Abstract
The D1 protein is an integral component of the photosystem II reaction center complex. In the cyanobacterium Synechocystis sp. PCC 6803, D1 is synthesized with a short 16-amino acids-long carboxyl-terminal extension. Removal of this extension is necessary to form active oxygen-evolving photosystem II centers. Our earlier studies have shown that this extension is cleaved by CtpA, a specific carboxyl-terminal processing protease. The amino acid sequence of the carboxyl-terminal extension is conserved among D1 proteins from different organisms, although at a level lower than that of the mature protein. In the present study we have analyzed a mutant strain of Synechocystis sp. PCC 6803 with a duplicated extension, and a second mutant that lacks the extension, to investigate the effects of these alterations on the function of the D1 protein in vivo. No significant difference in the growth rates, photosynthetic pigment composition, fluorescence induction, and oxygen evolution rates was observed between the mutants and the control strain. However, using long-term mixed culture growth analysis, we detected significant decreases in the fitness of these mutant strains. The presented data demonstrate that the carboxyl-terminal extension of the precursor D1 protein is required for optimal photosynthetic performance.
Photosystem II (PSII) is a multisubunit pigment-protein complex located in the thylakoid membranes of cyanobacteria and chloroplasts. This complex is the major producer of oxygen in the biosphere. Upon excitation with light, the reaction center chlorophylls (Chl) of PSII release electrons, which move via pheophytin and two plastoquinone molecules on the acceptor side of PSII (Barber, 1998). The oxidized reaction center is subsequently reduced by electrons that arrive from water via a cluster of four manganese ions and a redox-active Tyr residue on the donor side of this photosystem. The PSII complex in cyanobacteria in vivo contains nearly 20 different polypeptides (Debus, 2000). At the core of PSII is a heterodimer of two homologous polypeptides D1 and D2. Closely associated with them are other intrinsic and extrinsic membrane proteins, namely cytochrome b559, CP47, CP43, and a number of smaller polypeptides (Vermaas, 1993; Lorkovic et al., 1995; Pakrasi, 1995).
D1 and D2 are integral membrane components that provide binding sites for a number of important redox-active cofactors in PSII (Debus, 2000). Although D2 is relatively stable, D1 is rapidly turned over in vivo during a damage-repair cycle after excitation of the reaction center with light (Aro et al., 1993). This protein is usually synthesized as a precursor (pD1) with a carboxyl-terminal extension, and then processed on the carboxyl side of residue 344 (Ala), resulting in the removal of this extension (Takahashi et al., 1988). Analysis of the sequence of the psbA gene encoding the D1 protein in a wide variety of photosynthetic organisms has shown that the amino acid residues of the mature D1 protein are highly conserved. The carboxyl-terminal extension, which is cleaved off during processing, is less conserved than the mature protein, but still shows a high degree of homology between different species (Table I). The processing of pD1 is absolutely required for the assembly of the manganese cluster involved in PSII-mediated oxygen evolution (Taylor et al., 1988). This extension is cleaved by the CtpA protease, which has been identified in a few organisms (Shestakov et al., 1994; Fujita et al., 1995; Oelmüller et al., 1996; Trost et al., 1997). The deduced amino acid sequence of CtpA exhibits significant homology with that of a tail-specific protease (Tsp) in Escherichia coli, which has been classified as a new type of Ser protease (Keiler and Sauer, 1995).
Table I.
Aligned sequences of the carboxy-terminal extension of pD1 proteins from different organisms
| Organism | Sequence of the Extension Starting from Position 345 | Length | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synechocystis 6803 psbA2/3 | S | G | E | Q | A | P | V | A | L | T | A | P | A | V | N | G | 16 |
| Synechocystis 6714 psbA1 | * | * | * | * | * | * | * | * | * | * | * | * | * | I | * | * | 16 |
| Fremyella diplosiphon | A | * | * | V | * | * | * | * | * | * | * | * | * | I | * | * | 16 |
| Synechococcus 7942 psbA1 | A | * | * | A | T | * | * | * | * | * | * | * | T | I | H | * | 16 |
| Synechococcus 7942 psbA2/3 | A | * | * | A | T | * | * | * | * | * | * | * | * | I | * | * | 16 |
| Anabaena 7120 psbA1 | A | * | * | V | * | * | * | * | * | * | * | * | * | I | * | * | 16 |
| Anabaena 7120 psbA2/3 | A | * | * | V | * | * | * | * | I | S | * | * | * | I | * | * | 16 |
| Cyanophora paradoxa | * | * | * | V | M | * | * | * | * | * | * | * | S | I | * | A | 16 |
| Ectocarpus siliculosus | * | N | * | I | L | * | * | * | I | S | * | * | S | V | V | * | 16 |
| Prochlorothrix hollandica | A | V | K | – | – | – | – | – | – | – | * | * | S | I | I | * | 9 |
| Chlamydomonas reinhardtii | * | – | N | – | – | – | – | – | – | T | N | S | S | S | * | N | 8 |
| Marchantia polymorpha | A | V | * | – | – | – | – | – | – | – | * | * | * | V | * | * | 9 |
| Pinus contorta | A | V | * | – | – | – | – | – | – | – | S | I | S | I | G | * | 9 |
| Winter rye | A | V | * | – | – | – | – | – | – | – | V | * | S | I | * | * | 9 |
| Barley | A | V | * | – | – | – | – | – | – | – | V | * | * | I | * | * | 9 |
| Arabidopsis | A | V | * | – | – | – | – | – | – | – | * | * | S | T | * | * | 9 |
| Broad bean | A | V | D | – | – | – | – | – | – | – | * | * | S | I | S | * | 9 |
| Rice | A | L | * | – | – | – | – | – | – | – | V | * | S | L | * | * | 9 |
| Spinach | A | I | * | – | – | – | – | – | – | – | * | * | S | T | * | * | 9 |
The first sequence is that of the pD1 protein from Synechocystis 6803. Identical residues are represented by asterisks, and where the sequences differ, the amino acids are designated by their one-letter codes. “–” denotes a gap.
