Abstract
Recovery of hydraulic conductivity after the induction of embolisms was studied in woody stems of laurel (Laurus nobilis). Previous experiments confirming the recovery of hydraulic conductivity when xylem pressure potential was less than −1 MPa were repeated, and new experiments were done to investigate the changes in solute composition in xylem vessels during refilling. Xylem sap collected by perfusion of excised stem segments showed elevated levels of several ions during refilling. Stem segments were frozen in liquid N2 to view refilling vessels using cryoscanning electron microscopy. Vessels could be found in all three states of presumed refilling: (a) mostly water with a little air, (b) mostly air with a little water, or (c) water droplets extruding from vessel pits adjacent to living cells. Radiographic probe microanalysis of refilling vessels revealed nondetectable levels of dissolved solutes. Results are discussed in terms of proposed mechanisms of refilling in vessels while surrounding vessels were at a xylem pressure potential of less than −1 MPa. We have concluded that none of the existing paradigms explains the results.
Over the past two decades, scientists have found substantial evidence that the vulnerability of xylem to cavitation is an important factor in the adaptation of plants to the environment (Tyree and Sperry, 1989; Cochard et al., 1992; Salleo and Lo Gullo, 1993). The cavitation (drought-induced embolism) of xylem has been detected in stems (Cochard and Tyree, 1990), leaves (Kikuta et al., 1997), and roots (Mencuccini and Comstock, 1997) and has appeared to limit effectively the possible distribution areas of plant species (Cochard et al., 1992). For example, the vulnerability of Holm oak to xylem embolism caused by both drought and freeze stress (Lo Gullo and Salleo, 1993) provides a convincing explanation for the distribution versus elevation and latitude of this species (Pignatti, 1982) in the Mediterranean region.
The threshold xylem pressure for cavitation is close to the typical midday xylem pressure of many species in the field (Kikuta et al., 1997). Such a narrow safety margin (Sperry, 1995) is intrinsically dangerous for plant survival under adverse environmental conditions but might be of some advantage in buffering leaf water status (Dixon et al., 1984; Salleo et al., 1997) and in inducing stomatal closure (Sperry, 1995).
Debate still exists about the possible mechanisms involved in xylem refilling after cavitation events induced by drought (Tyree and Cochard, 1996) and freezing (Sperry, 1995) stress. The existing paradigm suggests that embolism removal must occur by gas dissolution in the surrounding water. Henry's law states that the solubility of a gas in water is proportional to the partial pressure of the gas species adjacent to the water. Since water in plants is saturated with air at atmospheric pressure, the paradigm requires that the embolism be above atmospheric pressure for the gases to dissolve. Some experiments on angiosperms and gymnosperms fit this paradigm (Tyree and Yang 1992; Yang and Tyree, 1992; Lewis et al., 1994). These studies reported that embolism dissolution ceases when gas pressure is at or below atmospheric pressure and that the rate of dissolution is limited, as expected, by the rate at which gases diffuse from air bubbles (as governed by Fick's law).
Surface tension at the air/water interface of a gas bubble permits the gas to be at a higher pressure than the liquid. For an embolism in a xylem conduit, the pressure of the gas (Pg) is given by:
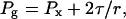 |
1 |
where Px is the threshold xylem pressure, r is the radius of curvature of the air/water interface, and τ is the surface tension of water in the vessel. Therefore, gas in an embolism will exceed atmospheric pressure whenever the threshold xylem pressure is greater than −2τ/rv, where rv is the radius of the vessel lumen containing the bubble. In a vessel lumen 20 μm in diameter, −2τ/rv is about −15 kPa; therefore, previous reports of embolism dissolution in stem segments at a threshold xylem pressure of −2τ/rv fit within existing paradigms (Borghetti et al., 1991; Tyree and Yang, 1992; Yang and Tyree, 1992). This condition (Px > −2τ/rv) can be expected in plants that develop root pressure at night (Ewers et al., 1991; Fisher et al., 1997) or in the early spring (Sperry et al., 1987). In a recent survey Fischer et al. (1997) reported measurements of root pressures in 109 tropical species that showed the maximum positive xylem pressures to be on average about 20 to 100 kPa. Root pressure can facilitate the recovery of embolized xylem conduits in herbs, shrubs, and small trees but is unlikely to do the same in tall trees, because root pressure is dissipated by guttation and gravity. Even without guttation, gravity dissipates root pressure at the rate of 10 kPa for every meter in height above the ground. Moreover, root pressures were absent in about 20 of the 109 species studied, with some taxonomic trend among them. Although root pressures are probably more widespread than previously thought, they have never been recorded in gymnosperms (Milburn and Kallarackal, 1991) or in most forest trees (Kramer, 1983).
Bubbles have been shown to dissolve when the threshold xylem pressure is less than −2τ/rv in stem segments of Scots pine (i.e. when Px = −40 kPa; Edwards et al., 1994). The authors of that study thought that their observations violated existing paradigms, but Lewis et al. (1994) argued that they did not because stem segments of Scots pine were perfused with degassed water. Hence, given sufficient time, the gas bubble pressure should decline to that of a perfect vacuum because of Henry's law. Under these unusual conditions, bubble collapse could occur down to about −120 kPa, depending on current atmospheric pressure and rv. These conditions would rarely be obtained in nature, because the water in plants and soils would be nearly saturated with air at atmospheric pressure.
Do embolisms dissolve in plants without root pressure? Recent studies (Salleo and Lo Gullo, 1989; Canny, 1995a, 1995b, 1997) have reported embolism repair under conditions when the threshold xylem pressure is much less than −2τ/r. The best documented case of embolism recovery under water stress was reported by Salleo et al. (1996). Studies were conducted on laurel (Laurus nobilis) in dry soil (predawn [ΨL] equal to −1 MPa). Therefore, the threshold xylem pressure should have been less than −1 MPa before embolisms were induced. One-year-old twigs were cavitated by air injection in a pressure collar and recovered from embolism 20 min after pressure release. Xylem refilling was greatly increased in twigs treated with 50 mm KCl or with KCl plus 1 mm IAA solutions put in contact with the exposed phloem of subsequently cavitated twigs. In the same study, it was shown that xylem refilling was prevented or strongly reduced by girdling the stem before inducing cavitation. The shorter the time interval between girdling and measurement of PLC, the larger the PLC recovery.
