Abstract
We observed induction of additional trichome formation on the adaxial surface of mature leaves of Arabidopsis after massive doses (1–3 kilograys) of γ-radiation from cobalt-60. A typical increase in trichome number was observed in the seventh leaf when the full expansion of the fifth leaf was irradiated. Under normal growth conditions, trichome numbers on the adaxial surface of seventh leaf of the Arabidopsis ecotypes Columbia (Col) and Landsberg erecta (Ler) were 122.5 ± 22.7 and 57.5 ± 14.5, respectively. However, γ-radiation induced additional trichome formation and the numbers rose to 207.9 ± 43.7 and 95.0 ± 27.1 in Col and Ler, respectively. In Col the shape of new trichomes was intact and their formation was spatially maintained at equal distances from other trichomes. In Ler trichome morphology was aberrant and the formation was relatively random. Treatment with antioxidants before γ-irradiation suppressed the increase in trichome number, and treatment with methyl viologen and light induced small trichomes. These results suggest that γ-radiation-induced trichome formation is mediated by active oxygen species generated by water radiolysis. γ-Radiation-induced trichome formation was blocked in the trichome mutants ttg-1, gl1-1, and gl2-1. These results suggest that γ-radiation-induced trichome formation is mediated by the normal trichome developmental pathway.
Massive doses of ionizing radiation have been shown to induce physiological changes in plants, such as enhancement of respiration, increase in ethylene production (Young, 1965; Abdel-Kader et al., 1968; Lee et al., 1968; Akamine and Goo, 1971; Romani, 1984), induction of enzyme activities (particularly for phenolic metabolisms; Riov et al., 1970; Pendharkar and Nair, 1975; Frylink et al., 1987), and accumulation of Suc (Hayashi and Aoki, 1985) and specific protein species (Ferullo et al., 1994). Cellular macromolecular components such as cell walls, membranes, and DNA are also markedly affected by ionizing radiation (Casarett, 1968). These effects are considered a consequence of both the direct interactions between the ionizing radiation and the macromolecular structures and the indirect action of AOS generated by water radiolysis.
We used Arabidopsis to elucidate the mechanisms of stress recognition, signal transduction, and the initiation of plant self-protective programs at the molecular level because this plant provides certain key advantages for genetic and molecular studies. We observed that Arabidopsis displayed particular responses within several days after irradiation with γ-rays (1–3 kGy) from cobalt-60 (1.17 and 1.33 MeV). These responses included accumulation of anthocyanin in the aerial part of plant, induction of new trichome formation on the adaxial surface of mature leaves, radial expansion of root cell layers, and elongation of root hairs. In this paper we characterize γ-radiation-induced trichome formation.
Arabidopsis trichomes are single-cell-originated epidermal hairs that serve as an appropriate model for plant cell differentiation and cell elongation. The trichomes are present on the surfaces of the leaves, stems, and sepals and on the margins of leaves and sepals. Plant growth does not require trichomes, because plants that lack trichomes appear to be fully viable and fertile (Koornneef et al., 1983). We have taken a genetic approach to studying trichome development. More than 70 trichome mutants representing 21 different genes were isolated (Lee-Chen and Steinitz-Sears, 1967; Feenstra, 1978; Koornneef et al., 1982; Haughn and Somerville, 1988; Huelskamp et al., 1994; Marks and Esch, 1994). Genetic and molecular biological studies have revealed that the GL1 (GLABROUS1) and TTG (TRANSPARENT TESTA GLABROUS) genes are required for the initiation of trichome development.
The GL1 gene has been cloned and shown to encode a putative myb-class transcription factor (Oppenheimer et al., 1991; Larkin et al., 1994b). GL1 transcripts are present at a low level throughout the protoderm, with much higher levels of expression in developing trichomes and presumptive trichome precursor cells (Larkin et al., 1993). Mutations in TTG also affect anthocyanin synthesis, integument development (Koornneef, 1981), and root-hair patterning (Galway et al., 1994). The observation that expression of the maize R gene in ttg mutant plants complemented ttg mutation functionally (Lloyd et al., 1992) suggested that TTG encodes a homolog of the maize R gene or the gene that regulates the expression of an R homolog. However, recent cloning of the TTG gene revealed no sequence homology to the R gene (A.R. Walker and J.C. Gray, personal communication).
