Abstract
Parvoviruses (family Parvoviridae) are small, single-stranded DNA viruses. Many parvoviral pathogens of medical, veterinary and ecological importance have been identified. In this study, we used high-throughput sequencing (HTS) to investigate the diversity of parvoviruses infecting wild and domestic animals in Brazil. We identified 21 parvovirus sequences (including twelve nearly complete genomes and nine partial genomes) in samples derived from rodents, bats, opossums, birds and cattle in Pernambuco, São Paulo, Paraná and Rio Grande do Sul states. These sequences were investigated using phylogenetic and distance-based approaches and were thereby classified into eight parvovirus species (six of which have not been described previously), representing six distinct genera in the subfamily Parvovirinae. Our findings extend the known biogeographic range of previously characterized parvovirus species and the known host range of three parvovirus genera (Dependovirus, Aveparvovirus and Tetraparvovirus). Moreover, our investigation provides a window into the ecological dynamics of parvovirus infections in vertebrates, revealing that many parvovirus genera contain well-defined sub-lineages that circulate widely throughout the world within particular taxonomic groups of hosts.
Keywords: parvovirus, Parvoviridae, ssDNA viruses, zoonotic viruses
1. Introduction
Parvoviruses are small, linear and non-enveloped viruses with single-stranded DNA (ssDNA) genomes ~5–6 kilobases (kb) in length [1]. All parvoviruses possess at least two major genes, a non-structural (NS) gene encoding the viral replicase and a capsid (VP) gene encoding the structural proteins of the virion [2]. The Parvoviridae family is divided into two subfamilies. All parvoviruses that infect vertebrates fall into one subfamily (Parvovirinae), which currently contains 41 viral species, classified into eight genera [1].
Parvoviruses cause disease in humans and domestic animals. For example, parvovirus B19—a species in the genus Erythroparvovirus—causes “erythema infectiosum” in children and polyarthropathy syndrome in adults [2], while canine parvovirus—a member of the genus Protoparvovirus—can cause haemorrhagic enteritis in dogs, with lethality in around 80% of cases [3].
In recent years, high throughput sequencing (HTS) approaches have been instrumental in the discovery of many novel parvovirus species [4,5,6,7]. Consequently, the known diversity of parvovirus species has expanded greatly and recent studies have suggested that the parvovirus host range may encompass the entire animal kingdom [8]. To understand the natural biology of vertebrate parvoviruses—that is, their dynamics in natural hosts, propensity to cause disease and zoonotic potential—it is important to document their distribution and diversity across a wide range of vertebrate species and populations. In this study, we used an HTS approach to investigate parvovirus infections among wild mammals and birds in Brazil.
2. Materials and Methods
2.1. Samples
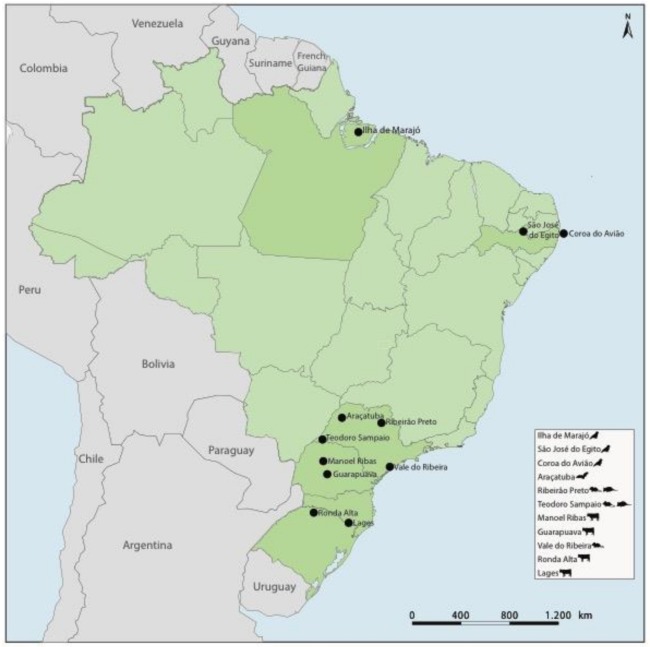
A total of 1073 specimens obtained from 21 different animal species were collected between 2007 and 2016 from rural areas of Pará, Pernambuco, São Paulo, Paraná, Santa Catarina and the Rio Grande do Sul states in Brazil. Individual specimens were distributed in 60 pools based on the species, sample type (i.e., tissue, blood, sera and cloacal swab), date and place of collection (Table S1). The species of wild animals were identified using morphological characteristics keys as previously described [9,10,11]. The geographical distribution of the pools is shown in Figure 1.
Figure 1.
Geographic locations of collected samples in Brazil.
