Abstract
Background
Urinary NGAL (uNGAL)-associated acute kidney injury (AKI) is common following liver transplant (LT), but whether early AKI predicts chronic kidney disease (CKD) and mortality remains uncertain.
Methods
Adults with LT from 2008–2010 in a previously published prospective cohort evaluating serial uNGAL pre- and post-LT were retrospectively assessed to evaluate uNGAL as a predictor of long-term outcomes post-LT. The primary outcomes were post-LT CKD, defined as MDRD estimated glomerular filtration rate (GFR)<60 mL/min/1.73m2 for 3 continuous months, and death.
Results
uNGAL at 24-hours post-reperfusion was significantly higher among patients who developed CKD. Multivariable modeling for the development of CKD demonstrated that uNGAL at 24-hours post-reperfusion, 24h post--LT renal function, initial calcineurin inhibitor and age were independent predictors of the development of CKD at in this cohort with long term follow up post-LT. Further, this association was stronger in those with preserved pre-LT renal function, a population where renal outcomes are often difficult to predict.
Conclusions
We propose that perioperative uNGAL may identify patients at risk for CKD and allow for targeted early implementation of renal-sparing strategies.
Introduction
Chronic kidney disease (CKD) is a frequent complication of liver transplantation (LT), affecting the majority of patients who survive beyond the first 6 months1. CKD post-LT is associated with significant morbidity and mortality, including increased risk of cardiovascular events2, allograft dysfunction2, and death3. The impact of CKD on overall survival is not limited to those with end stage renal disease (ESRD), as even a decrease of 30% of the estimated glomerular filtration rate (eGFR) between 3 and 12 months after LT has been associated with significantly higher mortality rates4. Identifying patients at risk for CKD is critical in order to implement renal-sparing strategies early post-LT, as well as more intensive cardiovascular and liver allograft monitoring in order to improve outcomes.
To date, there is no reliable method to predict post-LT renal outcomes, especially in patients with preserved renal function at the time of LT. A number of studies have identified risk factors including pre-operative creatinine5, diabetes mellitus6, coronary artery disease6, primary graft non-function6, and hepatitis C (HCV) infection7. Further, it has been shown that acute kidney injury (AKI) immediately8,9 and 3 months post-LT10 predict long-term renal outcomes. However, serum creatinine and creatinine-based equations for estimating glomerular filtration rate before and after LT are imprecise, especially in the setting of end stage liver disease or when assessing dynamic renal function in the perioperative setting11. Novel, more sensitive approaches to identify AKI in the perioperative setting that predict the development of post-LT CKD are needed.
Urinary neutrophil gelatinase-associated lipocalin (uNGAL), a protein expressed by injured kidney tubular epithelia12,13, identifies AKI in a number of clinical scenarios, including in patients with cirrhosis and post-LT14–17. In addition, pre-LT NGAL serum levels may be predictive of renal function in the 3 months following LT18. However, it remains unknown how NGAL-associated perioperative AKI impacts long-term outcomes including the development of CKD and mortality.
Materials and Methods
Study Subjects
Adult patients undergoing LT between August 2008 and January 2010 at Columbia University Medical Center were eligible for enrollment in a previously published prospective cohort study investigating the ability of uNGAL to predict perioperative AKI15. Patients with pre-operative renal failure requiring renal replacement therapy were excluded. In the present analysis, this cohort was retrospectively assessed for long-term renal and clinical outcomes up to 5 years post-LT. This protocol was approved by the Columbia University Institutional Review Board.
Sample collection and processing
In the previously described prospective cohort study15, urine was collected at the following time points: after induction of anesthesia prior to incision, immediately after portal reperfusion of the liver graft and then 3, 18, and 24 hours post-reperfusion. The urine was immediately spun at 2000 g and the supernatant was frozen at −80°C. uNGAL was determined using a commercially available ELISA (Antibodyshop, Gentofte, Denmark). The limit of detection for this assay is between 0.5 and 4.0 pg/mL and intra-assay variation in the urine is 2.1% (range1.3–4.0).
Immunosuppression
Standard immunosuppression consisted of a calcineurin inhibitor (CNI), combined with mycophenolate and corticosteroids given as an initial bolus and then taper. Patients with significant perioperative renal insufficiency or other contraindications to CNI received basiliximab induction (n= 27) followed by delayed CNI initiation. In addition, some patients were converted to a mammalian target of rapamycin (mTOR) inhibitor (everolimus=3 or sirolimus=10) during the study period based on clinician judgment. This occurred after the development of CKD in most (76.9%) cases.
