Abstract
IL-31, which is a member of the IL-6 family of cytokines, is produced mainly by activated CD4+ T cells, in particular activated Th2 cells, suggesting a contribution to development of type-2 immune responses. IL-31 was reported to be increased in specimens from patients with atopic dermatitis, and IL-31-transgenic mice develop atopic dermatitis-like skin inflammation, which is involved in the pathogenesis of atopic dermatitis. However, the role of IL-31 in development of contact dermatitis/contact hypersensitivity (CHS), which is mediated by hapten-specific T cells, including Th2 cells, is not fully understood. Therefore, we investigated this using IL-31-deficient (Il31−/−) mice, which we newly generated. We demonstrated that the mice showed normal migration and maturation of skin dendritic cells and induction of hapten-specific T cells in the sensitization phase of FITC-induced CHS, and normal induction of local inflammation in the elicitation phase of FITC- and DNFB-induced CHS. On the other hand, those mice showed reduced scratching frequency and duration during FITC- and/or DNFB-induced CHS. Our findings suggest that IL-31 is responsible for pruritus, but not induction of local skin inflammation, during CHS induced by FITC and DNFB.
Introduction
IL-31, which is a member of the IL-6 family of cytokines, is produced mainly by activated CD4+ T cells, in particular activated Th2 cells, mast cells, macrophages and dendritic cells1–4.Human and mouse IL-31 genes are located on chromosome 12q24.31 and chromosome 5, respectively, and their amino acid sequences show 31% homology1. Expression of IL-31 mRNA was reported in various human tissues, such as the testis, bone-marrow, skeletal muscle and kidney1. The receptor for IL-31 is a heterodimer, consisting of IL-31 receptor A (IL-31 RA) and oncostatin M receptor (OSMR)1. In humans and/or mice, IL-31 RA mRNA expression is found in various tissues, such as the testis, bone marrow, skin and dorsal root ganglia, and in various cells, such as activated monocytes, macrophages, dendritic cells (DCs), eosinophils, basophils and keratinocytes, while OSMR mRNA is broadly expressed in many tissues5–7. In cultures of various human cell lines such as keratinocytes1, intestinal and bronchial epithelial cell lines8,9, DCs7, monocytes and macrophages10, IL-31 has been shown to induce expression of such cytokines as IL-6, IL-8 and/or TNF and such chemokines as CCL2, CCL4, CCL5, CCL17 and/or CCL22.
IL-31 has been implicated to be involved in the pathogenesis of such allergic disorders as rhinitis, asthma and dermatitis5. Indeed, increased levels of IL-31 were observed in nasal secretions of patients with allergic rhinitis11. IL-31 levels in sera or PBMCs were significantly increased in patients with asthma12, and IL-31 can activate human bronchial epithelial cell lines, as described above. However, ovalbumin-induced airway inflammation developed normally in mice treated with anti-IL-31RA neutralizing Ab13, suggesting that IL-31 is not crucial for induction of allergic airway inflammation in that model.
In regard to skin diseases, elevated levels of IL-31 were observed in such specimens as plasma, sera and/or cutaneous biopsies from patients with atopic dermatitis14,15 or contact dermatitis16. In particular, IL-31 was implicated in itching by inducing activation of sensory nerve cells5. In support of that notion, transgenic mice (Tg mice), which express IL-31 systemically or specific for lymphocytes, spontaneously develop skin inflammation resembling human atopic dermatitis and show severe scratching behavior accompanied by exfoliation of epidermis1. Expression of IL-31 mRNA was increased in inflamed lesions of skin from mice that developed allergic dermatitis resembling human atopic dermatitis17. Administration of anti-IL-31 or anti-IL-31RA neutralizing Ab resulted in attenuation of scratching behavior, but not the severity of skin inflammation, in that mouse model18,19 or in patients with atopic dermatitis20. On the other hand, the role of IL-31 in the development of contact dermatitis/contact hypersensitivity (CHS) is not well understood. CHS is a T-cell-mediated cutaneous allergic response21. Several studies using type-2-cytokine-deficient mice showed that type-2 cytokines are important for the development of CHS (reviewed in22), suggesting that IL-31 may be involved in induction of CHS. Il31ra−/− mice showed enhanced type-2 immune responses due to hyperresponsiveness to OSM by increased formation of OSM receptors (the complex of gp130 and OSMR) rather than due to failure to form IL-31 receptors (the complex of IL-31RA and OSMR)13. Accordingly, we deemed that murine strain to be unsuitable for examining the role of IL-31 in CHS, and we instead used IL-31-deficient (Il31−/−) mice, which we newly generated.
