Abstract
Background
The anti-programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) monoclonal antibody has a good effect in the treatment of non-small cell lung cancer (NSCLC), but not all PD-1/PD-L1 positive patients can get benefit from it. Compensatory expression of other immune checkpoints may be correlated with the poor efficacy of anti-PD-1/PD-L1 monoclonal antibodies. The inhibitory human leukocyte antigen (HLA)/killer cell Ig-like receptor (KIR) can effectively block the killing effect of natural killer (NK) cells on tumors. Our previous studies have confirmed that high expression of KIR was correlated with poor prognosis of NSCLC. Inhibitory KIR expression was positively correlated with the expression of PD-1.
Methods
The expressions of KIR 2D (L1, L3, L4, S4) (BC032422/ADQ31987/NP_002246/NP_036446, Abcam) and PD-1 (NAT 105, Cell marque) proteins was assessed by immunohistochemistry.
Results
The expression of inhibitory KIR in tumor cells or tumor infiltrating lymphocytes (TILs) is associated with PD-1 expression. Among PD-1 positive patients, 76.3% were KIR 2D (L1, L3, L4, S4) positive on tumor cells, and 74.6% were KIR 2D (L1, L3, L4, S4) positive on TILs. We compared the expression of inhibitory KIR before and after treatment with nivolumab in 11 patients with NSCLC. We found that five (45.5%) patients had positive expression of inhibitory KIR in tumor tissue after being treated with anti-PD-1 monoclonal antibodies, two of whom exhibited a significant increase in expression of inhibitory KIR, and three showed no change.
Conclusions
PD-1 expression was correlated with KIR 2D (L1, L3, L4, S4) on tumor cells or TILs. The resistance to anti-PD-1 monoclonal antibody treatment might be related to KIR. The inhibitory HLA/KIR could combine with the PD-1/PD-L1 signaling pathway negatively regulating NSCLC tumor immunity.
Keywords: non-small cell lung cancer, immune therapy, HLA/KIR, PD-1/PD-L1, tumor immune escape
Introduction
Lung cancer is one of the most common cancers in the world.1 Most lung cancer patients are diagnosed at an advanced stage.2 In addition to traditional chemotherapy, targeted therapy has become a common treatment for advanced non-small cell lung cancer (NSCLC). However, only patients with a driver mutation can get benefit from it. Moreover, resistance to the targeted therapy is inevitable.3–5 Therefore, searching for a safer and more effective treatment is necessary.
Cancer immunotherapy has developed dramatically in recent years. Blocking immune checkpoints, such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), to activate T cell immune response to tumors has become a new anti-cancer strategy.6–11 PD-1/PD-L1 is an important pathway in tumor immune escape. When PD-1 binds to PD-L1, inhibitory signals can be delivered to activate T cells to inhibit cytotoxic T lymphocytes (CTLs).6 High expression of PD-L1 is indicative of poor prognosis in malignant tumors such as kidney, ovarian and lung cancer.12–14 In our previous studies, we analyzed the expression of PD-1 and PD-L1 in NSCLC patient surgical tumor tissues and found that patients with higher expression of PD-L1 had poorer prognosis.15 Checkmate 017, 057, Keynote-010, 024 and OAK showed that anti-PD-1/PD-L1 monoclonal antibodies (nivolumab, pembrolizumab and atezolizumab) could not only improve the objective response rate (ORR), but also prolong the overall survival (OS) in NSCLC patients. Based on those studies, the US Food and Drug Administration (FDA) has approved anti-PD-1/PD-L1 monoclonal antibodies to be the standard treatment for NSCLC patients.16–20
Although anti-PD-1/PD-L1 monoclonal antibodies can achieve a good response in advanced NSCLC, not all patients with PD-1/PD-L1 positive expression will benefit from them. The efficacy of PD-1/PD-L1 inhibitors was about 20% in advanced NSCLC patients.17,18,20 As with targeted therapy, resistance to immunotherapy is an inevitable problem.21 In a malignant melanoma study, 15 of 42 patients (35%) treated with anti-PD-1 monoclonal antibodies developed resistance. The resistance mechanism may be related to the mutation of Jana kinase 1 (JAK1), JAK2 and β2-microglobulin (B2M).22 Another study found that anti-PD-1 monoclonal antibody treatment resistance significantly increased the expression of T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), which suggested that the resistance to anti-PD-1 monoclonal antibody treatment might be related to other immunological checkpoints. The compensatory high expression of other immunological checkpoints might be involved in the resistance mechanisms to anti-PD1/PD-L1 monoclonal antibodies.23 Therefore, it is logical to consider whether the combination of anti-PD-1/PD-L1 monoclonal antibodies with other immune checkpoint inhibitors may effectively overcome anti-PD-1/PD-L1 monoclonal antibody resistance.