To analyze the mechanism of this unique enzymatic process and to understand the role of the carboxyl-terminal extension, we have constructed and studied two mutant strains that have different compositions of the extension region. So far it has been shown that Asp-342, Leu-343, and Ala-345 of pD1 play important roles in substrate recognition by CtpA (Nixon et al., 1992; Taguchi et al., 1993; Yamamoto and Satoh, 1998), but little is known about the function of other residues in the C-terminal extension. Moreover, although the deduced amino acid sequence of the extension is conserved between different organisms (Table I), the physiological significance of the presence of this C-terminal extension remains unclear. In particular, mutant strains of a green alga, Chlamydomonas reinhardtii, and a cyanobacterium, Synechocystis sp. PCC 6803, in which stop codons have been introduced at position 345 (+1 position of the carboxyl-terminal extension), are able to grow normally under photoautotrophic conditions (Lers et al., 1992; Nixon et al., 1992).
In the present study we have analyzed a Synechocystis 6803 mutant with a duplicated extension and a mutant that does not have the extension to investigate the effects of these alterations on the function of the D1 protein in vivo. The data presented below demonstrate that the carboxyl-terminal extension of the pD1 protein is required for optimal photosynthetic performance of Synechocystis 6803 cells.
RESULTS
Construction of Synechocystis 6803 Mutant Strains and Their Immunoblot Analysis
To analyze the role of the carboxyl-terminal extension of the D1 protein we have constructed a number of mutant strains that have different compositions of the extension region (Table II). The basic strategy for the generation of such mutants is depicted in Figure 1. One of them (MatD1) lacks the carboxyl-terminal extension. The second mutant (DoubleExt) contains an approximate duplication of the extension and produces the D1 protein with a carboxyl-terminal extension that is 29 residues long. We have also constructed a control strain that has a wild-type extension, but like MatD1 and DoubleExt mutants, contains an insertion of the Km− resistance cassette downstream of the psbA2 gene. Two other copies of the psbA gene (psbA1 and psbA3) in Synechocystis 6803 are absent in all of these strains.
Table II.
Compositions of the carboxyl-terminal extension of the pD1 proteins in various strains
| Strain | Composition of the Extension |
|---|---|
| Wild type and WTK | SGEQAPVALTAPAVNG |
| DoubleExt | SGEQAPVALTAPAVGEQAPVALTAPAVNG |
| MatD1 | The extension is absent |
The insertion in the DoubleExt mutant is underlined.
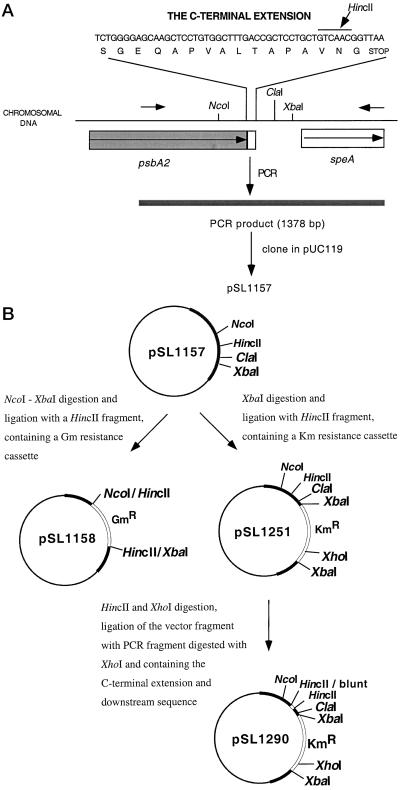
Figure 1.
The general scheme for the construction of the mutants. Various restriction sites are indicated. A, Partial nucleotide sequence of the psbA2 gene in the wild-type strain, which corresponds to the carboxyl-terminal extension of the D1 protein. The oligonucleotide primers used for PCR amplification are shown by small horizontal arrows. B, Construction of plasmids for the generation of various mutant strains. See text for further details.
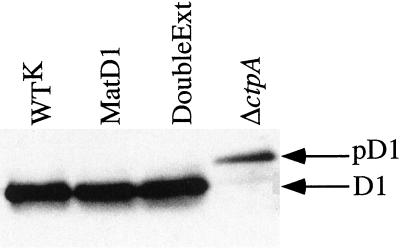
As shown in Figure 2, the D1 protein in the DoubleExt mutant was processed without any apparent problem, whereas only the pD1 form of this protein was found in the ΔctpA mutant strain.
Figure 2.
Immunoblot analysis of membrane proteins from cells of the control strain and mutants. The blot was probed with antibodies raised against the D1 protein. Positions of the mature D1 protein and its precursor form (pD1) are shown on the right.
