ABSTRACT
Ertapenem is a carbapenem antibiotic with activity against Mycobacterium tuberculosis. Dose simulations in a hollow-fiber infection model showed that 2,000 mg once daily is an appropriate dose to be tested in clinical studies. Before using this dose in a phase II study, the aim of this prospective pharmacokinetic study was to confirm the pharmacokinetics of 2,000 mg once daily in tuberculosis (TB) patients. Twelve TB patients received a single intravenous dose of 2,000 mg ertapenem as a 30-min infusion. Blood samples were collected at 0, 0.5, 1, 2, 3, 4, 8, 12, and 24 h postadministration. Drug concentrations were measured using a validated liquid chromatography-tandem mass spectrometry assay. A large interindividual variation in the pharmacokinetics of ertapenem was observed. The median (interquartile range) area under the plasma concentration-time curve to infinity (AUC0–∞) was 2,032 (1,751 to 2,346) mg · h/liter, the intercompartmental clearance (CL12) was 1.941 (0.979 to 2.817) liters/h, and the volume of distribution in the central compartment (V1) was 1.514 (1.064 to 2.210) liters. A more than dose-proportional increase in AUC was observed compared to results reported for 1,000 mg ertapenem in multidrug-resistant TB patients. Based on a MIC of 1.0 mg/liter, 11 out of 12 patients would have reached the target value of unbound drug exceeding the MIC over 40% of the time (f40% T>MIC). In conclusion, this study shows that 2,000 mg ertapenem once daily in TB patients reached the expected f40% T>MIC for most of the patients, and exploration in a phase 2 study can be advocated.
KEYWORDS: ertapenem, tuberculosis, pharmacokinetics, MIC
INTRODUCTION
The World Health Organization (WHO) estimated that there were approximately 600,000 new cases of rifampin-resistant tuberculosis (RR-TB), of which 490,000 were multidrug-resistant tuberculosis (MDR-TB), in 2016 (1). MDR-TB means resistance to at least two first-line anti-TB drugs, namely rifampin and isoniazid. MDR-TB is treated with a combination of second-line anti-TB drugs, which are more toxic and less effective than the first-line anti-TB drugs (1, 2). In addition, MDR-TB treatment lasts up to 20 months, in contrast to first-line anti-TB treatment, which takes 6 months (1). A shorter treatment duration of 9 to 12 months recommended by the WHO in May 2016 is only suitable for the relatively small subset of patients with pulmonary MDR- or RR-TB that is not resistant to fluoroquinolones, second-line injectables, or pyrazinamide (1). The success rate of MDR-TB treatment is approximately 50% worldwide, which calls for improvement (1). There is a need for new treatment options, especially for patients with additional resistance to two important classes of second-line drugs, fluoroquinolones and injectable aminoglycosides or capreomycin, referred to as extensively drug-resistant TB (XDR-TB) (1, 3).
Ertapenem is a carbapenem antimicrobial drug that belongs to the group of β-lactam antibiotics (4). Ertapenem is not included in the MDR-TB treatment guidelines, in contrast to two other carbapenems, i.e., imipenem and meropenem (5, 6). The efficacy of carbapenems against Mycobacterium tuberculosis is attributable to the inactivation of l,d-transpeptidases, which are responsible for M. tuberculosis peptidoglycan cross-linking. The efficacy of treatment with ertapenem is expected to be related to the free, unbound concentration based on the debated inability of protein-bound ertapenem to distribute to the infection site and bind to the target bacterial penicillin-binding protein (7–9). An early bactericidal activity study, including both free and bound ertapenem measurements, could be of help to answer the question of whether unbound or total drug determines the efficacy of ertapenem. The antibacterial activity of carbapenems is mainly determined by the time that the plasma concentration exceeds the MIC (T>MIC) (10–12). Based on previous studies in mice and in a hollow-fiber model, it is expected that free 40% time above the MIC (f40% T>MIC) is the most important pharmacodynamic (PD) parameter (12, 13). M. tuberculosis can inactivate ertapenem with the enzyme β-lactamase, and for this reason ertapenem needs to be combined with the β-lactamase inhibitor clavulanic acid (4, 5, 14).