Combination treatments using anti-PD-1/PD-L1 monoclonal antibodies with other treatments including chemotherapy, anti-angiogenic medicines and immune therapy are the focus of multiple recent studies. The CheckMate-012 study reported the results of the combination therapy of nivolumab and ipilimumab (anti-CTLA-4 monoclonal antibodies).24 The benefit observed from combining nivolumab and ipilimumab may be due to synergistic mechanisms of increasing T cell activity. Our previous studies have confirmed that high expression of killer cell Ig-like receptor (KIR) was correlated with poor prognosis of NSCLC, and inhibitory KIR expression was positively correlated with the expression of PD-1. In this study, we found the correlation between PD-1 and KIR expression and analyzed whether the resistance of anti-PD-1 monoclonal antibody treatment is related to KIR.
Methods
Patients
Primary tumor specimens were obtained from 130 NSCLC surgical patients, Chan Lab, University of Colorado, USA from June 2008 to October 2013. The patients had not undergone radiation or chemotherapy before surgery. The surgical histology reports were reviewed and the lymph node and lung cancer stages were categorized by the 7th edition International Association for the Study of Lung Cancer (IASLC) TNM staging system. Another 11 NSCLC patients treated with nivolumab were enrolled. The protocol was approved by the Shanghai Pulmonary Hospital, Tongji University. All patients were competent to provide their consent. All the patients provided written informed consent for the use of their tumor specimens in research. The University of Colorado approved the use of the tumor specimens and histological reports.
IHC for PD-1 and KIR 2D (L1, L3, L4, S4)
The immunohistochemistry (IHC) method for PD-1 was described in our published paper.15 The IHC method for KIR 2D (L1, L3, L4, S4) was described in our published paper.25
Statistical analysis
We performed statistical analysis by SPSS 17.0. Chi-square tests were used to analyze correlation between KIR protein expression and PD-1. All statistics were 2-sided and statistical significance was defined as P<0.05.
Results
Patient characteristics
Of the patients, 82 (71.0%) were less than 70 years old and 48 (29.0%) were more than 70 years old; 60 (48.4%) were male and 70 (51.6%) were female. The median age was 58 years old. There were 39 (21.0%) who had never smoked, while 91 (79.0%) were smokers; 22 (19.4%) had stage I–II lung cancer, while 108 (80.6%) were stage III–IV; 67 (67.7%) had adenocarcinoma and 49 (25.8%) had squamous cell carcinoma (SCC) (Table 1).
Table 1.
Patient characteristics
| Characteristic | Total |
|---|---|
| Age (years), median | 58 |
| <70, n (%) | 82 (71.0%) |
| ≥70, n (%) | 48 (29.0%) |
| Gender, n (%) | |
| Male | 60 (48.4%) |
| Female | 70 (51.6%) |
| Smoking status, n (%) | |
| Non-smoker | 39 (21.0%) |
| Smoker | 91 (79.0%) |
| Lung cancer staging, n (%) | |
| I–II | 22 (19.4%) |
| III–IV | 108 (80.6%) |
| Pathology, n (%) | |
| SCC | 49 (25.8%) |
| Adenocarcinoma | 67 (67.7%) |
| NSCLC NOS/mixed | 14 (6.5%) |
Abbreviations: NSCLC, non-small cell lung cancer; NOS, not otherwise specified; SCC, squamous cell carcinoma.
Correlation between PD-1 and KIR expression
Among PD-1 positive patients, 76.3% were KIR 2D (L1, L3, L4, S4) positive on tumor cells, and 74.6% were KIR 2D (L1, L3, L4, S4) positive on tumor infiltrating lymphocytes (TILs). High expression of PD-1 was significantly correlated with higher expression of KIR 2D (L1, L3, L4, S4) on tumor cells (P=0.040) and TILs (P=0.018) (Table 2).
Table 2.
