Abstract
Apparent redundancy is a recurrent theme in innate immunity in various domains including inflammatory cytokines, chemokines and pattern recognition receptors. While sharing core function, different mediators may subserve distinct functions related for instance to production and release (e.g. IL-1α versus IL-1β), predominantly local versus systemic function (e.g. PTX3 versus C-reactive protein) or fine tuning of innate and adaptive responses (chemokines). Based on hard-wired phagocyte recruitment and regulation by a wide spectrum of chemokines and conventional or atypical receptors, I argue that trafficking of phagocytic cells is a robust output of the chemokine system, resistant to genetic or environmental variation. In general, I speculate that the apparent overlap and redundancy observed in core functions represents an evolutionary strategy to preserve robust essential core outputs in the face of genetically or environmentally caused variation.
Introduction
Cytokines and chemokines exhibit a considerable degree of overlap of function. A classic example is provided by IL-1 for which two isoforms (α and β) have long been known to exist [1, 2]. IL-1α and IL-1β interact with the same receptor complex, activate the MyD88 pathway [3] and elicit the same pattern of responses in different cell types [4]. Moreover, the IL-1 spectrum of responses has long been known to overlap with that of TNF, with the notable exception of cell killing and of divergent actions in the regulation of hematopoiesis.
Apparent redundancy is a recurrent theme among cytokines (e.g. IL-17), growth factors and chemokines [5–9]. The chemokine superfamily, with its complexity and promiscuous usage of receptors, is perhaps the most striking example of apparent redundancy. Dissection of the regulation of production and action of seemingly redundant mediators reveals that in nature of fact each mediator has unique features which underlie its selection in evolution. Here, I argue that, while recognizing the unique functions of mediators with overlapping fingerprints, the existance of a shared retained function or set of function reflects darwinian selection of evolutionary essential core functions. As I argued in the past [10], it is my tenet that redundancy represents a strategy to preserve essential core functions in the face of evolutionary pressure.
Specialization and division of labour
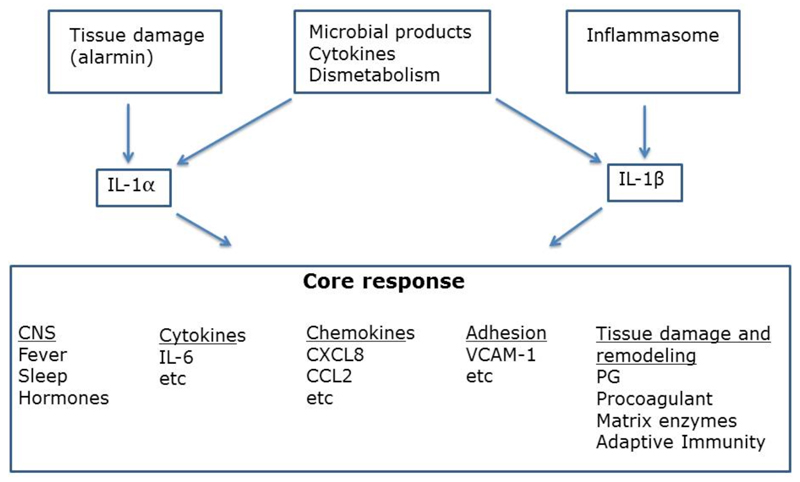
I will dwell on two molecular families whose members share essential structural features to illustrate specialization and division of labour as a recurrent theme. Another contribution in this issue of the Journal provides an original perspective on the general theme of robustness dwelling on tissue repair [11]. IL-1α and IL-1β are two cytokines with limited sequence homology but similar folding and three dimensional structures. They interact with the same signaling receptor, a heterodimer composed of IL-1R1 and an IL-1R accessory protein [2, 4, 12, 13]. Multimerization of the adaptor protein MyD88 leads to IRAK4 activation and ultimately NFkB activation. IL-1 is a prototypic inflammatory cytokine which activates a cascade of mediators with amplification and negative regulation loops. Moreover, IL-1 plays a key role in the activation and orientation of lymphoid cell mediated innate and adaptive immune responses [4]. Then why two IL-1s?