Carbapenems are generally well tolerated. A systematic study showed that less than 15% of all adverse events that occur during treatment that contained carbapenems, together with other anti-TB drugs, were attributable to carbapenems (15). Like for the older carbapenems, adverse events associated with ertapenem are often transient and mild (16). In contrast to imipenem and meropenem, ertapenem only needs to be administered once daily due to its longer half-life (4). Based on the in vitro activity and its long half-life, ertapenem is a promising drug in the treatment of MDR- and XDR-TB (4, 13, 15).
A hollow-fiber model is an in vitro model to simulate the human pharmacokinetics with a cartridge containing M. tuberculosis, mimicking the target site of infection. The European Medicines Agency has accepted hollow-fiber system models to be used in dose finding and regimen selection for the treatment of M. tuberculosis (17). Additionally, it could be used to define pharmacokinetic/pharmacodynamic (PK/PD) targets and show target attainment when sufficient data are available on human pharmacokinetics of anti-TB drugs (13).
In this study, we want to verify the hypothesis of van Rijn et al. that 2,000 mg ertapenem would be the most suitable dose for the treatment of TB (13). Therefore, the primary goals of this study were to determine the pharmacokinetics of 2,000 mg ertapenem to verify the hypothesis, compare the exposure of 2,000 mg ertapenem in TB patients to the exposure of 200 mg · h/liter used in the hollow-fiber model study, and to determine the f40% T>MIC (13).
RESULTS
Patients.
In total, 12 culture-confirmed patients with drug-susceptible TB received a single intravenous infusion of 2,000 mg ertapenem. Patients were mostly men (92%) with a median (interquartile range [IQR]) age of 36 (26 to 42) years and a BMI of 20.4 (18.5 to 23.7) kg/m2 (Table 1). Ten out of 12 patients had predominantly pulmonary TB, one patient had TB colitis besides pulmonary TB, and one patient had TB peritonitis.
TABLE 1.
Patient characteristics
| Parameter | Value for drug-susceptible TB patientsa (n = 12) |
|---|---|
| Ertapenem dose (mg) | 2,000 |
| Sex [n (%)] | |
| Male | 11 (92) |
| Female | 1 (8) |
| Age (yr) | 36 (26–42) |
| wt (kg) | 65.0 (56.8–77.3) |
| BMI (kg/m2) | 20.4 (18.5–23.7) |
| BSA (m2) | 1.80 (1.67–1.96) |
| Serum creatinine at baseline (mmol/liter) | 68 (59–75) |
| Dose/wt (mg/kg) | 30.8 (25.9–35.2) |
| Ethnicity [n (%)] | |
| Black | 7 (58) |
| Caucasian | 4 (33) |
| Asian | 0 (0) |
| Other | 1 (8) |
Data are expressed as medians (interquartile ranges) unless stated otherwise.
One patient experienced nausea after receiving 2,000 mg ertapenem. The patient did not vomit. Another patient experienced pain at the infusion site. This occurred 3 h after infusion. Afterwards the drip was placed in the other arm, where the pain also occurred after approximately 5 h.
Pharmacokinetics.
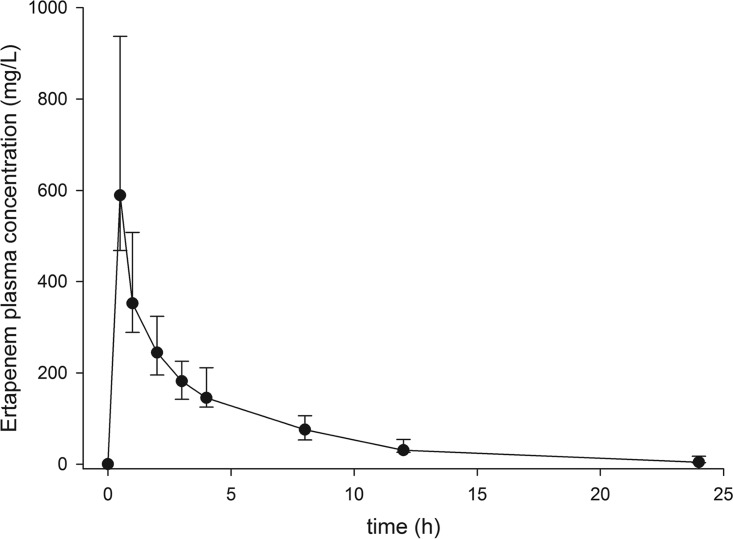
The pharmacokinetics of all patients is shown in Table 2. There is a large interindividual variation in the pharmacokinetics, especially in the area under the plasma concentration-time curve (AUC) (Fig. 1), which could be caused by a large variation in intercompartmental clearance (CL12) and volume of distribution (V).