Correlation between KIR 2D L1–3 and PD-1 expression
| Characteristic | PD-1 on TILs
|
||
|---|---|---|---|
| Negative | Positive | P-value | |
| KIR 2D on tumor cells, n (%) | |||
| Negative | 30 (42.3%) | 14 (23.7%) | 0.040 |
| Positive | 41 (57.7%) | 45 (76.3%) | |
| KIR 2D on TILs, n (%) | |||
| Negative | 33 (46.5%) | 15 (25.4%) | 0.018 |
| Positive | 38 (53.5%) | 44 (74.6%) | |
Abbreviations: KIR, killer cell Ig-like receptor; PD-1, programmed death-1; TILs, tumor infiltrating lymphocytes.
KIR expression before and after anti-PD-1 monoclonal antibody treatment
We compared the expression of inhibitory KIR before and after treatment with nivolumab in 11 patients with NSCLC. We found that five (45.5%) patients had positive expression of inhibitory KIR in tumor tissue after being treated with anti-PD-1 monoclonal antibodies, two of whom exhibited a significant increase in expression of inhibitory KIR and three showed no change (Figure 1).
Figure 1.
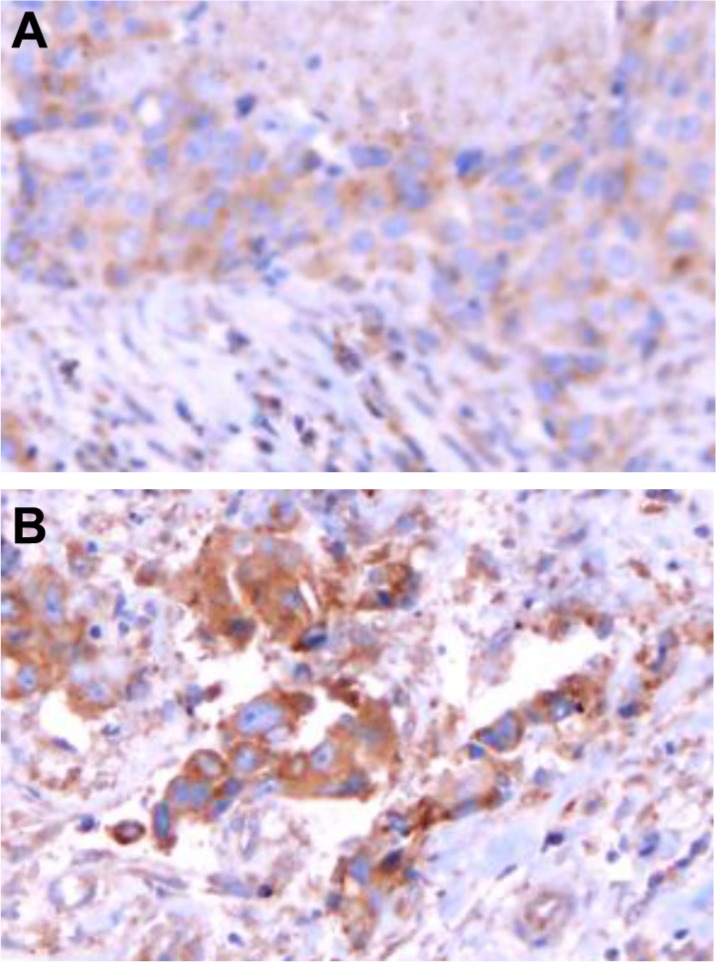
Inhibitory KIR expression on NSCLC before and after treatment with anti-PD-1 monoclonal antibodies. (A) Inhibitory KIR expression in NSCLC before treatment with anti-PD-1 monoclonal antibodies. (B) Inhibitory KIR expression in NSCLC after treatment with anti-PD-1 monoclonal antibodies.
Abbreviations: KIR, killer cell Ig-like receptor; NSCLC, non-small cell lung cancer; PD-1, programmed death-1.
Discussion
To our knowledge, this is the first study to analyze the correlations between PD-1 and KIR. We observed KIR expression before and after anti-PD-1 monoclonal antibody treatment. We found that the resistance to anti-PD-1 monoclonal antibody treatment might be related to KIR. The inhibitory HLA/KIR could combine with the PD-1/PD-L1 signaling pathway, negatively regulating NSCLC tumor immunity.
