Abstract
Background
Unplanned rehospitalizations (UR) within 30 days of discharge are common following lung transplantation. It is unknown whether UR represent preventable gaps in care or necessary interventions for complex patients. The objective of this study was to assess the incidence, causes, risk factors, and preventability of UR following initial discharge after lung transplantation.
Methods
This was a single-center prospective cohort study. Subjects completed a modified Short Physical Performance Battery (SPPB) to assess frailty at listing and at initial hospital discharge after transplantation and the State-Trait Anxiety Inventory (STAI) at discharge. For each UR a study staff member and the patient’s admitting or attending clinician used an ordinal scale (0, not; 1, possibly; 2, definitely preventable) to rate readmission preventability. A total sum score ≥2 defined a preventable UR.
Results
Of the 90 enrolled patients, 30 (33.3%) had an UR. The single most common reasons were infection (7 (23.3%)) and atrial tachyarrhythmia (5 (16.7%)). Among the 30 UR, 9 (30.0%) were deemed preventable. UR that happened before day 30 were more likely to be considered preventable than those between days 30–90 (30.0% versus 6.2%, p = 0.04). Discharge frailty, defined as SPPB<6, was the only variable associated with UR on multivariable analysis (OR = 3.4, 95% CI = 1.1–11.8, p = 0.04).
Conclusions
Although clinicians do not rate the majority of UR following lung transplant as preventable, discharge frailty is associated with UR. Further research should identify whether modification of discharge frailty can reduce UR.
Introduction
Unplanned rehospitalizations (UR) within 30 days of discharge are increasingly used as hospital quality of care markers with various penalties and inducements for reaching readmission prevention benchmarks.1,2 UR are common following solid organ transplantation, particularly for lung transplant recipients where single center incidence estimates range from 29.8 to 45.4%. 3–5 Among Medicare recipients undergoing lung transplantation, UR after initial discharge approach 42% depending on center volume.7 A growing body of retrospective research suggests that these rehospitalizations may be associated with increased mortality, subsequent readmission, and intensive care unit (ICU) utilization.6–8 As such, there has been ongoing interest in using UR as a quality marker for lung transplantation programs.8,9
It is unclear, however, to what extent UR represent preventable gaps in quality of care or necessary steps to provide timely inpatient interventions to complex patients.10 Although retrospective data have consistently identified similar reasons for UR within 30 days of initial discharge—infection, arrhythmias, and pleural space/postsurgical complications—there have been no prior studies of the extent to which these rehospitalizations are preventable. Extrapolating from limited data in the kidney transplant literature where preventability estimates are as low as 8.0%, it is possible that lung transplant health care professionals, patients, and caregivers can only prevent a small percentage of UR.11
Previous research in lung transplant recipients has primarily focused on “static” risks for UR such as native lung disease, sex, length of ICU and total hospital stay, and lung allocation score (LAS) rather than potentially modifiable factors. For example, frailty has been associated with UR in kidney transplant recipients and may represent a modifiable risk factor in lung transplant patients.12 Similarly, the presence of significant patient anxiety has been associated with increased risk for readmission after thoracic surgery and may also be modifiable.13 Identifying the extent to which UR are preventable and are associated with modifiable risk factors is an essential step in evaluating resource utilization following lung transplant.
The primary aim of this study was to examine prospectively the incidence, causes, direct costs, and preventability of UR within 30 days following discharge after lung transplantation. The secondary aim was to examine whether discharge frailty and anxiety were associated with UR.
Methods
Patient Population and Study Design
This was a prospective cohort study conducted at the Hospital of the University of Pennsylvania (HUP) from March 1st 2016 through February 28th 2017. Lung transplant recipients who survived to initial discharge were eligible to participate. We did not exclude re-transplant or multi-organ transplant patients. Study subjects provided informed consent for participation and the HUP institutional review board approved this study.
Clinical Variable Data Collection
We collected potential explanatory demographic and clinical risk factors for UR based on variables previously identified in lung and other solid organ transplants to be associated with UR.3,11,14 These included age, sex, number of hospitalizations in the year prior to transplant, concerns regarding social support at the time of listing or discharge as identified by a moderate or limited Stanford Integrate Psychosocial Assessment for Transplant score in social support domains, native lung disease leading to transplant (categorized as interstitial pulmonary fibrosis (IPF) versus non-IPF), most recent 6 minute walk test distance and percent predicted forced expiratory volume in 1 second (FEV1) prior to transplantation, and LAS at the time of transplantation. We also collected data on cytomegalovirus mismatch status (donor positive, recipient negative), the presence of any grade 3 primary graft dysfunction at any time point, or acute kidney injury (defined as rise in creatinine >20% from baseline) during the initial transplant hospitalization, the length of ICU and total hospitalization; and the number of standing medications at discharge.