The above results could fit with the existing paradigm if the threshold xylem pressure were locally greater than −2τ/r in refilling vessels while full vessels were at −1 MPa. These conditions could be obtained if refilling involved the excretion of osmotica (salts and/or organic molecules) from living cells surrounding the cavitated vessel. If the π of the sap in the refilling vessel were more than 1 MPa, the threshold xylem pressure of the sap would be at a pressure greater than 0. Similar suggestions were made by Grace (1993). The purpose of this study was to repeat the experiments of Salleo et al. (1996) and to look for the existence of solutes in stem segments in the process of refilling. In the absence of sufficient osmotica, we would have to hypothesize a new paradigm for refilling. Alternative paradigms are presented.
MATERIALS AND METHODS
Inducing Xylem Cavitation and Measuring Stem Hydraulic Conductivity
Experiments were conducted on potted 7-year-old plants of a Mediterranean species of laurel (Laurus nobilis L.) belonging to the group of trees called “laurel-type trees” (Lausi et al., 1989), which are the typical components of the Laurisilva forest. These plants typically grow in zones with a high RH, especially in the summer (Kamer, 1974).
About 50 laurel plants were grown in a greenhouse in the Botanical Garden of Trieste, Italy, under natural light. Plants were in 2.5-L pots, were about 1 m tall, and had six to eight branches per plant emerging near ground level. Plants were divided into two groups: 15 plants received constant irrigation to near full hydration and 35 plants were deprived of watering until the ΨL of their 1-year-old twigs declined to −1.0 ± 0.03 MPa at predawn. ΨL was measured by covering at least five leaves per plant with aluminum foil and black plastic bags and covering the whole plant with bags for at least 2 h to equilibrate ΨL with soil Ψ. ΨL was then estimated using a pressure chamber (Tyree and Hammel, 1972), which measures the xylem pressure (ΨL plus πx) of nontranspiring leaves. In our experiments πx was small, so the xylem pressure was a good estimate of ΨL. In transpiring leaves there is a gradient of ΨL along the leaf blade from the xylem to the evaporating surface. When the previously transpiring leaf is placed in the pressure chamber, the xylem pressure assumes an average value of ΨL plus πx. During the experiments, irrigated plants were maintained at a ΨL between −0.05 and −0.07 MPa.
A pressure differential of 2.24 MPa was applied across the interconduit pit membranes of 1-year-old twigs by combining the negative pressure developed in the xylem as a consequence of water shortage (Px = −1.0 ± 0.03 MPa) with positive air pressure applied from outside. Twigs were pressurized using a pressure collar about 80 mm in length tightly fitted at their midsection (Salleo et al., 1992, 1996). Air pressures up to +1.24 MPa were applied so that the total ΔP across the interconduit pit membranes was 2.24 MPa.
Several pressure/volume curves performed on leaves of laurel plants using the pressure chamber technique (Tyree and Hammel, 1972; Salleo, 1983) showed that ΨL at the turgor loss point was −2.26 ± 0.32 MPa. Therefore, the applied ΔP simulated a condition causing large xylem cavitation (Salleo and Lo Gullo, 1993). Twigs were pressurized by increasing the pressure with a rate of about 70 kPa min−1. Once the desired air pressure was reached, it was maintained constant for 20 min and then decreased at the same rate.
Twigs were cut off under distilled filtered water 2 or 20 min after the complete pressure release and tested for PLC (see below). The former time interval was selected to measure the initial impact of xylem cavitation on the hydraulic conductivity of twigs; the latter (20 min) is sufficient for cavitated xylem conduits of laurel twigs to refill partially (Salleo et al., 1996). The plants had six to eight similar branches emerging near ground level. Only one branch per plant was subjected to pressurization in the pressure collar to minimize rehydration of tissues after release of water from embolized stems: Water released in one stem should travel to all stems, roots, and soil soon after embolism formation. Water released by embolisms can cause a rehydration of ΨL of 100 to 200 kPa during bench-top dehydration of excised branches (Dixon et al., 1984; Salleo et al., 1997). Intact pressurized twigs were enclosed in black plastic bags with the entire plant to minimize transpiration until they were cut off and tested for PLC. In some cases xylem pressure values were measured after the pressure collar treatment to confirm predawn values, and no significant changes were found.
Twigs were cut off at their junction plane with older stems and were recut at both sides using new razor blades. Only about 20 mm of stem was removed at the distal twig side to leave the majority of the conduits as intact as possible. Twigs (0.35–0.47 m in length) were then connected to the equipment for measuring hydraulic conductivity, and the axial flow was measured under a pressure of 10 kPa, alternating measurements with flushes at 175 kPa to remove the emboli and providing the maximum conductivity (KMAX). The first measurement provided the initial conductivity (Ki), and the PLC was calculated as:
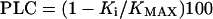 |
2 |
The technique used for measuring PLC was first described by Sperry et al. (1988) and was reported in detail in later studies (Salleo and Lo Gullo, 1993; Salleo et al., 1996).
The perfusion solution was 50 mm KCl filtered through 0.1-μm filters. At least 10 twigs from well-irrigated plants (control twigs) and 10 twigs from prestressed plants were tested for PLC.
Measuring Xylem Refilling
Xylem refilling in prestressed plants was measured in terms of the decrease in PLC in twigs allowed to recover spontaneously from cavitation (untreated twigs) and in twigs stimulated to refill by a KCl/IAA solution. IAA was first dissolved in 1.5 × 10−3 L ethanol and then in a 50 mm KCl solution to a final volume of 0.1 L, prepared with double-distilled water filtered to 0.1 μm. The final concentration of IAA was 1 mm.
The epidermis of twigs to be treated with the hormone was peeled off over a surface of about 9 mm2 at a middle internode. Three areas were prepared about 20 mm apart from each other and at different angles with respect to the vertical twig axis.