In addition to T cell immunity, natural killer (NK) cells also play a critical role in tumor immunity. NK cells account for about 10%–15% of the total number of human peripheral blood lymphocytes, and are mainly involved in immune surveillance to eliminate tumors, viral infections and other abnormal cells.26 In the case of tumors and viral infections, the low expression of human leukocyte antigen (HLA) on the cell surface triggers the NK cells to attack. KIR (CD158) is in the HLA-binding receptor family.27,28 The binding of inhibitory KIR to HLA can decrease the killing response of NK cells to abnormal cells, promoting susceptibility to many diseases. For example, inhibitory KIR expression was significantly increased in malignant melanoma;29 the upregu-lation of KIR 2DL4 expression increased the invasive and metastatic activity of breast cancer.30 Inhibitory KIR can also promote the development of multiple myeloma by blocking the process of NK cell immunity and T cell cytotoxicity.31 Moreover, inhibitory KIR could downregulate tumor immunity in metastatic colon cancer, acute myeloid leukemia, invasive cervical cancer and other malignant tumors.32–34
Since inhibitory KIR plays a critical role in regulating NK cell activation, the therapeutic strategy to block inhibitory KIR function may promote the anti-tumor effect of NK cells.35 Numerous related clinical trials are ongoing now including the Phase I clinical study (NCT00552396) initiated by Innate Pharma in patients with multiple myeloma (anti-KIR monoclonal antibodies, 1-7F9) and the phase I clinical study (NCT01256073) of anti-KIR monoclonal antibodies (1-7F9) in acute myeloid leukemia patients. The prospect of combining therapy of KIR inhibition with other immunotherapy is under investigation: Bristol-Myers Squibb initiated Phase I clinical trials (NCT01750580)40 to observe the safety of Lirilumab (BMS-986015, anti-KIR monoclonal antibodies) combined with ipilimumab (anti-CTLA-4 monoclonal antibodies) in patients with progression of advanced solid tumors. Meanwhile, another Phase I clinical trial to observe the safety of Lirilumab (BMS-986015, anti-KIR monoclonal antibodies) combined with nivolumab (anti-PD-1 monoclonal antibodies) in advanced solid tumors is also in progress (NCT01714739).41 We detected inhibitory KIR in 62 newly diagnosed NSCLC patients by IHC and found out that it expressed in both tumor cells and TILs. The high expression of inhibitory KIR in tumor cells or in TILs was correlated with poor prognosis.25 These results suggested that the inhibitory HLA/KIR signaling pathway might be involved in tumor immune escape in NSCLC. Blocking inhibitory HLA/KIR might be a new option for NSCLC treatments.
Both inhibitory HLA/KIR and PD-1/PD-L1 pathways can affect tumor immunity via cytokines. Blocking the inhibitory HLA/KIR pathway induced NK cells to secrete interferon-γ (IFN-γ) in an in vitro study.36 In acute myeloid leukemia, the blockade of inhibitory KIR leads to increased expression of tumor necrosis factor (TNF-α).33 In patients with multiple myeloma, when anti-inhibitory KIR medicine was used, the expression of IFN-γ, TNF-α, interleukin-6 (IL-6), macrophage inflammatory protein-1 macrophage inflammatory protein-1-β, MIP-1-β and IL-1β increased.31 In patients with acute myeloid leukemia, the same cytokine changes were observed when using inhibitory KIR blockade medicine.33 It was reported that IFN-γ could upregulate the expression of PD-L1 on tumor cells. Subcutaneous injection of IFN-γ in an ovarian cancer mouse model could induce the expression of PD-L1, promoting tumor growth.37 In multiple myeloma, IFN-γ could also upregulate the expression of PD-L1.38 Similarly, in the melanoma mouse model, IFN-γ secreted by CD 8+T cells could promote the expression of PD-L1,39 indicating a potential circular signaling pathway. In this study, we analyzed the expressions of KIR 2DL1–3 and PD-1 protein in 130 cases of newly diagnosed NSCLC tumors. We found that the expression of inhibitory KIR in tumor cells or TILs is associated with PD-1 expression. High expression of PD-1 was significantly correlated with higher expression of KIR 2D (L1, L3, L4, S4) on tumor cells and TILs. These results confirm that there was a significant positive correlation between inhibitory KIR and PD-1, which further suggested that there was crosstalk between the HLA/KIR and the PD-1/PD-L1 signaling pathways. Further, we compared the expression of inhibitory KIR before and after treatment with nivolumab in 11 patients with NSCLC. We found that five (45.5%) patients had positive expression of inhibitory KIR in tumor tissue after being treated with anti-PD-1 monoclonal antibodies, two of whom exhibited a significant increase in expression of inhibitory KIR, and three showed no change. Therefore, we hypothesize the resistance of anti-PD-1 monoclonal antibody treatment might be related to KIR.