Finally we recorded discharge destination, categorized as home or an acute rehabilitation facility/long-term acute care hospital (LTACH). We included this variable as a prior study identified LTACH discharge as the highest risk factor for UR among lung transplant recipients.3 This study, however, could not evaluate whether this was because patients were seen regularly by LTACH physicians and were readmitted prophylactically for evaluation of a developing problem or because of differences in frailty or illness severity among patients discharged to LTACH versus home. At HUP, all patients not discharged to rehab or an LTACH are seen within 72 hours by a transplant pulmonlogist or advanced practioner. They are all enrolled in an outpatient physical therapy program at HUP 3 times a week and are seen by a transplant pulmonologist or advanced practioner at least weekly during the first month. At these visits they have routine blood work, pulmonary function tests, and chest imaging.
These included age, sex, number of hospitalizations in the year prior to transplant, concerns regarding social support at the time of listing or discharge as identified, native lung disease leading to transplant (categorized as interstitial pulmonary fibrosis (IPF) versus non-IPF), most recent 6 minute walk test distance and percent predicted forced expiratory volume in 1 second (FEV1) prior to transplantation, and LAS at the time of transplantation. We also collected data on cytomegalovirus mismatch status (donor positive, recipient negative), the presence of grade 3 primary graft dysfunction, or acute kidney injury (defined as rise in creatinine >20% from baseline) during the initial transplant hospitalization, the length of ICU and total hospitalization; the number of standing medications at discharge; and discharge destination, categorized as home or an acute rehabilitation facility/long-term acute care hospital (LTACH).
Within 48 hours prior to or following discharge, enrolled patients completed a modified Short Physical Performance Battery (SPPB), a 2-component assessment that includes chair stands and balance.15 The modified SPPB is scored on an 8 point scale that can be treated ordinally (8 points = not frail; 7 points = prefrail; ≤6 points = frail) or continuously. All patients also had a baseline SPPB performed by a trained physical therapist at the time of listing as part of routine pretransplant evaluation. Within 48 hours prior to discharge, enrolled patients also completed the state-trait anxiety inventory (STAI), a self-reported assessment tool that measures anxiety around a specific situation (state-anxiety) and a subject’s disposition toward anxiety (trait-anxiety).16 The state-scale and the trait-scale each include 20 items scored on a 4 point Likert response scale with higher scores indicated increased anxiety (Cronbach’s alpha 0.86–0.95). Although there is no specific threshold in the STAI that defines anxious versus nonanxious, the maximum score is 80 and mean scores in the general population run between 31 and 34.
Outcome Definition
The primary study outcome was whether an UR occurred within 30 days following initial discharge. Scheduled or planned admissions that took place within 30 days of a discharge—for example, for a scheduled dialysis catheter change—were not considered UR. Within 72 hours of rehospitalization, the attending or admitting/referring clinician identified the primary reason for readmission and assigned it to 1 of 9 categories derived from a previously published study (Figure 1).3 For each UR, one member of the study staff and the patient’s admitting or attending clinician used an ordinal scale (0, not; 1, possibly; 2, definitely preventable) to rate preventability using previously described definitions of preventability.17 Study staff and admitting/attending clinicians were blind to each other’s judgments and there was good correlation between study staff and admitting/attending clinician assessment of preventability (Cohen’s kappa = 0.65, p<0.001). A total sum score ≥2 defined a preventable UR. For each preventable UR, the admitting or attending clinician identified the primary reason for why the admission could have been prevented using a previously validated instrument in solid organ transplant (Figure 1).11 Costs were broken down into charges, defined as professional charges and hospital charges, and payments defined as professional payments and hospital payments received, as captured from the administration’s Horizon Performance Management database.
Figure 1.