2.2. Preparation of Pools, Viral Genome Sequencing and Assembly
Tissue samples were individually homogenized with Hank’s balanced salt solution using the TissueLyser system (Qiagen, Germantown, MD, USA). Then, the homogenized tissue, sera and cloacal swabs were centrifuged for 5 min at 10,000 g and the pools were prepared as previously described [12]. The viral genomes were extracted with a QIAamp viral RNA mini kit (Qiagen, USA) and stored at −80 °C. Subsequently, the nucleic acid was quantified using a Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, NM, USA) and the purity and integrity of nucleic acid of samples were measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
The ssDNAs were converted to dsDNA and sequenced in high-throughput sequencing using the RAPID module with the TruSeq RNA Universal kit (Illumina, San Diego, CA, USA) protocol and standard multiplex adaptors. A paired-end, 150-base-read protocol in the RAPID module was used for sequencing on an Illumina HiSeq 2500 instrument, as recommended by the manufacturer. Sequencing was performed at the Life Sciences Core Facility of the University of Campinas, Brazil. A total of 7,059,398 to 94,508,748 paired-end reads per pool were generated with 64.85% to 91.45% of bases ≥ Q30 with a base call accuracy of 99.9% (Table S1). The sequencing reads were assembled using the de novo approach in the metaViC pipeline (https://github.com/sejmodha/MetaViC) [12]. The parvovirus contigs longer than length of 200 nucleotides and supercontigs were merged and classified using DIAMOND against NCBI RefSeq protein database [13,14].
2.3. Genome Characterization
Genome size, coding potential and molecular protein weight were assessed with Geneious 9.1.2 (Biomatters, Auckland, New Zealand). The annotations of protein domains were performed using the Conserved Domain Database [15]. The nucleotide sequences determined in this study have been deposited in GenBank under the accession numbers listed in Table 1.
Table 1.
Sequences information, sources, sample, location, location, date and environment of viruses identified in wild animals from Brazil.
| Genus | Viral Species | Strain | Genome | Size (nt) | Host Species | Sample | Samples Per Pool | Location | Date | GenBank |
|---|---|---|---|---|---|---|---|---|---|---|
| Tetraparvovirus | Rodent tetraparvovirus | 1135 | Nearly complete | 5494 | Necromys lasiurus | Blood | 59 | Ribeirão Preto, SP | 2008 | MG745669 |
| Tetraparvovirus | Rodent tetraparvovirus | 3542 | Nearly complete | 5494 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745670 |
| Tetraparvovirus | Didelphimorphs tetraparvovirus | 4113 | Nearly complete | 5420 | Didelphis albiventris | Serum | 14 | Teodoro Sampaio, SP | 2009 | MG745671 |
| Aveparvovirus | Passeriform aveparvovirus | 29 | Nearly complete | 5368 | Coryphospingus pileatus | Cloacal Swab | 4 | São José do Egito, PE | 2010 | MG745672 |
| Bocaparvovirus | Rodent bocaparvovirus | 1 | Nearly complete | 5227 | Necromys lasiurus | Blood | 58 | Ribeirão Preto, SP | 2008 | MG745673 |
| Protoparvovirus | Rodent protoparvovirus | 9424 | Nearly complete | 5219 | Necromys lasiurus | Blood | 58 | Ribeirão Preto, SP | 2008 | MG745674 |
| Protoparvovirus | Rodent protoparvovirus | 284 | Nearly complete | 5196 | Akodon montensis | Blood | 41 | Ribeirão Preto, SP | 2009 | MG745675 |
| Protoparvovirus | Rodent protoparvovirus | 119 | Nearly complete | 4998 | Calomys tener | Blood | 38 | Ribeirão Preto, SP | 2008 | MG745676 |
| Dependoparvovirus | Chiropteran dependoparvovirus 2 | 246 | Nearly complete | 4894 | Desmodus rotundus | Kidney | 8 | Araçatuba, SP | 2010 | MG745677 |
| Protoparvovirus | Rodent protoparvovirus | 2 | Nearly complete | 4898 | Necromys lasiurus | Blood | 59 | Ribeirão Preto, SP | 2008 | MG745678 |
| Tetraparvovirus | Ungulate tetraparvovirus | MR | Nearly complete | 5368 | Bos taurus | Blood | 15 | Manoel Ribas, PR | 2016 | MG745679 |