The ectopic expression of GL1 with the cauliflower mosaic virus 35S promoter does not lead to increased trichome formation, nor does it bypass the requirement for TTG, whereas the ectopic expression of both GL1 and R induced an increase in the trichome number (Larkin et al., 1994a). These results suggest that the GL1 and TTG gene products cooperate in promoting trichome initiation. The GL2 (GLABRA2) gene is necessary for subsequent phases of trichome morphogenesis, such as cell expansion, branching, and maturation of the trichome cell wall (Koornneef et al., 1982; Marks and Esch, 1994; Rerie et al., 1994). The GL2 gene has been also cloned and shown to have sequence similarity to homeodomain proteins (Rerie et al., 1994). Detailed expression analysis using anti-GL2 antibodies and the GUS reporter gene fused to the GL2 promoter revealed that GL2 expression persists in mature trichomes. A requirement of GL1 for GL2 expression in vivo has been suggested (Szymanski et al., 1998). An examination of the distribution of trichomes early in development disclosed that trichomes are initiated adjacent to other trichomes much less frequently than would be expected by chance.
Genetic and molecular biological studies have revealed the key genes related to the developmental process of trichome formation and have started the elucidation of the functions of these genes and their interactions. However, stress-induced trichome formation has never been found. In this paper we describe, for the first time to our knowledge, the induction of new trichomes after γ-irradiation. We also examined the conditions of new trichome formation thus induced and found that the AOS generated by water radiolysis may also contribute to the induction of new trichomes.
MATERIALS AND METHODS
Arabidopsis Strains and Growth Conditions
We used the wild-type Arabidopsis strains Columbia (Col) and Landsberg erecta (Ler). The Arabidopsis Research Center (Ohio State University, Columbus) provided the seeds of the mutants gl1-1, gl1-2, gl2-1, gl3-1, rhd2-1, and ttg-1. Sterilized seeds were sown on 1% agar medium containing 1× Murashige and Skoog basal salts (Sigma), 2% Suc, 100 mg/L inositol, 1 mg/L thiamine, 0.5 mg/L nicotinic acid, and 0.5 mg/L pyridoxine and grown with 24 h of continuous illumination (400–500 μW m−2 s−1) at 21°C.
γ-Irradiation and Trichome Enumeration
Eighteen days after germination, when the fifth leaf had expanded fully, the whole bodies of plants growing on agar medium were irradiated with γ-rays from cobalt-60 using a γ-cell (3.0 kGy h−1, 1300 TBq; Nordion, Ontario, Canada). After irradiation the plants grew alongside unirradiated plants in the same conditions as before. Three days after irradiation, leaves were taken from the plant and fixed in 4% paraformaldehyde and 2.5% glutaraldehyde aqueous solution, pH 7.2 (Wako Chemicals, Richmond, VA) at 4°C overnight. Water was substituted with 99.5% ethanol for 4.5 h and then with 99% 2-methyl-2-propanol for 4 h (both from Wako Chemicals). The leaves were then frozen at −20°C for 30 min and dried for 8 h in a freeze dryer (model ES-2030, Hitachi, Tokyo). Leaf trichomes were coated with an ion sputterer (model E-1010, Hitachi) for 90 s. The trichomes were counted with a scanning electron microscope.