Results
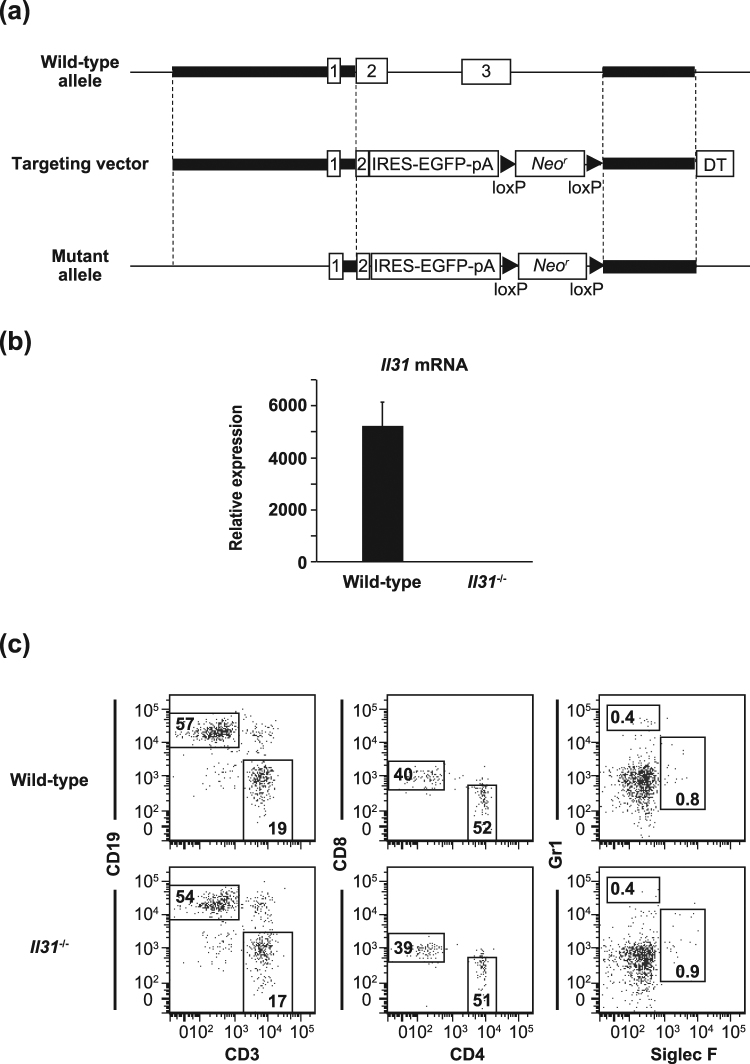
Generation of Il31−/− mice
Il31ra−/− mice were reported to have not only loss of IL-31 function but also elevated responsiveness to OSM13, suggesting that it is necessary to elucidate the exact roles of IL-31 in vivo by using Il31−/− mice rather than Il31ra−/− mice. Therefore, we newly generated Il31−/− mice by replacing Il31 genes with a cassette consisting of IRES-EGFP and a neomycin resistance gene, flanked by loxP sequences (Fig. 1a). Il31−/− mice were born at the expected Mendelian ratio, fertile, and did not show any gross phenotypic abnormalities under specific-pathogen-free housing conditions (data not shown). Expression of Il31 mRNA was below the limit of detection by qPCR in the lung and in PMA-ionomycin-stimulated spleen cells of Il31−/− mice (Fig. 1b and data not shown). No apparent abnormalities were found in the proportions of immune cells in the thymus, LNs, spleen and/or bone marrow between wild-type and Il31−/− mice (Fig. 1c, and data not shown).
Figure 1.
Generation of Il31−/− mice. (a) IL-31 gene targeting strategy. The region containing a part of exon 2 and exon 3 of Il31 was replaced with a cassette consisting of IRES-EGFP and a neomycin resistance gene (Neor), flanked by loxP sequences. (b) Expression of Il31 mRNA in lung tissue from wild-type (n = 8) and Il31−/− (n = 8) mice by qPCR. The data show the mean + SEM. (c) Leukocyte profiles in the spleen from wild-type and Il31−/− mice (n = 3). The percentages of CD19+ CD3− and CD19− CD3+ cells and of Gr1+ Siglec F− and Gr1- Siglec F+ cells in 7-AAD-negative spleen cells, and the percentages of CD8+ CD4− and CD8− CD4+ cells in 7-AAD-negative CD3+ spleen cells are shown. Representative flow cytometry data are shown.
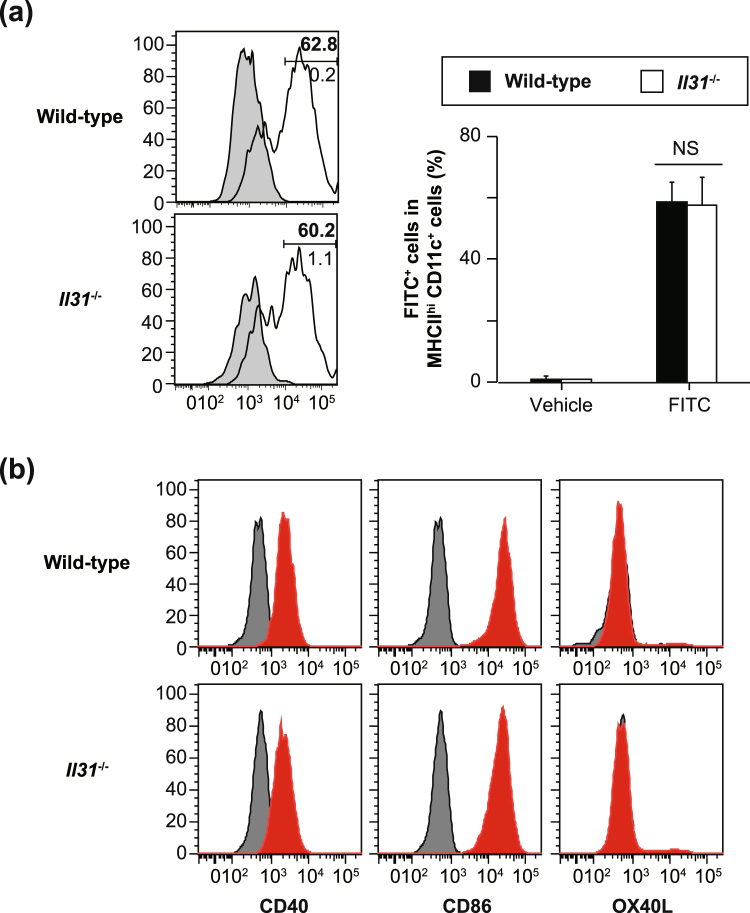
IL-31 is not essential for skin DC function or hapten-specific LN cell response in the sensitization phase of CHS
Treatment of mice that developed atopic dermatitis-like skin inflammation with anti-IL-31 or anti-IL-31RA neutralizing Ab resulted in attenuation of scratching behavior, but not the severity of skin inflammation18,19. On the other hand, the role of IL-31 in the development of CHS is not well understood. After epicutaneous exposure to haptens, skin DCs capture the haptens and migrate from the skin into draining LNs, followed by antigen presentation to naïve T cells to induce hapten-specific T cells21. To elucidate the role of IL-31 in skin DC migration and maturation, wild-type and Il31−/− mice were treated epicutaneously with FITC. Twenty-four hours later, the proportion of FITC-positive cells among MHC class IIhi CD11c+ cells in draining LNs and their expression of co-stimulatory molecules were determined by flow cytometry. The proportions of FITC-positive cells were comparable between the wild-type and Il31−/− mice (Fig. 2a). Such co-stimulatory molecules as CD86, CD40 and OX40L on the FITC-positive cells were also similarly expressed in the Il31−/− and wild-type mice (Fig. 2b).