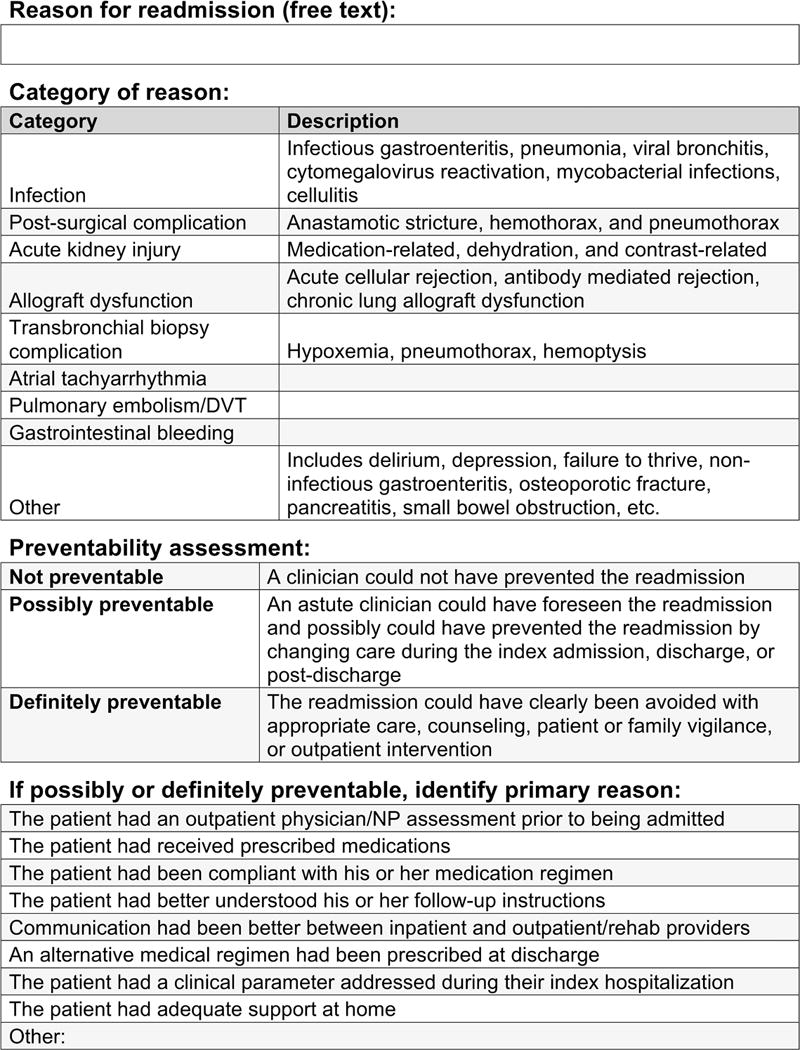
Readmission assessment tool.
Statistical analysis
We used simple descriptive statistics to identify percentages, medians, and quartiles for demographic and clinical variables. We used Fisher exact tests (for categorical variables), Student t tests (for normally distributed continuous variables), and Wilcoxon rank sum tests (for nonnormally distributed continuous variables) to compare selected demographic and clinical characteristics for patients with and without an UR. Given the small sample size we only included variables with p value < 0.15 in a multivariable logistic regression model to identify predictors of any UR. Finally, as a secondary analysis, for patients with at least 90 days of follow-up, we compared whether UR before day 30 were more likely to be categorized as preventable than those between days 30–90.
All analyses were performed using Stata (Version 14, Stata Corp, College Station, Texas). Study data were collected and managed using Research Electronic Data Capture, an electronic data capture tool hosted at HUP.18
Results
Overall study cohort
Between March 1st 2016 and February 28th 2017 there were 92 patients eligible for study participation, 90 of whom consented to study enrollment. One patient declined for unspecified reasons and another declined secondary to the perceived complexity of the STAI. Demographic and clinical characteristics of this cohort are listed in Table 1.
Table 1.
Demographic and Clinical Characteristics of Study Cohort (N = 90).