TABLE 2.
Pharmacokinetic parameters of 2,000 mg ertapenem in tuberculosis patients
| Pharmacokinetic parametera | Median (IQR) |
|---|---|
| AUC0–∞ (h · mg/liter) | 2032 (1751–2346) |
| CL12 (liters/h) | 1.941 (0.979–2.817) |
| V1 (liters) | 1.514 (1.064–2.210) |
| V2 (liters) | 2.984 (1.912–3.428) |
| Vss (liters) | 4.560 (3.857–5.160) |
| k10 (/h) | 0.677 (0.539–0.863) |
| k12 (/h) | 1.690 (0.482–2.666) |
| k21 (/h) | 0.718 (0.530–0.863) |
| t1/2 1 (h) | 0.225 (0.162–0.519) |
| t1/2 2 (h) | 3.859 (3.620–4.301) |
| MRT (h) | 3.991 (3.840–5.494) |
| MIT (h) | 0.296 (0.250–0.342) |
AUC0–∞, area under the concentration-time curve from 0 h to infinity; CL12, intercompartmental clearance; V1, volume of distribution of the central compartment; V2, volume of distribution in the peripheral compartment; Vss, volume of distribution at steady-state; k12 and k21, first-order intercompartmental transfer rate constants between the central and peripheral compartments; k10, elimination rate constant; t1/2 1 and 2, distribution and elimination half-lives; MRT, mean resident time; MIT, mean input time.
FIG 1.
Plasma-concentration time curve for all 12 patients receiving 2,000 mg ertapenem.
Free 40% time above the MIC.
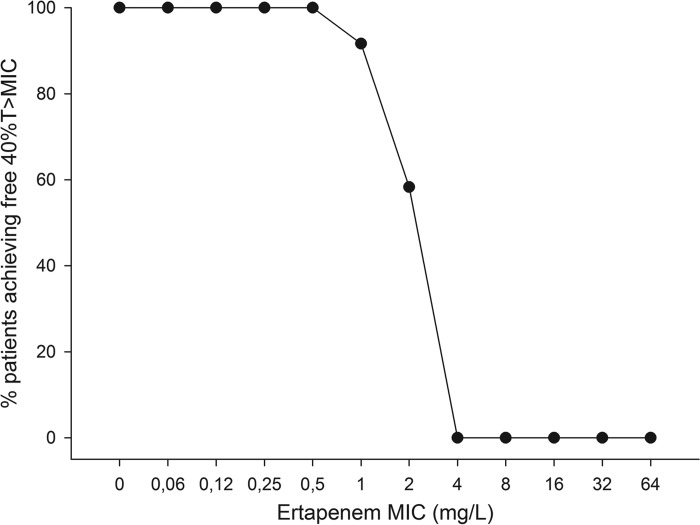
Based on a MIC of 0.5 mg/liter and a percentage of unbound ertapenem of 5%, all patients exceeded the minimum f40% T>MIC (range, 10.32 to 22.88 h). This means that all TB patients would have a sufficient therapeutic concentration if the MIC was 0.5 mg/liter or less. If the MIC was 1.0 mg/liter, 11 out of 12 patients would have exceeded the minimum of f40% T>MIC (range, 8.08 to 20.96 h). At a MIC of 2.0 mg/liter, 7 out of 12 patients exceeded the minimum of f40% T>MIC (range, 5.62 to 14.88 h). The percentage of patients achieving f40% T>MIC at various MIC values is shown in Fig. 2.
FIG 2.
Percent target attainment for patients receiving 2,000 mg ertapenem at various MICs. 40% T>MIC, 40% of time above the MIC.
