Abstract
Bendamustine + rituximab (BR) has demonstrated high response rates in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). However, progression-free survival (PFS) after BR is <18 months. This study was designed to determine if maintenance lenalidomide after BR induction could improve PFS in R/R CLL/SLL. Thirty-four patients with R/R CLL/SLL who had received 1-5 prior chemotherapy regimens were treated with 6 cycles of BR induction. Patients achieving at least a minor response received twelve 28-day cycles of lenalidomide 5-10 mg/day. The primary endpoint was PFS. The median age was 67 years, with a median of 2 prior therapies. Eleven had confirmed presence of 17p and/or 11q deletions. Twenty-five (74%) completed 6 cycles of induction BR (response rate 56%). Nineteen (56%) patients received maintenance lenalidomide; only 6 patients completed the intended 12 cycles, highlighting the limited feasibility of lenalidomide in this setting due to primarily hematologic and infectious toxicities. The observed median PFS of 18.3 months is not significantly different from that of BR induction in R/R CLL/SLL without maintenance therapy (15.2 months). It is possible that lenalidomide maintenance may be more feasible and effective in the front-line setting, which is being tested in an ongoing trial (NCT01754857).
Keywords: Chronic lymphocytic leukemia, small lymphocytic lymphoma, rituximab, bendamustine, lenalidomide, maintenance
Introduction
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) represent different clinical manifestations of the same chronic lymphoproliferative disease, with a typically indolent but incurable course.(Tsimberidou, et al 2007) Multiple chemotherapy-based treatments have proven beneficial in the treatment of CLL/SLL, including alkylating agents, nucleoside analogues, anthracyclines, and combinations of these agents with rituximab. However, once patients are refractory to nucleoside analogues and/or alkylating agent-based regimens, treatment options become limited.(Tam, et al 2014) Patients with CLL/SLL who have become refractory to fludarabine have poor outcomes, with median survivals of 8-10 months previously reported.(Keating, et al 2002)
Bendamustine has favorable activity in CLL/SLL, with response rates of 88% in up-front therapy of disease and 59% in the relapsed/refractory (R/R) setting.(Fischer, et al 2012, Fischer, et al 2011) A report by the German CLL Study Group observed a median progression-free survival (PFS) of 15.2 months after therapy with bendamustine + rituximab (BR) in the R/R setting.(Fischer, et al 2011) Although these data show encouraging activity of bendamustine for relapsed disease, durations of remission remain shorter than those observed in other non-Hodgkin lymphoma (NHL) subtypes.
Lenalidomide has demonstrated single-agent activity with acceptable toxicity in CLL/SLL.(Chanan-Khan, et al 2006, Chen, et al 2011, Ferrajoli, et al 2008, Witzig, et al 2009) The unique mechanism of action of lenalidomide in CLL/SLL appears to preserve drug activity in the setting of disease refractory to chemotherapy.(Chanan-Khan, et al 2006, Ferrajoli, et al 2008, Witzig, et al 2009) Other disease models, such as myeloma, have demonstrated activity and tolerance of lenalidomide as a maintenance strategy following a chemotherapy-based induction.(Attal, et al 2012) This study is designed to evaluate the activity of lenalidomide as a continuous maintenance therapy after BR induction therapy, with the goal of prolonging PFS.
Patients and methods
Patients
The study was approved by the Human Subjects Committee at the University of Wisconsin and by the Institutional Review Board at each participating Wisconsin Oncology Network (WON) institution. All patients signed an informed consent document describing the investigational nature of the proposed treatments.
Patients were eligible for this trial if they had histologically confirmed CLL or SLL (defined as lymphocyte count <5000/uL) that was progressive after at least 1 prior chemotherapy regimen. Patients were required to be ≥18 years of age with an ECOG performance status of ≤2. Patients could not have received more than 5 prior regimens, and retreatment with an identical regimen was not counted as a separate treatment regimen. Rituximab-refractory disease was permitted, as was prior exposure to bendamustine and lenalidomide, if disease was not refractory to these agents at enrollment. Refractory disease was defined as failure to achieve at least a partial response to the prior therapy, or disease progression ≤6 months after achieving a response.
Patients were required to have measurable or evaluable disease, with no known central nervous system involvement or evidence of HIV disease or chronic infection with hepatitis B or C. Laboratory requirements included a calculated creatinine clearance ≥40 mL/min, neutrophil count ≥1500/μL and platelets ≥100,000/μL. Low blood counts were not exclusionary if related to splenomegaly or disease replacement of bone marrow. Women who were pregnant or breast-feeding were excluded. Patients were required to be disease-free of a prior malignancy for ≥2 years, with the exception of squamous or basal cell skin carcinoma, in situ breast or cervical cancer, or localized prostate cancer treated definitively with hormonal therapy, radiotherapy, or surgery.