Figure 2.
Normal skin DC function in Il31−/− mice. (a) The proportion of FITC-positive cells among 7-AAD-negative MHC class IIhi CD11c+ cells in draining LNs from wild-type (n = 4) and Il31−/− (n = 4) mice 24 hours after sensitization with FITC or vehicle alone was determined by flow cytometry. Representative flow cytometric data are shown. Shaded areas = LNs obtained from the vehicle-treated left ear (lower number [%]), and solid lines = LNs obtained from the FITC-treated right ear (upper number [%]). The data show the mean + SEM. (b) The expression levels of CD86, CD40 and OX40L on 7-AAD-negative MHC class IIhi CD11c+ FITC-positive cells in draining LNs from wild-type and Il31−/− mice 24 hours after sensitization with FITC or vehicle alone were determined by flow cytometry. Representative flow cytometric data are shown for wild-type (n = 6) and Il31−/− (n = 4) mice. Shaded areas = isotype-matched control Ig staining, and solid lines = specific mAb staining.
We next elucidated whether the lack of IL-31 influenced hapten-specific T-cell induction in the sensitization phase of CHS. After epicutaneous sensitization of Il31−/− and wild-type mice with FITC, draining LN cells were harvested and cultured in the presence and absence of FITC. After 48 and 72 hours of culture, the levels of IFN-γ, IL-4 and IL-17 in the culture supernatants were comparable in the two mouse strains (Fig. 3). Therefore, these observations suggest that, for the sensitization phase of CHS, IL-31 is not essential for such skin DC functions as migration, maturation and hapten-specific T-cell induction.
Figure 3.
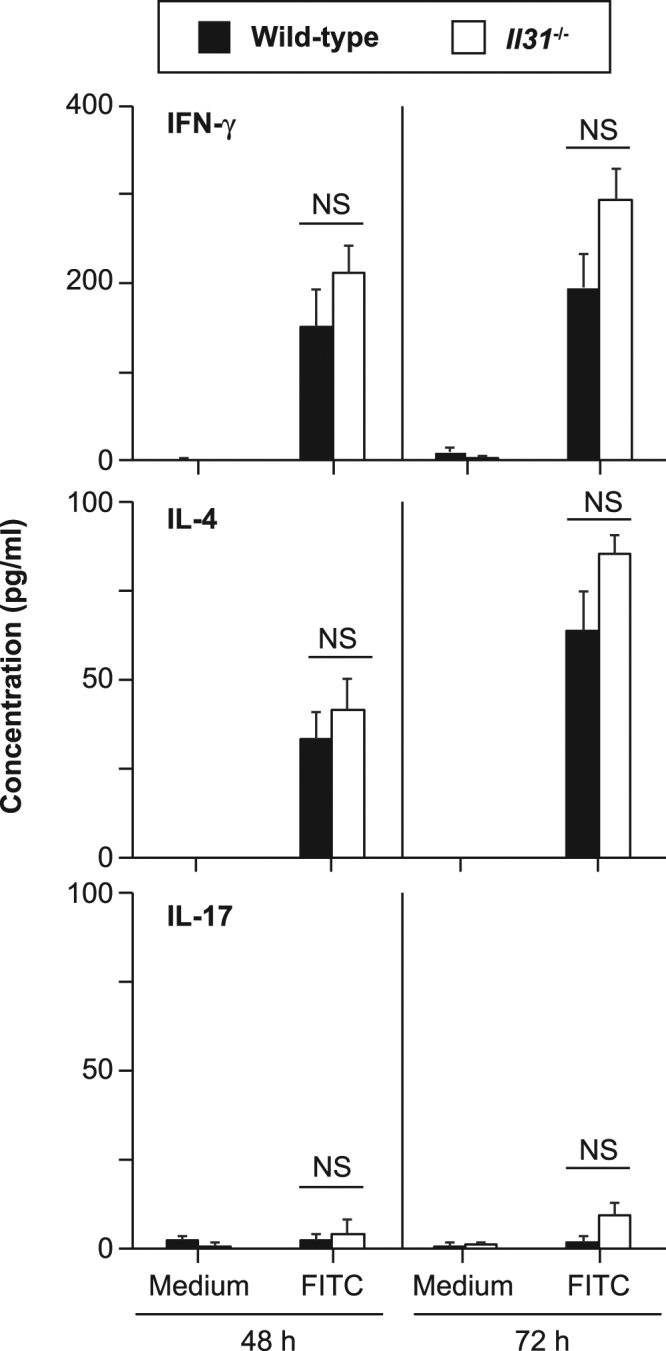
Normal hapten-specific LN cell responses in Il31−/− mice. (a) LN cells from FITC-sensitized wild-type (n = 5) and Il31−/− (n = 5) mice were cultured in the presence and absence of 40 μg/ml FITC for 48 and 72 hours. The levels of IFN-γ, IL-4 and IL-17 in the culture supernatants were determined by ELISA. The data show the mean + SEM.