| Erythroparvovirus | Ungulate erythroparvovirus 1 | Ronda Alta | Nearly complete | 5220 | Bos taurus | Blood | 6 | Ronda Alta, RS | 2016 | MG745680 |
| Protoparvovirus | Rodent protoparvovirus | 1594 | Partial | 2255 | Didelphis albiventris | Blood | 32 | Ribeirão Preto, SP | 2012–2013 | MG745681 |
| Bocaparvovirus | Rodent bocaparvovirus | 4093 | Partial | 2844 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745682 |
| Protoparvovirus | Rodent protoparvovirus | 8 | Partial | 1679 | Calomys tener | Blood | 34 | Ribeirão Preto, SP | 2009, 2012–2013 | MG745683 |
| Protoparvovirus | Rodent protoparvovirus | 888 | Partial | 1606 | Oligoryzomys nigripes | Blood | 20 | Ribeirão Preto, SP | 2012–2013 | MG745684 |
| Protoparvovirus | Rodent protoparvovirus | 23 | Partial | 1566 | Akodon montensis | Blood | 55 | Ribeirão Preto, SP | 2008 | MG745685 |
| Bocaparvovirus | Rodent bocaparvovirus | 422 | Partial | 1362 | Necromys lasiurus | Blood | 52 | Ribeirão Preto, SP | 2009 | MG745686 |
| Protoparvovirus | Rodent protoparvovirus | 1010 | Partial | 1283 | Oligoryzomys nigripes | Blood | 20 | Ribeirão Preto, SP | 2012–2013 | MG745687 |
| Protoparvovirus | Rodent protoparvovirus | 66 | Partial | 1099 | Akodon montensis | Blood | 55 | Ribeirão Preto, SP | 2008 | MG745688 |
| Protoparvovirus | Rodent protoparvovirus | 38 | Partial | 1067 | Calomys tener | Blood | 34 | Ribeirão Preto, SP | 2009,2012-2013 | MG745689 |
Legend: SP (São Paulo State), PR (Paraná State), PE (Pernambuco State), RS (Rio Grande do Sul State).
2.4. Phylogenetic Analysis
Maximum likelihood (ML) phylogenetic trees were reconstructed using alignments of non-structural (NS) proteins and viral proteins (VPs), identified in the present study with representative members of the Parvovirinae subfamily [1]. Multiple sequence alignment (MSA) was carried out using RevTrans 2.0 [16] with manual adjustment. The alignments of the core of the NS and VP protein ML trees were inferred using IQ-TREE version 1.4.3 software based on an LG+F+G4 protein substitution model to the core of an NS protein with 145 amino acids and an LG+F+I+G4 protein substitution model to the core of a VP protein with 245 amino acids, both with 1000 replicates [17,18]. Statistical support for individual nodes was estimated via bootstrap replicates. Phylogenetic trees were visualized using Figtree 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). Nucleotide divergence calculations were performed using the Sequence Demarcation Tool (SDT) version 1.2 in muscle mode [19].
3. Results
Using HTS, we identified 21 parvovirus sequences in samples derived from rodents, bats, opossums, birds and cattle in Pernambuco, São Paulo, Paraná and Rio Grande do Sul states in Brazil (Figure 1). These sequences comprised twelve nearly complete genomes and nine partial genomes (Table 1) and included the first examples of parvoviruses identified in opossums, New World bats and sigmondontine rodents. Parvovirus sequences recovered in our study were classified on the basis of (i) phylogeny and (ii) pairwise distance.
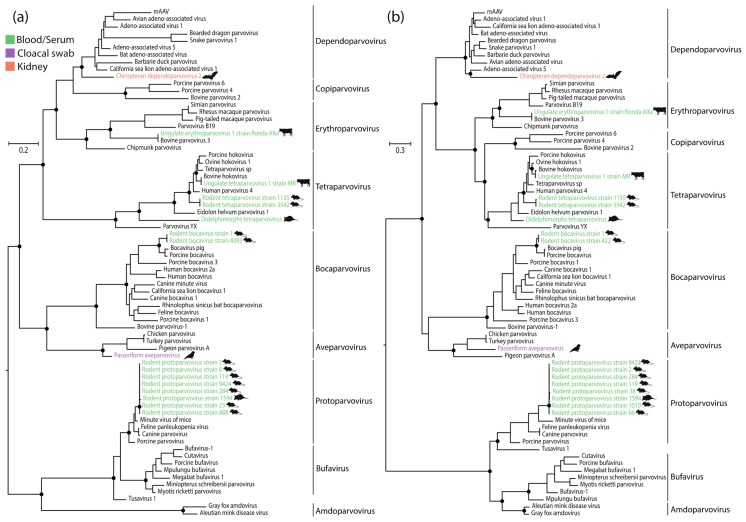
To investigate the phylogenetic relationships between the novel parvoviruses and those described previously, we inferred ML phylogenetic trees from alignments of 71 NS proteins and 71 VP peptide sequences. Phylogenies revealed eight distinct clades corresponding to recognized genera, each having high bootstrap support (values > 75%). The sequences recovered in this study were grouped into six distinct genera (Figure 2). In most cases, the newly identified sequences grouped robustly within the established diversity of their respective genera. Only the Dependoparvovirus-like sequence identified in our study was grouped in a basal position with respect to previously characterized taxa in both NS and VP trees.
Figure 2.
