1016/j.parint.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.