RESULTS
γ-Radiation Induces Additional Trichome Formation in Arabidopsis
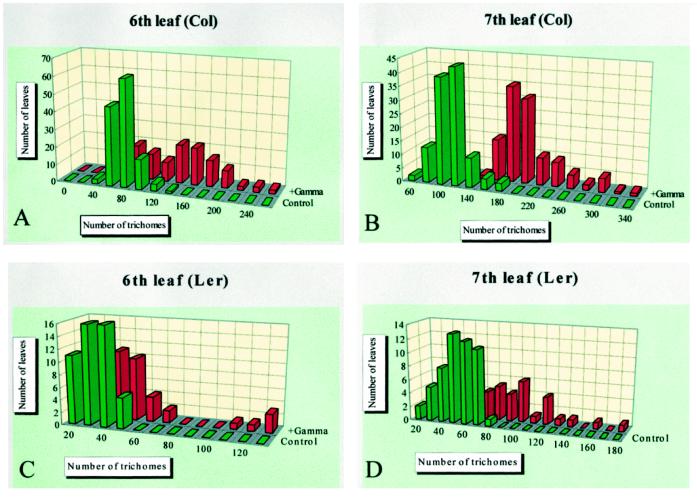
Leaf trichomes occur mainly on the adaxial surface of the early rosette leaves of Arabidopsis. Under our growth conditions (see Methods), their number on the seventh leaf surface was 122.5 ± 22.7 and 57.5 ± 14.5 in the control Col and Ler, respectively. We found that massive doses (1–3 kGy) of γ-radiation from cobalt-60 to Arabidopsis induced additional trichome formation. The numbers of trichomes on the adaxial surface of sixth and seventh leaves of unirradiated plants are distributed on the normal probability curve (Fig. 1). When the fifth leaf was fully expanded plants were irradiated at 2 kGy, and 3 d later the trichome numbers of the sixth and seventh leaves were counted. As indicated in Figure 1, the peak trichome number increased nearly 2-fold in both leaves of both ecotypes. However, there were differences in the responses between the sixth and seventh leaves and between ecotypes: The seventh leaf showed a clearer response than the sixth leaf, and the increase in trichome number in Col was more obvious than in Ler. Furthermore, no increase in trichome number was observed in the first to fourth leaves, and the fifth leaf showed a very small response (data not shown).
Figure 1.
Increase of trichome number after γ-radiation. Trichome numbers on the adaxial surfaces of sixth and seventh leaves of Col and Ler were counted and their distribution is indicated in green. When the fifth leaf was fully expanded, about 18 d post germination, 2 kGy of γ-rays from cobalt-60 were given. Three days later, the trichome numbers on the sixth and seventh leaves were counted and their distribution is shown in red.
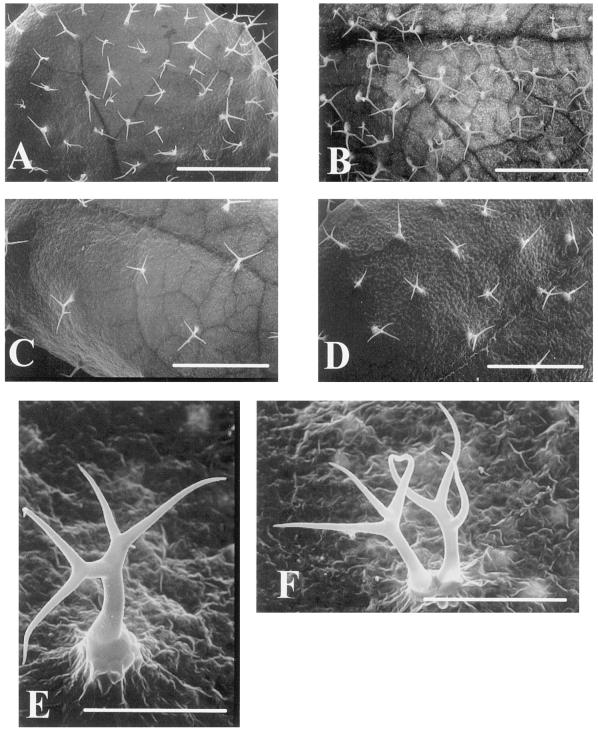
These results suggest that the increase in trichome number is dependent on the age of the leaf and on the ecotype. The timing of radiation is very important to the ability of the epidermal cells to differentiate into trichomes. We observed this difference between ecotypes not only in the number of trichomes but also in the shape and spatial distribution of new trichomes. Scanning electron microscope images of trichomes are shown in Figure 2. In Col the spatial distribution and shape of the trichomes maintained a similarity to those under normal growth conditions during the process of trichome formation. However, in Ler we saw abnormally branched trichomes and an aberrant trichome similar to those observed in the Triptychon mutant (Huelskamp et al., 1994). We also saw twinned trichomes and four branched trichomes similar to those in the unirradiated Ler shown in Figure 2, E and F (approximately 0.5% of normally existing trichomes in Ler), indicating that the characteristics of trichomes in Ler developed successively after γ-irradiation.
Figure 2.