There were some limitations in our study. First, the size of the sample was small. Second, we lacked some data including ORR and OS to predict the prognosis. Third, we should separate NK cells from other immune cells. Further, we will prospectively analyze KIR expression on TILs and NK cells, and analyze the correlation between KIR with ORR and OS in a large scale study.
Based on the above data, we propose that inhibitory HLA/KIR can block NSCLC tumor immunity, and inhibition of HLA/KIR combined with PD-1/PD-L1 can mediate NSCLC tumor immune escape. The combined effect between NK cell immunity in the inhibitory HLA/KIR pathway and T cell immunity in the PD-1/PD-L1 pathway deserves further investigation.
Acknowledgments
This study was supported in part by a grant from Shanghai Pujiang Program (17PJD036); and “Major disease clinical skills enhancement program of three-year action plan for promoting clinical skills and clinical innovation in municipal hospitals”, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A). The fundamental research funds for the central universities (Tongji University, China [2017]).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29:2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 4.Pennell NA. Integration of EGFR inhibitors and conventional chemotherapy in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2011;12:350–359. doi: 10.1016/j.cllc.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 13.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013;14:157–163. doi: 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Rozeboom L, Rivard CJ, et al. PD-1, PD-L1 Protein expression in non-small cell lung cancer and their relationship with tumor-infiltrating lymphocytes. Med Sci Monit. 2017;23:1208–1216. doi: 10.12659/MSM.899909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, Bunn PA, Zhou C, Chan D. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget. 2016;7:82104–82111. doi: 10.18632/oncotarget.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 27.Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol Ther. 2009;8:2211–2220. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdy AK, Campbell KS. Natural killer cells and cancer. Regulation by the killer cell Ig-like receptors (KIR) Zhongguo Fei Ai Za Zhi. 2010;13:731–736. doi: 10.3779/j.issn.1009-3419.2010.07.14. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naumova E, Mihaylova A, Stoitchkov K, Ivanova M, Quin L, Toneva M. Genetic polymorphism of NK receptors and their ligands in melanoma patients: prevalence of inhibitory over activating signals. Cancer Immunol Immunother. 2005;54:172–178. doi: 10.1007/s00262-004-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueshima C, Kataoka TR, Hirata M, et al. The killer cell Ig-like receptor 2DL4 expression in human mast cells and its potential role in breast cancer invasion. Cancer Immunol Res. 2015;3:871–880. doi: 10.1158/2326-6066.CIR-14-0199. [DOI] [PubMed] [Google Scholar]
- 31.Benson DM, Jr, Cohen AD, Jagannath S, et al. A phase I trial of the anti-KIR antibody IPH2101 and Lenalidomide in patients with relapsed/refractory multiple myeloma. Clin Cancer Res. 2015;21:4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Re V, Caggiari L, De Zorzi M, et al. Genetic diversity of the KIR/HLA system and outcome of patients with metastatic colorectal cancer treated with chemotherapy. PLoS One. 2014;9:e84940. doi: 10.1371/journal.pone.0084940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vey N, Bourhis JH, Boissel N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 34.Martin MP, Borecki IB, Zhang Z, et al. HLA-Cw group 1 ligands for KIR increase susceptibility to invasive cervical cancer. Immunogenetics. 2010;62:761–765. doi: 10.1007/s00251-010-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nature Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 36.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 39.Spranger S, Spaapen RM, Zha Y, et al. Upregulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov [homepage on the Internet] Bristol-Myers Squibb; 2012. [updated July 22, 2015]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01750580?term=NCT01750580&rank=1. [Google Scholar]
- 41.ClinicalTrials.gov [homepage on the Internet] Bristol-Myers Squibb; 2012. [updated March 16, 2018]. Available from: https://www.clinical-trials.gov/ct2/show/NCT01714739?term=NCT01714739&rank=1. [Google Scholar]


