Abstract
We present the first reported work that explores the potential of radiomics to predict patients who are at risk for developing immunotherapy-induced pneumonitis. Despite promising results with immunotherapies, immune-related adverse events (irAEs) are challenging. Although less common, pneumonitis is a potentially fatal irAE. Thus, early detection is critical for improving treatment outcomes; an urgent need to identify biomarkers that predict patients at risk for pneumonitis exists. Radiomics, an emerging field, is the automated extraction of high fidelity, high-dimensional imaging features from standard medical images and allows for comprehensive visualization and characterization of the tissue of interest and corresponding microenvironment. To this end, in this pilot study, we sought to determine whether radiomics has the potential to predict development of pneumonitis. We performed radiomic analyses using baseline chest computed tomography images who did (N=2) and did not (N=30) develop immunotherapy-induced pneumonitis. We extracted 1860 radiomic features in each patient. Maximum relevance and minimum redundancy feature selection method, anomaly detection algorithm, and leave-one-out cross-validation identified radiomic features that were significantly different and predicted subsequent immunotherapy-induced pneumonitis (accuracy, 100% [p=0.0033]). This study suggests that radiomic features can classify and predict those patients at baseline who will subsequently develop immunotherapy-induced pneumonitis, further enabling risk-stratification that will ultimately lead to better treatment outcomes.
Keywords: immunotherapy, immune-related adverse event, pneumonitis, radiomics
In recent years, immunotherapies have revolutionized the treatment of advanced cancer [1]. Immunotherapeutic agents produce remarkable responses by the process of immunomodulation, and several checkpoint inhibitors are now Food and Drug Administration (FDA)–approved for certain types of advanced cancer: ipilimumab for melanoma; nivolumab for melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, and Hodgkin lymphoma; pembrolizumab for melanoma, NSCLC, and squamous cell carcinoma of head and neck; atezolizumab for urothelial carcinoma and NSCLC; and, combination of nivolumab plus ipilimumab for BRAF V600 mutant and wild-type advanced melanoma [2]. However, unrestrained modulation of the immune system decreases self-tolerance and produces a unique spectrum of toxic effects called immune-related adverse events (irAEs) [3].
The irAEs are diverse and have important clinical implications [3]. The most common irAEs include dermatitis, enterocolitis, hepatitis, transaminitis, endocrinopathies, pneumonitis, and uveitis. Among these, pneumonitis is less common, but is a concern because it is life threatening [4]. Due to its auto-immune mechanism, treatment-related pneumonitis is termed an “event of special interest [5,6] ”. Pneumonitis of all grades has been increasingly reported in patients treated with programmed death 1 (PD-1) pathway inhibitors, with incidences ranging from 1.1% to 10.6%, 1.6% on monotherapy and 6.6% on combination therapy[7]. Pneumonitis may be a class-related toxic effect and seen mostly in patients on PD-1 inhibitors [8]; but, anecdotal cases have been reported with other immunotherapeutic agents such as ipilimumab. Although most cases are mild and managed successfully with drug-holding and/or steroids, treatment-related deaths and long-term respiratory morbidity due to pneumonitis are reported [6,9–11]. Early detection is therefore key to optimal patient management.
With recent approval of several immunotherapeutic agents for certain indications and extensive investigations across several tumor types, the clinical use of immunotherapeutic agents is on the rise. Besides serious clinical consequences, irAEs such as pneumonitis are increasingly recognized as a contributing factor to treatment noncompliance, discontinuation, or dose modification, which may compromise the clinical efficacy of the immunotherapeutic treatment. This underscores the need for prompt identification and effective management of pneumonitis. Further, the clinical diagnosis of pneumonitis is difficult as the clinical presentation can be similar to infectious pneumonia and radiologic patterns of pneumonitis may vary greatly from case to case. The time to onset of pneumonitis ranges widely from 9 days to 24 months [12,4]. This along with varying clinical presentations and the wide spectrum of radiographic patterns caused by pneumonitis [4] presents a challenging situation for the treating physician. A critical need exists to identify biomarkers for early prediction of pneumonitis that will allow for risk stratification before initiation of therapy and close monitoring during treatment. Unfortunately, there is no known way to predict which patients are likely to develop pneumonitis caused by immunotherapy.