| Recipient Characteristics | |
|---|---|
| Age, mean ± SD (years) | 53.5 ± 14.1 |
| Male sex, n (%) | 51 (56.7%) |
| Pulmonary fibrosis, n (%) | 52 (57.8%) |
| Concern about social support, n (%) | 17 (18.9%) |
| FEV1, mean ± SD (% predicted) | 34.9 (18.1%) |
| Six minute walk test, mean ± SD (feet) | 979.2 ± 423.3 |
| Number of hospitalizations in year before transplantation, median (IQR) | 1 (0-2) |
| SPPB on listing, n (%) | 8 (8-8) |
| Waitlist time, median (IQR) (days) | 51 (13-139) |
| Lung allocation score, median (IQR) | 39.7 (35.9-55.1) |
| Multi-organ transplant, n (%) | 2 (2.2) |
| Retransplant, n (%) | 2 (2.2) |
| Donor Characteristics and Posttransplant Course | |
| Increased risk donor, n (%) | 20 (22.2%) |
| Cytomegalovirus donor positive recipient negative, n (%) | 27 (30.0%) |
| Single lung transplant, n (%) | 17 (20.0%) |
| Any grade 3 primary graft dysfunction, n (%) | 12 (13.3%) |
| Acute kidney injury during index hospitalization, n (%) | 42 (46.7%) |
| Intensive care unit length of stay, median (IQR) (days) | 6 (3-14) |
| Index hospitalization length of stay, median (IQR) (days) | 18 (13-26) |
| SPPB at discharge, n (%) | 4 (4-6) |
| A-state score at discharge, median (IQR) | 36 (27-46) |
| A-trait score at discharge, median (IQR) | 33 (25-41) |
| Number of standing medications at discharge, median (IQR) | 18 (16-20) |
| Initial discharge to LTACH or rehabilitation facility, n (%) | 21 (23.3%) |
FEV1, forced expiratory volume in 1 second; IQR, interquartile range; LTACH, long term acute care hospital; SPPB, short performance physical battery, SD, standard deviation
The median number of hospitalizations in the year prior to transplant was 1 (interquartile range [IQR] = 0–2). All enrolled patients had a SPPB of either 7 or 8 at the time of listing, indicating that they were not frail, with a median SPPB of 8. The median time between listing and transplantation was 51 days and the median LAS at the time of transplant was 39.7. The median length of ICU stay was 6 days and the median total length of hospitalization was 18 days. The median SPPB at discharge was 4 (IQR = 4–6) and 63 patients (70.0%) had a SPPB<6. The median A-state score was 36 and the median A-trait score was 33 (out of a total possible 80 points).
Characteristics of patients with early unplanned rehospitalization
Among the 90 patients in the overall cohort, there were 30 (33.3%) UR within 30 days of initial discharge. Comparison of characteristics of patients with and without UR is shown in Table 2. Concern about social support identified at listing or at initial discharge, the number of hospitalizations in the year before transplantation, length of ICU stay, and total length of hospitalization were not associated with UR. Similarly, neither A-state nor A-trait score at discharge were associated with UR. A diagnosis of pulmonary fibrosis leading to transplant (73.3% vs 50.0%, p = 0.04) and a SPPB<6 (86.7% vs 61.7%, p = 0.02) were significantly associated with UR.
Table 2.
Univariate Comparison between Patients with And without Unplanned Rehospitalization within 30 Days of Discharge Following Lung Transplantation.
| No UR (n=60) |
UR (n=30) |
p | |
|---|---|---|---|
| Age, mean ± SD (years) | 53.0 ± 16.1 | 53.7 ± 13.1 | 0.83 |
| FEV1, mean ± SD (% predicted) | 35.4 ± 16.6 | 34.6 ± 18.9 | 0.85 |
| Male sex, n (%) | 34 (56.7) | 17 (56.7) | 1.00 |
| Pulmonary fibrosis, n (%) | 30 (50.0) | 22 (73.3) | 0.04 |
| Concern about social support, n (%) | 12 (20.0) | 5 (16.7) | 0.78 |
| Six minute walk test, mean ± SD (feet) | 923.4 ± 412.7 | 1007.2 ± 429.2 | 0.38 |
| Number of hospitalizations in year prior to transplantation, median (IQR) | 1 (0-2) | 0 (0-2) | 0.52 |
| SPPB<8 at transplant listing, n (%) | 12 (20.3) | 9 (20.0) | 0.43 |
| Lung allocation score >50 | 14 (23.3) | 12 (40.0) | 0.14 |
| Cytomegalovirus donor positive recipient negative, n (%) | 20 (33.3) | 7 (23.3) | 0.46 |
| Any grade 3 primary graft dysfunction, n (%) | 7 (11.7) | 5 (16.7) | 0.52 |
| Acute kidney injury during index hospitalization, n (%) | 31 (51.7) | 11 (36.7) | 0.26 |
| Intensive care unit length of stay, median (IQR) (days) | 7 (4-13) | 5 (3-15) | 0.66 |
| Index hospitalization length of stay, median (IQR) (days) | 17 (13-22) | 21 (13-26) | 0.32 |
| SPPB<6 on discharge, n (%) | 37 (61.7) | 26 (86.7) | 0.02 |
| SPPB at discharge, median (IQR) | 5 (4-6) | 4 (4-5) | 0.28 |
| A-state score at discharge, median (IQR) | 38 (26-45) | 35 (27-46) | 0.82 |
| A-trait score at discharge, median (IQR) | 33 (25-41) | 33 (25-41) | 0.97 |
| Initial discharge to LTACH or rehabilitation facility, n (%) | 16 (26.7) | 5 (16.7) | 0.43 |
FEV1, forced expiratory volume in 1 second; IQR, interquartile range; LTACH, long term acute care hospital; SPPB, short performance physical battery; SD, standard deviation; UR, unplanned rehospitalization
We then developed a multivariable model of potential risk factors for UR. Because LAS>50 and diagnosis of pulmonary fibrosis were significantly correlated (phi correlation coefficient = 0.25, p = 0.02) our final model included only SPPB<6 and LAS >50. On multivariable analysis, SPPB<6 remained significantly associated with UR (odds ratio [OR] = 3.5, 95% confidence interval [CI] = 1.1–11.8, p = 0.04) but LAS>50 was not (OR = 1.7, 95% CI = 0.6–4.5, p = 0.30). Including place of discharge (home vs acute rehabilitation or LTAC facility) in the model did not change the association between SPPB<6 and UR (OR = 5.1, 95% CI = 1.4–17.6 p = 0.01).