The pathways leading to release of IL-1α and IL-1β are shared and in part distinct (Fig. 1). IL-1β is only produced on demand and release requires processing by caspase 1. In contrast, IL-1α is stored preformed in a variety of cell types, skin keratinocytes in particular, in amounts sufficient to cause death of the organism. Moreover, the pathway of regulated secterion of IL-1α does not involve caspase 1. IL-1α therefore behaves as an “alarmin” with its uncontrolled release signaling tissue damage. Thus the pathways of release are a major distinguishing feature between IL-1α and IL-1β, which share fundamental core functions.
Fig. 1.
Core functions and division of labour between IL-1α and IL-β
Monoclonal antibodies targeting IL-1α and IL-1β are currently undergoing clinical evaluation. Anti IL-1α has shown promising results in the treatment of cancer cachexia, while anti-IL-1β has been shown to prevent atherosclerosis-related pathology and lung cancer incidence and mortality in the large (10061 patients) Cantos trial [14, 15]. It will be interesting to assess whether and to what extent the clinical activity of blocking IL-1α and IL-1β mirrors the involvement of different pathways of production in different pathological conditions.
Pentraxins are an evolutionary conserved family of molecules which includes C reactive protein (CRP), serum amyloid P component (SAP) and the long pentraxin PTX3 [16–18]. Pentraxins serve as fluid phase pattern recognition molecule which recognize microbial moieties and apoptotic cells and trigger effector functions including complement activation and opsonic disposal of targeted cells. Moreover, they have a regulatory function on inflammatory reactions. CRP (or SAP in the mouse) is produced as an acute phase protein in huge amounts in the liver downstream of an IL-1-IL-6 cascade. In contrast, PTX3 is stored in neutrophil granules or produced in tissues on demand, leaking in the systemic circulation in small amounts. Therefore we have argued that CRP and PTX3 serve complementary functions mediating resistance primarily at a systemic and local tissue level, respectively.
Redundancy for robust outputs
Chemokines are a complex superfamily of cytokines characterized by shared structural organization [5, 7–10]. The eponymous function of chemokines is induction of directional cell migration, chemotaxis. The chemokine system is complex, with over 50 ligands originating from 48 distinct genes (47), 18 receptors and four atypical chemokine receptors serving as decoy and scavengers, transporters or presenters [6, 19]. With two notable exceptions (e.g. CXCR4 and its ligand CXCL12; CX3CR1 and its ligand CX3CL1), chemokine receptors are promoscuous in that two or more ligands recognize the same receptor and one ligand frequently recognizes more than one receptor. These general features of the system reflect a division of labour among receptors and the need for fine tuning of leukocyte traffic in innate and adaptive immunity [8, 9]. Interestingly, phagocytes express a wide, possibly the widest, repertoire of chemokine receptors and the capacity to attract monocytes is a common denominator of a substantial fraction of chemokines belonging to the CC, CXC and CX3C families. Moreover, virtually all inflammatory CC and CXC chemokines are trapped and scavenged by only 2 atypical receptors, ACKR1 and ACKR2. I therefore argue that attraction of phagocytes is a robust output of the chemokine system, to be retained in face of genetic variation or environmental changes.