Squares of thin blotting paper previously wetted with about 6 μL of the KCl/IAA solution were applied to the exposed twig cortex, and plastic sheets were used to maintain the paper in situ and to prevent evaporation. The pressure collar was then fitted tightly to twigs, including the three peeled areas, and twigs were pressurized at the proper pressure (see above). This procedure, which has been described in detail elsewhere (Salleo et al., 1996), was expected to facilitate the penetration of the hormone into the stem under a phloem-to-xylem pressure gradient. A small volume of water could move from the wetted filter paper to the plant, but less then 60 μL entered the plants because the filter papers were still wet at the end of the pressure collar treatment. Based on the water content and the pressure/volume curves of the leaves, we estimated that ΨL would have increased by less than 20 kPa (assuming that none of the water entered the soil or remained in the stems). This calculation was confirmed by pressure bomb measurements made after the pressure collar treatments; no significant change in balance pressure of excised leaves was observed 5 or 20 min after the pressure collar treatment compared with the initial balance pressure (P < 0.01; n = 12).
To determine whether the solution applied to the exposed stem cortex could be transported radially inward, five experiments were performed in which a dye was added to the saline/hormone solution. Fast-Green (1% [w/v] in aqueous solution) was used for this because this dye binds to cellulose, staining the living parenchyma cells (including the rays and the paratracheal parenchyma). Twenty minutes after pressure release, twigs were cross-sectioned by hand and observed under a microscope.
Osmolality and Ion Content of Xylem Sap
Twigs from well-watered plants (controls) were cut off under distilled filtered water. About 3 mm of their distal cut end was girdled (i.e. the bark was removed) to prevent any contamination of xylem sap with phloem exudate. Twigs were then connected to the equipment used for measuring hydraulic conductivity and perfused with double-distilled water filtered to 0.1 μm at P = 10 kPa. Serial sap samples of 14 to 15 μL were collected every 2 min from the cut distal end of twigs using plastic microcaps with stoppers. The osmolality of these samples was measured using a microosmometer (model 110, Fiske Associates, Norwood, MA). The osmolality of samples from control twigs was approximately constant in different plants at 21.4 ± 2.61 mOsm kg−1 during the first 10 min (i.e. the first five samples) and then declined progressively to zero as distilled water began to mix with natural xylem sap. The mean osmolality of the early four sap samples could therefore be safely taken as “native” xylem sap. This procedure allowed us to estimate the original xylem sap osmolality before perfusion of the twigs with other experimental solutions.
Pressurized twigs treated with KCl/IAA solutions were cut off under distilled filtered water using the same procedure reported above, connected to the hydraulic equipment, and perfused with a Gly solution adjusted to 20 ± 2 mOsm kg−1 (i.e. to the osmolality of xylem sap measured in well-irrigated plants). Gly was chosen as the solute because amino acids are common components of xylem sap (Van Bel, 1995) and do not interfere with the inorganic ion content.
Two serial sap samples of 10 to 12 μL were collected 2, 5, 10, 15, 20, and 25 min after pressure release (about 1.5 min was sufficient for cutting a twig off and connecting it to the hydraulic equipment). The osmolality of the samples was measured as reported above in at least 10 twigs from different plants.
In similar experiments, an equal number of sap samples was collected for measuring K+, Na+, Ca2+, and Mg2+ content using the atomic absorption spectrometer (model 5000, Perkin-Elmer) at the Department of Chemical Sciences of the University of Trieste.
Cryoscanning Electron Microscopy
Pressurized twigs were cut off in a bath of liquid N2 at 2, 5, and 20 min after pressure release. Frozen twig pieces were obtained by fracturing the pressurized twig segments. The pieces were immediately put into vials, inserted in a cryogenic dry shipper (Artic Shipper, Thermoline, Dubuque, IA), and sent to the Biology Department of Carleton University (Ottawa, Canada), where they were held in cryostorage at the temperature of liquid N2 until examination (by M.T.T. and A.N. with the assistance of M. Canny).
Samples were sectioned on a cryomicrotome (model CR 2000, Research and Manufacturing, Tucson, AZ) under liquid N2. Transverse or longitudinal faces of the stems were planed roughly with a glass knife and then very smoothly with a diamond knife; both procedures were done at −80°C (Huang et al., 1994). The specimen was then transferred under liquid N2 to a cryotransfer system (model CT 1300, Oxford Instruments, Eynsham, Oxford, UK) and then to the cryostage in a scanning electron microscope (model JSM 6400, JEOL). The face of the specimen was etched slightly by warming it slowly to −90°C while observing the specimen at 1 kV. Etching was stopped, and the specimen was recooled as soon as cell outlines began to appear. It was then coated with aluminum (50 nm) in the preparation chamber, returned to the sample stage (−170°C), and observed at 7 kV.
We performed microanalysis with the Link eXL LZ4 system with the Be window (Oxford Instruments). The voltage was 15 kV, the working distance was 35 mm, the takeoff angle was 33°, and the probe current was 1.00 nA. Spectra were accumulated until 80,000 counts for Al had been reached. Counts for elements were obtained as percent ratios of the Al count. These ratios were divided by the lifetime to correct for any local variations in the thickness of the Al coating. Absolute values for elements were obtained with the appropriate standards. For more details of these procedures see articles by Canny and Huang (1993), Huang et al. (1994), and McCully (1994).
RESULTS
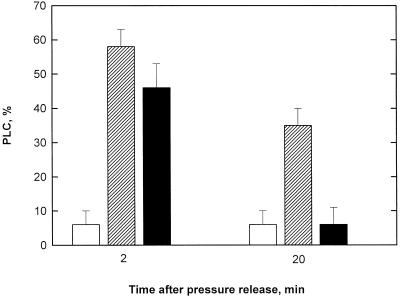
The pressure differential applied to the interconduit pit membranes of laurel twigs (2.24 MPa from Px = −1.0 MPa and P = +1.24 MPa) induced 1-year-old twigs to cavitate extensively so that 2 min after pressure release, the PLC increased from 6.5% ± 2.75% in twigs of well-watered plants to about 55% in untreated and KCl/IAA-treated twigs of prestressed plants (Fig. 1). Twenty minutes after pressure release, however, untreated twigs had significantly recovered from cavitation: PLC had decreased to about 34% spontaneously (Fig. 1).
Figure 1.
PLC ± sd (n = 10) measured in 1-year-old twigs of laurel. White columns, Control twigs; hatched columns, untreated twigs; black columns, KCl/IAA-treated twigs. Values are 2 and 20 min after release of the pressurization that induced the cavitations.