A radiomics-based approach, which utilizes quantitative image analytics to identify associations between imaging features and clinical outcomes and uses these associations to develop prediction models [13–15], may offer a solution. Radiomics, an emerging field within medical imaging, is the automated extraction of high fidelity, high-dimensional imaging features from standard medical images and allows for comprehensive visualization and characterization of the tissue of interest and corresponding microenvironment [16]. Studies have demonstrated the prognostic power of radiomic features and their potential to serve as an important foundation for decision support tools [17,15]. Therefore, we sought to employ radiomics to predict patients who would develop immunotherapy-induced pneumonitis using pretreatment imaging studies.
MATERIALS AND METHODS
Patients and Samples
We reviewed the electronic medical records of patients with advanced cancer who were treated on early phase immunotherapy clinical trials that included at least one immunotherapeutic agent in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center (MDACC) between January 1, 2010, and July 31, 2015. This retrospective study was approved by the Institutional Review Board (IRB) at MDACC and informed consent was waived. All patients included in this analysis however, provided written informed consent before enrollment on an IRB-approved immunotherapy clinical trial (immune checkpoint inhibitors, cytokines, vaccines, or immunotherapy-based combinations) during this period.
Two investigators (T.F. and M.A.B.) independently reviewed the electronic medical record and collected clinical and demographic data. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 [18]. Any disagreements were resolved through discussion with a third investigator (A.N.). We defined pneumonitis as the presence of infiltrates on thoracic imaging and clinical symptoms of cough, shortness of breath, or wheezing and the absence of microbiological evidence of infection and the absence of volume overload as assessed by echocardiography, right heart catheterization, or response to diuretics in patients who were currently receiving immunotherapy. Patients with pneumonitis were taken as cases for our study. Patients without pneumonitis were randomly selected as control patients. Radiographic pretreatment baseline computed tomography (CT) scans of both patient groups were obtained for radiographic analysis.
Image Acquisition
The image acquisition was performed using scanners from MDACC; scanner manufactures included General Electric Healthcare, Philips Healthcare, and Siemens Healthcare. All scans were acquired using MDACC’s CT chest protocol (120kVp, slice thickness range 2–2.5mm, 512 x 512 matrix, and pixel spacing 0.78mm) and standard image reconstruction, which attempts to balance image noise and sharpness.
Radiographic Analysis
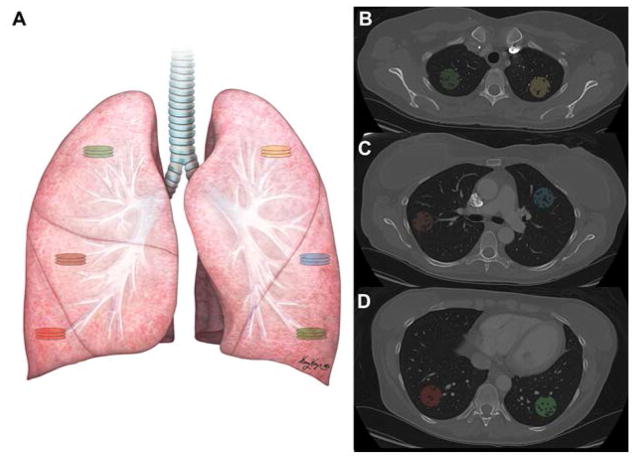
We performed radiomic analyses using baseline post-contrast chest CT images of patients who developed treatment-induced pneumonitis (cases) and a subset with no clinical or radiological finding of pneumonitis (controls). Prior to radiomic feature extraction, image segmentation was performed using 3D Slicer Version 4.3.1 (http://slicer.org) [19]. Owing to a lack of strong evidence in the literature for regional predilection of pneumonitis in the lungs, 3 volumes of interest (VOIs) were segmented in each lung. Taking into consideration the basic anatomy of the lungs (3 lobes in the right lung and 2 lobes in the left), 1 VOI per lobe was outlined for the right lung, and 3 mirrored contralateral VOIs were segmented on the left lung (Fig 1). Regions of interest (ROI), with radii ranging from 14 mm to 15 mm, were obtained from 3 consecutive slices, comprising a single VOI for a total of 18 ROI per patient lung. Enhancing blood vessels within the lung parenchyma were subtracted from the VOIs. All VOIs were manually segmented by an imaging expert and then reviewed by a board-certified radiologist (R.R.C., 8 years experience) both whom were blinded to the patients’ final diagnosis.