Maximum likelihood (ML) phylogenies showing the evolutionary relationships of newly identified parvoviruses. (a) Phylogenetic tree of non-structural (NS) proteins; (b) Phylogenetic tree of viral proteins (VPs). Phylogenies are midpoint rooted for clarity of presentation. The scale bar indicates evolutionary distance in substitutions per amino acid site. Black lines indicate genera within the Parvovirinae subfamily. Black circles indicate nodes with maximum likelihood bootstrap support levels >75%, based on 1000 bootstrap replicates. Taxa names of parvoviruses identified in our study are coloured according to sample type, as shown in the key. Silhouettes indicate host species groups.
According to the species demarcation criteria of the International Committee on Taxonomy of Viruses (ICTV), parvoviruses in the same species should share >85% amino acid sequence identity across the entire NS polypeptide sequence [1]. On this basis, the 21 genomes described in this study represent six novel species of parvoviruses and two that have been described previously—Ungulate erythroparvovirus 1 and ungulate tetraparvovirus 1 (Figures S1 and S2).
We identified a novel species of protoparvovirus in sigmondontine rodents. This virus, which was detected in samples from several distinct animals and species (Table 1), is quite similar to the minute virus of mice (MVM) but is sufficiently distinct based on ICTV criteria to be considered a distinct species. We also identified novel tetraparvoviruses in the opossum and hairy-tailed bolo mouse and a novel dependoparvovirus in tissue samples derived from common vampire bats (Desmodus rotundus). We identified a novel bocaparvovirus species—rodent bocaparvovirus—in two distinct sample pools obtained from hairy-tailed bolo mice and a novel aveparvovirus in the grey pileated finch in São José do Egito, Pernambuco State, Brazil. We also identified strains of two parvoviruses that were previously detected in cattle—Ungulate erythroparvovirus 1 and Ungulate tetraparvovirus 1—identified in cattle serum of Ronda Alta in the Rio Grande do Sul State and Manoel Ribas in Paraná State, both located in South of Brazil.
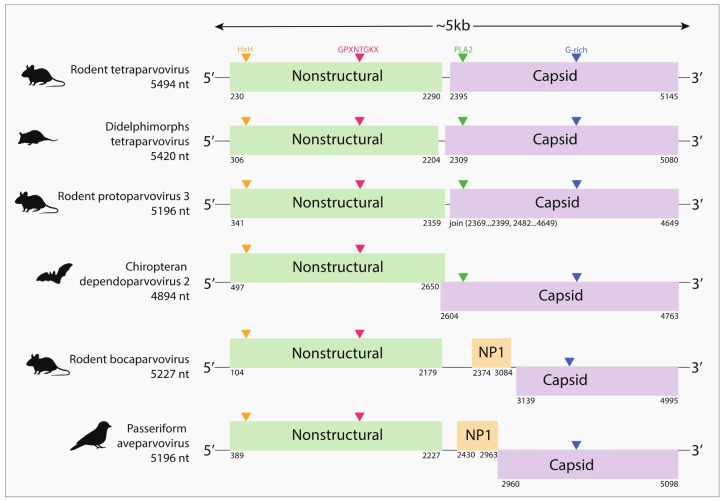
All the viruses identified in our study have typical parvovirus genome structures encoding NS and VP proteins. The deduced NS protein sequences from these viruses contain the “HxH” domain, which is similar to “HIH,” a metal binding domain previously described in the endonuclease domain [20,21]. This domain is a catalytic unit of the endonuclease, which was described to cleave one of the strands of dsDNA in viral cycle replication [2]. Also, we identified helicase motifs including Walker motifs [22], which are involved in viral DNA synthesis (Figure S3) [2,21]. Most of the capsid proteins also possess a glycine-rich (G-rich) region required for cellular entry [23] and the PLA2 motif involved in the viral release from the endosome and entry into the nucleus [24]. However, we did not identify a PLA2 motif in passeriform aveparvovirus or rodent bocaparvovirus. Interestingly, we observed that one species—chiropteran dependoparvovirus 2—encodes NS and VP as overlapping open reading frames (ORFs), with a shared region of 47 nucleotides (Figure 3).
Figure 3.
Genome structures of nearly complete coding sequences of newly identified parvoviruses. The length of the determined nucleotide sequences of the viral sequences are shown on the left. Boxes indicate the open reading frames (ORFs) and the number represents the respective position of their ORFs.
Notably, the rodent bocaparvoviruses and passeriform aveparvovirus contain a putative additional ORF (NP1). This gene is located in the middle of the viral genome and overlaps with the C-terminus region of the NS ORF but in a different reading frame (Figure 3). In the case of the rodent bocaparvoviruses, this ORF may correspond to the NP1 protein, which has been reported to play a role in efficient replication for human and canine bocaparvoviruses [25,26,27] and in immune evasion for porcine bocaparvoviruses [28].