DISCUSSION
To our knowledge, this is the first study showing the pharmacokinetics of 2,000 mg ertapenem in TB patients. A dose of 2,000 mg ertapenem had a better ability to reach the f40%T>MIC, the PK/PD parameter related to microbial kill (13). The dose was based on the results from the study of van Rijn et al., who showed in a hollow-fiber model that 2,000 mg ertapenem might be more effective in the treatment of M. tuberculosis than 1,000 mg (18). Another study, however, with healthy young volunteers, has been published on the pharmacokinetics of 2,000 mg (19). The results from the study of Majumdar et al. were used for comparison with the results from this study (19). The patient characteristics of the healthy volunteers were significantly different from those of our TB patients, except for the distribution of males and females in both studies. The AUC from time zero to infinity (AUC0–∞) and peak concentration (Cmax) were shown to be twice as high in TB patients as in healthy volunteers (19). The higher AUC0–∞ could be due to the lower plasma clearance in TB patients, which was 33 times lower than that in healthy volunteers. Also, the volume of distribution in TB patients was half that in healthy volunteers, explaining the higher plasma concentration of ertapenem (20).
As can be seen from the results, the volume of distribution in the central compartment (V1) is lower than the total plasma volume of around 3 liters. This shows that there was incomplete distribution at the time of taking the first plasma sample, which may be caused by slow distribution of ertapenem after an infusion of 30 min. Because of this incomplete distribution, the V1 value cannot be calculated correctly. Another explanation for the high Cmax is the sampling from the same arm. In order to eliminate this possibility, we took samples from two of the patients at two time points from the other arm, but this gave the same analytical results, contributing to the assumption that ertapenem had incomplete distribution immediately after administration. There were no specific differences in patient characteristics or treatment that could explain this slow distribution.
In the study of healthy volunteers, it was shown that there was a slightly less than dose-proportional increase in AUC over a dose range of 0.5 to 3 g ertapenem (19). This is due to saturation of plasma protein binding, which causes the unbound fraction of ertapenem to increase at higher plasma concentrations. Comparing the studies with 1,000 mg and 2,000 mg ertapenem in TB patients, 2,000 mg showed a more than dose-proportional increase in AUC, since the mean AUC is more than three times as high for 2,000 mg as it is for 1,000 mg (18). The clearance is higher for 1,000 mg than for 2,000 mg, but the volume of distribution is not significantly different in this case (18). The only difference between the two patient groups, except for the dose of ertapenem given, is the age of the patients (18).
A dose of 2,000 mg ertapenem in TB patients showed nonlinear pharmacokinetic behavior, which is thought to be caused by saturation of the major metabolic pathway according to the significantly reduced clearance (21). The major metabolic pathway in the case of ertapenem has been shown to be the formation of the beta-lactam ring-open metabolite by dehydropeptidase-1, located in the renal tubules. Around 37% of ertapenem is metabolized to the beta-lactam ring-open metabolite and excreted by the kidneys (9). In earlier studies it was shown that the renal clearance of unbound ertapenem can exceed the glomerular filtration rate, which is hypothesized to be the result of tubular secretion. The mean renal clearance of unbound ertapenem was 207 ml/min, which is higher than the creatinine clearance, indicating that ertapenem undergoes glomerular filtration and net tubular secretion (7). A similar phenomenon of saturation of renal clearance is also seen with piperacillin (22).
Additionally, the volume of distribution at steady state (Vss) is lower than expected. The Vss of 2,000 mg ertapenem was shown to be around 9.5 liters for healthy volunteers (19); however, we found a Vss of 4.560 liters in TB patients. One reason for this could be the difference in body composition, which was shown to be the case previously (19). Another possibility is that it was difficult to estimate the Vss correctly due to nonlinear pharmacokinetic behavior (23).
A study by Chen et al. showed that obese patients had lower AUCs than normal-weight patients (24). Our TB patients had a significantly lower BMI than the healthy volunteers, and this could have explained the higher AUC. The PK variability of 1,000 as well as 2,000 mg ertapenem in healthy volunteers was also lower than that in TB patients, which could be due to the disease state (18, 19). Three patients received 2,000 mg ertapenem during a 1-h infusion instead of half an hour, as was stated in the protocol. However, the PK of these patients did not significantly differ from those of the other patients, as the volume of distribution was between 5.321 and 5.808 and the clearance was between 0.813 and 1.295. Therefore, the longer infusion time is thought not to have influenced the pharmacokinetic results.