Fig. 1. Depiction of Regions (ROI) and Volumes of Interest (VOI) in the lungs.
(a) One VOI containing 3 consecutive slices was taken in each lobe in the right lung, and 3 ROIs (whose levels correspond to those of the VOIs in the right lung) were outlined in the left lung. (b–d) Post-contrast lung computed tomography images depicting the segmented VOIs in the upper (b), middle (c), and lower (d) sections of the right and left lungs. Each VOI is indicated with a different color
Radiomic texture features based on histogram and gray level co-occurrence matrix (GLCM) were extracted [20–23]. In the first step, re-quantization of the image gray levels was performed. Initially, the number of gray levels in the CT images was high. By re-quantizing the gray levels into a smaller number we achieved a reduction in noise. Since the ideal number of gray levels has not been established, we used various gray levels (8, 16, 32, 64, 256 gray levels). In the second step, 10 histogram and 60 GLCM features were extracted per gray level. The histogram-based features provide information about the intensity distribution within a VOI. The 10 histogram-based features extracted were minimum, maximum, mean, standard deviation, skewness, kurtosis, and the percentiles (1%, 5%, 95%, and 99%). The GLCM-based features provide information about the distribution of pairs of voxels separated by given distance at a specific direction [20]. In our implementation, we considered a distance of 1 (neighboring voxels) and 4 angular directions (in-plane rotations; θ=0, 45, 90, 135 degrees); thus, 4 GLCMs were calculated per VOI. For each GLCM, the following 20 features were extracted: autocorrelation, contrast, correlation, cluster shade, cluster prominence, dissimilarity, energy, entropy, homogeneity, maximum probability, variance, sum average, sum variance, sum entropy, difference variance, difference entropy, information measure of correlation 1, information measure of correlation 2, inverse difference moment, and normalized inverse difference moment [20–22]. To obtain invariant measures of the GLCM-based features across different rotations, we obtained the average, range, and angular variance of the features calculated for different θ values, thereby resulting in 60 invariant GLCM-based features for each VOI for a specific gray level. Accounting for the number of gray levels (5) and the segmented VOIs (6 VOIs per patient), a total of 1860 features were computed for each patient.
A maximum relevance minimum redundancy (MRMR) feature selection method was applied to identify features associated with immunotherapy-induced pneumonitis. With the MRMR method, each feature is ranked according to its relevance to the outcome and redundancy with other features [24]. Selected features were normalized by subtracting the mean and dividing by the standard deviation. To predict the immunotherapy-induced pneumonitis cases, we used an unsupervised anomaly detection algorithm [25]. Initially, the selected normalized features of each patient were fitted to a multivariate normal distribution that captures the trends and relationships present in the data. Then, based on the probabilities of each patient to be an outlier (i.e., not being modeled by the multivariate normal distribution), an optimal predictive threshold was identified to separate patients with immunotherapy-induced pneumonitis from patients without pneumonitis. The MRMR approach was implemented in R using the R package “mRMRe” and the Anomaly Detection Algorithm in OCTAVE [26]. Support vector machine as previously described and developed by our group for large-scale radiomic analysis was not used due to inherent disadvantages in this method when using small datasets [27]. The Anomaly Detection Algorithm method is a form of classification to predict whether data is typical for the distribution and can be used to detect rare events that can have great significance and deviate from past events [28,29]. Leave-one-out cross-validation (LOOCV) using all the features selected for entire dataset was also performed to determine generalizability of our statistical results.
RESULTS
A total of 290 patients were treated on early phase immunotherapy-based clinical trials between January 1, 2010, and July 31, 2015.