4. Discussion
Brazil has a great diversity and abundance of wildlife and is considered a hotspot for the potential emergence of novel zoonotic viruses [29]. However, parvovirus studies in Brazil have focused predominantly on canine parvovirus and human parvovirus B19 [2,30]. In this study, we used an HTS approach to investigate parvovirus infections among wild mammals and birds from Brazil that were apparently without symptoms or disease. We identified 21 parvovirus sequences, representing six novel—and two previously described—parvovirus species. We report the first examples of parvoviruses in samples derived from Sigmondontinae rodents, opossums and New World bats. Interestingly, almost all the viruses detected here were sequenced from serum or blood samples suggesting that viremia may have been a factor in their identification.
We detected strains of ungulate tetraparvovirus—a virus in the genus Tetraparvovirus—in cattle from the South of Brazil. Ungulate tetraparvovirus 2—formerly known as porcine hokovirus—has previously been identified in swine in Brazil [31]. However, ungulate tetraparvovirus 1—formerly known as bovine hokovirus—has not previously been reported outside Asia. This virus, which was originally identified in bovine spleen samples obtained from food markets in Hong Kong, has also been identified in domestic yaks (Bos grunniens) in northwestern China [32,33]. The identification of this virus in an entirely distinct population (Brazilian cattle) not only establishes that it occurs outside Asia but also suggests it may be present in cattle populations throughout the world. In addition, we identified novel species of tetraparvovirus in samples obtained from rodents and from an opossum. Interestingly, the opossum sequence grouped basal relative to the largest Tetraparvovirus clade, which contains isolates from diverse eutherian mammals. Further sampling may reveal whether this basal position reflects the broad co-divergence of tetraparvoviruses and mammals dating back to the common ancestor of marsupials and eutherians. Such ancient origins of the Tetraparvovirus genus are consistent with evidence from endogenous viral element (EVE) sequences that parvoviruses have been infecting mammals for millions of years [34,35].
Recently, studies have reported numerous novel dependoparvoviruses in samples derived from Asian bats [36,37]. Here, we provide the first report of a dependoparvovirus in a New World bat—the vampire bat (Desmodus rotundus). In trees based on Rep, this virus groups basally within the Dependoparvovirus genus, consistent with these viruses potentially having an ancestral origin in bats, as has been proposed previously [36].
Currently, only one species is recognised in the genus Aveparvovirus. This virus (Galliform aveparvovirus 1) infects chickens and turkeys and is widespread in poultry farms in the United States and Europe [38,39]. We identified a novel Aveparvovirus species in samples derived from pileated finch (Coryphospingus pileatus), an indigenous (and non-migratory) South American bird, suggesting that viruses belonging to the Aveparvovirus genus may circulate widely among avian species, including wild as well as domestic birds.
We detected Ungulate erythroparvovirus 1 (genus Erythroparvovirus) in Brazilian cattle. Since this virus—to the best of our knowledge—has only been described as a contaminant of commercial bovine serum [40], our study is the first to report detection of Ungulate erythroparvovirus 1 in cattle populations.
We also identified a novel protoparvovirus species infecting sigmodontine rodents in Brazil. Sigmodontine rodent protoparvovirus was identified in several species of rodents (all belong to the subfamily) that we captured in the Ribeirão Preto region of São Paulo State. These viruses are closely related to the Minute virus of mice (MVM), a common pathogen of laboratory mice [41] but, following official taxonomic criteria, they are sufficiently divergent from MVM (>85% in NS and >73% aa in VP) to be considered a distinct species within the Protoparvovirus genus.
Bocaparvoviruses are associated with pathogenic conditions in human, bovine and canine hosts [2,42]. Rodent bocaparvoviruses have recently been reported [43] but relatively little is known about their broader distribution. We identified novel rodent bocaparvoviruses in sigmodontine rodents that are closely related to bocaparvoviruses recently reported in brown rats (Rattus rattus) in China [43] (data not shown). Together, these findings suggest a broad distribution for rodent bocaparvoviruses.
Parvoviruses that infect domestic and wild carnivores (including amdoviruses and protoparvoviruses) have been studied fairly extensively in the field. These studies have shown that groups of closely related parvoviruses circulate widely among species in the order Carnivora, with the barriers to transmission between species within the order apparently being relatively low [44,45,46]. The findings of our study suggest that this pattern might be reflected more broadly in parvovirus ecology, with many parvovirus genera containing sublineages that circulate within particular taxonomic groups of hosts (and are largely restricted to this host group). For example, the phylogenetic relationships shown in Figure 1 indicate that closely related protoparvoviruses circulate widely among rodents and that closely related tetraparvoviruses circulate widely in ungulates. With further sampling of parvovirus diversity, it should quickly become apparent whether these inferences are accurate.