Epidemiology and cost of unplanned rehospitalizations
The median time to unplanned rehospitalization was 9 days (IQR = 4–18) and the median length of rehospitalization was 7 days (IQR = 3–13) (Table 3). The single most common reasons for rehospitalization were infection (7 (23.3%)) and atrial tachyarrhythmia (5 (16.7%)). The total charges for all UR were $7,716,374 and the total payments for all UR were $1,500,473.
Table 3.
Characteristics of Unplanned Rehospitalization within 30 Days of Initial Discharge (N = 30).
| Time to rehospitalization, median (IQR) (days) | 9 (4-18) |
| Length of rehospitalization, median (IQR) (days) | 7 (3-13) |
| Preventable, n (%) | 9 (30.0%) |
| Professional and hospital charges for all rehospitalizations, median (IQR) (dollars) | $87555 ($59511-198390) |
| Professional and hospital payments for all rehospitalizations, median (IQR) (dollars) | $13048 ($7583-27272) |
| Professional and hospital charges for preventable rehospitalizations, median (IQR) (dollars) | $85193 ($70272-207953) |
| Professional and hospital payments for preventable rehospitalizations, median (IQR) (dollars) | $17398 ($11712-35627) |
| Reason for readmission | |
| Infection, n (%) | 7 (23.3%) |
| Atrial tachyarrhythmia, n (%) | 5 (16.7%) |
| Postsurgical complication, n (%) | 4 (13.3%) |
| Acute kidney injury, n (%) | 3 (10.0%) |
| Gastrointestinal bleeding, n (%) | 2 (6.7%) |
| Other, n (%) | 9 (30.0%) |
| Failure to thrive | 2 (22.2%) |
| Small bowel obstruction | 1 (11.1%) |
| Seizure | 1 (11.1%) |
| Long bone fracture | 1 (11.1%) |
| Noninfectious diarrhea | 1 (11.1%) |
| Vertebral body fracture | 1 (11.1%) |
| Inadequate pain control | 1 (11.1%) |
| Incidental pneumomediastium | 1 (11.1%) |
| Allograft dysfunction, n (%) | 0 (0.0%) |
| Transbronchial biopsy complication, n (%) | 0 (0.0%) |
| Pulmonary embolism/deep vein thrombosis, n (%) | 0 (0.0%) |
IQR=interquartile range
Among the 30 UR, 9 (30.0%) were judged preventable, 78% of which occurred in the first week following discharge (Table 3). For these UR the median preventability score was 3 and 6 UR had a score of ≥3. The most common reasons cited for preventability were “if communication had been better between inpatient and outpatient/rehabilitation providers” (3 (33.3%)) and “if an alternative medical regimen had been prescribed at discharge” (2 (22.2%)) (Table 4). There was no difference in median total charges ($85,193 ($70,272–207,953) vs $89,917 ($57,908–137,425), p = 0.61) or median total payments between preventable and not preventable UR ($17,398 ($11,712–35,627) vs $11,308 ($7334–19,567), p=0.32). Among enrolled patients with at least 90 days of follow-up, UR that happened before day 30 following discharge were more likely to be preventable than those that occurred between days 30–90 (30.0% versus 6.2%, p = 0.04).