Photoautotrophic Competence, Pigment Composition, and Photosynthetic Electron Transfer Activities of the Mutants
We have compared the photosynthetic properties of the mutants (Table III). WTK, MatD1, and DoubleExt strains were able to grow photoautotrophically, approximately at the same rate as the wild-type strain. Moreover, under high light (200 μmol m−2 s−1) or low temperature conditions (20°C), the mutants grew at rates similar to those of the control strain (data not shown). The mutants also showed approximately similar Chl and phycobillin content (data not shown), similar yield of Chl a fluorescence, and similar whole chain, as well as PSII-mediated oxygen evolution rates (Table III).
Table III.
Growth and photosynthetic properties of the control and mutant strains
| WTK | MatD1 | DoubleExt | |
|---|---|---|---|
| Doubling time in dim light (h) | 12.2 ± 0.9 | 12.7 ± 0.8 | 12.3 ± 1.0 |
| Variable fluorescencea | 0.42 ± 0.07 | 0.47 ± 0.12 | 0.45 ± 0.15 |
| Rates of electron transfer (% of WTK) | |||
| Whole chainb | 100 | 100 ± 5 | 98 ± 12 |
| PSIIc | 100 | 96 ± 10 | 95 ± 10 |
Each value is the mean ± sd of at least three independent measurements.
Determined as the maximum flash-induced fluorescence yield normalized to the initial level of fluorescence, (FM − F0)/F0, in the presence of 40 μm DCMU.
Determined by polarographic measurements of rates of oxygen evolution in the presence of 10 mm NaHCO3 in BG11. The WTK samples evolved 207 ± 15.5 μmol O2 mg−1 Chl h−1.
Determined by polarographic measurements of rates of oxygen evolution in the presence of 0.5 mm 2,6-dichloro-p-benzoquinone and 1 mm K3Fe(CN)6 in BG11. The WTK samples evolved 543 ± 92.6 μmol O2 mg−1 Chl h−1.
Analysis of Growth in Mixed Culture Conditions
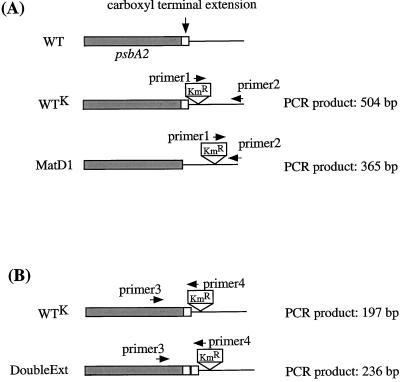
To analyze the environmental fitness of the mutants, mixed culture experiments were conducted. At the beginning of such an experiment, the mixed culture contained an equal number of cells from both strains. If a mutation decreases the fitness of any mutant strain in comparison with the control strain, the latter should overgrow the mutant (Bustos and Golden, 1992; Ouyang et al., 1998; Taguchi et al., 1998). Figure 3 shows the schematic diagrams of the psbA2 gene region in the constructed mutants in comparison with the control strain. To detect the number of mutated copies of the psbA2 gene relative to the wild-type copies from the control strain, PCR analysis was performed. Using specific primers, we were able to distinguish between PCR fragments that corresponded to the control strain and PCR fragments that corresponded to a mutant strain.
Figure 3.
Schematic diagram of the psbA2 gene and its downstream region in constructed mutants in comparison with that from the control strain. The oligonucleotide primers used for PCR amplification in long-term mixed-culture experiments are shown as horizontal arrows. Sizes of PCR products are shown on the right side. A, WTK and MatD1 strains; B, WTK and DoubleExt strains.
In the case of the control strain and the MatD1 mutant, primers specific for the 3′ region of the KmR cassette and for the sequence immediately downstream from the psbA2 gene were used (5′-GTC AGC AAC ACC TTC TTC AC-3′ and 5′-TGG TAG AGT TGC GAG GGC AAT CAT C-3′; Fig. 3A). Because the KmR cassette was inserted in different locations in these strains (with respect to the end of the cloned fragment, 504 bases upstream in the WTK strain, and 365 bases upstream in the MatD1 strain), the corresponding PCR products from the two strains, had different sizes and were easily distinguishable (Fig. 4A).
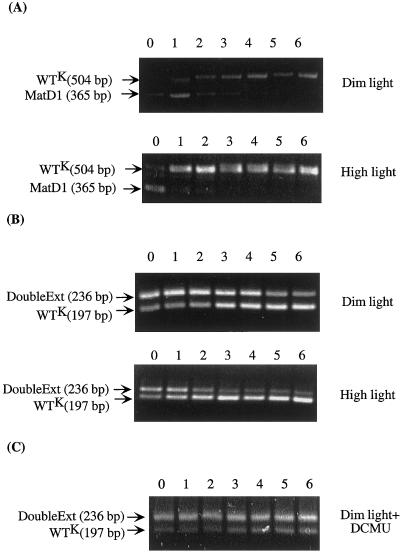
Figure 4.
Patterns of amplified PCR products, which were separated by agarose gel electrophoresis, from the mixed-culture cells. Names of the strains and size of PCR products are marked on the left side of the corresponding bands. The conditions under which the mixed cultures were grown are indicated on the right side. The numbers 0 to 6 refer to the number of times of subculturing. A, WTK and MatD1 mixed-culture. B, WTK and DoubleExt mixed-culture. C, WTK and DoubleExt mixed culture in the presence of DCMU and Glc.