IL-31 is involved in pruritus, but not inflammation, during CHS
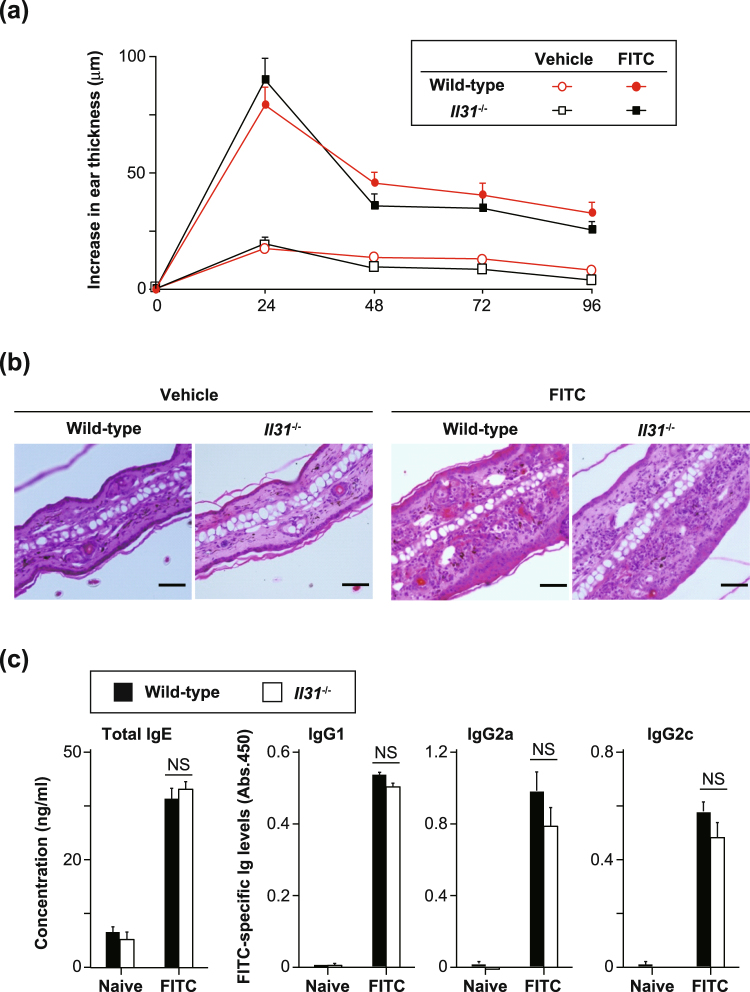
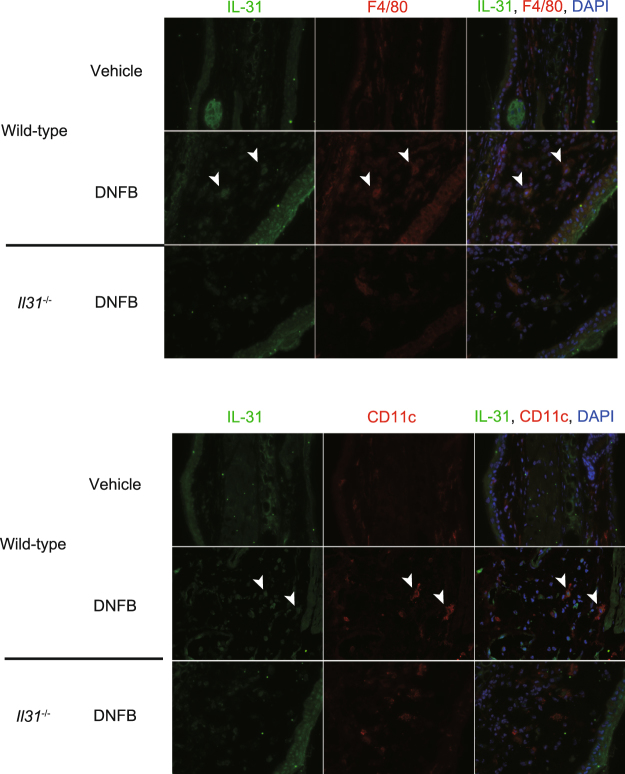
We next investigated whether IL-31 is involved in the pathogenesis of FITC-induced CHS. The degree of skin inflammation was assessed by measuring the thickness of the ear skin after challenge with FITC. The ear thickness was comparable between wild-type and Il31−/− mice at the indicated time points after the initial epicutaneous application of FITC (Fig. 4a). The degree of skin inflammation assessed by histological analysis was also similar between the wild-type and Il31−/− mice at 24 hours after challenge with FITC or vehicle (Fig. 4b). We also used ELISA to examine the levels of FITC-specific IgG subsets and total IgE in sera from naïve and FITC-treated mice. After FITC challenge, the levels of FITC-specific IgG1, IgG2b and IgG2c and total IgE in sera from Il31−/− mice increased similarly to those from wild-type mice (Fig. 4c). On the other hand, the scratching frequency and duration were significantly decreased in Il31−/− mice compared with wild-type mice at 24 hours after FITC challenge (Fig. 5). Similar phenotypes were also observed for Il31−/− mice during DNFB-induced CHS. Although the ear thickness was slightly, but not significantly, increased in Il31−/− mice compared with wild-type mice at the indicated time points after the initial epicutaneous application of DNFB (Fig. 6a), the scratching frequency and duration were significantly decreased in Il31−/− mice compared with wild-type mice at 24 hours after DNFB challenge (Fig. 6b). GFP+ cells were hardly detected in LNs from Il31gfp/+ mice during DNFB-induced CHS (data not shown). We found that a part of F4/80+ and CD11c+ cells, but not CD3+ cells, Gr1+ cells and tryptase+ cells (data not shown), were producers of IL-31 in skin from wild-type mice, but not Il31−/− mice, at 24 h after DNFB challenge (Fig. 7). The signals in keratinocytes from wild-type mice were due to non-specific binding of the anti-IL-31 Ab, since they were detected even in Il31−/− mice (Fig. 7). These observations suggest that IL-31 is important for induction of pruritus, but not inflammation, in CHS induced by FITC and DNFB.
Figure 4.
IL-31 is not essential for skin inflammation during FITC-induced CHS. (a) Wild-type (n = 47) and Il31−/− (n = 41) mice were epicutaneously sensitized and challenged with FITC. The ear skin thickness of the mice was measured at the indicated time points after challenge with FITC or vehicle alone. The data show the mean + SEM. (b) Sections of ear skin from wild-type and Il31−/− mice at 24 hours after challenge with FITC or vehicle alone were stained with hematoxylin and eosin. Representative data are shown. Scale bar = 50 μm. (c) Ninety-six hours after challenge with FITC, sera were collected from wild-type (n = 5 [naïve], and n = 21 [FITC]) and Il31−/− (n = 5 [naïve], and n = 15 [FITC]) mice. The levels of FITC-specific IgG1, IgG2a and IgG2c and total IgE in the sera were determined by ELISA. The data show the mean + SEM.