5. Conclusions
In this study, we used a sequencing-based approach to characterize parvovirus infections in wild and domestic animals in Brazil. Our findings extend the known biogeographic range of previously characterized parvovirus species and the known host range of three parvovirus genera (Dependovirus, Aveparvovirus and Tetraparvovirus). More broadly, our findings indicate that many parvovirus genera contain well-defined sub-lineages that circulate widely throughout the world within particular taxonomic groups of hosts.
Acknowledgments
We thank Meire Christina Seki, Janaína Menegazzo Gheller, Luiz Gustavo Betim Góes, Cristiano de Carvalho, Wagner André Pedro, Luciano M. Thomazelli, Fábio Maués, Marcello Schiavo Nardi, Severino M. de Azevedo Júnior, Roberto Rodrigues, Renata Hurtado and Felipe Alves Morais, Márcio Schaefer, Mario Figueiredo, Felipe Morais, Jaqueline R. Silva, Paulo Paulosso, Dercílio Pavanelli, Edison Montilha, Armando F. A. Nascimento, José Teotônio for help in fieldwork. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (Grant number. 13/14929-1 and Scholarships No. 17/13981-0; 12/24150-9; 15/05778-5; 16/01414-1; 14/20851-8; 06/00572-0; 08/06411-4; 11/06810-9; 11/22663-6; 16/02568-2, 06/00572-0; 09/05994-9 and 11/13821-7). Robert James Gifford was supported by the Medical Research Council of the United Kingdom (Grant number MC_UU_12014/10).
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4915/10/4/143/s1, Table S1: Samples information, host, sources, sample type, location, date, reads and %Bases ≥Q30. Figure S1: Heatmap of pairwise amino acid identities of the NS protein of parvoviruses identified in this study and representative members of the Parvovirinae subfamily based on ICTV criteria. The viruses described in this study are highlighted in bold. Figure S2: Heatmap of pairwise amino acid identities of the VP protein of parvoviruses identified in this study and representative members of the Parvovirinae subfamily based on ICTV criteria. The viruses described in this study are highlighted in bold. Figure S3: Alignment of helicase enzymatic motif showing walker’s motifs.
Author Contributions
William Marciel Souza and Robert James Gifford conceived and designed the experiments; William Marciel Souza, Tristan Dennis, Marcílio Jorge Fumagalli, Marilia Farignoli Romeiro and Luiz Carlos Vieira performed the experiments; William Marciel Souza, Tristan Dennis and Robert James Gifford analyzed the data; Sejal Modha, Márcio Roberto Teixeira Nunes and Luiz Tadeu Moraes Figueiredo contributed reagents/materials/analysis tools; Gilberto Sabino-Santos Jr, Felipe Gonçalves Motta Maia, Gustavo Olszanski Acrani, Adriano de Oliveira Torres Carrasco, Luzia Helena Queiroz, Jansen Araujo, Tatiana Lopes Ometto, Edison Luiz Durigon collected samples and performed fieldwork; William Marciel Souza, Tristan Dennis, Luiz Tadeu Moraes Figueiredo and Robert James Gifford wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cotmore S.F., Agbandje-McKenna M., Chiorini J.A., Mukha D.V., Pintel D.J., Qiu J., Soderlund-Venermo M., Tattersall P., Tijssen P., Gatherer D., et al. The family Parvoviridae. Arch. Virol. 2014;159:1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu J., Soderlund-Venermo M., Young N.S. Human parvoviruses. Clin. Microbiol. Rev. 2017;30:43–113. doi: 10.1128/CMR.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miranda C., Thompson G. Canine parvovirus: The worldwide occurrence of antigenic variants. J. Gen. Virol. 2016;97:2043–2057. doi: 10.1099/jgv.0.000540. [DOI] [PubMed] [Google Scholar]
- 4.Hargitai R., Pankovics P., Kertesz A.M., Biro H., Boros A., Phan T.G., Delwart E., Reuter G. Detection and genetic characterization of a novel parvovirus distantly related to human bufavirus in domestic pigs. Arch. Virol. 2016;161:1033–1037. doi: 10.1007/s00705-015-2732-4. [DOI] [PubMed] [Google Scholar]
- 5.Li L., Giannitti F., Low J., Keyes C., Ullmann L.S., Deng X., Aleman M., Pesavento P.A., Pusterla N., Delwart E. Exploring the virome of diseased horses. J. Gen. Virol. 2015;96:2721–2733. doi: 10.1099/vir.0.000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza W.M., Romeiro M.F., Fumagalli M.J., Modha S., de Araujo J., Queiroz L.H., Durigon E.L., Figueiredo L.T., Murcia P.R., Gifford R.J. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 2017;98:225–229. doi: 10.1099/jgv.0.000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Liu Z., Wang Y., Li W., Fu X., Lin Y., Shen Q., Wang X., Wang H., Zhang W. A novel rodent chapparvovirus in feces of wild rats. Virol. J. 2016;13:133. doi: 10.1186/s12985-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francois S., Filloux D., Roumagnac P., Bigot D., Gayral P., Martin D.P., Froissart R., Ogliastro M. Discovery of parvovirus-related sequences in an unexpected broad range of animals. Sci. Rep. 2016;6:30880. doi: 10.1038/srep30880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonvicino C.R., Oliveira J.A., D’Andrea P.S. Guia dos Roedores do Brasil, com Chaves para Gêneros Baseadas em Caracteres Externos. 1st ed. Centro Pan-Americano de Febre Aftosa-Pan-American Health Organization//World Health Organization; Rio de Janeiro, Brazil: 2008. p. 120. [Google Scholar]