For the experiment with the control strain and the DoubleExt mutant, primers corresponding to the 3′ region of the psbA2 gene and the 5′ region of the KmR cassette were used (5′-CCA CAA CTT CCC CCT AGA TCT AGC GTC TGG GGA GC-3′ and 5′-CGG ACT CCC CGT CGA CCG ATG GCA ATC-3′; Fig. 3B). For the control strain, the size of the PCR product was 197 bp, whereas an insertion of 39 nucleotides (corresponding to a duplication of 13 amino acid residues in the extension region) increased the size of the PCR fragment to 236 bp in the DoubleExt mutant strain (Fig. 4B).
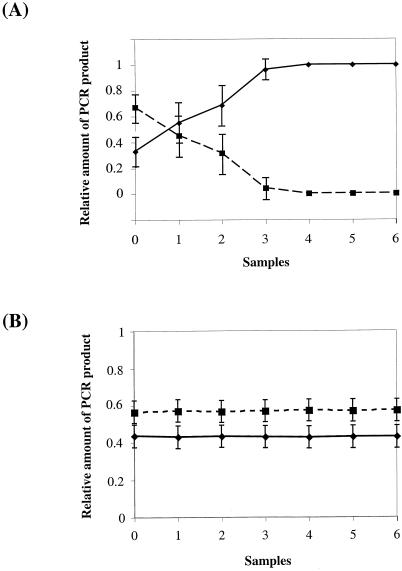
Figure 4, A and B shows the patterns of amplified PCR products for the mixed-culture experiment from the sample taken immediately after the mixing (“0”) and after different numbers of subculturing. The results clearly indicate that in comparison with the control strain, the amount of PCR products of the MatD1 and the DoubleExt mutants was progressively reduced under dim light growth condition. Under high light (200 μE m−2 s−1) growth conditions, this ratio changed even more dramatically. Using the ScionImage program (National Institutes of Health), we quantified the density of each corresponding band (Fig. 5A). It is evident that the relative amount of the PCR product resulting from the MatD1 cells was nearly zero after only three rounds of subculturing, indicating that the MatD1 mutant was out-competed by the control strain under the mixed culture condition. Similar results were obtained for the DoubleExt mutant.
Figure 5.
The relative amounts of the PCR products in the mixed-culture experiments. Each experiment was repeated three times and standard deviations are indicated. A, Relative amounts of PCR products for WTK (solid line) and MatD1 (dashed line) mutants in the mixed culture experiment under high light. B, Relative amounts of PCR products from WTK (solid line) and DoubleExt (dashed line) mutants in the mixed culture experiment under dim light in the presence of DCMU and Glc. See text and Figure 4 for further details.
To examine the behaviors of the mutants under heterotrophic conditions, a similar experiment was carried out in the presence of 5 mm Glc and 10 μm of 3-(3, 4-dichlorophenyl)-1,1-dimethylurea (DCMU; Sigma Chemical, St. Louis), an inhibitor of PSII activity. In the experiment with the control strain and the DoubleExt mutant, there was no significant change in the ratio of two PCR products between the initial and final samples (Fig. 4C). Quantification of the density of the bands also showed that there was no significant change in the ratio of the PCR products between the samples for the mixed-culture experiment in the presence of DCMU (Fig. 5B). This indicates that the change in the ratio of the PCR products of the two strains mixed together in the absence of DCMU was due to a difference in the photosynthetic activity in the respective cells. Similar results were obtained for the MatD1 strain (data not shown).
Response of the Mutants to High Light Stress
It has been demonstrated that the light-induced turnover of the D1 protein is closely related to photodamage of the PSII complex (Barber, 1998; Constant et al., 2000). To analyze the possible effects of the D1 extension mutations on such a damage-repair cycle, the photochemical efficiency of PSII during the high light treatments of intact cells was monitored as variable fluorescence/dark-level fluorescence (FV/F0). The control strain and the mutants behaved similarly during the photoinhibition period of 30 min during which the FV/F0 ratio decreased by 50%. During recovery from photoinhibition, restoration of FV/F0 was monitored for 90 min in dim light. During this period the FV/F0 of the control strain and of the mutants recovered up to 80% with similar kinetics (data not shown).
The photoinhibition treatment was also conducted in the presence of 20 μm lincomycin, an inhibitor of translational initiation, followed by the recovery in the absence of the inhibitor. In these experiments also, we observed approximately similar rates of photoinhibition and recovery for the control and the mutant strains (data not shown).
DISCUSSION
An uncommon feature of the D1 protein is its carboxyl-terminal extension, which has to be processed by a specific protease CtpA to assemble the active manganese cluster for water oxidation (Taylor et al., 1988; Shestakov et al., 1994; Inagaki et al., 1996; Oelmüller et al., 1996). The extension has been found to be present in all eukaryotic and cyanobacterial photosynthetic organisms examined so far (Table I), with the exception of Euglena gracilis (Svensson et al., 1991).