Scanning electron micrographs of the leaf surface. A, Adaxial surface of the seventh leaf of Col. Bar = 1 mm. B, Adaxial surface of the seventh leaf of γ-radiated Col. Bar = 1 mm. C, Adaxial surface of seventh leaf of Ler. Bar = 1 mm. D, Adaxial surface of γ-radiated Ler. Bar = 1 mm. E, Trichome with aberrant branching observed on the adaxial surface of γ-radiated Ler. Bar = 0.2 mm. F, Twin trichome observed on the adaxial surface of γ-radiated Ler. Bar = 0.2 mm.
AOS Contribute to γ-Radiation-Induced Additional Trichome Formation
To dissect the process of γ-radiation-induced trichome formation physiologically, we examined the components contributing to the induction process. At least two events may contribute to this process: DNA damage and the generation of AOS such as hydroxide, superoxide, and hydroxyl radicals. γ-Radiation is known to induce both genomic and organellar DNA double-stranded breakage and to generate AOS through water radiolysis. To determine whether AOS participate in γ-radiation-induced trichome formation, we performed two types of experiments: (a) treatment with an antioxidant (a radical scavenger) before γ-irradiation, and (b) treatment with methyl viologen under light. If the radicals generated by γ-radiation contributed to γ-radiation-induced trichome formation, then antioxidant treatment before γ-irradiation should suppress trichome formation. Likewise, treatment with methyl viologen under light (which increases the cytoplasmic concentration of superoxide radicals by trapping electrons generated by PSI; Nakano and Asada, 1980) should induce new trichomes to form on the leaf surfaces.
We used pyrrolidine dithiocarbamate, n-propyl gallate, and nordihydroguaiaretic acid as antioxidants. One hundred microliters of aqueous solution in three different concentrations was dropped into the basal region of the plants once a day for 3 d before dosing them with 2 kGy of γ-radiation. Three days after irradiation, trichomes were counted; the results are shown in Table I. Addition of an antioxidant without γ-radiation did not cause a critical change in trichome number. Although 1 mm of pyrrolidine dithiocarbamate could suppress the increase of trichome number, 0.01 mm nordihydroguaiaretic acid could not. A 10-fold increase (0.1 mm) of the acid completely suppressed it, however. We obtained similar results with n-propyl gallate.
Table I.
Average number of trichomes on the adaxial surface of the seventh leaf following treatment with the antioxidants pyrrolidine dithiocarbamate (PDTC), nordihydro-guaiaretic acid (NDGA), and n-propyl gallate (n-PG) before γ-radiation
| Treatment | Col | Ler |
|---|---|---|
| Normal growth | 122.5 ± 22.7 | 57.5 ± 14.5 |
| γ-Radiation (2 kGy) | 207.9 ± 43.7 | 95.0 ± 27.1 |
| PDTC (1 mM) | 135.8 ± 31.3 | 49.8 ± 11.6 |
| PDTC (1 mm) + γ-radiation | 151.0 ± 26.3 | 55.5 ± 9.1 |
| NDGA (0.01 mm) | 124.5 ± 28.4 | 40.3 ± 7.6 |
| NDGA (0.01 mm) + γ-radiation | 164.5 ± 38.5 | 55.3 ± 10.0 |
| NDGA (0.1 mm) | 127.3 ± 14.8 | 40.3 ± 7.1 |
| NDGA (0.1 mm) + γ-radiation | 124.3 ± 20.7 | 41.3 ± 2.1 |
| n-PG (0.01 mm) | 129.0 ± 43.2 | 51.0 ± 11.8 |
| n-PG (0.01 mm) + γ-radiation | 125.5 ± 17.9 | 51.3 ± 18.1 |
| n-PG (0.1 mm) | NT | 52.0 ± 8.5 |
| n-PG (0.1 mm) + γ-radiation | 134.0 ± 13.7 | 46.8 ± 6.3 |
Data are means ± se of 100 plants.