- 10.Ridgely R.S., Tudor G. The Birds of South America. University of Texas Press; Austin, TX, USA: 1994. p. 940. [Google Scholar]
- 11.Sikes R.S., Gannon W.L. Guidelines of the American society of mammalogists for the use of wild mammals in research. J. Mammal. 2011;92 doi: 10.1644/10-MAMM-F-355.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Souza W.M., Fumagalli M.J., de Araujo J., Sabino-Santos G., Jr., Maia F.G.M., Romeiro M.F., Modha S., Nardi M.S., Queiroz L.H., Durigon E.L., et al. Discovery of novel anelloviruses in small mammals expands the host range and diversity of the anelloviridae. Virology. 2018;514:9–17. doi: 10.1016/j.virol.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary N.A., Wright M.W., Brister J.R., Ciufo S., Haddad D., McVeigh R., Rajput B., Robbertse B., Smith-White B., Ako-Adjei D., et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion and functional annotation. Nucleic Acids Res. 2016;44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using diamond. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 15.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., et al. Cdd: Ncbi’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernersson R., Pedersen A.G. Revtrans: Multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 2003;31:3537–3539. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. Modelfinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhire B.M., Varsani A., Martin D.P. Sdt: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE. 2014;9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kivovich V., Gilbert L., Vuento M., Naides S.J. The putative metal coordination motif in the endonuclease domain of human parvovirus B19 NS1 is critical for NS1 induced s phase arrest and DNA damage. Int. J. Biol. Sci. 2012;8:79–92. doi: 10.7150/ijbs.8.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki M., Gonzalez G., Wada Y., Setiyono A., Handharyani E., Rahmadani I., Taha S., Adiani S., Latief M., Kholilullah Z.A., et al. Divergent bufavirus harboured in megabats represents a new lineage of parvoviruses. Sci. Rep. 2016;6:24257. doi: 10.1038/srep24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker S.L., Wonderling R.S., Owens R.A. Mutational analysis of the adeno-associated virus type 2 Rep68 protein helicase motifs. J. Virol. 1997;71:6996–7004. doi: 10.1128/jvi.71.9.6996-7004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellanos M., Perez R., Rodriguez-Huete A., Grueso E., Almendral J.M., Mateu M.G. A slender tract of glycine residues is required for translocation of the VP2 protein N-terminal domain through the parvovirus MVM capsid channel to initiate infection. Biochem. J. 2013;455:87–94. doi: 10.1042/BJ20130503. [DOI] [PubMed] [Google Scholar]
- 24.Ros C., Gerber M., Kempf C. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J. Virol. 2006;80:12017–12024. doi: 10.1128/JVI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q., Deng X., Yan Z., Cheng F., Luo Y., Shen W., Lei-Butters D.C., Chen A.Y., Li Y., Tang L., et al. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog. 2012;8:e1002899. doi: 10.1371/journal.ppat.1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y., Chen A.Y., Cheng F., Guan W., Johnson F.B., Qiu J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009;83:3956–3967. doi: 10.1128/JVI.02569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou W., Cheng F., Shen W., Engelhardt J.F., Yan Z., Qiu J. Nonstructural protein NP1 of human bocavirus 1 plays a critical role in the expression of viral capsid proteins. J. Virol. 2016;90:4658–4669. doi: 10.1128/JVI.02964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R., Fang L., Wu W., Zhao F., Song T., Xie L., Li Y., Chen H., Xiao S. Porcine bocavirus NP1 protein suppresses type I IFN production by interfering with IRF3 DNA-binding activity. Virus Genes. 2016;52:797–805. doi: 10.1007/s11262-016-1377-z. [DOI] [PubMed] [Google Scholar]