Twigs treated with the KCl/IAA solution (Fig. 1) recovered much better than untreated twigs (PLC returned to about 8%, which is near the same level recorded in watered plants), confirming that the KCl/IAA solution stimulated xylem refilling.
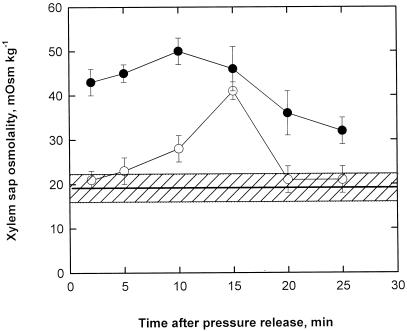
During the 20 min needed for twigs to recover from PLC, the osmolality of the xylem sap collected was elevated above the control (nonembolized) plants. The osmolality of xylem sap in embolized plants was higher in twigs treated with KCl/IAA than in untreated embolized twigs. The osmolality of xylem sap from untreated twigs increased gradually from about 20 to greater than 40 mOsm kg−1 (Fig. 2), reaching a peak 15 min after pressure release. During the subsequent 5 min, the sap osmolality declined again to about 20 mOsm kg−1, corresponding to that measured in control twigs (Fig. 2).
Figure 2.
Time course of xylem sap osmolality measured in untreated twigs (○) and KCl/IAA-treated twigs (•) from prestressed plants. The horizontal hatched area represents the osmolality measured in control twigs from well-watered plants. Values are means ± sd (n = 5).
A sap osmolality of 43 mOsm kg−1 was measured in KCl/IAA-treated twigs 2 min after pressure release (Fig. 2). Such a high value was maintained approximately constant for up to 15 min after pressure release, with peak values up to about 50 mOsm kg−1. Between 15 and 25 min after pressure release, the osmolality of xylem sap from KCl/IAA-treated twigs declined simultaneously but gradually with the analogous decrease measured in untreated twigs but at higher levels; 25 min after pressure release the sap osmolality measured in KCl/IAA-treated twigs was still about 35 mOsm kg−1 versus only 20 mOsm kg−1 measured in untreated twigs.
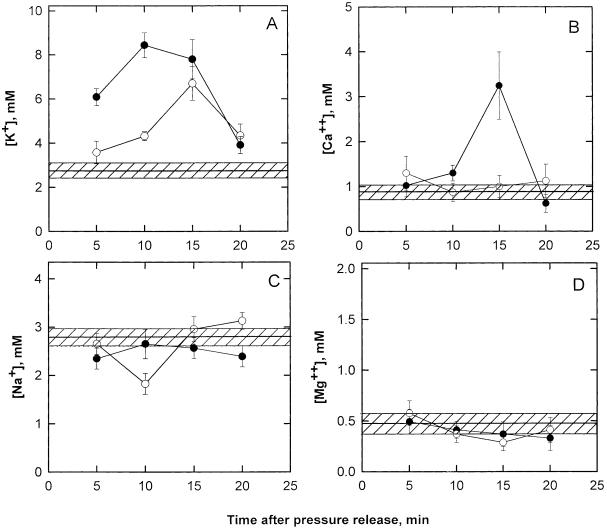
The [K+] of xylem sap in twigs of well-irrigated plants was between 2.7 and 3.1 mm (Fig. 3A). Twigs spontaneously recovering from PLC showed a significant increase in [K+]. Ten minutes after pressure release, [K+] was greater than that measured in irrigated plants, and 5 min later it was as high as 6.5 mm (i.e. more than twice as much). Five minutes after pressure release, twigs treated with KCl/IAA solutions had a [K+] as high as 6.2 mm, and 5 min later, up to 8.4 mm. Changes in the [K+] of xylem sap in treated and untreated twigs showed the same time course as that measured for sap osmolality (i.e. in both cases [K+] increased up to 15 min after pressure release and then decreased).
Figure 3.
Time course of [K+] (A), [Ca2+] (B), [Na+] (C), and [Mg2+] (D) in the xylem sap of untreated twigs (○) and KCl/IAA-treated twigs (•) from prestressed plants. The horizontal hatched area represents the ion content of xylem sap from well-watered plants. Values are means ± sd (n = 5).
Changes in [Ca+2] (Fig. 3B) showed differences between untreated and KCl/IAA-treated twigs in that [Ca2+] in the xylem sap of the former equaled that of twigs from irrigated plants, whereas KCl/IAA-treated twigs had a xylem sap significantly enriched in Ca2+ (10 min after pressure release [Ca2+] was about 1.8 mm versus about 0.75 mm in control and untreated twigs). [Ca2+] reached a peak of as much as 3.3 mm simultaneously with peaks in sap osmolality and [K+] 15 min after pressure release.
No significant differences between control and prestressed twigs were measured in terms of [Na+] (Fig. 3C), whereas [Mg2+] in the xylem sap of prestressed twigs (regardless of whether or not they were treated with hormone) was slightly but significantly lower than that measured in control twigs (Fig. 3D).