Figure 5.
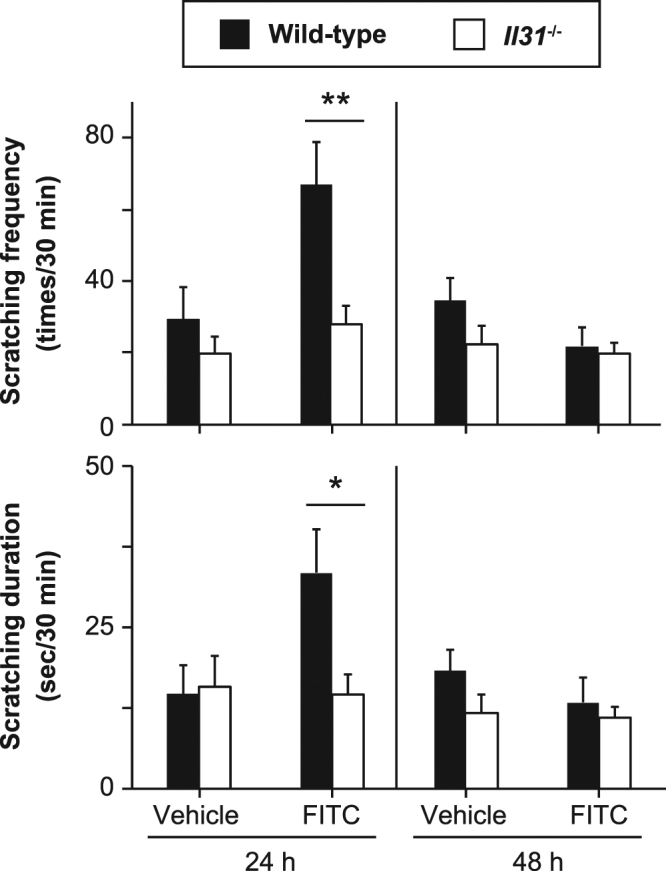
IL-31 is important for pruritus during FITC-induced CHS. (a) Scratching behavior (number of times and duration) in wild-type (n = 12–14) and Il31−/− (n = 12–15) mice was assessed at 24 and 48 hours after challenge with FITC or vehicle alone. The data show the mean + SEM. *p < 0.05 and **p < 0.01.
Figure 6.
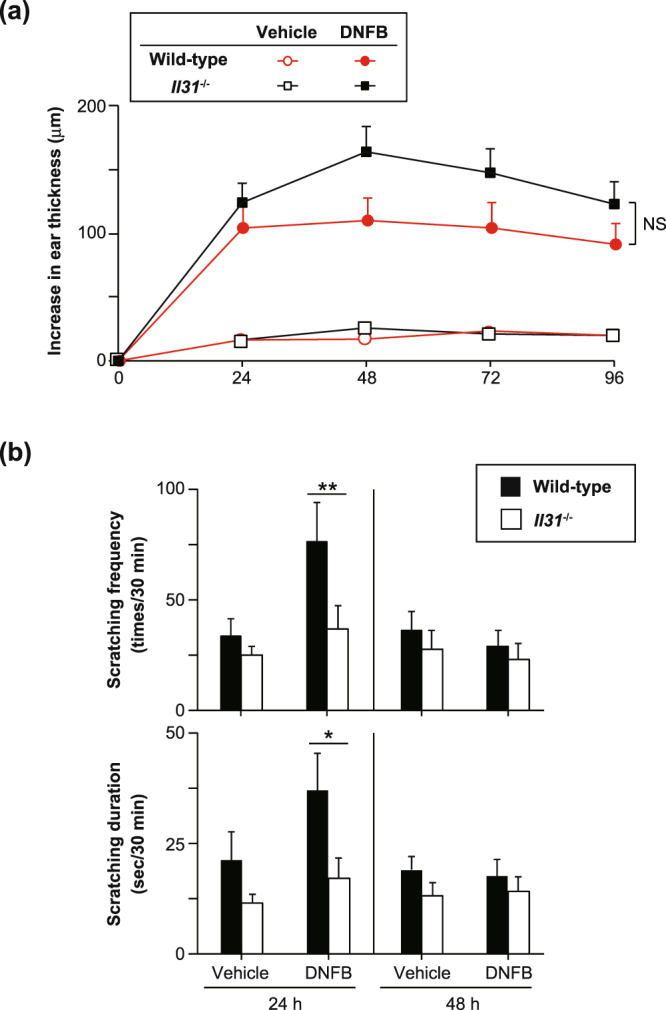
IL-31 is important for pruritus, but not skin inflammation, during DNFB-induced CHS. (a) Wild-type (n = 12) and Il31−/− (n = 12) mice were epicutaneously sensitized and challenged with DNFB. The ear skin thickness of the mice was measured at the indicated time points after challenge with DNFB or vehicle alone. The data show the mean + SEM. (b) Scratching behavior (number of times and duration) in wild-type (WT; n = 8–13) and Il31−/− (n = 8–12) mice was assessed at 24 and 48 hours after challenge with DNFB or vehicle alone. The data show the mean + SEM. *p < 0.05 and **p < 0.01.
Figure 7.
IL-31 is produced by macrophages and dermal DCs in the skin during DNFB-induced CHS. Wild-type and Il31−/− mice were epicutaneously sensitized and challenged with DNFB. 24 h after the epicutaneous challenge with DNFB or vehicle alone, the ear skin was collected. IL-31 expression in sections of the ear skin was detected by immunohistochemistry. Arrowhead(s) = representative IL-31+ F4/80+ cells or IL-31+ CD11c+ cells.