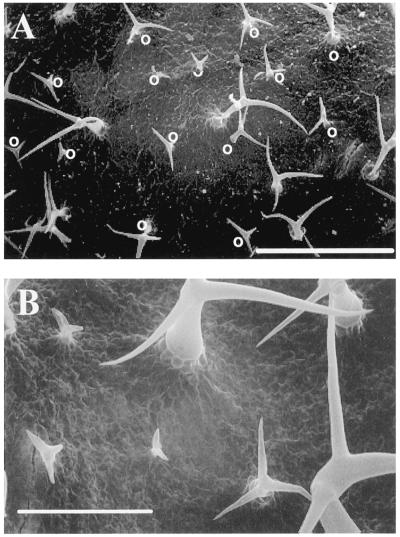
One hundred milliliters of 2 μm methyl viologen was added to the full expansion of the fifth leaf of experimental plants, and the same volume of water was added to the control plants. The plants then grew under light for 3 d, and we counted the trichomes on the seventh leaf. As shown in Figure 3, treatment with methyl viologen, but not with water, induced new but small, branched, trichome-like hairs among the preexisting trichomes. The surface of the methyl-viologen-treated leaf (Col) is shown in Figure 3A and a magnified view appears as Figure 3B. Table II shows the results of counting these hairs. These data suggest that the number of preexisting mature-sized trichomes did not change after the methyl-viologen treatment; however, it was only after the treatment that we observed the small trichome-like structures.
Figure 3.
Scanning electron micrographs of the leaf surface of plant treated with methyl viologen. A, Adaxial surface of the seventh leaf of Col treated with methyl viologen and grown for 3 d under light. Small trichome-like hairs are indicated with “O.” Bar = 0.5 mm. B, Magnified view of A. Bar = 0.2 mm.
Table II.
Average number of trichomes on the adaxial surface of the seventh leaf 3 d after treatment with methyl viologen
| Treatment | Mature Trichome | Small Trichome |
|---|---|---|
| Methyl viologen (n = 70) | 117.8 ± 21.5 | 56.5 ± 20.4 |
| Water (n = 20) | 120.0 ± 39.5 | 0 |
Data are the means ± se of 100 plants.
These results indicate that treatment with antioxidants before γ-irradiation suppresses additional trichome formation, and treatment with methyl viologen induces trichome-like hair formation. Because treatment with methyl viologen did not induce DNA damage, AOS, rather than damaged DNA, must have contributed to the γ-radiation-induced trichome formation.
Genes Contributing to Normal Trichome Formation Also Contribute to γ-Radiation-Induced Trichome Formation
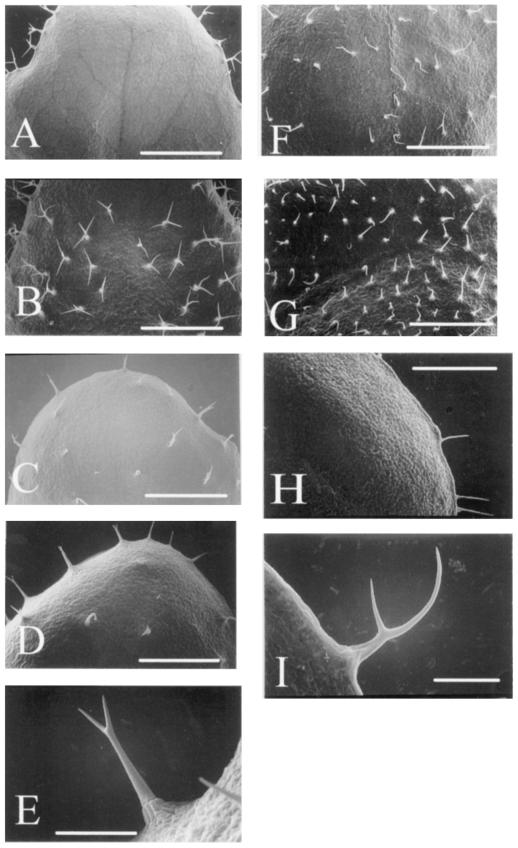
Genetic analyses revealed that the GL1 and TTG genes are required for the initiation of trichome development. GL2 and GL3 genes are thought to be the targets of GL1 and TTG genes. The trichome phenotypes of gl2 and gl3 mutants are quite similar: Mutants in both genes have fewer than normal trichomes and the trichomes are less branched than normal (Larkin et al., 1994b). Under our growth conditions the root-hair-defective mutant rhd2-1 (Schiefelbein and Somerville, 1990) also exhibited a glabrous phenotype. To determine whether these genes are required for γ-radiation-induced trichome formation, γ-radiation was given to these mutants (Table III). The allele gl1-1 has a deletion of the entire coding region (Oppenheimer et al., 1991). Additional trichome formation after γ-irradiation was completely suppressed. On the other hand, gl1-2 is a relatively leaky allele, because only a few amino acids in the C-terminal region are deleted from the GL1 protein (Esch et al., 1994). γ-Radiation given to this mutant induced the increase of trichome number in the margin and the formation of new trichomes on the surface; see Figure 4, A and B.