Treatment
Patients were treated with bendamustine 90 mg/m2 IV on days 1 & 2 and rituximab 375 mg/m2 IV on day 1 every 28 days for 6 treatment cycles. Administration of rituximab on day 2 of cycle 1 was permitted in patients with high tumor burden at risk for cytokine release syndrome. Patients determined to be at increased risk for tumor lysis syndrome prior to induction chemotherapy were required to receive allopurinol 300 mg orally once or twice daily for 14 days at the start of induction therapy.
Lenalidomide maintenance was started 6-12 weeks after cycle 6, day 1 of induction chemotherapy, once adequate hematologic recovery was observed (neutrophils ≥1500/uL, platelets ≥75K/uL). Patients received lenalidomide 5 mg orally daily on days 1-28 of each 28-day cycle for up to 12 treatment cycles. If there were no unacceptable toxicities and hematologic parameters were met (neutrophils ≥1000/uL and platelets ≥50K/uL), lenalidomide was permitted to escalate to 10 mg orally daily during cycles ≥2. Patients entering maintenance with reduced renal function (creatinine clearance ≥40 and <60 mL/min) started lenalidomide at 5 mg every other day, and were allowed to dose escalate in 5 mg increments in subsequent cycles if the treatment was well-tolerated without any dose modifications or interruptions due to toxicity. Patients who required dose modification for toxicity during lenalidomide maintenance cycles were permitted to dose re-escalate in subsequent cycles.
Protocol therapy was discontinued in the event of unacceptable toxicity, disease progression, or patient and/or physician discretion. Response assessments were performed after cycles 3 and 6 of induction chemotherapy, and following every 3 cycles of maintenance therapy.
Supportive care
Patients received anti-emetic and anti-infective prophylaxis per the local institutional standard. Patients were permitted to receive growth factor support during both induction and maintenance therapy at the discretion of the treating physician.
Allopurinol for tumor lysis syndrome prophylaxis was mandatory in all patients with the initiation of cycle 1 of maintenance lenalidomide. Allopurinol prophylaxis was also required during any cycle in which lenalidomide was dose-escalated or after any prolonged interruption of lenalidomide therapy.
Prophylactic anticoagulation and/or aspirin therapy was permitted in patients deemed to be increased risk for thrombosis, but was not required.
Dose modifications
Dose modifications for toxicity were specified in the protocol. Sequential dose modification of bendamustine was permitted to dose levels of 70 mg/m2, 50 mg/m2, and 40 mg/m2. Reasons for dose reduction of bendamustine included occurrence of neutropenic fever or infection, grade 4 neutropenia or thrombocytopenia lasting ≥7 days, grade ≥3 toxicities not resolving to ≤grade 2 within 2 weeks, or delay of a cycle of induction chemotherapy >2 weeks due to any toxicity.
Lenalidomide dose interruption was required for hematologic toxicities of grade 3 neutropenia with fever or infection, and grade 4 neutropenia or thrombocytopenia. After a dose interruption, patients were permitted to resume lenalidomide at the next lower dose level (i.e., to 5 mg daily from 10 mg daily, to 5 mg every other day from 5 mg daily) if neutrophils recovered to ≥750/uL and platelets recovered to ≥25K/uL prior to day 21 of the cycle. If neutrophils and platelets were not recovered to ≥750/uL and ≥25K/uL within 4 weeks of dose interruption, then lenalidomide was discontinued. The inability to recover from non-hematologic toxicities to grade ≤2 within 4 weeks required discontinuation of lenalidomide.
Criteria for response
Patients were considered evaluable for response if they had completed at least 3 cycles of induction chemoimmunotherapy and undergone an initial response evaluation or had completed at least 1 cycle of therapy and demonstrated disease progression prior to the first scheduled disease assessment. Patients were considered evaluable for toxicity if they had completed at least 1 cycle of therapy. Response and progression in cases of SLL were evaluated using the International Working Group Criteria for response in NHL.(Cheson, et al 1996) Response and progression in cases of CLL were evaluated using the NCI-sponsored CLL Working Group guidelines for CLL.(Cheson, et al 2007)
Statistical considerations
The primary endpoint of this study was progression-free survival (PFS), defined as the number of days from the day of first study drug administration to the day the patient experienced disease progression or death. The study was designed to test the null hypothesis that the median PFS with induction BR and maintenance lenalidomide is at most 18 months versus the alternative hypothesis that median PFS is >18 months, at a one-sided significance level of 0.10 with a power of 80%. A target sample size of 34 was chosen based on an expected accrual period of 30 months, a minimum follow-up period of 12 months, and an anticipated median PFS of at least 32 months. An observed median PFS of 26.7 months or longer would lead to rejection of the null hypothesis in favor of the alternative hypothesis. PFS was summarized using point estimates of the median time to progression or death, and associated 95% confidence intervals, and its survival distribution estimated using Kaplan-Meier method.
Secondary endpoints included the objective response observed with induction BR and overall survival (OS), defined as the number of days from the day of first study drug administration until death from any cause. The proportions of subjects with a complete and partial response were reported along with the corresponding 95% confidence intervals which were calculated using the binomial distribution. Descriptive statistics were generated to summarize toxicity data. Data analysis was performed using SAS (SAS Institute), version 9.4 and R (R-project.org.).