Discussion
IL-31 is preferentially produced by Th2 cells in Th subsets, and excessive IL-31 production in IL-31 Tg mice resulted in development of dermatitis due to enhancement of type–2 immune responses1. By contrast, Il31ra−/− mice showed enhanced type–2 immune responses during nematode infection23, suggesting that IL-31R signaling has a suppressive role in type-2 immune responses. Regarding the apparent discrepancies in phenotype between IL-31 Tg mice1 and Il31ra−/− mice23, the latter were reported to be hyperresponsive to OSM due to increased formation of OSM receptors (gp130 and OSMR) caused by the lack of IL-31R (IL-31RA and OSMR), resulting in OSM-mediated suppression of type–2 immune responses13. Therefore, to investigate the precise role of IL-31 in vivo, we newly generated Il31−/− mice for the present study. Using those mice, we demonstrated for the first time that IL-31 is important for induction of pruritus, but not inflammation, in CHS induced by FITC and DNFB.
IL-31 is considered to be involved in the pathogenesis of type–2 cytokine-associated allergic disorders such as rhinitis, asthma and dermatitis5. In support of that notion, the levels of IL-31 were increased in patients with allergic rhinitis11, asthma12 and atopic dermatitis14,15. DNFB- and/or FITC-induced CHS was attenuated in Il4−/− mice24,25 and/or stat6−/− mice26, suggesting that type-2 cytokines are important for induction of DNFB- and/or FITC-induced CHS. In addition, the levels of IL-31 were also elevated in patients with contact dermatitis16. These observations suggest that IL-31 may be involved in induction of type-2-cytokine-associated CHS. However, we demonstrated that Il31−/− mice showed normal migration and maturation of skin DCs and induction of hapten-specific T cells in the sensitization phase of FITC-induced CHS, and normal induction of local inflammation in the elicitation phase of FITC- and DNFB-induced CHS, indicating that IL-31 is not essential for induction of skin inflammation during FITC- or DNFB-induced CHS.
As a unique function of IL-31, it is known to be involved in itching via signal activation of IL-31 receptors on sensory nerve cells5,27. Indeed, IL-31 Tg mice show severe scratching behavior accompanied by exfoliation of epidermis1. Administration of anti-IL-31 or anti-IL-31RA neutralizing Ab resulted in attenuation of scratching behavior, but not the severity of skin inflammation, in mice that developed atopic dermatitis-like skin inflammation18,19. Consistent with this, we showed that Il31−/− mice had reduced scratching frequency and duration in FITC- and/or DNFB-induced CHS, indicating that IL-31 is responsible for pruritus in the setting. However, the underlying mechanism of this remains unclear and should be further investigated in future studies.
In summary, we demonstrated here that IL-31 is involved in pruritus reactions without affecting induction of local skin inflammation in CHS.
Methods
Mice
C57BL/6 N wild-type mice were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Il31−/− mice on the C57BL/6 N background were generated as follows. The Il31 gene was disrupted by replacement of the region from a part of exon 2 to a part of the intron behind exon 3 with a cassette consisting of IRES-EGFP and a neomycin resistance gene, flanked by loxP sequences (Fig. 1a). Homologous regions were amplified by PCR using the following primers: 5′-CAACCTATTCCTAGTTCCCTCACC-3′ and 5′-AAAGCAGCACCAGGGTAGGCTTCG-3′ to generate a 6-kb fragment, and 5′- TGAATGAGGGGAACAGAAAATTACC-3′ and 5′-TGATGAATAATGATATTCCTTACC-3′ to generate a 2-kb fragment. The targeting vector was electroporated into C57BL/6 N ES cells (EGR-101, kindly provided by Dr. Masaru Okabe, Osaka University). Male chimeric mice were obtained from three distinct targeted clones and mated with C57BL/6 N female mice. Genotyping of Il31−/− mice was performed by PCR using the following primers: common 1 (5′-GACAACCCTTTATATATTGCCTGG-3′), WT 1 (5′-GCTTCCATAGCTGGCTTTATATCG-3′) and IL-31 KO (5′-CGACACCGGCCTTATTCCAAGCGG-3′). The common 1 and WT 1 primers were used for detection of wild-type alleles (~550 bp), and the common and IL-31 KO primers were used for detection of mutant alleles (~390 bp). For generation of Il31egfp/egfp mice, a plasmid carrying Cre cDNA (pCAG-Cre, kindly provided by Dr. Jun-ichi Miyazaki, Osaka University) was injected into fertilized eggs from Il31−/− mice to deplete the neomycin resistance gene, and it was flanked by loxP sequences. The eggs were then transferred into pseudopregnant female mice. Genotyping of Il31egfp/egfp mice was performed by PCR using the following primers: common 2 (5′-TGGGAAGATACATGAAACCAATCC-3′), WT 2 (5′-CACTGGCTGCTCTTCCAGAGGACC-3′) and Neor (5′-GACGTGCTACTTCCATTTGTCACG-3′). The common and WT primers were used for detection of wild-type alleles (~493 bp), and the common and Neor primers were used for detection of mutant alleles (no band). All mice were housed in a specific-pathogen-free environment at The Institute of Medical Science, The University of Tokyo. The animal protocol for experiments was approved by the Institutional Review Board of the Institute (A11-28 and A14-10), and all experiments were conducted according to the ethical and safety guidelines of the Institute.
Quantitative PCR
Total RNA was prepared from lungs using Sepasol (Nacalai Tesque, Inc.) and treated with DNase (TURBO DNA-free-kit; Thermo Fisher Scientific Inc., MA). cDNA was synthesized from the isolated RNA by RT-PCR (PrimeScript RT Reagent kit; TAKARA BIO Inc., Shiga, Japan). Quantitative real-time PCR was performed with SYBER Premix Ex Taq (TAKARA BIO Inc.) or SYBER Premix DimerEraser (TAKARA BIO Inc.) using a CFX384TM Touch Real-time PCR Detection System (BioRad Laboratories, Inc., Hercules, CA). Relative gene expression was determined against HPRT gene expression. The following PCR primers were designed: forward primer 5′-ATACAGCTGCCGTGTTTCAG-3′ and reverse primer 5′-AGCCATCTTATCACCCAAGAA-3′ for Il31 mRNA; and forward primer 5′-GGCCAGACTTTGTTGGATTTG-3′ and reverse primer 5′-CGCTCATCTTAGGCTTTGTATTTG-3′ for Hprt mRNA.