In spite of intensive studies, little is known to date about the functional and/or structural roles of the C-terminal extension of the pD1 protein. Nixon and coworkers studied a mutant strain of Synechocystis 6803 in which a stop codon was introduced at position 345 (+1 position of the carboxyl-terminal extension; Nixon et al., 1992). The mutant grew normally under photoautotrophic conditions. A similar mutant was constructed in a green alga C. reinhardtii (Lers et al., 1992). This strain was also indistinguishable from the wild type in terms of photosynthetic performance. On the basis of these data it was concluded that the carboxyl-terminal extension of pD1 is not required for photosynthetic competence of oxygenic organisms. However, this conclusion does not explain why the extension is present and conserved in all cyanobacteria, algae, and plants that have been studied from a functional point of view. The data presented here clearly demonstrate that the carboxyl-terminal extension of the pD1 protein is required for optimal photosynthetic performance of Synechocystis 6803.
To investigate the function of the carboxyl-terminal extension of the D1 protein we have analyzed two mutants carrying alterations in the extension (Table II). One of these mutants, MatD1, synthesizes the mature D1 protein directly rather than processing it by the CtpA protease. A variety of conditions was tested, but in agreement with previously published results (Lers et al., 1992; Nixon et al., 1992) we were not able to detect any significant difference in growth rates and PSII activity between the control strain and this mutant that lacks the extension (Table III).
The same characteristics were tested for the second mutant, DoubleExt. The carboxyl-terminal extension of this mutant contains an insertion of 13 amino acid residues that are identical to the central part of the normal extension peptide, thus almost duplicating the size of the extension. The resultant fragment is 29 residues long, the longest carboxyl-terminal extension of the D1 protein that has been described so far. Despite the unusual length of the extension, the CtpA enzyme was able to process it to produce mature D1 protein (Fig. 2). The DoubleExt mutant demonstrated similar rates of growth and photosynthetic activities in comparison with the control strain (Table III). Therefore, the duplication of the extension does not result in significant alterations in growth or photosynthetic characteristics.
In the present study we have used a long-term mixed-culture approach (Bustos and Golden, 1992; Ouyang et al., 1998; Taguchi et al., 1998) to compare relative fitness of the constructed mutant strains. At the beginning of the experiment the mixed culture contains an equal number of cells from both competing strains. If a mutation in one of the strains decreases its environmental fitness, it will be overgrown by the second strain. This approach allows comparisons of two strains over many generations and for a much longer period of time than other methods used in this study or those used in other studies of similar mutant strains (Lers et al., 1992; Nixon et al., 1992). During this longer time period subtle differences build up and become more noticeable. Using this approach we have demonstrated that the MatD1 mutant, which lacks the extension, exhibits a decrease in fitness (Figs. 4A and 5A). Moreover, high light conditions greatly decreased that fitness of this mutant. These data conclusively demonstrate that the presence of the extension enhances the environmental fitness of the wild-type strain in comparison with the MatD1 mutant.
Similar results were obtained for the DoubleExt mutant (Fig. 4B). That the DoubleExt mutant has reduced fitness shows that the structure of the extension, and not just its existence, is also important for cellular fitness. There are a few possible explanations for this fact. It can be explained by a slower processing rate of pD1 by CtpA, which reduces the rate of formation of active PSII in cells. Another possible explanation is that the duplication of the extension changes the structure of the terminus, impairing its ability to perform its function properly. We have also considered a possibility that the mutations introduced into the carboxyl-terminal extension of the D1 protein might affect metabolic processes other than photosynthesis. However, this possibility was ruled out since there was no decrease in the fitness of this mutant strain in the mixed culture experiment performed in the presence of DCMU, a PSII inhibitor (Figs. 4C and 5B).
To investigate the cause of the lower fitness of the mutant strains, we studied whether the mutations significantly affect the rate of degradation of the D1 protein during photoinhibition or the rate of D1 integration into PSII during the period of recovery from high light stress. The possibility of a slower rate of processing of pD1 by the CtpA protease was also considered for the DoubleExt mutant. However, no significant difference was detected between the control and the mutant strains in the rates of photoinhibition, as well as recovery from it. It is possible that any difference in rates of D1 turnover between the control strain and the mutants is so slight that we cannot detect it by the photoinhibition experiments.
In conclusion we have confirmed that the carboxyl-terminal extension of the pD1 protein is not essential for photosynthetic growth and PSII activity in Synechocystis sp. PCC 6803. At the same time we have shown that the cells carrying mutations in the extension have a distinct phenotype, which is characterized by lower fitness. The data presented in this manuscript conclusively demonstrate that not only the presence of the extension, but also its structure is important for optimal photosynthetic performance. We suggest a few possible functions of the extension. It could protect the pD1 protein during its integration into the thylakoid membrane and PSII, covering the active center of the protein and preventing damage from photons before the D1 protein is able to function. Another possibility is that the extension somehow takes part in D1 integration into membranes and/or PSII. In this case the absence of the extension or alteration of its structure will lead to a slower integration rate of pD1 into membranes and/or PSII. Other explanations, including participation of the extension in an initial step in the process of manganese binding in PSII, are also possible.