Results
Patients
Thirty-four patients from the University of Wisconsin and seven WON institutions were enrolled from October 2009 to November 2011. The median age of patients was 67 years (range 48-86), with 71% men (Table 1). The majority of patients had an ECOG performance status of ≤1 (94%). Patients had received a median of 2 prior therapies (range 1-4). The majority of patients had received prior rituximab (79%) and fludarabine (68%), with 41% of patients receiving prior therapy with fludarabine, cyclophosphamide, and rituximab (FCR). Three patients (9%) had received prior therapy with bendamustine. Cytogenetic profiling by FISH analysis was available in 24 patients (71%), with 11/24 (46%) demonstrating presence of 17p and/or 11q deletions (positivity for 17p defined as >6.5% of nuclei with presence of deletion; 11q defined as >4.5% of nuclei with presence of deletion).
Table 1.
Baseline patient characteristics (n=34).
| Age median (range) | 67 (48-86) | |
| Age <60 | 5 (15%) | |
| Age ≥60 | 29 (85%) | |
| Age ≥70 | 11 (32%) | |
|
| ||
| Sex | ||
| Men | 24 (71%) | |
| Women | 10 (29%) | |
|
| ||
| ECOG PS | ||
| 0 | 16 (47%) | |
| 1 | 16 (47%) | |
| 2 | 2 (6%) | |
|
| ||
| Staging: | ||
| CLL (Rai staging) | 26 | |
| Stage III/IV | 14 (54%) | |
| Stage I/II | 12 (46%) | |
| SLL (Ann Arbor staging) | 8 | |
| Stage III/IV | 7 (88%) | |
| Stage I/II | 1 (12%) | |
|
| ||
| Median prior therapies (range) | 2 (1-4) | |
| 1 | 15 (44%) | |
| 2 | 9 (26%) | |
| 3 | 9 (26%) | |
| 4 | 1 (3%) | |
|
| ||
| Prior therapies: | ||
| Rituximab | 27 (79%) | |
| Fludarabine | 23 (68%) | |
| FCR† | 14 (41%) | |
| Bendamustine | 3 (9%) | |
| Cyclophosphamide | 11 (32%) | |
| Alemtuzumab | 2 (6%) | |
| Ofatumumab | 1 (3%) | |
| Chlorambucil | 1 (3%) | |
|
| ||
| Refractory to most recent therapy | Yes | 4 (12%) |
| No | 30 (88%) | |
|
| ||
| Elevated LDH | Yes | 20 (59%) |
| No | 14 (41%) | |
|
| ||
| Cytogenetics (by FISH analysis)* | 24/34 | (71%) |
| 13q†† | 2/24 | (8%) |
| Trisomy 12†† | 8/24 | (33%) |
| 11q‡ | 6/24 | (43%) |
| 17p‡ | 6/24 | (43%) |
| Normal | 3/24 | (13%) |
Available in 24 patients at baseline.
Fludarabine, cyclophosphamide, rituximab.
13q and trisomy 12 reported when present as isolated abnormalities.
1 patient had FISH analysis positive for both 11q and 17p deletions.
Responses to induction chemotherapy
Twenty-five of 34 patients (74%) completed 6 cycles of BR induction therapy. Two patients died after cycle 1 of BR from pneumonia and heart failure, respectively. Seven patients (21%) completed 2-5 cycles of induction BR chemotherapy. Reasons for discontinuing induction BR prematurely included: progressive/transformed disease (n=2), infection (n=2; cellulitis, cytomegalovirus reactivation), bowel perforation, elevated liver function tests, and stable disease in setting of failure to thrive.
The overall response rate (ORR) was 56% (95% confidence interval 38-73%), with 7 complete (21%) and 12 partial (35%) responses (Table 2). Nine patients achieving stable disease (26%) were eligible to proceed to maintenance therapy. Among the 11 patients with higher-risk 11q and/or 17p deletions, the ORR was similar at 55% (Table 2).
Table 2.
Response rates to BR induction therapy.
| Enrolled population (n=34)† | ||
|---|---|---|
| Response | n (%) | 95% CI |
| CR | 7 (21) | 9-38% |
| PR | 12 (35) | 20-54% |
| SD | 9 (26) | 13-44% |
| ORR | 19 (56) | 38-73% |
| Subgroup with known 11q or 17p deletions (n=11) | ||
| Response | n (%) | 95% CI |
| CR | 1 (9) | 0-41% |
| PR | 5 (45) | 17-77% |
| SD | 4 (36) | 11-69% |
| ORR | 6 (55) | 23-83% |
CI=confidence interval, CR=complete response, ORR=overall response rate, PR=partial response, SD=stable disease
3 patients were not evaluable for response, but included in the intent-to-treat analysis.