Light Microscopy
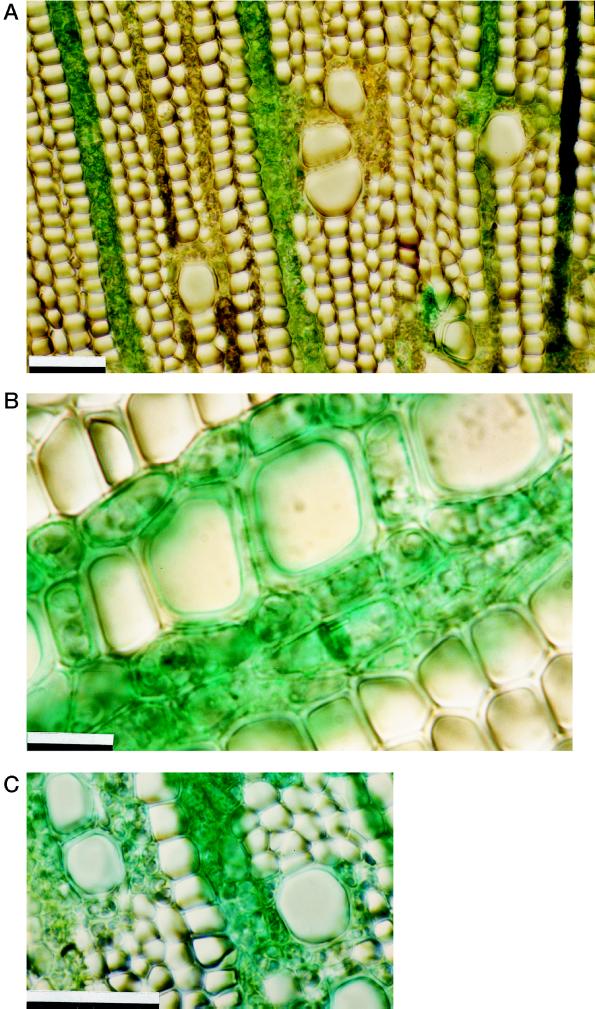
Anatomical observations (Fig. 4) showed that solutions applied to the exposed cortex of twigs penetrated under pressure into the secondary xylem via the rays. In fact, when Fast-Green dye was added to the hormone solution, the rays showed up as green in cross-sections cut 20 min after pressure release (Fig. 4A). In particular, the rays of 1-year-old twigs appeared to be numerous, multiseriate, and in close contact with the parenchyma cells surrounding xylem conduits or vasicentric parenchyma (Carlquist, 1988; Fahn, 1990) (Fig. 4, B and C). Rays and vasicentric parenchyma were stained green, showing that solutions applied to the exposed twig cortex had migrated via the rays and vasicentric parenchyma to the secondary xylem within 20 min after pressure release.
Figure 4.
Cross-sections of 1-year-old twigs in which Fast-Green dye was applied to the exposed cortex before pressurization. A, Green-stained rays showing that the dye was transported to the wood via the rays; B, detail of secondary wood (note green-stained multiseriate rays and parenchyma cells surrounding vessels); and C, detail of green-stained vasicentric parenchyma. Scale bars = 50 μm.
Electron Microscopy
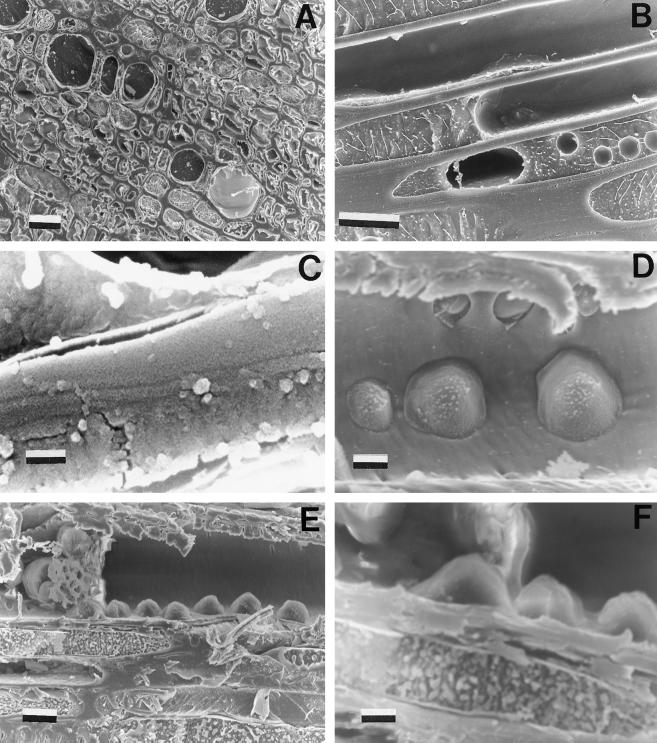
A general view of xylem with cavitated and functional conduits is presented in Figure 5A. Three cavitated xylem conduits of twigs spontaneously recovering from PLC (untreated twigs) and sampled 20 min after pressure release are shown in Figure 5B. One empty vessel is shown, together with two partly embolized conduits containing one large or many small emboli. An enlarged detail of the air/water interface is clearly visible in Figure 5C, where a thin sap layer (about 0.2 μm thick) can be seen adhering to the conduit wall. Figure 5, D and E, shows water droplets entering an embolized xylem conduit, with living cells in close contact with the conduit. In Figure 5F, water droplets can be seen entering a conduit through pits.
Figure 5.
A, Full (functional) and empty (dysfunctional) xylem conduits observed under a cryoscanning electron microscope. Twig samples were collected from prestressed plants. Scale bar = 100 μm. B, Cavitated vessel with a large bubble in it and a conduit with many small bubbles. Scale bar = 10 μm. C, Detail of a cavitated conduit showing the air/water interface and a sap layer persisting adherent to the conduit wall. Scale bar = 1 μm. D, Cavitated conduit at the beginning of refilling (note the numerous water droplets entering the conduit through the pits). Scale bar = 5 μm. E, Cavitated conduit at the beginning of refilling (same as D) showing the parenchyma cells close to the conduit. Scale bar = 10 μm. F, Detail of D at a higher magnification (note water droplets entering the conduit). Scale bar = 5 μm.
In cross-section views, some vessels can be seen with water droplets emerging (as in Fig. 5F) or filled mostly or completely with water. Cells completely filled with water in the cross-sectional plane still may have been filling somewhere else in the vessel. Consequently, all vessels were subjected to elemental analysis. No detectable quantities of C, K, Ca, Na, P, or Cl were found. In many cases, we analyzed living xylem parenchyma or ray cells adjacent to same vessels used for elemental analysis. Detectable quantities of C, K, Ca, and P were found (Table I). Limits of detection were defined as twice the sd of elemental analysis performed on distilled water in calibration curves. These detection limits were essentially the same as the 95% confidence interval of the linear regressions of the calibration curves.
Table I.
Elemental analysis of cell contents in laurel stems
| Element | C | K | Ca | Na | P | CI |
|---|---|---|---|---|---|---|
| % | mmol | |||||
| Living cell (n = 13) | ||||||
| Mean | 7.6 | 314 | 20 | NDa | 80 | ND |
| sd | 1.8 | 84 | 7 | – | 26 | – |
| Vessel (n = 18) | ||||||
| Mean | ND | ND | ND | ND | ND | ND |
| Detection limit | 0.7 | 14 | 17 | 36 | 17 | 17 |
Values are given as means ± sd.
ND, Not detectable (the detectable limits are defined as values that exceed two times the sd of measurements made on distilled water and are given at the end of the table).