Table 4.
Reasons for Preventable Unplanned Rehospitalization within 30 Days of Initial Discharge (N = 9)
| Communication had been better between inpatient and outpatient/inpatient rehabilitation providers | 3 (33.3%) |
| An alternative medical regimen had been prescribed at discharge | 2 (22.2%) |
| The patient had an outpatient physician/advanced practioner assessment prior to being admitted | 1 (11.1%) |
| The patient had better understood his or her follow-up instructions | 1 (11.1%) |
| The patient had a clinical parameter addressed during their index hospitalization | 1 (11.1%) |
| Other | 1 (11.1%) |
| The patient had adequate support at home | 0 (0.0%) |
| The patient had received prescribed medications | 0 (0.0%) |
| The patient had been compliant with his or her medication regimen | 0 (0.0%) |
Discussion
This is the first prospective cohort study in solid organ transplantation of the incidence, causes, costs, and preventability of UR within 30 days of initial discharge. It is also the first study in lung transplantation to use validated frailty and anxiety instruments to assess the impact of these factors on UR. Our primary findings were that a third of lung transplant recipients have an UR within 30 days of discharge and that almost a third of these readmissions are preventable. Among the variables we considered, discharge frailty was significantly associated with UR.
In the last year, there have been 6 studies published on rehospitalization following lung transplantation.3–6,19 Consistent with this growing literature, where the incidence of UR has been reported between 29.8–45.4%, 30.0% of patients in our cohort had an UR. Similarly, we found that infection, atrial arrhythmias, and postsurgical complications were the single most common reasons for UR.6 Readmissions occurred relatively soon after transplant (median 9 days) and were relatively short, with almost half being 5 days or less in duration. The financial burden associated with these readmissions adds increased cost to the health system.
STAI scores in our cohort were in keeping with previously reported pre and postlung transplant values, which have ranged from 36–42.20–22 Despite evidence in the nontransplant literature that anxiety predicts readmission, we did not find that state or trait anxiety scores were associated with UR.13,23 This is consistent with studies in lung transplant that have found no relationship between STAI score and other posttransplantation outcomes.20–22 Since patients with chronic lung diseases have high levels of anxiety pretransplant, it may be that recipients have developed skills or social support networks to cope with these symptoms.24 We did not have significant numbers of patients with very high levels of anxiety (STAI>50) and were therefore unable to assess whether severe anxiety was associated with UR.
We were, however, able to consider several other factors not previously examined in the lung transplant rehospitalization literature, including concern about social support at listing or discharge and number of hospitalizations in the year prior to transplantation. Despite the perception that patients without significant social support or who have a pattern of frequent hospitalizations are more likely to be readmitted, we did not find such an association between these variables and UR. It may be that clinicians identified these patients as being high risk for readmission leading to closer follow-up or monitoring after initial discharge or that our study was underpowered to detect small effects.
Discharge frailty was strongly associated with UR and this study adds to the growing body of literature on the importance of frailty on transplant outcomes.12,25–27, Notably, none of the patients who survived to discharge and were eligible for study enrollment were frail at listing. but a significant number (70.0%) were frail at discharge. Although not a focus of this study, we note that all of patients in our center who were frail at listing died before initial hospital discharge. The rate of discharge frailty in our cohort was consistent with the 82–86% frailty rates found among medical ICU survivors.28,29 We also do not know whether frailty developed while on the waitlist, during the index hospitalization, or both; the extent to which frailty could have been prevented; and whether modifying frailty would reduce UR. Importantly, however, frailty was associated with UR independently of discharge to a LTACH or rehabilitation facility suggesting that these rehospitalizations are not just a function of place of discharge.3,5 Our center requires recipients to attend outpatient pulmonary rehabilitation at our hospital 3 days a week for up to 12 weeks following initial discharge. This program anecdotally appears to improve frailty trajectories but the actual impact on UR is unknown.