The bacteriostatic target of f40% T>MIC was reached in all patients at a MIC of 0.5 mg/liter, 92% of the patients at a MIC of 1 mg/liter, and 58% at a MIC of 2 mg/liter. Target attainment was considerably higher in this study compared with what was observed in the study of 1,000 mg ertapenem in MDR-TB patients, where only 2 out of 12 patients reached f40% T>MIC at a MIC of 1 mg/liter (18). Van Rijn et al. used an AUC of 200 mg · h/liter to simulate the pharmacokinetics and determine the target attainment; however, in our study a median AUC0–∞ of 2,032 mg · h/liter was found (13). In that study, around 63% of the patients would achieve f40% T>MIC at a MIC of 2 mg/liter. In the current study, we show that 58% of the patients reached the target value at a MIC of 2 mg/liter. We have used the European Committee on Antimicrobial Susceptibility Testing (EUCAST) values for the MIC of ertapenem instead of determining the MIC distributions. This is due to the fact that the EUCAST-approved methods require at least 7 days of incubation, and ertapenem was shown to degrade at rates of more than 20-fold the doubling time of M. tuberculosis under the acidic incubation conditions, which causes the results to show resistance even though this is not the case (14).
Although intramuscular injection can be preferred in settings with limited resources, in our setting it is not licensed and therefore not recommended. A previous study showed that 1,000 mg intramuscularly injected ertapenem had an AUC0–∞ similar to that of a 30-min intravenous infusion of 1,000 mg ertapenem (541.8 versus 597.4 μg · h/ml), as well as a similar renal clearance (10.9 versus 12.7 ml/min) and the same half-life of 3.8 h (25). The only significantly different PK parameter between intravenous and intramuscular injection was shown to be the Cmax (25). However, it remains to be seen whether a 2,000-mg intramuscular injection can be administered or should be given as two separate injections. A study is needed to specifically address the possibility of the intramuscular administration of 2,000 mg ertapenem.
In conclusion, 2,000 mg ertapenem once daily in TB patients reached f40% T>MIC in most patients. Therefore, this dose is suitable for examination in a phase 2 study to test its efficacy and tolerability. This study shows promise for the use of 2,000 mg ertapenem as an option in the treatment of MDR- and XDR-TB.
MATERIALS AND METHODS
Ethics.
This prospective pharmacokinetic study was conducted at the Tuberculosis Center Beatrixoord of the University Medical Center Groningen (Haren, The Netherlands). The study was approved by the Medical Ethical Review Board of the University Medical Center Groningen (METc; M16.200922). Written informed consent was obtained from all subjects included in this study.
Inclusion and exclusion criteria.
We planned to enroll 12 patients between 18 and 64 years of age with drug-susceptible TB. The M. tuberculosis isolate (including M. africanum) should be drug susceptible, either proven by culture or confirmed with molecular testing. Patients were excluded if they had a previous anaphylactic reaction to ertapenem or another β-lactam antibiotic, renal insufficiency (estimated glomerular filtration rate [eGFR] of ≤30 ml/min), pregnancy, HIV infection, and/or a body weight of 40 kg or less.
Sampling.
Ertapenem was administered as a single intravenous infusion of 2,000 mg ertapenem in 30 min. Blood samples (2 ml) were collected at 0, 0.5, 1, 2, 3, 4, 8, 12, and 24 h postinfusion. During plasma sampling patients were on a continuous saline drip. Plasma samples were stored at −80°C until analysis. A validated liquid chromatography-tandem mass spectrometry method was used to determine the total ertapenem concentration in plasma (26).
Pharmacokinetics.
The area under the concentration-time curve up to 24 h after infusion (AUC0–24) was determined with a two-compartment pharmacokinetic method using the KINFIT module of MW\Pharm 3.82 (Mediware, Zuidhorn, The Netherlands).
Determination of the T>MIC of the unbound fraction.