Flow cytometry
Spleen cells were incubated with anti-mouse CD16/CD32 mAb (2.4G2; BD Biosciences, San Jose, CA) in FACS buffer (HBSS containing 2% FCS) for FcR blocking for 15 min on ice, and then incubated with BV421-conjugated anti-mouse CD3 mAb (145-2C11; Biolegend, San Diego, CA), APC-conjugated anti-mouse CD8 mAb (53-6.7; Biolegend), PE-conjugated anti-mouse CD4 (GK1.5 Biolegend) or Siglec F (E50-2440; BD Biosciences) mAb, PE-Cy7-conjugated anti-mouse CD19 mAb (6D5; Biolegend) or Gr1 (RB6-8C5; Affymetrix eBioscience, San Diego, CA) mAb for 20 min on ice. The expression profile of each cell surface marker on 7-aminoactinomycin D (7-AAD; Sigma-Aldrich Co. LLC, St. Louis, MO) -negative cells was analyzed on a MACSQuant Analyser (Miltenyi Biotec, Bergisch Gladbach, Germany) with MACSQuantify Software (Miltenyi Biotec) and FlowJo software (Tree Star Inc., Ashland, OR).
Skin DC migration
Skin DC migration was determined as described elsewhere28. In brief, mice were epicutaneously treated with 40 µl of 0.5% (w/v) FITC isomer I (Sigma-Aldrich Co. LLC) in a 1:1 mixture of acetone (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and dibutyl phthalate (Sigma-Aldrich Co. LLC) (20 µl to each surface of the left ear) and the vehicle alone (20 µl to each surface of the right ear). Twenty-four hours later, submaxillary lymph nodes (LNs) were separately collected from both the FITC-treated left and vehicle-treated right ears. After incubation with anti-mouse CD16/CD32 mAb (2.4G2, BD Biosciences) for 15 minutes on ice, LN cells were stained with PE-conjugated anti-mouse CD11c mAb (HL3, BD Biosciences) and APC-conjugated anti-mouse MHC Class II (I-A/I-E) mAb (M5/114.15.2, Affymetrix eBioscience) for 20 minutes on ice. The percentage of FITC-positive cells among 7-AAD-negative MHC Class IIhi CD11c+ cells was determined using a FACS Verse (BD Biosciences), and data analyses were performed using FlowJo software (Tree Star Inc.).
Skin DC maturation
A shaved area on the back and both surfaces of both ears of mice were painted with 150 µl and 20 µl of 0.5% FITC isomer I, respectively. Twenty-four hours later, inguinal, axillary and submaxillary LNs were harvested and pooled. LN single-cell suspensions were prepared and incubated with anti-mouse CD16/CD32 mAb for 15 minutes on ice. The cells were then stained with APC-conjugated MHC-class II mAb, PE-Cy7-conjugated anti-mouse CD11c mAb, and PE-conjugated mAb for CD86 (GL-1, Biolegend), CD40 (3/23, Biolegend) or OX40L (RM134L, Biolegend) for 20 minutes on ice. The expression levels of CD86, CD40 and OX40L on 7-AAD-negative MHC Class IIhi CD11c+ FITC+ cells were determined using a FACS Verse (BD Biosciences), and data analyses were performed using FlowJo software (Tree Star Inc.).
Induction of contact hypersensitivity (CHS)
CHS was induced with FITC and DNFB as described elsewhere28,29. Briefly, 2 days after shaving the back hair with electrical clippers, the back skin was treated with 150 µl of 1.0% FITC isomer I in a 1:1 mixture of acetone and dibutyl phthalate or 25 µl of 0.5% DNFB (SIGMA) in a 4:1 mixture of acetone and olive oil. Five days later, the respective mice were challenged with 40 µl of 0.5% FITC isomer I or 0.2% DNFB (each surface of the left ear) and 40 µl of the vehicle alone (each surface of the right ear). The ear thickness was measured before and after FITC challenge using dial thickness gauges (Ozaki MFG. Co., Ltd., Tokyo, Japan).
Measurement of levels of serum immunoglobulins
Sera were collected from mice 4 days after FITC challenge during FITC-induced CHS. The levels of FITC-specific Igs in the sera were determined by ELISA as described elsewhere29. In brief, 96-well flat-bottom plates (Thermo Fisher Scientific Inc.) were coated with 20 µg/ml FITC-conjugated OVA at 4 °C overnight. After the wells were blocked with PBS containing 1% FBS, optimally diluted serum samples (IgG1 = 1/100; IgG2b = 1/100; IgG2c = 1/10) were placed in the wells, and the plates were incubated at room temperature for 2 hours. After washing, HRP-conjugated anti-mouse IgG1, IgG2b or IgG2c mAb (Bethyl Laboratories, Inc., TX) was added, followed by incubation at room temperature for 1 hour. For enzymatic reaction, TMB substrate (Nacalai Tesque Inc.) was used as a substrate. The reaction was stopped by addition of 0.2 M H2SO4, and then the absorbance (450 nm) was measured using a VersaMax (Molecular Devices, LLC, Sunnyvale, CA). Data show the absorbance value at 450 nm. The levels of total IgE in sera were determined using an ELISA kit (Bethyl Laboratories, Inc.) in accordance with the manufacturer’s instructions.
FITC-specific LN cell responses
FITC-specific LN cell responses were determined as described elsewhere28,29. Briefly, mice were epicutaneously treated with 1.0% FITC isomer I in a 1:1 mixture of acetone and dibutyl phthalate on both the left and right ears (20 μl on one surface of each ear). Six days later, submaxillary LNs were collected. The LN cells were cultured in the presence and absence of 40 μg/ml FITC at 37 °C for 72 hours. The levels of cytokines in each culture supernatant were determined with mouse IFN-γ, IL-4 and IL-17A ELISA kits obtained from Biolegend (San Diego, CA) or Peprotech (Rocky Hill, NJ).