Table III.
Characteristics of trichome mutants and their responses to γ-radiation
| Mutant | Ecotype | n | Unirradiated | γ-Radiateda |
|---|---|---|---|---|
| gl1-1 | Ler | 10 | 0 | 0 |
| gl1-2 | Col | 35 | 32.3 ± 5.8a | 58.4 ± 4.6a |
| 0b | 78.3 ± 13.4b | |||
| gl2-1 | Ler | 32 | 12.7 ± 3.8 | 16.9 ± 5.3 |
| gl3-1 | Ler | 28 | 50.6 ± 12.3 | 88.7 ± 15.4 |
| ttg-1 | Ler | 10 | 4.3 ± 1.2 | 4.2 ± 1.3 |
| rhd2-1 | Col | 10 | 0 | 4.6 ± 2.5 |
Trichomes counted at the leaf margin.
Trichomes counted on the leaf surface.
Figure 4.
Scanning electron micrographs of the leaf surface of trichome mutants treated with γ-radiation. A, Adaxial surface of the seventh leaf of the gl1-2 mutant. Bar = 1 mm. B, Adaxial surface of the seventh leaf of γ-radiated gl1-2 mutant. Bar = 1 mm. C, Adaxial surface of the seventh leaf of the gl2-1 mutant. Bar = 1 mm. D, Adaxial surface of the seventh leaf of γ-radiated gl2-1 mutant. Bar = 1 mm. E, Immaturely branched trichome observed on the surface of leaf shown in D. Bar = 0.2 mm. F, Adaxial surface of the gl3-mutant. Bar = 1 mm. G, Adaxial surface of γ-radiated gl3-1 mutant. Bar = 1 mm. H, New trichomes induced by γ-radiation of the ttg-1 mutant. Bar = 1 mm. I, Immature trichome induced by γ-radiation of the rhd2-1 mutant. Bar = 0.2 mm.
The allele gl2-1 shows reduced trichome number and less-branched trichomes (Fig. 4E). An increase of less-branched trichomes was not clear after γ-radiation (Table III; Fig. 4, C and D). Although gl3-1 showed a phenotype similar to gl2-1, the number of less-branched trichomes in gl3-1 increased after γ-radiation (Table III; Fig. 4, F and G). A small number of trichomes existed along the margin of the allele ttg-1, but γ-irradiation could not induce new trichome formation (Table III; Fig. 4H). The rhd2-1 mutant was hairless on the adaxial surface of leaves; however, γ-radiation induced the formation of a few unbranched trichomes (Fig. 4I). These results suggest that the GL1, TTG, and GL2 genes are required not only for the normal development of trichomes, but also for γ-radiation-induced trichome formation. The mutation of GL3 and RHD2 genes could not suppress γ-radiation-induced trichome formation.
DISCUSSION
We gave a massive dose of γ-radiation to Arabidopsis plants and found that the number of leaf trichomes increased. This phenomenon was typically observed on the adaxial surface of the seventh leaf after the fifth leaf had fully expanded and the plant was then irradiated. The response of the ecotypes Col and Ler were compared. Col showed a much clearer response than Ler. This difference may be related to the difference in original trichome number in both ecotypes (Larkin et al., 1993). To elucidate which locus is required for the response, crossing of both ecotypes and quantitative trait analysis is necessary.
The fact that treatment with antioxidants before γ-radiation suppressed trichome formation, whereas methyl viologen treatment under light induced it, suggests that γ-radiation-induced trichome formation is mediated by AOS. We are not sure whether normal (developmental) trichome formation is also mediated by AOS. Different SOD-like activities in Col and Ler revealed by electron spin resonance analysis (data not shown) might explain the different responses of these ecotypes to γ-radiation. Under normal growth conditions the SOD-like activity in Ler is twice as high as that in Col. If superoxide molecules were required for developmental trichome formation, the difference in trichome number between the two ecotypes might be due to the presence of SOD-like enzyme activity and surviving superoxide molecules in leaves. To examine this possibility, we are currently producing transgenic plants that express the SOD enzyme ectopically in Col to determine the relationship between the expression of SOD activity and the leaf trichome number.