Murine and human data are consistent with the hypothesis that from the point of view of fundamental phagocyte function robustness is a feature of the chemokine system. Mice deficient for inflammatory chemokines or chemokine receptors have relatively subtle phenotypes [20], unlike CXCR4/CXCL12 deficiency which results in profound alterations in the brain, heart, intestine and hematopoietic system [21]. In the perspective of rubustness it is interesting that genetic deficiency in any one inflammatory chemokine receptor results in a wave of perturbation of the system. Since chemokine receptors also scavenge their ligands, deficiency of a given receptor (e.g. CCR2) results in higher levels of the ligands recognized (e.g. the monocyte chemotactic proteins MCPs1,2,3) some of which (e.g. MCP-2 and 3) are then available to interact with other cognate signaling receptors (e.g. CCR1 and CCR5). Inflammatory chemokine receptors of the CXC and CC family recognizes more than one ligand but by far the widest repertoire of ligands is bound by atypical receptors ACKR1 and ACKR2 [6, 22]. ACKR2 binds all inflammatory chemokines with the exception of one of the isoforms of CCL3. It acts as a decoy and scavenger which dampens inflammation and promotes resolution. ACKR1 has more complex functions in hematopoiesis and transport of chemokines [23], but it also can scavenge its ligands. Thus one lesson to be learned from atypical receptors, ACKR2 in particular, is that dampening chemokine-driven inflammation requires disposal of a wide repertoire of inflammatory ligands.
Human data are consistent with the general view of robustness of the chemokine system, from the perspective of phagocyte function. Subjects homozygous for the Δ32 mice mutation of CCR5 are protected against HIV but have no developmental abnormality or increased susceptibility to disease. Although it is likely that the spread and persistence of Δ32 CCR5 reflects evolutionary advantage likely related to resistance to unknown pathogens, its lack of health impact is consistent with other members of the family taking over its function. Development of chemokine inhibitors as anti-inflammatory agents has been disappointing. I surmise that failure of anti-chemokine strategies to control inflammation is a reflection of the robustness of the chemokine system in terms of monocyte-macrophage trafficking.
Concluding remarks
Under many circumstances, apparent redundancy among members of cytokine families and pattern recognition receptors actually reflects specialized functional localization, as illustrated by the two examples discussed here. However several lines of evidence suggest that robustness of phagocyte recruitment is a built-in feature of the chemokine system allowing tissue remodeling, disposal of debris and a minimum immune resistance. The same general consideration may apply to core functions shared by cytokines and growth factors. Robust outputs of cytokine networks may allow retention of sufficient core function in the face of genetic, epigenetic or environment-induced variation of individual components.
Acknowledgements
Support of Italian Association for Cancer Research (AIRC IG-19014 and AIRC 5x1000), Ministero della Salute (RF-2011-02348358 and RF-2013-02355470), Ministry of Education, University and Research (PRIN 2015YYKPNN) and European Research Council (ERC – N° 669415), is gratefully acknowledged.
References
- [1].Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews Drug discovery. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- [4].Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [6].Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature reviews Immunology. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- [7].Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual review of immunology. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- [8].Murphy PM. Chemokines and the molecular basis of cancer metastasis. The New England journal of medicine. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- [9].Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mantovani A. The chemokine system: redundancy for robust outputs. Immunology today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- [11].Truchetet ME, Pradeau T. Re-thinking our understanding of immunity: robustness in the tissue reconstruction system. Seminars in immunology. 2018 doi: 10.1016/j.smim.2018.02.013. in press. [DOI] [PubMed] [Google Scholar]
- [12].Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O'Neill L, et al. IL-1 family nomenclature. Nature immunology. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- [14].Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- [15].Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- [16].Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annual review of immunology. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- [17].Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annual review of immunology. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- [18].Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. PTX3, a key component of humoral innate immunity at the interface between defense and tissue remodeling. Physiological Reviews. 2017 in press. [Google Scholar]
- [19].Graham GJ, Locati M, Mantovani A, Rot A, Thelen M. The biochemistry and biology of the atypical chemokine receptors. Immunology letters. 2012;145:30–38. doi: 10.1016/j.imlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- [20].Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, et al. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- [22].Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacological reviews. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duchene J, Novitzky-Basso I, Thiriot A, Casanova-Acebes M, Bianchini M, Etheridge SL, et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nature immunology. 2017;18:753–761. doi: 10.1038/ni.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]