Incidence of irAEs
Of the 290 patients on the study, 98 (33.8%) had at least 1 any grade irAE, including 16 with grade 3–4 irAEs. There were no irAE-related deaths. Two (0.7%) patients developed pneumonitis; one grade 2 and the other grade 3 pneumonitis. Both these were included as the cases in our study.
Radiographic Analysis for Pneumonitis
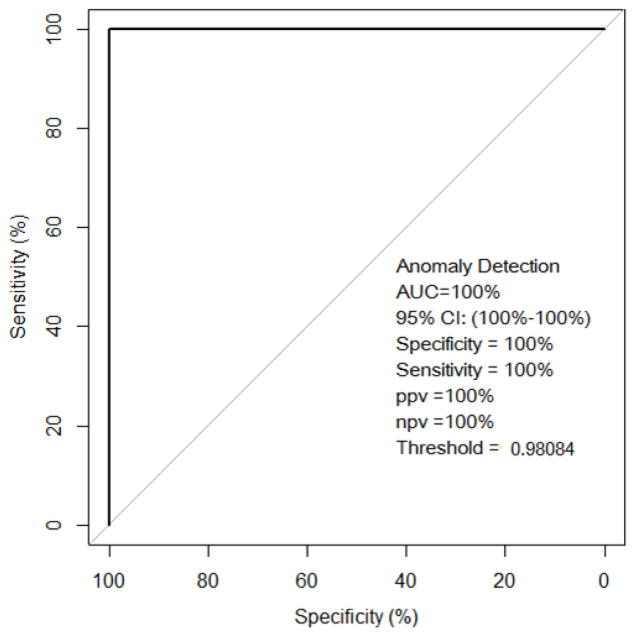
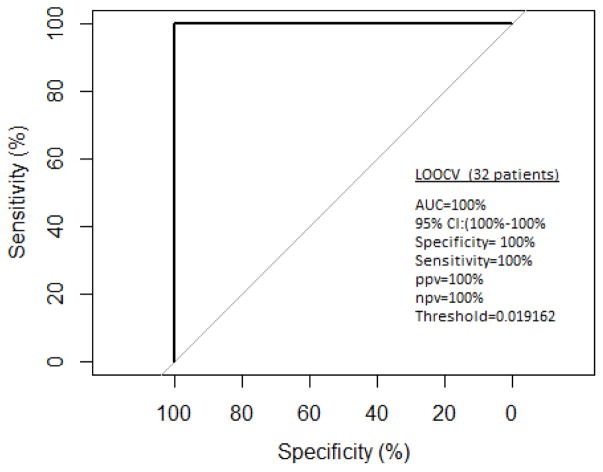
Two patients who developed immunotherapy-induced pneumonitis and 30 randomly selected control patients with no clinical manifestation of pneumonitis and no apparent findings (no pre-existing confounding lung pathology such as consolidation, pneumonia, or radiation-induced fibrosis) on post-contrast chest CT were included in the radiographic analysis (Table 1). As described in the Methods section, 1860 radiomic features were obtained for each patient using first and second order texture analysis. Features were ranked using the MRMR method and the top two features selected for subsequent use by the Anomaly Detection algorithm were skewness (measure of histogram symmetry; skewness is zero for symmetric histogram) and angular variance of sum of squares (measure of dispersion). Patients who subsequently developed immunotherapy-induced pneumonitis had higher values of skewness and angular variance of sum of squares. The top two features were combined because together they improved the predictive accuracy over the accuracy obtained individually with each feature. The anomaly detection algorithm identified the 2 patients who developed immunotherapy-induced pneumonitis outliers with respect to the control patients (Fig 2). LOOCV using all the features selected for entire dataset demonstrated high sensitivity, specificity, and accuracy (100%) (Fig 3).
Table 1.