Responses to maintenance therapy
Of the 25 patients who completed BR induction chemotherapy, 19 patients (56% of enrolled population) started maintenance therapy with lenalidomide (Figure 1). Reasons for not initiating maintenance lenalidomide included patient refusal (n=1), progressive disease (n=1), persistent cytopenias (n=3), and infection (n=1).
Figure 1.
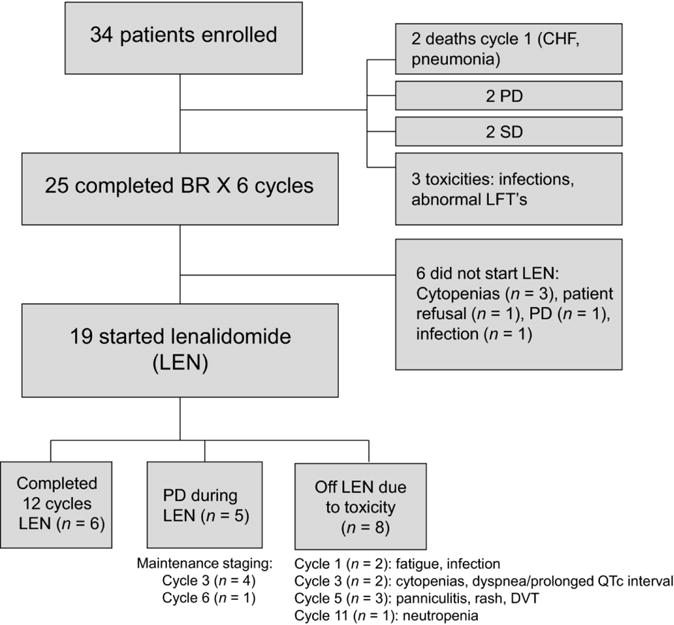
CONSORT diagram of patient treatment administration per protocol.
One patient improved from a partial to complete response during the course of maintenance therapy. Seven patients maintained a PR following induction therapy throughout maintenance, and 4 patients maintained a CR following induction therapy throughout maintenance. Five patients experienced progressive disease (PD) during maintenance therapy.
Tolerance of maintenance therapy
Six of 19 patients treated with maintenance lenalidomide completed all 12 planned cycles of maintenance therapy. The primary reasons for early discontinuation of maintenance therapy were PD (n=5) and toxicity (n=8). Toxicities leading to early discontinuation of lenalidomide are described in Figure 1.
Early progression was a common reason for discontinuation of lenalidomide during maintenance cycles 1-3. Of 8 patients discontinuing lenalidomide during maintenance cycles 1-3, 4 had evidence of PD at the time of initial disease assessment post-maintenance cycle 3 and the remaining 4 discontinued due to primary toxicities of neutropenia, thrombocytopenia, and infection. Four additional patients received 5-6 cycles of maintenance lenalidomide, and 1 patient received 11 cycles of lenalidomide.
A total of 122 lenalidomide maintenance cycles were administered, which is 54% of the intended 228 cycles among the 19 patients starting maintenance therapy. Sixty-two cycles (51%) were administered at dose level 1 (5 mg/day), 39 cycles (32%) were administered at dose level 2 (10 mg/day), and 21 cycles (17%) were administered at dose level −1 (5 mg every other day). Twenty-four of the administered 122 cycles (20%) required dose interruption or dosing delays due to toxicities.
Toxicities during induction bendamustine and rituximab
Toxicities were reported using the Common Terminology Criteria for Adverse Events, version 3.0. Dose modifications were required in 14 (41%) patients, most commonly for neutropenia (n=12), thrombocytopenia (n=3), and weight loss/failure to thrive (n=3). Grade 3-4 toxicities were primarily hematologic, with neutropenia in 20 patients (59%), anemia in 1 (3%), and thrombocytopenia in 7 (21%). Febrile neutropenia occurred in 5 patients (15%). Infections with or without neutropenia were common; grade 2 infections occurred in 12 patients (35%), and grade 3-4 infections occurred in 6 patients (18%). One death from pneumonia occurred during cycle 1 of BR. Grade 2 rash occurred in 5 patients (15%). Other common grade 2 toxicities included nausea (5%), stomatitis (6%), emesis (6%), and hyperglycemia (21%).
Toxicities during lenalidomide maintenance
Hematologic toxicities and infections were the mostly commonly reported adverse events during maintenance therapy (Table 3). Grade 3-4 neutropenia occurred in 47% (9/19) of patients, and grade 3 thrombocytopenia occurred in 1 (5%) patient.
Table 3.