There was a significantly higher rate of preventable UR in our study (30.0%) compared to a prior report of 8.0% in a retrospective chart review study in kidney transplant.11,30 Asking clinicians involved in the patient’s care to assess preventability near the time of admission is likely a more valid measurement than reconstructing the hospitalization process from the medical record. The 30% incidence in our study was in keeping with estimates in the limited data on preventable readmissions in nontransplant populations such as percutaneous coronary interventions (42.6%) and the general surgical (20.4%) and medical populations (26.9%).17,31,32 Ideally, as many preventable readmissions as possible would be avoided, but it may be that the opportunity costs involved in building frameworks to limit these readmissions could not be justified. Defining an acceptable preventable readmissions rate is, therefore, at least partly a health systems issues. In this context, it would be useful to have multicenter data to define average preventable readmissions rates, whereby unacceptable rates could be defined by relative outlier. Our finding that preventable readmissions are more likely to occur before 30 days than between 30–90 days is, however, consistent with health policy strategies that target 30 day readmissions. Given, however, that 78% of preventable UR occurred in the first week following discharge, this may be a more appropriate benchmark if confirmed in a multi-institutional setting.33 Finally, results also suggest that interventions targeting inpatient and outpatient communication may improve UR rates, as has been noted in the nontransplant literature.34
Our study has several limitations. First, this was a single institution study and generalizability may be limited because of practice patterns or reasons for readmission specific to our patient population. Second, we were not powered to identify variables with small or moderate effect sizes on UR and we did not have data on other variables such as nutritional status, gastroesophageal reflux, duration of air leak, or body fat composition that may be associated with readmission.5,19,35 Finally, we were not able to identify variables specifically associated with preventable readmissions. As we accumulate additional follow-up time, we anticipate being able to assess the relationship between UR and subsequent mortality.
Conclusions
Although the majority of UR following lung transplant are not preventable, discharge frailty is associated with UR. Further research should identify whether modification of discharge frailty reduces UR.
Acknowledgments
Funding
Support for this research was provided by National Institutes of Health grants (5T32-HL-007633-30, K23-HL-121406, and K24-HL-115354). The sponsors had no role in the design of the study, the collection and analysis of the data, or preparation of the manuscript.
Abbreviations
- CI
confidence interval
- FEV1
forced expiratory volume in 1 second
- IPF
interstitial pulmonary fibrosis
- HUP
Hospital of the University of Pennsylvania
- ICU
intensive care unit
- LAS
lung allocation score
- LTACH
long-term acute care hospital
- OR
odds ratio
- SPPB
short physical performance battery test
- STAI
state-trait anxiety inventory
- UR
unplanned rehospitalizations
Footnotes
Authorship
AMC, DZ, LG, and JMD participated in research design, writing of the paper, performance of the research, data analysis, and approval of the final manuscript. VNA, JDC, MC, DH, JL, MM, NP, MP, EEC, and CB participated in research design, performance of the research, and approval of the final manuscript.
Disclosure statement
The authors declare no conflicts of interest.
References
- 1.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–5. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 2.H.R. 1845 (112th): Medicare IVIG Access and Strengthening Medicare and Repaying Taxpayers Act of 2012. Available at: https://www.govtrack.us/congress/bills/112/hr1845/text. Accessed March 14, 2017.
- 3.Courtwright AM, Salomon S, Fuhlbrigge A, et al. Predictors and outcomes of unplanned early rehospitalization in the first year following lung transplantation. Clinic Transplant. 2016;30:1053–8. doi: 10.1111/ctr.12787. [DOI] [PubMed] [Google Scholar]
- 4.Osho AA, Castleberry AW, Yerokun BA, et al. Clinical predictors and outcome implications of early readmission in lung transplant recipients. J Heart Lung Transplant. 2016 doi: 10.1016/j.healun.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alrawashdeh M, Zomak R, Dew MA, et al. Pattern and predictors of hospital readmission during the first year after lung transplantation. Am J Transplant. 2016 doi: 10.1111/ajt.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lushaj E, Julliard W, Akhter S, et al. Timing and frequency of unplanned readmissions after lung transplantation impact long-term survival. Ann Thorac Surg. 2016;102:378–84. doi: 10.1016/j.athoracsur.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 7.Mooney JJ, Weill D, Boyd JH, Nicolls MR, Bhattacharya J, Dhillon GS. Effect of transplant center volume on cost and readmissions in Medicare lung transplant recipients. Ann Am Thorac Soc. 2016;13:1034–41. doi: 10.1513/AnnalsATS.201601-017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J, Singer P, Raviv Y, et al. Outcome of lung transplant recipients requiring readmission to the intensive care unit. J Heart Lung Transplant. 2011;30:54–8. doi: 10.1016/j.healun.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Vigneswaran WT, Helenowski M, Bhorade SM, Lamounier F, Alex C, Garrity ER., Jr Early readmission is a predictor of overall survival following isolated lung transplantation. Internat Surg. 2010;95:299–304. [PubMed] [Google Scholar]