Assessment of scratching behaviour
SCLABA-Real (NOVELTEC) was used to assess scratching behavior (frequency and duration) at 24 and 48 hours after challenge with FITC or DNFB during FITC- or DNFB-induced CHS.
Histology
Twenty-four hours after FITC or vehicle challenge, the ear skins were harvested, fixed in Carnoy’s fluid and embedded in paraffin. Sections were prepared and stained with hematoxylin-eosin.
Immunohistochemistry
Twenty-four hours after epicutaneous challenge with DNFB as described above, the ear skin was frozen in OCT compound (Sakura Finetek, Tokyo, Japan). Sections (4.5-μm thick) were prepared using a cryostat (Leica) and dried on aminosilane-coated slide glasses (Matsunami Glass, Osaka, Japan). The sections were fixed in 4% paraformaldehyde at room temperature for 10 min and incubated with Blocking One Histo (Nacalai Tesque, Inc.) and Avidin/Biotin Blocking Kit (Vector Laboratories, CA) at room temperature for 10 min. They were then incubated with primary Abs, rabbit anti-mouse CD3 Ab (ab5690; Abcam plc., Tokyo, Japan), hamster anti-mouse CD11c Ab (ab33484; Abcam plc.), rat anti-mouse F4/80 (MCA497GA; Bio-Rad), biotin-conjugated goat anti-mouse IL-31 Ab (BAF3028; R&D Systems), rat anti-mouse Ly-6G/-6C Ab (ab2557; Abcam plc.) or rabbit anti-mouse tryptase Ab (ab134932; Abcam plc.), at 4 °C overnight. After washing, the sections were incubated with secondary Abs/reagent, Alexa fluor® 594-conjugated donkey anti-rabbit Ab (ab150064; Abcam plc.), Alexa fluor® 594-conjugated donkey anti-rat Ab (ab150156; Abcam plc.), Alexa fluor® 594-conjugated goat anti-hamster Ab (A21113: Thermo Fisher Scientific), or Alexa fluor® 488-conjugated streptavidin (S11223: Thermo Fisher Scientific), at 4 °C for 60 min. Nuclei were stained with DAPI (R37606; Thermo Fisher Scientific). Images were acquired using a fluorescent microscope (BZX-700, KEYENCE, Tokyo, Japan) and analyzed with a software (BZ-analysis application, KEYENCE).
Statistics
A two-tailed Mann-Whitney U test was performed for statistical significance using Graphpad Prism software (Graphpad Prism). P values of less than 0.05 were considered statistically significant.
Acknowledgements
We thank Masako Fujiwara and Yoshiko Shimamoto for their skilled technical assistance. We also thank Lawrence W. Stiver (Tokyo, Japan) for his critical reading of the manuscript. This work was supported by Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency (S.N.).
Author Contributions
A.T. and S.N. designed the experiments. A.T., K. Sato, S.Y., T.N., T.S., T.Y., K.A. and H.M. performed the experiments. A.N., S.Y., K. Sudo and S.N. generated the Il31−/− mice. K. Matsuda and H.M. assessed the scratching behavior. K. Matsumoto, J.K. and K.O. provided resources. A.T. and S.N. wrote the manuscript. All authors reviewed the article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dillon SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 2.Ishii T, et al. Pivotal role of mast cells in pruritogenesis in patients with myeloproliferative disorders. Blood. 2009;113:5942–5950. doi: 10.1182/blood-2008-09-179416. [DOI] [PubMed] [Google Scholar]
- 3.Niyonsaba F, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184:3526–3534. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen C, et al. Ultraviolet B radiation and reactive oxygen species modulate interleukin-31 expression in T lymphocytes, monocytes and dendritic cells. Br J Dermatol. 2011;165:966–975. doi: 10.1111/j.1365-2133.2011.10487.x. [DOI] [PubMed] [Google Scholar]
- 5.Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14:423. doi: 10.1007/s11882-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 6.Hermanns HM, Oncostatin M. and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26:545–558. doi: 10.1016/j.cytogfr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Horejs-Hoeck J, et al. Dendritic cells activated by IFN-gamma/STAT1 express IL-31 receptor and release proinflammatory mediators upon IL-31 treatment. J Immunol. 2012;188:5319–5326. doi: 10.4049/jimmunol.1101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dambacher J, et al. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut. 2007;56:1257–1265. doi: 10.1136/gut.2006.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip WK, et al. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122:532–541. doi: 10.1111/j.1365-2567.2007.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 induces pro-inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins. Allergy. 2010;65:712–721. doi: 10.1111/j.1398-9995.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 11.Baumann R, et al. The release of IL-31 and IL-13 after nasal allergen challenge and their relation to nasal symptoms. Clin Transl Allergy. 2012;2:13. doi: 10.1186/2045-7022-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei Z, et al. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. 2008;63:327–332. doi: 10.1111/j.1398-9995.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 13.Bilsborough J, Mudri S, Chadwick E, Harder B, Dillon SR. IL-31 receptor (IL-31RA) knockout mice exhibit elevated responsiveness to oncostatin M. J Immunol. 2010;185:6023–6030. doi: 10.4049/jimmunol.0902769. [DOI] [PubMed] [Google Scholar]
- 14.Sonkoly E, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Raap U, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122:421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Neis MM, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Takaoka A, et al. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180–181. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Grimstad O, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasutani K, et al. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br J Pharmacol. 2014;171:5049–5058. doi: 10.1111/bph.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruzicka T, et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med. 2017;376:826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 23.Perrigoue JG, et al. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigmann B, et al. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase of the elicitation reaction. Scand J Immunol. 1997;45:308–314. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]
- 25.Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. Inhibition of allergic contact dermatitis to DNCB but not to oxazolone in interleukin-4-deficient mice. J Invest Dermatol. 1999;112:476–482. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 26.Yokozeki H, et al. Signal transducer and activator of transcription 6 is essential in the induction of contact hypersensitivity. J Exp Med. 2000;191:995–1004. doi: 10.1084/jem.191.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cevikbas F, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suto H, et al. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 29.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]