Adverse events during induction BR (worst grade toxicity per patient, n=34).
| Gr 3 (%) | Gr 4 (%) | Gr 5 (%) | |
|---|---|---|---|
| Hematologic toxicities: | |||
| Leukopenia | 5 (15%) | 5 (15%) | — |
| Neutropenia | 6 (18%) | 14 (41%) | — |
| Anemia/hemoglobin | 1 (3%) | — | — |
| Thrombocytopenia | 5 (15%) | 2 (6%) | — |
| Infections: | |||
| Febrile neutropenia | 4 (12%) | — | — |
| Infections | 10 (30%) | 1 (3%) | 1 (3%) |
| Non-hematologic toxicities: | |||
| Failure to thrive | 1 (3%) | — | — |
| Fatigue | 2 (6%) | — | — |
| Nausea | 1 (3%) | — | — |
| Emesis | 2 (6%) | — | — |
| Anorexia | 1 (3%) | — | — |
| Diarrhea | 3 (9%) | — | — |
| Dysphagia | 1 (3%) | — | — |
| Heart failure | — | — | 1 (3%) |
| Hyperglycemia | 1 (3%) | — | — |
| Hypokalemia | 1 (3%) | — | — |
| Hypophosphatemia | 2 (6%) | — | — |
| Elevated AST/ALT | 4 (12%) | — | — |
| Hyponatremia | 1 (3%) | — | — |
| Tumor lysis syndrome | 1 (3%) | — | — |
Grade 3-4 infections were observed in 5 (26%) patients, and 1 (5%) patient experienced grade 3 febrile neutropenia. Most of the infectious complications involved sinus, pulmonary, and soft tissue infections. Other common grade 1-2 toxicities included fatigue (32%), rash (26%), nausea (21%), pain (43%), cough (21%), and elevated transaminases (21%).
Progression-free and Overall Survival
Accrual occurred from October 2009 through November 2011, with long-term follow-up through January 1, 2015, and a median follow-up of 36.5 months. Seventeen (50%) patients have died during the study follow-up period. CLL/SLL was the cause of death in 9 patients (26%), including 2 patients with biopsy-proven transformation to high-grade lymphoma. Five deaths (15%) occurred as the result of infections, 2 deaths resulted from cardiac causes, and 1 death was the result of toxicity from subsequent therapy.
All patients were included in the analysis according to the intention-to-treat principle. The median PFS was 18.3 (95% CI, 10.6-24.3) months (Figure 2A). Progression-free survival for the 24 patients with baseline CLL FISH mutation analysis data available (Figure 2B) showed no difference between those with the high-risk 11q or 17p deletions (median 17.5 months, 95% CI, 8.7-22.1) and those without these high-risk deletions (median 12.8 months, 95% CI, 6.5-24.3). The three-year PFS rate was 23% (95% CI 12-43%), and was not different based on the risk profile of baseline cytogenetics (18% for those with high-risk deletions vs. 15% for those without).
Figure 2.

A. Progression-free survival in the intention-to-treat population (n=34). Events include observed progression or death.
B. Progression-free survival based on cytogenetic subgroup analysis by FISH. Events include observed progression or death.
C. Overall survival in the intention-to-treat population (n=34).
The median OS survival was 42.8 months (95% CI undefined, Figure 2C). There was no difference in OS between those with the high-risk deletions versus those without.
Discussion
Bendamustine-based chemotherapy has provided another effective therapy option for patients with relapsed CLL/SLL, although median durations of remission have been <18 months.(Fischer, et al 2011) Maintenance therapy has previously been explored with agents such as rituximab in other indolent NHL subtypes, with resulting improvement in median PFS and time to next therapy.(Forstpointner, et al 2006, Hochster, et al 2009, Salles, et al 2011, van Oers, et al 2010) Rituximab is a less appealing option as a single-agent maintenance therapy in CLL/SLL given the low level of CD20-expression in CLL/SLL and low single-agent response of rituximab in CLL/SLL. Although some data have suggested a potential benefit with maintenance therapy with rituximab after induction chemotherapy in CLL/SLL,(Abrisqueta, et al 2013, Hainsworth, et al 2003) other agents with novel mechanisms of action in CLL/SLL deserve further exploration.