- 10.Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. New Eng J Med. 2012;366:1366–9. doi: 10.1056/NEJMp1201598. [DOI] [PubMed] [Google Scholar]
- 11.Harhay M, Lin E, Pai A, et al. Early rehospitalization after kidney transplantation: assessing preventability and prognosis. Am J Transplant. 2013;13:3164–72. doi: 10.1111/ajt.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams‐DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–5. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tully PJ, Baker RA, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. J Behavior Med. 2008;31:281–90. doi: 10.1007/s10865-008-9153-8. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Hosmer A, Parks T, Sonnenday CJ, Sharma P. Predictors of early hospitalization after deceased donor liver transplantation. Dig Dis Sci. 2015;60:3242–7. doi: 10.1007/s10620-015-3753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Spielberger CE, Gorsuch RL. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychology Press; 1970. [Google Scholar]
- 17.Wasfy JH, Strom JB, Waldo SW, et al. Clinical preventability of 30-day readmission after percutaneous coronary intervention. J Am Heart Assoc. 2014;3:e001290. doi: 10.1161/JAHA.114.001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Info. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo WK, Goldberg HJ, Burakoff R, Feldman N, Chan WW. Increased proximal acid reflux is associated with early readmission following lung transplantation. Neurogastroenterol Motil. 2016;28:251–9. doi: 10.1111/nmo.12720. [DOI] [PubMed] [Google Scholar]
- 20.Cohen L, Littlefield C, Kelly P, Maurer J, Abbey S. Predictors of quality of life and adjustment after lung transplantation. Chest. 1998;113:633–44. doi: 10.1378/chest.113.3.633. [DOI] [PubMed] [Google Scholar]
- 21.Smith PJ, Blumenthal JA, Trulock EP, et al. Psychosocial predictors of mortality following lung transplantation. Am J Transplant. 2016;16:271–7. doi: 10.1111/ajt.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeulen KM, Ten Vergert EM, Verschuuren EA, Erasmus ME, van der Bij W. Pre-transplant quality of life does not predict survival after lung transplantation. J Heart Lung Transplant. 2008;27:623–7. doi: 10.1016/j.healun.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Volz A, Schmid JP, Zwahlen M, Kohls S, Saner H, Barth J. Predictors of readmission and health related quality of life in patients with chronic heart failure: a comparison of different psychosocial aspects. J Behavior Med. 2011;34:13–22. doi: 10.1007/s10865-010-9282-8. [DOI] [PubMed] [Google Scholar]
- 24.Singer HK. The psychological impact of end-stage lung disease. Chest. 2001;120:1246–52. doi: 10.1378/chest.120.4.1246. [DOI] [PubMed] [Google Scholar]
- 25.Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Resp Crit Care Med. 2015;192:1325–34. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson ME, Vakil AP, Kandel P, et al. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant. 2016;35:173–8. doi: 10.1016/j.healun.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Kelm DJ, Bonnes SL, Jensen MD, et al. Pre-transplant wasting (as measured by muscle index) is a novel prognostic indicator in lung transplantation. Clin Transplant. 2016;30:247–55. doi: 10.1111/ctr.12683. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin MR, Reid MC, Westlake AA, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29:401–8. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollack LR, Goldstein NE, Gonzalez WC, et al. The frailty phenotype and palliative care needs of older survivors of critical illness. J Am Geriatric Soc. 2017 doi: 10.1111/jgs.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldfield NI, McCullough EC, Hughes JS, et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30:75–91. [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton C, Marshall L, Lloyd T, Garcea G, Berry D, Kelly M. Leicestershire surgical readmissions survey. Journal of Clin Excel. 2002;4:33–41. [Google Scholar]
- 32.Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Int Med. 2016;176:484–93. doi: 10.1001/jamainternmed.2015.7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin DL, Bang H, Manickam RN, Romano PSD. Rethinking thirty-day hospital readmissions: Shorter intervals might be better indicators of quality of care. Health Affairs. 2016;35:1867–75. doi: 10.1377/hlthaff.2016.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Ann Rev Med. 2014;65:471–85. doi: 10.1146/annurev-med-022613-090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad O, Browning M, Baz M, et al. Impact of body fat composition on hospital length of stay and one year readmission rate in patients post bilateral lung transplantation. Am J Resp Crit Care Med. 2016;193:A4671. [Google Scholar]