The concentration-time curve of each patient was used to determine whether the f40% T>MIC is reached with a single intravenous infusion of 2,000 mg ertapenem. For this simulation, we plotted MIC distributions from 0 to 64 mg/liter, for which the amount of time that the concentration-time curve is above the MIC was determined. Since there are no clinical breakpoints available for ertapenem in the treatment of M. tuberculosis, the PK/PD breakpoints of ertapenem were used as recommended by EUCAST. These PK/PD breakpoints are set by EUCAST and are derived from the relationship between the PK/PD index and the MIC for other bacteria by using Monte Carlo simulations and the variability in both exposure and MIC (27, 28). Additionally, the results were discussed for the f40% T>MIC at a MIC of 2.0 mg/liter, as this was shown to be a more accurate MIC for ertapenem based on hollow-fiber study results (13). The percentage of unbound ertapenem used for this study was 5% (4, 18). The percentage of unbound ertapenem increases disproportionately with doses above 2,000 mg, when total drug concentrations are higher than 150 mg/liter (7). However, we consider the worst-case scenario of 5% unbound ertapenem at all plasma concentrations. The target value of T>MIC was ≥40% (i.e., 9.6 h). The f40% T>MIC was calculated by multiplying the plasma concentration by 20, because of the 5% protein binding, to get the target MIC. The curve then was plotted with MW/Pharm 3.82 (Mediware, Zuidhorn, The Netherlands), from which we could see how long the curve exceeded the target MIC.
Statistics.
Statistical analysis of patient characteristics in studies with ertapenem compared with those from our study was performed using Analyze-it for Microsoft Excel, version 2.30. Patient characteristics and PK parameters were compared using Mann-Whitney U test for age, BMI, body surface area (BSA), serum creatinine levels at baseline, and dose/total bodyweight. The different groups were compared using Fisher's exact test for sex and Pearson's chi-square test for ethnicity.
ACKNOWLEDGMENTS
We thank the Beatrixoord Noord Nederland Foundation (210.161) for their funding.
We also thank everyone involved in this study for their contributions.
We have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1. [Google Scholar]
- 2.Ramachandran G, Swaminathan S. 2015. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf 38:253–269. doi: 10.1007/s40264-015-0267-y. [DOI] [PubMed] [Google Scholar]
- 3.Alffenaar JC, Akkerman OW, Anthony RM, Tiberi S, Heysell S, Grobusch MP, Cobelens FG, Van Soolingen D. 2017. Individualizing management of extensively drug-resistant tuberculosis: diagnostics, treatment, and biomarkers. Expert Rev Anti Infect Ther 15:11–21. doi: 10.1080/14787210.2017.1247692. [DOI] [PubMed] [Google Scholar]
- 4.Cordillot M, Dubée V, Triboulet Dubost L, Marie A, Hugonnet JE, Arthur M, Mainardi JL. 2013. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veziris N, Truffot C, Mainardi JL, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2600. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. World Health Organization, Geneva, Switzerland: ISBN-13:978-92-4-154963-9. [Google Scholar]
- 7.Nix DE, Majumdar AK, DiNubile MJ. 2004. Pharmacokinetics and pharmacodynamics of ertapenem: an overview for clinicians. J Antimicrob Chemother 53(Suppl S2):ii23–ii28. doi: 10.1093/jac/dkh205. [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt O, Brunner M, Schmidt S, Grant M, Tang Y, Derendorf H. 2006. Penetration of ertapenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers measured by in vivo microdialysis. J Antimicrob Chemother 58:632–636. doi: 10.1093/jac/dkl284. [DOI] [PubMed] [Google Scholar]
- 9.Hammond ML. 2004. Ertapenem: a group 1 carbapenem with distinct antibacterial and pharmacological properties. J Antimicrob Chemother 53(Suppl 2):ii7–ii9. doi: 10.1093/jac/dkh203. [DOI] [PubMed] [Google Scholar]
- 10.Kristoffersson AN, David-Pierson P, Parrott NJ, Kuhlmann O, Lave T, Friberg LE, Nielsen EI. 2016. Simulation-based evaluation of PK/PD indices for meropenem across patient groups and experimental designs. Pharm Res 33:1115–1125. doi: 10.1007/s11095-016-1856-x. [DOI] [PubMed] [Google Scholar]