Lenalidomide is an oral agent with novel immunomodulatory and antiangiogenic properties in CLL/SLL, although the exact mechanisms of activity are unknown. Lenalidomide has been demonstrated to inhibit VEGF and TNF-α-induced endothelial cell migration.(Dredge, et al 2005) In addition, lenalidomide stimulates T-cell proliferation and the production of IL-2, IL-10 and IFN-γ. The resulting increased production of IL-2 and IFN-γ leads to augmentation of NK cell number and function.(Ramsay and Gribben 2009) Lenalidomide appears to restore defective T-cell actin cytoskeletal signaling synapses and enhance the antigen-presenting function of CLL cells via upregulation of co-stimulatory molecules.(Aue, et al 2009, Ramsay, et al 2012, Ramsay and Gribben 2009) In patients receiving lenalidomide for first-line therapy of CLL, the signature of gene expression was significantly modified following lenalidomide exposure and enriched for immune-associated genes, notably TNF-α, lymphocytic activation gene-3, and IFN-induced genes such as 2,5-oligoadenylate synthetase.(Chen, et al 2011)
Lenalidomide has previously demonstrated single-agent activity in R/R CLL/SLL (ORR 32-47%), including fludarabine-refractory disease.(Chanan-Khan, et al 2006, Ferrajoli, et al 2008) Lenalidomide 10-25 mg/day resulted in objective responses in 31% of patients with R/R CLL/SLL and presence of the adverse prognostic indicators 11p or 17p deletion.(Ferrajoli, et al 2008) In 25 previously untreated patients with CLL, treatment with lenalidomide 10 mg/day on days 1-21 of each 28 day cycle resulted in a partial response in 56% of patients and ≥50% reduction in peripheral lymphocytosis in 80% of patients.(Chen, et al 2011) Previous reports have also observed an association between likelihood of objective response in NHLs in the setting of lower tumor burden, generating further interest in the use of lenalidomide as a maintenance therapy after an initial chemotherapy cytoreduction.(Tuscano, et al 2007, Wiernik, et al 2007) Lenalidomide as a continuous dosing schedule may be advantageous, as prior reports described rebound lymphocytosis during breaks from drug dosing.(Chen, et al 2011) Based on these previous observations of lenalidomide activity and tolerability, the hypothesis was proposed that lenalidomide maintenance therapy to treat minimal residual disease following induction BR would improve PFS in R/R CLL/SLL. In addition, these novel mechanisms of anti-tumor activity and immunomodulatory properties of lenalidomide were hypothesized to provide benefit to patients with heavily pretreated or chemotherapy-refractory disease and patients with higher-risk genetic mutations.
The median PFS of 18.3 months observed in this study is not significantly different from the median PFS reported by the German CLL Study Group experience with BR chemotherapy without maintenance therapy (15.2 months) in R/R CLL.(Fischer, et al 2011) There are several potential explanations for this finding. The population enrolled to this study did not represent a particularly favorable-risk population, and the toxicities of BR induction therapy in previously treated patients were demonstrated. As a result, a significant number of patients (44%) were unable to initiate maintenance therapy (Figure 1). Therefore, it is difficult to fully assess the potential benefit of maintenance therapy given the smaller number of patients who were able to initiate maintenance lenalidomide. The induction regimen may have been insufficient to provide enough cytoreduction for maintenance therapy to achieve sustained disease control. As over 40% of the enrolled population had previously received FCR chemotherapy, there was less likelihood of achieving deep responses given the lower rates of complete response with BR versus FCR chemotherapy.(Tam, et al 2008)
It is also possible that the low dosing strategy employed was insufficient to achieve anti-tumor activity for the residual disease present after induction therapy. In the single-agent studies of lenalidomide in R/R CLL/SLL, dosing levels of lenalidomide were 10-25 mg/daily. Other dosing strategies with lenalidomide utilizing some breaks in therapy (i.e., dosing on days 1-21 of each 28 day cycle) may also minimize toxicity and improved tolerability compared with the continuous dosing employed in this study. Conversely, the high rate of discontinuation of lenalidomide during maintenance therapy due to toxicities (8 of 19 patients) suggest that further dose-escalation of lenalidomide may not be feasible in the R/R population.
Lenalidomide in combination with other agents may prove to be more effective as maintenance therapies in the treatment of CLL/SLL. Recently reported data investigating the activity of lenalidomide + rituximab in treatment of R/R CLL have shown impressive rates of activity, even in patients with high-risk genetic mutations and chemotherapy-refractory disease.(Badoux, et al 2013) In a report of 59 patients with R/R CLL, an ORR of 53% was observed in patients with presence of the 17p deletion, which was not significantly different from the 66% ORR in the overall population.(Badoux, et al 2013) The optimal timing of lenalidomide maintenance therapy may also be better suited for use in the front-line setting, when bone marrow reserve may be improved and toxicities may be less problematic after induction chemotherapy. Although the addition of rituximab to lenalidomide in the maintenance setting will likely lead to increased rates of neutropenia (Badoux, et al 2013), the severity of neutropenia may be less with lower risk for infectious complications when this combination is used in first-line therapy for CLL/SLL. These hypotheses are the basis for an ongoing study through the WON investigating BR induction therapy followed by rituximab + lenalidomide maintenance therapy for previously untreated CLL/SLL (NCT01754857). Formal assessment of CLL FISH mutation analysis is required for all patients, allowing for better assessment of the activity of lenalidomide + rituximab maintenance in a higher-risk subpopulation.
Lenalidomide as maintenance therapy did not improve PFS after BR induction therapy in this R/R population. The toxicities observed with induction BR chemotherapy combined with the low proportion of patients able to receive planned maintenance lenalidomide limited the feasibility of investigating the activity of lenalidmide in this R/R population. A follow-up study is ongoing to evaluate the combination of lenalidomide + rituximab as maintenance following BR induction in the previously untreated CLL/SLL population (NCT01754857), where the synergistic activity of lenalidomide + rituximab and presumed improved ability to tolerate toxicity of treatment as up-front therapy will be investigated.
Table 4.