Leaf age is also important; we observed an increase in trichome number in leaves that expanded after γ-irradiation but not in leaves that had already expanded. This may have been due to the extinction of the activator for trichome differentiation, aging of the leaf, or the presence of an inhibitor to trichome differentiation in aged leaves. If γ-radiation activates some genes required for trichome development, in situ expression analysis of genes such as GL1 and TTG may provide insight into the inducibility of trichome formation. Scanning electron microscope observation revealed that new trichomes developed not randomly but relatively equidistant to the already present trichomes. In the normal development of leaf trichomes a minimum distance between neighboring trichomes is maintained (Larkin et al., 1993) and this pattern-formation rule is maintained after γ-radiation.
γ-Irradiation of trichome mutants demonstrated that whereas the GL1 and TTG gene products are required for γ-radiation to induce trichomes, the GL3 and RHD2 gene products are not, suggesting that the GL3 gene product is required for at least two distinct functions: The phenotype of the gl3-1 mutation shows that GL3 is required for the establishment of the full complement of trichomes and for the branching of trichome cells. γ-Irradiation of gl3-1 induced the formation of unbranched trichomes, indicating that the maintenance of trichome number is suppressed by γ-radiation and suggesting that GL2 and GL3 individually contribute to the development of trichomes.
Another interesting observation was that the mutation of the RHD2 gene affected not only root hairs but also trichomes. Although Schiefelbein and Somerville (1990) have reported that none of the root-hair mutations affect trichome morphology, under our experimental conditions the rhd2-1 mutation produced a glabrous phenotype. It is possible that the same gene is required for both root-hair differentiation and trichome development, because they are both single-cell-originated and stalk-differentiation processes. The phenotype of the rhd2-1 mutation after γ-irradiation suggests that the functional defect of the rhd2-1 mutation was partially bypassed.
Many trichome mutants have now been isolated and intensive genetic studies have revealed the epistatic relationships of the genes and the cascades of gene action. Some useful promoter and reporter gene constructs were used to analyze the expression of the transcripts of key regulatory genes such as GL1 and GL2. In addition to these molecular tools, the finding of γ-radiation-induced trichome formation should prove useful for the analysis of trichome development in Arabidopsis.
ACKNOWLEDGMENTS
The Arabidopsis mutants used in this work were kindly provided by the Arabidopsis Biological Resource Center at Ohio State University. We are grateful to Chieko Yoshida, Kazuko Yagi, Keiko Takahashi, Ikuko Hasegawa, Rie Abe, Kazuko Toyoshima, Yumiko Iguchi, and Keiko Takeuchi for assistance with experimental procedures. We also thank Dr. Izumi Matsuda for providing important information about the use of the scanning electron microscope.
Abbreviations:
- AOS
active oxygen species
- kGy
kilograys
- SOD
superoxide dismutase
Footnotes
This study was supported by a grant from the Regional Links Research Program at Nagasaki of Japan Science and Technology Corporation. It was also partially funded by the Ministry of Agriculture, Forestry and Fisheries of Japan.