- 11.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47(Suppl 1):S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 12.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 13.Van Rijn SP, Srivastava S, Wessels MA, van Soolingen D, Alffenaar JC, Gumbo T. 2017. The sterilizing effect of ertapenem-clavulanate in a hollow fiber model of tuberculosis and implications on clinical dosing. Antimicrob Agents Chemother 61:e02039-16. doi: 10.1128/AAC.02039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava S, van Rijn SP, Wessels AM, Alffenaar JW, Gumbo T. 2016. Susceptibility testing of antibiotics that degrade faster than the doubling time of slow-growing mycobacteria: ertapenem sterilizing effect versus Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:3193–3195. doi: 10.1128/AAC.02924-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotgiu G, D'Ambrosio L, Centis R, Tiberi S, Esposito S, Dore S, Spanevello A, Migliori GB. 2016. Carbapenems to treat multidrug and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 17:373–383. doi: 10.3390/ijms17030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teppler H, Gesser RM, Friedland IR, Woods GL, Meibohm A, Herman G, Mistry G, Isaacs R. 2004. Safety and tolerability of ertapenem. Antimicrob Chemother 53(Suppl 2):ii75–ii81. doi: 10.1093/jac/dkh209. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency (EMA). 2016. Qualification opinion. In-vitro hollow fiber system model of tuberculosis (HSF-TB). EMA/CHMP/SAWP/47290/2015. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 18.van Rijn SP, van Altena R, Akkerman OW, van Soolingen D, van der Laan T, de Lange WC, Kosterink JG, van der Werf TS, Alffenaar JW. 2016. Pharmacokinetics of ertapenem in patients with multidrug-resistant tuberculosis. Eur Respir J 47:1229–1234. doi: 10.1183/13993003.01654-2015. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar AK, Musson DG, Birk KL, Kitchen CJ, Holland S, McCrea J, Mistry G, Hesney M, Xi L, Li SX, Haesen R, Blum RA, Lins RL, Greenberg H, Waldman S, Deutsch P, Rogers JD. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob Agents Chemother 46:3506–3511. doi: 10.1128/AAC.46.11.3506-3511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkett DJ. 1996. Pharmacokinetics made easy 11 designing dose regimens. Aust Prescr 19:76–81. doi: 10.18773/austprescr.1996.069. [DOI] [Google Scholar]
- 21.Ratain MJ, Plunkett WK Jr. 2003. Principles of Pharmacokinetics. In Kufe DW, Pollock RE, Weichselbaum RR, Blast RC Jr, Gansler TS, Holland JF, Frei E III (ed), Holland-Frei cancer medicine, 6th ed Decker, Hamilton, BC, Canada. [Google Scholar]
- 22.Vinks AA, Den Hollander JG, Overbeek SE, Jelliffe RW, Mouton JW. 2003. Population pharmacokinetic analysis of nonlinear behavior of piperacillin during intermittent or continuous infusion in patients with cystic fibrosis. Antimicrob Agents Chemother 47:541–547. doi: 10.1128/AAC.47.2.541-547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Nekka F, Li J. 2016. Steady-state volume of distribution of two-compartment models with simultaneous linear and saturated elimination. J Pharmacokinet Pharmacodyn 43:447–459. doi: 10.1007/s10928-016-9483-z. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS Jr. 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob Agents Chemother 50:1222–1227. doi: 10.1128/AAC.50.4.1222-1227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musson DG, Majumdar A, Birk K, Holland S, Wickersham P, Li SX, Mistry G, Fisher A, Waldman S, Greenberg H, Deutsch H, Rogers JD. 2003. Pharmacokinetics of intramuscularly administered ertapenem. Antimicrob Agents Chemother 47:1732–1735. doi: 10.1128/AAC.47.5.1732-1735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rijn SP, Wessels AM, Greijdanus B, Touw DJ, Alffenaar JW. 2014. Quantification and validation of ertapenem using a liquid chromatography-tandem mass spectrometry method. Antimicrob Agents Chemother 58:3481–3484. doi: 10.1128/AAC.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Committee on Antimicrobial Susceptibility Testing. 2017. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0. http://www.eucast.org/clinical_breakpoints/.
- 28.Mouton JW, Brown DF, Apfalter P, Cantón R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]