Adverse events during maintenance lenalidomide (worst grade toxicity per patient, n=19).
| Gr 1-2 (%) | Gr 3 (%) | Gr 4 (%) | |
|---|---|---|---|
| Hematologic toxicities: | |||
| Leukopenia | 4 (21%) | 6 (32%) | 1 (5%) |
| Neutropenia | 3 (16%) | 7 (37%) | 2 (11%) |
| Anemia/hemoglobin | 4 (21%) | — | — |
| Thrombocytopenia | 2 (11%) | 1 (5%) | — |
| Infections: | |||
| Febrile neutropenia | — | 1 (5%) | — |
| Infection: sinus, pulmonary, or ear | 5 (26%) | 2 (11%) | 1 (5%) |
| Infection: skin or soft tissue | 1 (5%) | 1 (5%) | — |
| Infection: other | 1 (5%) | 1 (5%) | — |
| Non-hematologic toxicities: | |||
| Constitutional symptoms: | |||
| Fatigue | 6 (32%) | — | 1 (5%) |
| Generalized weakness | 1 (5%) | — | — |
| Fever | 1 (5%) | — | — |
| Night sweats | 3 (16%) | — | — |
| Tumor flare | 1 (5%) | — | — |
| Lymphadenitis | 1 (5%) | — | — |
| Edema | 1 (5%) | — | — |
| Dermatologic: | |||
| Rash | 5 (26%) | ||
| Gastrointestinal: | |||
| Anorexia | 1 (5%) | — | — |
| Nausea | 4 (21%) | — | — |
| Vomiting | 1 (5%) | — | — |
| Constipation | 1 (5%) | — | — |
| Diarrhea | 1 (5%) | — | — |
| Dyspepsia | 1 (5%) | — | — |
| Xerostomia | 1 (5%) | — | — |
| Stomatitis | — | 1 (5%) | — |
| Esophagitis | 1 (5%) | — | — |
| Abdominal pain | — | 1 (5%) | — |
| Respiratory: | |||
| Cough | 4 (21%) | — | — |
| Dyspnea | 1 (5%) | — | — |
| Neurologic: | |||
| Headache | 3 (16%) | — | — |
| Sensory neuropathy | 1 (5%) | — | — |
| Musculoskeletal/pain: | |||
| Arthralgias | 1 (5%) | — | — |
| Musculoskeletal pain | 6 (32%) | — | — |
| Pain NOS | 2 (11%) | ||
| Cardiac/vascular: | |||
| Palpitations | 1 (5%) | — | — |
| Prolonged QTc interval | — | 1 (5%) | — |
| Pericarditis | — | 1 (5%) | — |
| Deep venous thrombosis | — | 1 (5%) | — |
| Laboratory values/chemistries: | |||
| Hypokalemia | — | — | 1 (5%) |
| Hypocalcemia | 1 (5%) | — | — |
| Hyphophosphatemia | 1 (5%) | — | — |
| Hyperglycemia | 4 (21%) | — | — |
| Hypogammaglobulinemia | 1 (5%) | 1 (5%) | — |
| Elevated alkaline phosphatase | 1 (5%) | — | — |
| Elevated AST/ALT | 4 (21%) | — | — |
Table 5.
Progression-free survival.
| 1-year (95% CI) |
2-year (95% CI) |
3-year (95% CI) |
|
|---|---|---|---|
| PFS in ITT population (n=34) | 0.62 (0.47-0.80) | 0.35 (0.22-0.56) | 0.23 (0.12-0.43) |
| PFS in patients with high-risk genetic mutations† (11/34) | 0.63 (0.41-0.99) | 0.18 (0.05-0.64) | 0.18 (0.05-0.64) |
| PFS in patients without high-risk genetic mutations (13/34) | 0.54 (0.32-0.89) | 0.31(0.14-0.70) | 0.15 (0.04-0.55) |
| PFS in patients with unknown genetic mutations (10/34) | 0.70 (0.47-1.00) | 0.60 (0.36-1.00) | 0.40 (0.19-0.85) |
ITT=intention to treat, CI=confidence interval.
High-risk genetic mutations defined as 17p or 11q deletions.
Acknowledgments
This work was supported by Celgene Corporation and by the Cancer Center Support Grant P30 CA014520 to the University of Wisconsin Carbone Cancer Center.
Footnotes
Conflicts of interest: Julie E. Chang, Research funding Celgene and Genentech.
Thomas Havighurst, No conflicts of interest to report.
KyungMann Kim, No conflicts of interest to report.
Jens Eickhoff, No conflicts of interest to report.
Anne M. Traynor, No conflicts of interest to report.
Rachel Kirby-Slimp, No conflicts of interest to report.
Lynn M. Volk, No conflicts of interest to report.
Jae E. Werndli, No conflicts of interest to report.
Ronald S. Go, No conflicts of interest to report.
Matthias Weiss, No conflicts of interest to report
Jules Blank, No conflicts of interest to report.
Brad S. Kahl, Consulting for Celgene and Genentech.
Author contributions:
Julie Chang, Trial design and implementation; data collection, data analysis, and manuscript preparation.
Thomas Havighurst, Data analysis, manuscript preparation.