MATERIALS AND METHODS
Bacterial Strains and Culture Conditions
The wild-type and mutant strains of Synechocystis sp. PCC 6803 were grown in BG11 medium (Allen, 1968) at 30°C unless indicated otherwise. The media for WTK, MatD1, and DoubleExt strains were supplemented with 50 μg/mL kanamycin, whereas the medium for the ΔpsbA mutant was supplemented with 5 mm Glc and 15 μg/mL gentamycin. The ΔctpA mutant strain (V.V. Bartsevich and H.B. Pakrasi, unpublished data), which lacks the CtpA protease and is unable to process the D1 protein, was grown in the presence of 25 μg/mL erythromycin and 5 mm Glc. Unless indicated otherwise, cultures were grown under 50 μE m−2 s−1 of fluorescent light, except for the ΔpsbA and ΔctpA mutants, which were grown under 5 μE m−2 s−1 of fluorescent light. Liquid cultures were grown with vigorous bubbling with filtrated room air. Growth of Synechocystis cells was quantified by measurement of light scattering at 730 nm on a spectrophotometer (model DW2000, SLM-Aminco, Urbana, IL).
The Escherichia coli strain TG1 [supE hsdD5 thi Δ(lac-proAB) F′ (traD36 proAB+ lacIq lacZDM15)] containing various recombinant plasmids was grown at 37°C in Luria-Bertani liquid or agar-solidified medium with appropriate antibiotics according to procedures described in Sambrook et al. (1989).
DNA Manipulation and Genetic Transformation
Basic DNA manipulation was performed according to the method of Sambrook et al. (1989). Enzymes used for recombinant DNA procedures were from New England Biolabs (Beverly, MA). PCR amplification was performed with KlenTaq1 polymerase obtained from W. Barnes (Washington University School of Medicine, St. Louis). Oligonucleotides were synthesized by Life Technology (Cleveland).
The plasmid pUC119 (Vieira and Messing, 1987) was used as a basic cloning vector. The mutant strains were constructed using insertion and deletion inactivation approaches. To create a mutant that lacks the psbA2 gene (that encodes the D1 protein), an 855-bp gentamycin resistantance cassette (GmR; Schweizer, 1993) was used. For the construction of other mutants, a 1.25-kb kanamycin resistantance cassette (KmR) from the pUC4K plasmid was used (Amersham Pharmacia Biotech, Piscataway, NJ).
Transformants of Synechocystis 6803 were selected in the presence of increasing amounts of gentamycin (5–15 μg/mL) or kanamycin (5–50 μg/mL) under low light (5 μE m−2 s−1) conditions. To monitor segregation of mutations in the cyanobacterial genome, PCR was performed on chromosomal DNA isolated from transformed strains. Chromosomal DNA from Synechocystis 6803 cells was isolated as described earlier (Williams, 1988). The presence of the specific mutations within the chromosome was confirmed by DNA sequence analysis.
Generation of Synechocystis 6803 Mutant Strains
The ΔpsbA strain was constructed using an insertional inactivation approach. The 3′ part of the coding region of the gene (corresponding to the carboxyl-terminal part of the protein starting from Leu-120) and its downstream region (651 bp downstream from the stop codon of the gene) were amplified from genomic DNA using synthetic oligonucleotides (5′-CCT CAT CGG CAT TTT CTG CTA CAT G-3′ and 5′-TGG TAG AGT TGC GAG GGC AAT CAT C-3′). The resultant PCR product (1,378 bp) was cloned into the pUC119 vector (Fig. 1A). The resulting plasmid (pSL1157) was digested with NcoI and XbaI, filled in with Klenow fragment, and ligated with an 855-bp HincII fragment containing a gentamycin-resistant cassette. This resulted in the plasmid pSL1158 in which the 3′ part of the psbA2 gene corresponding to the carboxyl-terminal part of the protein starting from Thr-292 and its downstream sequence was deleted (Fig. 1B). The recipient strain was the MatD1 (Mature D1) mutant, which was previously constructed in our laboratory (P.R. Anbudurai and H.B. Pakrasi, unpublished data), lacks psbA1 and psbA3 genes, has a stop codon replacing the Ser-345 codon in the psbA2 gene, and has an insertion of the kanamycin-resistant cassette 334 bp downstream from this stop codon. Transformation of this mutant with pSL1158 resulted in the ΔpsbA mutant strain, which did not have any functional D1 protein and was unable to grow photoautotrophically. This latter strain was used as a host for the construction of the control strain WTK (wild-type psbA2 gene, kanamycin-resistant) and the DoubleExt (double extension of the pD1 protein) mutant strain. To construct the control strain, the pSL1157 plasmid was digested with XbaI, filled in with Klenow fragment, and ligated with an 1.25-kb HincII fragment containing kanamycin-resistant cartridge, resulting in the pSL1251 plasmid (Fig. 1B). The pSL1251 plasmid was used to transform the ΔpsbA strain of Synechocystis 6803, resulting in the WTK strain, which has the wild-type carboxyl-terminal extension and the KmR cassette downstream from the gene.
For the construction of the DoubleExt mutant, pSL1251 was also digested with HincII and XhoI, and the vector fragment (4.55-kb) was eluted. The same plasmid was used as a template for a PCR using synthetic oligonucleotides (5′-GGG GAG CAA GCT CCT GTG GC-3′ and 5′-TGG TAG AGT TGC GAG GGC AAT CAT C-3′). The resulting PCR fragment (1,953 bp) corresponded to the 3′ terminal part of the psbA2 gene and its downstream sequence in the plasmid, which includes the KmR cassette. This PCR fragment was digested with XhoI. The larger fragment (1,286 bp) was eluted and ligated with the vector fragment mentioned above. The resulting plasmid (pSL1290) was used to introduce the desired mutation into the ΔpsbA strain of Synechocystis 6803, to generate the DoubleExt mutant strain.