LITERATURE CITED
- Abdel-Kader AS, Moris LL, Maxie EC. Physiological studies of gamma irradiated tomato fruits. I. Effects on respiratory rate, ethylene production and ripening. Proc Am Soc Hortic Sci. 1968;92:553–567. [Google Scholar]
- Akamine EK, Goo T. Respiration of gamma-irradiated fresh fruits. J Food Sci. 1971;36:1074–1077. [Google Scholar]
- Casarett AP. Radiation chemistry and effects of gamma radiation on the cell. In: Casarett AP, editor. Radiation Biology. Englewood Cliffs, NJ: Prentice-Hall; 1968. [Google Scholar]
- Esch JJ, Oppenheimer DG, Marks MD. Characterization of a weak allele of the GL1 gene of Arabidopsis thaliana. Plant Mol Biol. 1994;24:203–207. doi: 10.1007/BF00040586. [DOI] [PubMed] [Google Scholar]
- Feenstra WJ. Contiguity of linkage groups I and IV as revealed by linkage relationship of two newly isolated markers dis-1 and dis-2. Arabidopsis Inf Serv. 1978;15:35–38. [Google Scholar]
- Ferullo J-M, Nespoulous L, Triantaphylides C. Gamma-ray-induced changes in the synthesis of tomato pericarp protein. Plant Cell Environ. 1994;17:901–911. [Google Scholar]
- Frylink L, Dubery IA, Schabort JC. Biochemical changes involved in stress response and ripening behavior of gamma-irradiated mango fruit. Phytochemistry. 1987;26:681–686. [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville CR. Genetic control of morphogenesis in Arabidopsis. Dev Genet. 1988;9:73–89. [Google Scholar]
- Hayashi T, Aoki S. Effect of irradiation on the carbohydrate metabolism responsible for sucrose accumulation in potatoes. J Agric Food Chem. 1985;33:13–17. [Google Scholar]
- Huelskamp M, Misera S, Juergens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Koornneef M. The complex syndrome of ttg mutants. Arabidopsis Inf Serv. 1981;18:45–51. [Google Scholar]
- Koornneef M, Dellaert SW, van der Veen JH. EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L) Heynh. Mutat Res. 1982;93:109–123. doi: 10.1016/0027-5107(82)90129-4. [DOI] [PubMed] [Google Scholar]
- Koornneef M, van Eden J, Hanhart C, Stam P, Braaksma FJ, Feenstra WJ. Linkage map of Arabidopsis thaliana. J Hered. 1983;74:265–272. [Google Scholar]
- Larkin JC, Oppenheimer DG, Lloyd A, Paparozzi ET, Marks MD. The roles of GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell. 1994a;6:1065–1076. doi: 10.1105/tpc.6.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Marks DM. The GL1 gene and the trichome development pathway in Arabidopsis thaliana. In: Nover L, editor. Plant Transcription Factors. Berlin: Springer-Verlag; 1994b. pp. 259–275. [DOI] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Pollock S, Marks MD. Arabidopsis GLABROUS1 gene requires downstream sequences for function. Plant Cell. 1993;5:1739–1748. doi: 10.1105/tpc.5.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, McGlasson WB, Edwards RA. Effect of gamma radiation on tomato fruit picked at four stages of development. Radiat Bot. 1968;8:259–267. [Google Scholar]
- Lee-Chen S, Steinitz-Sears LM. The location of linkage groups in Arabidopsis thaliana. Can J Genet Cytol. 1967;9:381–384. [Google Scholar]
- Lloyd AM, Walbot V, Davis RW. Anthocyanin production in dicots activated by maize anthocyanin-specific regulators, R and C1. Science. 1992;258:1773–1775. doi: 10.1126/science.1465611. [DOI] [PubMed] [Google Scholar]
- Marks MD, Esch JJ. Morphology and development of mutant and wild type trichomes on the leaves of Arabidopsis thaliana. In: Bowman J, editor. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994. pp. 56–73. [Google Scholar]
- Nakano Y, Asada K. Spinach chloroplast scavenge hydrogen peroxide on illumination. Plant Cell Physiol. 1980;21:1295–1307. [Google Scholar]
- Oppenheimer DG, Hermann PL, Sivakumarans S, Esch J, Marks MD. A myb-related gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell. 1991;67:483–493. doi: 10.1016/0092-8674(91)90523-2. [DOI] [PubMed] [Google Scholar]
- Pendharkar MB, Nair PM. Induction of phenylalanine ammonia-lyase (PAL) in gamma irradiated potatoes. Radiat Bot. 1975;15:191–197. [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- Riov J, Monselise SP, Kahan RS. Radiation damage to grape fruit in relation to ethylene production and phenylalanine ammonia-lyase activity. Radiat Bot. 1970;10:281–286. [Google Scholar]
- Romani RJ. Respiration, ethylene, senescence, and homeostasis in an integrated view of postharvest life. Can J Bot. 1984;62:2950–2955. [Google Scholar]
- Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB, Jilk RA, Pollock SM, Marks MD. Control of GL2 expression in Arabidopsis leaves and trichomes. Development. 1998;125:1161–1171. doi: 10.1242/dev.125.7.1161. [DOI] [PubMed] [Google Scholar]
- Young RE. Effect of ionizing radiation on respiration and ethylene production of avocado fruit. Nature. 1965;205:1113–1114. doi: 10.1038/2051113a0. [DOI] [PubMed] [Google Scholar]