KyungMann Kim, Data analysis, manuscript preparation.
Jens Eickhoff, Trial design and implementation.
Anne M. Traynor, Trial design and implementation, data management.
Rachel Kirby-Slimp, Trial design and implementation, data management.
Lynn Volk, Trial design and implementation, data management.
Jae Werndli, Trial design and implementation, data management.
Ronald S. Go, Trial design and implementation.
Matthias Weiss, Trial design and implementation.
Jules Blank, Trial design and implementation.
Brad Kahl, Trial design and implementation; data collection, and manuscript preparation.
References
- Abrisqueta P, Villamor N, Terol MJ, Gonzalez-Barca E, Gonzalez M, Ferra C, Abella E, Delgado J, Garcia-Marco JA, Gonzalez Y, Carbonell F, Ferrer S, Monzo E, Jarque I, Muntanola A, Constants M, Escoda L, Calvo X, Bobillo S, Montoro JB, Montserrat E, Bosch F. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122:3951–3959. doi: 10.1182/blood-2013-05-502773. [DOI] [PubMed] [Google Scholar]
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L, Decaux O, Leyvraz S, Vekemans MC, Voillat L, Michallet M, Pegourie B, Dumontet C, Roussel M, Leleu X, Mathiot C, Payen C, Avet-Loiseau H, Harousseau JL, Investigators, I.F.M Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- Aue G, Njuguna N, Tian X, Soto S, Hughes T, Vire B, Keyvanfar K, Gibellini F, Valdez J, Boss C, Samsel L, McCoy JP, Jr, Wilson WH, Pittaluga S, Wiestner A. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94:1266–1273. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, Sargent R, Burger JA, Ferrajoli A. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31:584–591. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, Brandwein J, Pantoja M, Johnston J, Gibson S, Hernandez T, Spaner D, Trudel S. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on, L Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, Pfluger KH, Schott S, Goede V, Isfort S, von Tresckow J, Fink AM, Buhler A, Winkler D, Kreuzer KA, Staib P, Ritgen M, Kneba M, Dohner H, Eichhorst BF, Hallek M, Stilgenbauer S, Wendtner CM. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, Bottcher S, Staib P, Kiehl M, Eckart MJ, Kranz G, Goede V, Elter T, Buhler A, Winkler D, Kneba M, Dohner H, Eichhorst BF, Hallek M, Wendtner CM. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, Pott C, Seymour JF, Metzner B, Hanel A, Lehmann T, Hartmann F, Einsele H, Hiddemann W. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108:4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Litchy S, Barton JH, Houston GA, Hermann RC, Bradof JE, Greco FA. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, Zhang L, Colocci N, Frankel S, Horning SJ. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MJ, O’Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M, Lerner S, Kantarjian H. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- R-project.org. R Development CoreTeam R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: URL. Available from: http://www.R-project.org.2014. [Google Scholar]
- Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–1202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Ferme C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- SAS Institute, I.S.O., Cary, NC: SAS Institute, Inc., 2005.
- Tam CS, O’Brien S, Plunkett W, Wierda W, Ferrajoli A, Wang X, Do KA, Cortes J, Khouri I, Kantarjian H, Lerner S, Keating MJ. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab) Blood. 2014;124:3059–3064. doi: 10.1182/blood-2014-06-583765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimberidou AM, Wen S, O’Brien S, McLaughlin P, Wierda WG, Ferrajoli A, Faderl S, Manning J, Lerner S, Mai CV, Rodriguez AM, Hess M, Do KA, Freireich EJ, Kantarjian HM, Medeiros LJ, Keating MJ. Assessment of chronic lymphocytic leukemia and small lymphocytic lymphoma by absolute lymphocyte counts in 2,126 patients: 20 years of experience at the University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2007;25:4648–4656. doi: 10.1200/JCO.2006.09.4508. [DOI] [PubMed] [Google Scholar]
- Tuscano JM, Lossos IS, Justice G, Vose JM, Takeshita K, Ervin-Haynes A, Pietronigro D, Zeldis JB, Habermann TM. Lenalidomide Oral Monotherapy Produces a 53% Response Rate in Patients with Relapsed/Refractory Mantle-Cell Non-Hodgkin’s Lymphoma. ASH Annual Meeting Abstracts. 2007;110:2563. [Google Scholar]
- van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, Kimby E, van t Veer M, Vranovsky A, Holte H, Hagenbeek A. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, McBride K, Wride K, Pietronigro D, Takeshita K, Ervin-Haynes A, Zeldis JB, Habermann TM. Lenalidomide Response in Relapsed/Refractory Aggressive Non-Hodgkin’s Lymphoma Is Related to Tumor Burden and Time from Rituximab Treatment. ASH Annual Meeting Abstracts. 2007;110:2565. [Google Scholar]
- Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, Kaplan H, Voralia M, Pietronigro D, Takeshita K, Ervin-Haynes A, Zeldis JB, Vose JM. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol. 2009;27:5404–5409. doi: 10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]