An speA gene, which encodes Arg decarboxylase, is located downstream of the psbA2 gene (Fig. 1A). Insertions and deletions that were generated in the mutants and the control strain were introduced upstream of the speA gene so as not to influence its function.
Membrane Isolation, Protein Electrophoresis, and Immunodetection
Cellular membranes were isolated as described previously (Zak et al., 1999). Samples containing 5 μg of Chl a were subjected to electrophoresis on 16% to 20% (w/v) polyacrylamide gradient gels containing urea. Concentration of Chl a was measured after methanolic extraction (Lichtenthaler, 1987). Proteins were blotted into nitrocellulose filters, reacted with appropriate antisera, and the signals were visualized using enhanced chemiluminescence reagents (Pierce Chemical, Rockford, IL).
Measurement of Rates of Electron Transfer Reactions
Rates of photosynthetic electron transfer reactions from intact cyanobacterial cells were measured on a Clark-type oxygen electrode as described elsewhere (Mannan and Pakrasi, 1993). Samples in BG11 medium were adjusted to a final Chl a concentration of 5 μg/mL (Lichtenthaler, 1987). Whole-chain electron transport rates were measured in the presence of 10 mm sodium bicarbonate, whereas PSII-mediated rates were measured in the presence of 0.5 mm 2,6-dichloro-p-benzoquinone (Eastman-Kodak, Rochester, NY) and 1 mm K3Fe(CN)6 (Sigma).
Spectroscopic Analysis
Chl a fluorescence induction kinetics were measured as described by Meetam et al. (1999) on a fluorometer (FL-100, Photo Systems International, Brno, Czech Republic). For such measurements, samples were adjusted to a final Chl a concentration of 2 μg/mL. FV was calculated as differences between maximum fluorescence yield (FM) after cells were illuminated with actinic light, and F0. Before each measurement, cells were kept in dark for 2 min. Microsoft Excel 7.0 for Microsoft Windows 95 (Seattle) was used to analyze the data.
Mixed-Culture Experiments
Cells of the control strain and the mutants were grown in 100 mL of BG11 medium under normal light intensity (50 μE m−2 s−1 of fluorescent light) to an exponential phase. An equal number of cells of each mutant strain and control strain were mixed in fresh BG11 medium and grown under normal light (50 μE m−2 s−1) or under high light (200 μE m−2 s−1) to an exponential phase. An aliquot of each mixed culture (500 μL) was inoculated into 100 mL of fresh BG11 medium every 3 d. The rest of the cells were used for the isolation of total DNA. In each experiment such subculturing was repeated six times. PCR was carried out in a final volume of 50 μL of the reaction mix that contained 50 ng chromosomal DNA as template, 350 ng of each primer, 200 μm dNTP mixture, PCR buffer (50 mm Tris-HCl, pH 9.2, 16 mm ammonium sulfate, 2.5 mm MgCl2, and 0.1% [w/v] Tween 20), and 1 unit of KlenTaq1 polymerase. PCR amplification involved 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s.
Fluorescence Measurement during Photoinhibition Treatments and Recovery from Photoinhibition
An aliquot of cell suspension (20 μg Chl/mL) was treated in each experiment under 700 μE m−2 s−1 light. Fluorescence was monitored periodically by removing a 160-μL sample and diluting it to 2 μg Chl/mL. Recovery was followed when cells were incubated under 50 μE m−2 s−1 of light. Measurements were made every 10 min. The photochemical efficiency of PSII during the high light treatments of intact cells was monitored as FV/F0.
ACKNOWLEDGMENTS
We thank Dr. P.R. Anbudurai for the MatD1 strain, Dr. V.V. Bartsevich for the ΔctpA strain, and Drs. Wing-On Ng and Elena Zak for critical reading of the manuscript.
Footnotes
This work was supported by grants from National Institutes of Health (GM 45797 to H.B.P.) and from the International Human Frontier Science Program (to H.B.P. and S.V.S.). N.B.I. was partially supported by a training grant from the Monsanto Company to the Plant Biology Program at Washington University.