Baseline Characteristics of Patient Cohort included in the Radiographic Analysis
| Baseline Characteristics | Controls (n=30) | Patients (n=2) |
|---|---|---|
|
| ||
| Age at Initial Diagnosis (range), years | ||
| Median | 50.28 | 66.39 |
| Range | 24.83–71.48 | 65.32–67.46 |
| Sex | ||
| Male | 9 (30) | - |
| Female | 21 (70) | 2 (100) |
| ECOG performance status | ||
| 0 | 4 (13) | - |
| 1 | 24 (80) | 2 (100) |
| 2 | 2 (7) | - |
| No. of metastatic sites | ||
| ≤ 2 | 22 (73) | 1 (50) |
| > 2 | 8 (27) | 1 (50) |
| Tumor Type | ||
| Breast | 1 (3) | |
| Colorectal | 2 (7) | |
| Pancreatic | 2 (7) | |
| Gynecologic | 9 (30) | |
| Renal cell carcinoma | 5 (17) | |
| Melanoma | 2 (7) | |
| Sarcoma or GIST | 5 (17) | 1 (50) |
| Other rare tumors | 4 (13) | 1 (50) |
Note: All data are no. of patients (%) unless otherwise indicated.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GIST, gastrointestinal stromal tumor.
Fig. 2. Receiver operating characteristic (ROC) curve of the anomaly detection algorithm.
Thirty-two patients (30 patients without and 2 patients with immunotherapy-induced pneumonitis) were included in the analysis. The ROC demonstrated high accuracy for discriminating those patients likely to develop immunotherapy-induced pneumonitis
Fig. 3. Leave-one-out cross-validation.
LOOCV demonstrated high sensitivity, specificity and accuracy in predicting subsequent development of immunotherapy-induced pneumonitis
DISCUSSION
We present the first reported work that explores the potential of radiomics to predict patients who are at high risk of developing pneumonitis on immunotherapy-based trials. In our study, 98 of 290 (33.8%) patients with advanced cancer treated on early phase immunotherapy clinical trials had any-grade irAEs. Any-grade pneumonitis was reported in 2 patients treated with cell-based vaccine therapy for neuroendocrine cancer and leiomyosarcoma. In a meta-analysis that included 4,496 patients on PD-1 inhibitor trials, any-grade pneumonitis was reported in 2.7% of the patients on monotherapy and 6.6% on combination therapy [7]. The incidence was significantly higher in patients with NSCLC and renal cell carcinoma compared to melanoma. Importantly, pneumonitis-associated deaths were reported in patients treated on nivolumab [9], pembrolizumab [10], and, combination of ipilimumab and nivolumab [11]. This anecdotal observation lends support to the need for clinical suspicion and early detection of irAEs by treating physicians for optimal treatment outcomes.
The morbid nature and the uncertainty associated with irAEs led us to investigate the ability of radiomic analysis to identify patients who will develop immunotherapy-induced pneumonitis. Radiomic texture features measure the heterogeneity of image intensities in a specific region and highlight small differences that cannot be identified by the naked eye [16]. We postulated that patients who subsequently developed immunotherapy-induced pneumonitis have micro-tissue changes at their baseline scan that could be detected and quantified by radiomic analysis, and used to predict the development of pneumonitis. Our results showed that the most predictive features were skewness (measure of histogram symmetry; skewness is zero for symmetric histogram) and angular variance of sum of squares (measure of dispersion of the voxel intensity distribution; voxel intensities that differ from the average value of the VOI will be weighted higher, resulting in a higher value of the feature). Patients that subsequently developed immunotherapy-induced pneumonitis had high positive skewness values, indicating that the histograms were asymmetric and thus characterized by heterogeneous intensities. High angular variance of sum of squares also indicates heterogeneity as more voxels with intensities different from the average value of the VOI were observed in different angular directions. Our approach enabled us to discriminate patients who subsequently developed pneumonitis with 100% accuracy (p=0.0033). In a retrospective study done in 106 patients with esophageal cancer who received radiation therapy (RT), 12 radiomic features were identified that were significantly different between pre- and post-RT scan in patients with radiation pneumonitis [13]. Thus, despite the small number of patients included, our pilot feasibility study highlights the ability of radiomics to estimate the probability of developing pneumonitis and potentially risk-stratifying patients before therapy is initiated thus enabling a more personalized approach to treatment.