- 29.Olival K.J., Hosseini P.R., Zambrana-Torrelio C., Ross N., Bogich T.L., Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kailasan S., Agbandje-McKenna M., Parrish C.R. Parvovirus family conundrum: What makes a killer? Annu. Rev. Virol. 2015;2:425–450. doi: 10.1146/annurev-virology-100114-055150. [DOI] [PubMed] [Google Scholar]
- 31.Souza C.K., Streck A.F., Goncalves K.R., Pinto L.D., Ravazzolo A.P., de Barcellos D.E., Canal C.W. Phylogenetic characterization of the first ungulate tetraparvovirus 2 detected in pigs in brazil. Braz. J. Microbiol. 2016;47:513–517. doi: 10.1016/j.bjm.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau S.K., Woo P.C., Tse H., Fu C.T., Au W.K., Chen X.C., Tsoi H.W., Tsang T.H., Chan J.S., Tsang D.N., et al. Identification of novel porcine and bovine parvoviruses closely related to human parvovirus 4. J. Gen. Virol. 2008;89:1840–1848. doi: 10.1099/vir.0.2008/000380-0. [DOI] [PubMed] [Google Scholar]
- 33.Xu F., Pan Y., Wang M., Wu X., Tian L., Baloch A.R., Zeng Q. First detection of ungulate tetraparvovirus 1 (bovine hokovirus 1) in domestic yaks in northwestern china. Arch. Virol. 2016;161:177–180. doi: 10.1007/s00705-015-2638-1. [DOI] [PubMed] [Google Scholar]
- 34.Katzourakis A., Gifford R.J. Endogenous viral elements in animal genomes. PLoS Genet. 2010;6:e1001191. doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Fu Y., Xie J., Cheng J., Ghabrial S.A., Li G., Peng Y., Yi X., Jiang D. Widespread endogenization of densoviruses and parvoviruses in animal and human genomes. J. Virol. 2011;85:9863–9876. doi: 10.1128/JVI.00828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau S.K.P., Ahmed S.S., Tsoi H.W., Yeung H.C., Li K.S.M., Fan R.Y.Y., Zhao P.S.H., Lau C.C.C., Lam C.S.F., Choi K.K.F., et al. Bats host diverse parvoviruses as possible origin of mammalian dependoparvoviruses and source for bat-swine interspecies transmission. J. Gen. Virol. 2017;98:3046–3059. doi: 10.1099/jgv.0.000969. [DOI] [PubMed] [Google Scholar]
- 37.Hu D., Zhu C., Wang Y., Ai L., Yang L., Ye F., Ding C., Chen J., He B., Zhu J., et al. Virome analysis for identification of novel mammalian viruses in bats from southeast china. Sci. Rep. 2017;7:10917. doi: 10.1038/s41598-017-11384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marusak R.A., Guy J.S., Abdul-Aziz T.A., West M.A., Fletcher O.J., Day J.M., Zsak L., Barnes H.J. Parvovirus-associated cerebellar hypoplasia and hydrocephalus in day old broiler chickens. Avian Dis. 2010;54:156–160. doi: 10.1637/8976-070709-Case.1. [DOI] [PubMed] [Google Scholar]
- 39.Day J.M., Zsak L. Determination and analysis of the full-length chicken parvovirus genome. Virology. 2010;399:59–64. doi: 10.1016/j.virol.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allander T., Emerson S.U., Engle R.E., Purcell R.H., Bukh J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. USA. 2001;98:11609–11614. doi: 10.1073/pnas.211424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janus L.M., Mahler M., Kohl W., Smoczek A., Hedrich H.J., Bleich A. Minute virus of mice: Antibody response, viral shedding and persistence of viral DNA in multiple strains of mice. Comp. Med. 2008;58:360–368. [PMC free article] [PubMed] [Google Scholar]
- 42.Proenca-Modena J.L., Gagliardi T.B., Paula F.E., Iwamoto M.A., Criado M.F., Camara A.A., Acrani G.O., Cintra O.A., Cervi M.C., Arruda L.K., et al. Detection of human bocavirus mrna in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS ONE. 2011;6:e21083. doi: 10.1371/journal.pone.0021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau S.K., Yeung H.C., Li K.S., Lam C.S., Cai J.P., Yuen M.C., Wang M., Zheng B.J., Woo P.C., Yuen K.Y. Identification and genomic characterization of a novel rat bocavirus from brown rats in china. Infect. Genet. Evol. 2017;47:68–76. doi: 10.1016/j.meegid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Allison A.B., Kohler D.J., Fox K.A., Brown J.D., Gerhold R.W., Shearn-Bochsler V.I., Dubovi E.J., Parrish C.R., Holmes E.C. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J. Virol. 2013;87:2342–2347. doi: 10.1128/JVI.02428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrish C.R., Holmes E.C., Morens D.M., Park E.C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canuti M., Whitney H.G., Lang A.S. Amdoparvoviruses in small mammals: Expanding our understanding of parvovirus diversity, distribution and pathology. Front. Microbiol. 2015;6:1119. doi: 10.3389/fmicb.2015.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.