DISCUSSION
Xylem repair of embolism after water stress release is a complex process involving radial and axial wood parenchyma. The refilling of cavitated xylem appears to be due to water flowing into conduits surrounded by vasicentric parenchyma cells (Fig. 5, D–F).
The complete PLC recovery (Fig. 1) measured in twigs supplied with a saline/hormone solution suggests that refilling was accomplished by water flow along the phloem-to-xylem pathway. In contrast, root pressure causes recovery by water transport along the xylem-to-xylem pathway (Fisher et al., 1997). Root pressure is absent in laurel (Salleo et al., 1996).
During recovery of hydraulic conductivity, the osmolality of xylem sap increased to 43 mOsm kg−1. The [K+] in the sap collected from untreated twigs increased up to 6.5 mm (Fig. 3A). The other ions contributed 0.9, 2.6, and 0.5 mm, respectively, for Ca, Na, and Mg. The combined osmolality of these ions is approximately 10.5 mOsm kg−1, and if we assume an equal osmolality of anions, we can account for approximately one-half of the observed osmolality of 43 mOsm kg−1. The concentration of sap recovered from perfused stems could be diluted by water from full vessels and would not represent the concentration of solutes in refilling vessels. Therefore, we have to examine the microprobe results (Table I) to obtain an estimate of the maximum osmolality of the solute. All elements were below the detection limits of the microprobe. This is consistent with Figure 3, which shows that all ions were below the detection limit.
These results leave the existing paradigm in a very weak state. Even if all ions in Table I were present just below the detection limit, their osmolalities would add up to approximately 100 mOsm kg−1. If we partition 0.7% C in common organic molecules we might gain an additional 40 mOsm kg−1. Thus, the maximum π that could go undetected in our experiment is 0.34 MPa, which is insufficient to account for water flow into cavitated vessels from surrounding living cells at a Ψ of −1 MPa. Since the predawn ΨL was −1 MPa in our laurel twigs, we would expect functioning xylem vessels and surrounding living cells to be at a Ψ of less than −1 MPa. The Ψ of leaves distal to the stems did not rise above −1 MPa during our experiments. For water to flow into refilling vessel by osmosis, the π would have to be greater than 1 MPa.
Our results confirm previous studies (Salleo et al., 1996) and demonstrate that refilling of laurel vessels occurs when the threshold xylem pressure is likely to be less than −1 MPa. Clearly, a new paradigm is needed. Canny (1995b, 1997) introduced the concept of “tissue pressure” to explain how changes in the π of living cells in stems might exert a prolonged amelioration of the xylem pressure in adjacent xylem vessels. We feel that this theory is thermodynamically and mechanically impossible. Surrounding tissues cannot effect a lasting change in xylem pressure in the presence of continued transpiration. Canny proposed that starch-to-sugar conversion in stem tissues would cause a swelling of the living cells (true) and that the swelling of these tissues would apply a tissue pressure against the cell walls of vessels (also true). The stress of the additional tissue pressure would cause a transitory release of pressure in the vessel lumen because of the contraction of the vessel diameter under the stress of the increased tissue pressure. However, eventually (in seconds or less) the stress of the tissue pressure would be totally balanced by the strain (reduction in vessel wall dimensions). The water pressure in the lumen would soon return to the same or lower pressure value as additional water was withdrawn from the vessel by transpiration in the attached leaves. We conclude that tissue pressure cannot cause a permanent increase in xylem pressure.
Could transitory increases in xylem pressure be sufficient to cause refilling of vessels? Transitory changes in tissue pressure, if large enough, could return the xylem pressure of cavitated vessels to values above atmospheric for a brief period. In Canny's paradigm (1995b, 1997) some living cells undergo a starch-to-sugar conversion; this conversion raises their π and draws in water, which causes them to swell. These swelling (living) cells then compress other living cells, causing them to release water. This paradigm is unlikely for two reasons. First, there would have to be a countercurrent of water flow within adjacent regions of the stem cross-section. For living cells to swell, there would have to be water flow from full xylem vessels to the living cells that swell after the increase in π because of the starch-to-sugar conversion. At the same time, there would have to be water flow from other living cells, compressed by tissue pressure, to the cavitated vessels that might be adjacent to the full vessels. Since mass flow of water cannot occur in two directions simultaneously in the same space, the flow would have to occur in adjacent but separate places, which is unlikely. Second, there would be a large mechanical disadvantage in Canny's tissue-pressure mechanism. Some of the mechanical swell would be wasted, as some would be in a direction away from the cavitated vessels and might increase stem diameter without releasing water. Some swelling pressure would be taken up by strain (compression) of xylem vessels and would not release water. Therefore, only a fraction of the mechanical advantage would be exerted where it is needed, i.e. on living cells squeezed by tissue pressure.
As a counterproposal, we would suggest a more efficient paradigm. Canny (1995b, 1997) proposes an increase in π in some living cells to release water from other living cells. It makes much more sense to propose a decrease in π (e.g. by sugar-to-starch conversions) in all living cells surrounding a cavitated vessel. This would cause a rise in cell Ψ and the corresponding release of water and decrease in turgor pressure of the affected living cells. There would be no countercurrent in water flow (toward swelling cells and away from shrinking cells) and therefore no mechanical disadvantages.
Unfortunately, there are four serious problems with our paradigm and that of Canny (1995b, 1997).
The first problem is that in woody tissue the modulus of elasticity of cell walls is very high, so volume changes are quite small: as little as 0.1% volume change MPa−1 (Irvine and Grace, 1997). In woody stems, vessel lumina can account for up to 20% of the tissue volume (Tyree and Yang, 1992). Vessel lumina occupied 10.3% (sd = 2.1; n = 5) of the stem volume in our laurel twigs. Therefore, if 80% of the stem is filled with living cells and they all release 0.3% of their volume for a 3-MPa change in turgor pressure, that would be enough to refill only 2.4% of the vessel volume (= 0.3% × [80%/10%]). The potential volume of water that could be released by shrinking would therefore be too small.
The second problem is that the water released would not flow preferentially from shrinking (living) cells to cavitated vessels, it would flow to all vessels simultaneously. Actually, more water would flow from shrinking (living) cells to full vessels than to embolized vessels because the pressure drop from the shrinking (living) cells to the full vessels would be more than from the shrinking (living) cells to the embolized living cells. Based on these first two problems, it appears that tissue-volume changes would not be enough to account for the volume flows required to refill embolized vessels.