LITERATURE CITED
- Allen MM. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Barber J. Photosystem two. Biochim Biophys Acta. 1998;1365:269–277. doi: 10.1016/s0005-2728(98)00079-6. [DOI] [PubMed] [Google Scholar]
- Bustos SA, Golden SS. Light-regulated expression of the psbD gene family in Synechococcus sp. strain 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992;232:221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- Constant S, Eisenberg-Domovitch Y, Ohad I, Kirilovsky D. Recovery of photosystem II activity in photoinhibited Synechocystis cells: light-dependent translation activity is required besides light-independent synthesis of the D1 protein. Biochemistry. 2000;39:2032–2041. doi: 10.1021/bi9914154. [DOI] [PubMed] [Google Scholar]
- Debus RJ. The polypeptides of photosystem II and their influence on manganotyrosyl-based oxygen evolution. Met Ions Biol Syst. 2000;37:657–711. [PubMed] [Google Scholar]
- Fujita S, Inagaki N, Yamamoto Y, Taguchi F, Matsumoto A, Satoh K. Identification of the carboxyl-terminal processing protease for the D1 precursor protein of the photosystem II reaction center of spinach. Plant Cell Physiol. 1995;36:1169–1177. [Google Scholar]
- Inagaki N, Yamamoto Y, Mori H, Satoh K. Carboxyl-terminal processing protease for the D1 precursor protein: cloning and sequencing of the spinach cDNA. Plant Mol Biol. 1996;30:39–50. doi: 10.1007/BF00017801. [DOI] [PubMed] [Google Scholar]
- Keiler KC, Sauer RT. Identification of active site residues of the Tsp protease. J Biol Chem. 1995;270:28864–28868. doi: 10.1074/jbc.270.48.28864. [DOI] [PubMed] [Google Scholar]
- Lers A, Heifetz PB, Boynton JE, Gillham NW, Osmond CB. The carboxyl-terminal extension of the D1 protein of photosystem II is not required for optimal photosynthetic performance under CO2- and light-saturated growth conditions. J Biol Chem. 1992;267:17494–17497. [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–383. [Google Scholar]
- Lorkovic ZJ, Schroder WP, Pakrasi HB, Irrgang KD, Herrmann RG, Oelmüller R. Molecular characterization of PsbW, a nuclear-encoded component of the photosystem II reaction center complex in spinach. Proc Natl Acad Sci USA. 1995;92:8930–8934. doi: 10.1073/pnas.92.19.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan RM, Pakrasi HB. Dark heterotrophic growth conditions result in an increase in the content of photosystem II units in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol. 1993;103:971–977. doi: 10.1104/pp.103.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetam M, Keren N, Ohad I, Pakrasi HB. The PsbY protein is not essential for oxygenic photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 1999;121:1267–1272. doi: 10.1104/pp.121.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PJ, Trost JT, Diner BA. Role of the carboxy terminus of polypeptide D1 in the assembly of a functional water-oxidizing manganese cluster in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: assembly requires a free carboxyl group at C-terminal position 344. Biochemistry. 1992;31:10859–10871. doi: 10.1021/bi00159a029. [DOI] [PubMed] [Google Scholar]
- Oelmüller R, Herrmann RG, Pakrasi HB. Molecular studies of CtpA, the carboxyl-terminal processing protease for the D1 protein of the photosystem II reaction center in higher plants. J Biol Chem. 1996;271:21848–21852. doi: 10.1074/jbc.271.36.21848. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi HB. Genetic analysis of the form and function of Photosystem I and Photosystem II. Annu Rev Genet. 1995;29:755–776. doi: 10.1146/annurev.ge.29.120195.003543. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schweizer HP. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. Biotechniques. 1993;15:831–834. [PubMed] [Google Scholar]
- Shestakov SV, Anbudurai PR, Stanbekova GE, Gadzhiev A, Lind LK, Pakrasi HB. Molecular cloning and characterization of the ctpA gene encoding a carboxyl-terminal processing protease: analysis of a spontaneous photosystem II-deficient mutant strain of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1994;269:19354–19359. [PubMed] [Google Scholar]
- Svensson B, Vass I, Styring S. Sequence analysis of the D1 and D2 reaction center proteins of photosystem II. Z Naturforsch. 1991;46:765–776. doi: 10.1515/znc-1991-9-1008. [DOI] [PubMed] [Google Scholar]
- Taguchi F, Takahashi Y, Satoh K. Viability of Chlamydomonas mutants with amino acid substitutions in the precursor D1 protein at the carboxyl-terminal processing site: an analysis by mixed-culture growth experiments. Plant Cell Physiol. 1998;39:1324–1329. [Google Scholar]
- Taguchi F, Yamamoto Y, Inagaki N, Satoh K. Recognition signal for the C-terminal processing protease of D1 precursor protein in the photosystem II reaction center: an analysis using synthetic oligopeptides. FEBS Lett. 1993;326:227–231. doi: 10.1016/0014-5793(93)81796-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shiraishi T, Asada K. COOH-terminal residues of D1 and the 44-kDa CPa-2 at spinach photosystem II core complex. FEBS Lett. 1988;240:6–8. doi: 10.1016/0014-5793(88)80330-2. [DOI] [PubMed] [Google Scholar]
- Taylor MA, Packer JCL, Bowyer JR. Processing of the D1 protein of the photosystem II reaction center and photoactivation of a low fluorescence mutant (LF-1) of Scenedesmus obliquus. FEBS Lett. 1988;237:229–233. [Google Scholar]
- Trost JT, Chisholm DA, Jordan DB, Diner BA. The D1 C-terminal processing protease of photosystem II from Scenedesmus obliquus: protein purification and gene characterization in wild type and processing mutants. J Biol Chem. 1997;272:20348–20356. doi: 10.1074/jbc.272.33.20348. [DOI] [PubMed] [Google Scholar]
- Vermaas W. Molecular-biological approaches to analyze photosystem II structure and function. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:457–481. [Google Scholar]
- Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Williams JGK. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- Yamamoto Y, Satoh K. Competitive inhibition analysis of the enzyme-substrate interaction in the carboxy-terminal processing of the precursor D1 protein of photosystem II reaction center using substituted oligopeptides. FEBS Lett. 1998;430:261–265. doi: 10.1016/s0014-5793(98)00671-1. [DOI] [PubMed] [Google Scholar]
- Zak E, Norling B, Andersson B, Pakrasi HB. Subcellular localization of the BtpA protein in the cyanobacterium Synechocystis sp. PCC 6803. Eur J Biochem. 1999;261:311–316. doi: 10.1046/j.1432-1327.1999.00281.x. [DOI] [PubMed] [Google Scholar]