Recent advances in image analyses have revealed that digital medical images contain unlimited high-dimensional data than what has been traditionally extracted as standard of care[15]. In our application of radiomics to radiographic images, we highlight that routine imaging used in clinical practice for diagnosis and evaluation of therapeutic response harbors biologic information that has prognostic and predictive value. Thus, radiomics has potential as a cost-effective decision-support tool that can be utilized in the clinic to help guide treatment choices in an effort to improve patient outcomes.
Our pilot study has several limitations. First, it is a retrospective study with only two cases and 30 controls. However, the reported incidence of immunotherapy-related pneumonitis is low4. Also, our study lacks external validation. Prospective studies are underway in our institution to validate our proof of concept findings in a larger cohort and to evaluate if radiomic changes are also representative of clinical resolution of pneumonitis in these patients.
In conclusion, treatment-related pneumonitis is an uncommon, but potentially fatal irAE that demands clinical suspicion, early detection, and prompt management. To this end, the results of our study are relevant to clinical practice as oncologists are likely to face this challenge with increasing use of immunotherapeutic agents in treatment of patients with advanced cancer. Our results suggest that radiomic features can be used to predict patients who are likely to develop pneumonitis at baseline with high accuracy, sensitivity and specificity. This strategy will allow efficient risk stratification, close monitoring, and prompt management of treatment-related adverse events. This will potentially improve patient quality of life and optimize treatment outcomes in patients with advanced cancer. Future multi-center, prospective studies in large cohorts are required to validate the clinical utility and generalizability of our results.
Acknowledgments
Funding: This research was partially funded by the John S. Dunn Sr. Distinguished Chair in Diagnostic Imaging Fund, The University of Texas MD Anderson Brain Tumor Center Program, The University of Texas MD Anderson Cancer Center startup funding, and the Cancer Prevention and Research Institute of Texas Individual Investigator Research Award (RP160150; RRC). This work was also supported in part by The University of Texas MD Anderson Cancer Center support grant (P30 CA016672; KRH, RRC, FMB) and a K23 Career Development Award (K23AI117024; AS) from the National Institutes of Health.
Footnotes
Previous Presentation: This study was presented in part at the 28th EORTC – NCI – AACR Molecular Targets and Cancer Therapeutics Symposium in November 2016.
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest: The authors declare no competing financial interests.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: For this type of study formal consent is not required.
- Rivka R. Colen: Conception and design, Designed and performed imaging analysis, Statistical analysis, Interpretation of the results, Contributed to the initial draft, reviewed and approved the final manuscript
- Takeo Fujii: Collection and assembly of data, Contributed to the initial draft, reviewed and approved the final manuscript
- Mehmet Asim Bilen: Collection and assembly of data, Contributed to the initial draft, reviewed and approved the final manuscript
- Aikaterini Kotrotsou: Designed and performed imaging analysis, Interpretation of the results, Contributed to the initial draft, reviewed and approved the final manuscript
- Srishti Abrol: Collection and assembly of data, Designed and performed imaging analysis, Contributed to the initial draft, reviewed and approved the final manuscript
- Kenneth R. Hess: Conception and design, Statistical analysis, Interpretation of the results, Contributed to the initial draft, reviewed and approved the final manuscript
- Joud Hajjar: Contributed to the initial draft, reviewed and approved the final manuscript
- Maria E. Suarez-Almazor: Contributed to the initial draft, reviewed and approved the final manuscript
- Anas Alshawa: Collection and assembly of data, Contributed to the initial draft, reviewed and approved the final manuscript
- David S. Hong: Contributed patients, Contributed to the initial draft, reviewed and approved the final manuscript
- Dunia Giniebra-Camejo: Statistical analysis, Contributed to the initial draft, reviewed and approved the final manuscript