The third problem is that water would flow from living cells to cavitated vessels only if the Ψ of the living cell became more than the Ψ of the water in the filling vessel. For example, in our experiments predawn ΨL was −1 MPa, so it is likely that all living stem cells were at an initial Ψ of −1 MPa, and for every living cell Ψ = Pt − π, where Pt is turgor pressure. Let us assume that the π of the living cells is the same as that in leaves (π = 2.4 MPa); the turgor pressure would then have to be 1.4 MPa when the living cells are in equilibrium with a threshold xylem pressure of −1 MPa in functioning vessels. For water to flow from the living cells to the cavitated vessels, where xylem pressure is approximately 0 during filling, the cell π would have to fall rapidly by more than 1 MPa to raise the Ψ above 0 (e.g. from π = 2.4 to 1.3 for Ψ to reach +0.1 MPa). A similar argument applies to Canny's tissue-pressure hypothesis (1995b, 1997). Tissue pressure caused by swelling (living) cells would have to compress other living cells to raise their Pt by more than 1 MPa for Ψ to be above 0. Consequently, the swelling cells would have to raise their π by more than 1 MPa (e.g. from 2.4 to more than 3.4 MPa).
The final problem with the two paradigms is that filling vessels would sometimes be adjacent to full vessels. In the pit membrane between the full and empty vessel there would be a water meniscus that sustains the large pressure difference between the vessels. The problem is that during filling of the embolized vessel the water might reach the pit membrane in some of the bordered pits before it reaches all of the pits. If the meniscus in a full vessel is rejoined with the water in a partly filled vessel, why isn't the water sucked out as fast or faster than it enters? One possibility is that the cell walls of the over-arching bordered pits might be slightly hydrophobic (lignin in secondary walls is known to be hydrophobic). Water in the filling vessels might not pass through the bordered pits until the vessel lumen is completely full. At that point the water in the lumen might rise a few kilopascals above atmospheric pressure and thus push through all pit borders simultaneously.
The above problems suggest that our proposed paradigm and the existing paradigm by Canny are both very unlikely. The biggest problem might be the speed with which changes in π would have to occur. While π is decreasing, water would be simultaneously flowing out of the living cell; so for Ψ to reach greater than 0, the t1/2 for the π decrease would have to be less than the t1/2 for water equilibration between living cells. Pressure probe studies on leaf cells reveal the t1/2 to be 1 to 10 s. Since t1/2 is inversely proportional to bulk modulus of elasticity (ε), we would expect the t1/2 of woody cells to be even less. It seems improbable to us that π could change more than 1 MPa in less than 1 s.
In conclusion, we feel that our data confirm the refilling of embolized vessels in plants when some adjacent vessels are at a threshold xylem pressure much less than −2τ/r. However, to explain this refilling a new paradigm may be needed since all existing paradigms seem improbable. We are reminded of a quote from a famous fictional detective, “… when you have eliminated the impossible, whatever remains, however improbable, must be the truth.” (Doyle, 1986). It seems that we have eliminated some of the possible and some of the improbable paradigms, so for the moment we should be open to new suggestions, however improbable they may seem. Clearly, more work on the mechanism of xylem refilling will be required to answer the issues raised above.
NOTE ADDED IN PROOF
During the revision of this manuscript for publication, we became aware of some novel ideas of Holbrook and Zwieniecki (1999), who go into much more detail about the conditions that must pertain to the hydrophobic regions around pit pores during refilling of vessels. They also suggested an alternative method for water release, i.e. opening of membrane/water channels (aquaporins). The opening of water channels could release water without a large change in cell π if the reflection coefficient (ς) fell below 1 when aquapores open. If the vasicentric parenchyma cells had an original π of 2.4 MPa, a drop in ς from 1.0 to 0.5 would be sufficient to cause rapid release of water. A drop in ς would also make the membranes leaky to solutes. Such release of solutes would be consistent with our results if it was not too great, i.e. enough to be detected by atomic absorption spectrometry but not enough to be detected by x-ray microanalysis. The opening of aquapores might be fast enough to cause refilling of vessels, but the maximum volume of water that could be released would still be limited by the elasticity of living cells, so we would have to propose that these cells were much less lignified than the xylem vessel to make them change enough in volume. We should also not discount the possibility of refilling by release of solutes into refilling vessels in other species. We believe these ideas need careful consideration.
Abbreviations:
- π
osmotic pressure
- πx
π of the xylem
- PLC
percentage loss of hydraulic conductivity
- Ψ
water potential
- ΨL
leaf Ψ
Footnotes
This study was supported by the Italian Ministry of University and Technological and Scientific Research.