- Bettzy Stephen: Wrote the initial draft, reviewed and approved the final manuscript
- Vivek Subbiah: Contributed patients, reviewed and approved the final manuscript
- Ajay Sheshadri: Contributed to the initial draft, reviewed and approved the final manuscript
- Tito Mendoza: Contributed to the initial draft, reviewed and approved the final manuscript
- Siqing Fu: Contributed patients, Contributed to the initial draft, reviewed and approved the final manuscript
- Padmanee Sharma: Contributed to the initial draft, reviewed and approved the final manuscript
- Funda Meric-Bernstam: Contributed patients, Contributed to the initial draft, reviewed and approved the final manuscript
- Aung Naing: Conception and design Administrative support, Contributed patients, Collection and assembly of data, Interpretation of the results, Contributed to the initial draft, reviewed and approved the final manuscript
References
- 1.Dizon DS, Krilov L, Cohen E, Gangadhar T, Ganz PA, Hensing TA, Hunger S, Krishnamurthi SS, Lassman AB, Markham MJ, Mayer E, Neuss M, Pal SK, Richardson LC, Schilsky R, Schwartz GK, Spriggs DR, Villalona-Calero MA, Villani G, Masters G. Clinical cancer advances 2016: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2016;34(9):987–1011. doi: 10.1200/JCO.2015.65.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Hematology/Oncology (Cancer) approvals & safety notifications. 2016. [Google Scholar]
- 3.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss M, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 8.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 9.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–U2032. doi: 10.1200/Jco.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L Investigators K. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunliffe A, Armato SG, 3rd, Castillo R, Pham N, Guerrero T, Al-Hallaq HA. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys. 2015;91(5):1048–1056. doi: 10.1016/j.ijrobp.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–1069. doi: 10.1016/j.crad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colen RR, Piwnica-Worms D. Radiomics and radiogenomics in breast cancer. Breast Diseases. 2016;27(1):23–24. [Google Scholar]
- 17.Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5644. ARTN 4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. NIH; 2009. [Google Scholar]
- 19.Gering DT, Nabavi A, Kikinis R, Hata N, O’Donnell LJ, Grimson WEL, Jolesz FA, Black PM, Wells WM. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13(6):967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 20.Clausi DA. An analysis of co-occurrence texture statistics as a function of grey level quantization. Can J Remote Sens. 2002;28(1):45–62. [Google Scholar]
- 21.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. Ieee T Syst Man Cyb Smc. 1973;3(6):610–621. doi: 10.1109/Tsmc.1973.4309314. [DOI] [Google Scholar]
- 22.Soh LK, Tsatsoulis C. Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. Ieee T Geosci Remote. 1999;37(2):780–795. doi: 10.1109/36.752194. [DOI] [Google Scholar]
- 23.Papoulis A. Probability, Random Variables, and Stochastic Processes. McGraw-Hill; 1991. [Google Scholar]
- 24.Peng H, Long F, Ding C. Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell. 2005;27(8):1226–1238. doi: 10.1109/TPAMI.2005.159. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein M, Uchida S. A comparative evaluation of unsupervised anomaly detection algorithms for multivariate data. PLoS ONE. 2016;11(4):e0152173. doi: 10.1371/journal.pone.0152173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Jay N, Papillon-Cavanagh S, Olsen C, El-Hachem N, Bontempi G, Haibe-Kains B. mRMRe: an R package for parallelized mRMR ensemble feature selection. Bioinformatics. 2013;29(18):2365–2368. doi: 10.1093/bioinformatics/btt383. [DOI] [PubMed] [Google Scholar]
- 27.Anguita D, Ghio A, Greco N, Oneto L, Ridella S. Ieee Ijcnn. 2010. Model Selection for Support Vector Machines: Advantages and Disadvantages of the Machine Learning Theory. [Google Scholar]
- 28.Syed Z, Saeed M, Rubinfeld I. Identifying High-Risk Patients without Labeled Training Data: Anomaly Detection Methodologies to Predict Adverse Outcomes. AMIA Annu Symp Proc. 2010;2010:772–776. [PMC free article] [PubMed] [Google Scholar]
- 29.Christiansen P, Nielsen LN, Steen KA, Jorgensen RN, Karstoft H. DeepAnomaly: Combining Background Subtraction and Deep Learning for Detecting Obstacles and Anomalies in an Agricultural Field. Sensors (Basel) 2016;16(11) doi: 10.3390/s16111904. [DOI] [PMC free article] [PubMed] [Google Scholar]