LITERATURE CITED
- Borghetti M, Edwards WRN, Grace J, Jarvis PG, Raschi A. The refilling of embolised xylem in Pinus sylvestris L. Plant Cell Environ. 1991;14:357–369. [Google Scholar]
- Canny MJ. Potassium cycling in Helianthus: ions of the xylem sap and secondary vessel formation. Phil Trans R Soc Lond B. 1995a;348:457–469. [Google Scholar]
- Canny MJ. A new theory for the ascent of sap: cohesion supported by tissue pressure. Ann Bot. 1995b;75:343–357. [Google Scholar]
- Canny MJ. Vessel contents during transpiration, embolism and refilling. Am J Bot. 1997;84:1223–1230. [PubMed] [Google Scholar]
- Canny MJ, Huang CX. What is in the intercellular spaces of roots? Evidence from the cryo-scanning electron microscope. Physiol Plant. 1993;8:561–568. [Google Scholar]
- Carlquist S. Comparative Wood Anatomy: Systematic, Ecological and Evolutionary Aspects of Dicotyledon Wood. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Cochard H, Brèda N, Granier A, Aussenac G. Vulnerability to air embolism of three European oak species. Ann Sci For. 1992;49:225–233. [Google Scholar]
- Cochard H, Tyree MT. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990;6:393–407. doi: 10.1093/treephys/6.4.393. [DOI] [PubMed] [Google Scholar]
- Dixon MA, Grace J, Tyree MT. Concurrent measurements of stem density, leaf water potential and cavitation on a shoot of Thuja occidentalis L. Plant Cell Environ. 1984;7:615–618. [Google Scholar]
- Doyle AC (1986) Sherlock Holmes: The Complete Novels and Stories, Vol 1. Bantam Books, New York
- Edwards WRN, Jarvis PG, Grace J, Moncrieff JB. Reversing cavitation in tracheids of Pinus sylvestris L. under negative water potentials. Plant Cell Environ. 1994;17:389–397. [Google Scholar]
- Ewers FW, Fisher JB, Fichtner K (1991) Water flux and xylem structure in vines. In FE Putz, HA Mooney, eds, The Biology of Vines. Cambridge University Press, New York, pp 127–160
- Fahn A. Plant Anatomy. Oxford: Butterworth-Heinemann; 1990. [Google Scholar]
- Fisher JB, Angeles G, Ewers FW, Lòpez-Portillo J. Survey of root pressure in tropical vines and woody species. Int J Plant Sci. 1997;158:44–50. [Google Scholar]
- Grace J. Refilling of embolized xylem. In: Borghetti M, Grace J, Raschi A, editors. Water Transport in Plants under Climate Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 51–62. [Google Scholar]
- Holbrook NM, Zwieniecki MA. Embolism repair and xylem tension. Do we need a miracle? Plant Physiol. 1999;120:7–10. doi: 10.1104/pp.120.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CX, Canny MJ, Oates K, McCully ME. Planing frozen hydrated plant specimens for SEM observation and EDX microanalysis. Microsc Res Tech. 1994;28:67–74. doi: 10.1002/jemt.1070280108. [DOI] [PubMed] [Google Scholar]
- Irvine J, Grace J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of wood. Planta. 1997;202:455–461. [Google Scholar]
- Kamer F. Klima und Vegetation auf Tenerife, besondes im Hinblick auf den Nebelniederschlag. Scripta Geobotanica. 1974;7:1–78. [Google Scholar]
- Kikuta SB, Lo Gullo MA, Nardini A, Richter H, Salleo S. Ultrasound acoustic emissions from dehydrating leaves of deciduous and evergreen trees. Plant Cell Environ. 1997;20:1381–1390. [Google Scholar]
- Kramer PJ. Water Relations of Plants. London: Academic Press; 1983. [Google Scholar]
- Lausi D, Nimis PL, Tretiach M. Adaptive leaf structures in a Myrica-Erica stand on Tenerife (Canary Islands) Vegetation. 1989;79:133–142. [Google Scholar]
- Lewis AM, Harnden VD, Tyree MT. Collapse of water-stress emboli in the tracheids of Thuja occidentalis L. Plant Physiol. 1994;106:1639–1646. doi: 10.1104/pp.106.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Gullo MA, Salleo S. Different vulnerabilities of Quercus ilex L. to freeze- and summer drought-induced xylem embolism: an ecological interpretation. Plant Cell Environ. 1993;16:511–519. [Google Scholar]
- McCully ME. Accumulation of high levels of potassium in developing xylem elements in roots of soybean and some other dicotyledons. Protoplasma. 1994;183:116–125. [Google Scholar]
- Mencuccini M, Comstock J. Vulnerability to cavitation in populations of two desert species, Hymenoclea salsola and Ambrosia dumosa, from different climatic regions. J Exp Bot. 1997;48:1323–1334. [Google Scholar]
- Milburn JA, Kallarackal J (1991) Sap exudation. In AS Raghavendra, ed, Physiology of Trees. Wiley, New York, pp 385–402
- Pignatti S (1982) Flora d'Italia. Edagricole, Bologna, Italy
- Salleo S. Water relations of two Sicilian species of Senecio (groundsel) measured by the pressure bomb technique. New Phytol. 1983;95:179–188. [Google Scholar]
- Salleo S, Hinckley TM, Kikuta SB, Lo Gullo MA, Weilgony P, Yoon TM, Richter H. A method for inducing xylem embolism in situ: experiments with a field grown tree. Plant Cell Environ. 1992;15:491–497. [Google Scholar]
- Salleo S, Lo Gullo MA. Xylem cavitation in nodes and internodes of Vitis vinifera L. plants subjected to water stress: limits of restoration of water conduction in cavitated xylem conduits. In: Kreeb KH, Richter H, Hynckley TM, editors. Structural and Functional Responses to Environmental Stresses: Water Shortage. The Hague, The Netherlands: SPB Academic Publishing; 1989. pp. 33–42. [Google Scholar]
- Salleo S, Lo Gullo MA. Drought resistance strategies and vulnerability to cavitation of some Mediterranean sclerophyllous trees. In: Borghetti M, Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 99–113. [Google Scholar]
- Salleo S, Lo Gullo MA, De Paoli D, Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Salleo S, Nardini A, Lo Gullo MA. Is sclerophylly of Mediterranean evergreens an adaptation to drought? New Phytol. 1997;135:603–612. [Google Scholar]
- Sperry JS. Limitations of stem water transport and their consequences. In: Gartner BL, editor. Plant Stems: Physiology and Functional Morphology. San Diego, CA: Academic Press; 1995. pp. 105–124. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988;11:35–40. [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 1987;83:414–417. doi: 10.1104/pp.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Cochard H. Summer and winter embolism in oak: impact on water relations. Ann Sci For. 1996;53:173–180. [Google Scholar]
- Tyree MT, Hammel HT. The measurement of the turgor pressure and water relations of plants by the pressure bomb technique. J Exp Bot. 1972;23:267–282. [Google Scholar]
- Tyree MT, Sperry JS. The vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:19–38. [Google Scholar]
- Tyree MT, Yang S. Hydraulic conductivity recovery versus water pressure in xylem of Acer saccharum. Plant Physiol. 1992;100:669–676. doi: 10.1104/pp.100.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel AJE. The low profile directors of carbon and nitrogen economy in plants: parenchyma cells associated with translocation channels. In: Gartner BL, editor. Plant Stems: Physiology and Functional Morphology. San Diego, CA: Academic Press; 1995. pp. 205–222. [Google Scholar]
- Yang S, Tyree MT. A theoretical model of hydraulic conductivity recovery from embolism with comparison to experimental data on Acer saccharum. Plant Cell Environ. 1992;15:633–643. [Google Scholar]