Statistics
The primary outcome was the development of post-LT CKD, defined as Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate (GFR)<60 mL/min/1.73m2 for 3 continuous months19. Laboratory measures of renal function were collected at listing, immediately prior to LT, and then monthly until death or at a minimum of 4 years post-LT. The secondary outcome was post-LT mortality. AKI was defined by the Acute Kidney Injury Network criteria20 and by RIFLE (acronym indicating Risk of renal dysfunction; Injury to the kidney; Failure of kidney function, loss of kidney function and End-stage kidney disease) criteria21. Urine output was not captured in this study and was not included in the classification of AKI.
Continuous variables were compared between groups by t-test or rank-sum. Categorical variables were compared between groups by chi-square. Receiver operating characteristic (ROC) curves were generated to determine uNGAL cutoffs that maximized sensitivity and specificity. Uni- and multivariable Cox proportional-hazards models were used to evaluate relationships between perioperative uNGAL levels and time to CKD and death with a backwards elimination technique. A competing risk model was not completed for death, because each patient developed chronic kidney disease before death. Variables significant with p<0.2 in univariable analysis were considered for inclusion in multivariable models, and those not reaching significance of p<0.05 were sequentially eliminated. Post-LT CKD was treated as a time-varying covariate when included in models to predict post-LT mortality. Kaplan-Meier curves were generated and log-rank tests were utilized to determine significance. A p<0.05 was defined as significant. All analyses were performed with Stata 13.0 (College Station, TX).
Results
Patient Characteristics
Ninety-two patients were enrolled in this study. The mean age was 54.4 years, 65% were male, 59% were Caucasian, and 52% had HCV (Table 1).
Table 1.
Patient characteristics*
| All Patients (n=92) |
CKD (n=63) | Non-CKD (n=29) |
P-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 54.4 (11.5) | 56.9 (8.9) | 54.4 (11.5) | <0.01 |
|
| ||||
| Male (%) | 60 (65.2) | 39 (61.9) | 21 (72.4) | 0.33 |
|
| ||||
| BMI (Kg/m2) | 27.7 (6.0) | 28.7 (6.4) | 25.5 (4.4) | 0.02 |
|
| ||||
| BMI (Kg/m2) Category (%) | ||||
| < 25 | 32 (34.7) | 16 (25.4) | 16 (55.2) | |
| 25–30 | 35 (38.0) | 27 (42.9) | 8 (27.6) | |
| 30–35 | 13 (14.1) | 9 (14.3) | 4 (13.8) | 0.03 |
| 35–40 | 7 (7.6) | 6 (9.5) | 1 (3.4) | |
| >40 | 5 (5.4) | 5 (7.9) | 0 (0.0) | |
|
| ||||
| Diagnosis (%) | ||||
| Hepatitis C | 48 (52.2) | 42 (66.7) | 6 (20.7) | <0.01 |
| Hepatitis B | 6 (6.5)** | 4 (6.3) | 2 (6.9) | 0.92 |
| Alcohol | 11 (12.0) | 6 (9.5) | 5 (17.2) | 0.29 |
| Other | 28 (34.8) | 12 (19.0) | 16 (55.2) | <0.01 |
|
| ||||
| Race (%) | ||||
| Caucasian | 54 (58.7) | 37 (58.7) | 17 (82.8) | |
| Hispanic | 19 (20.7) | 12 (19.0) | 7 (24.1) | 0.73 |
| African American | 11 (12.0) | 9 (14.3) | 2 (6.9) | |
| Asian | 8 (8.7) | 5 (7.9) | 3 (10.3) | |
|
| ||||
| HCC (%) | 37 (40.2) | 31 (49.2) | 6 (20.7) | 0.01 |
|
| ||||
| Pre-LT MDRD eGFR (mL/min/1.73m2) (Median) | 93.4 | 83.4 | 109.4 | <0.01 |
|
| ||||
| Pre-LT MDRD eGFR <60 mL/min/1.73m2 (%) | 20 (21.7) | 17 (27.0) | 3 (10.3) | 0.07 |
|
| ||||
| DM (%) | ||||
| Before LT | 25 (27.2) | 19 (30.2) | 6 (20.7) | 0.34 |
| After LT | 32 (34.8) | 30 (47.6) | 11 (37.9) | 0.39 |
|
| ||||
| Hypertension (%) | ||||
| Before LT | 36 (39.1) | 29 (46.0) | 7 (24.1) | 0.046 |
| After LT | 60 (65.2) | 42 (66.7) | 18 (62.1) | 0.67 |
|
| ||||
| LDLT (%) | 13 (14.1) | 5 (7.9) | 8 (27.6) | 0.01 |
|
| ||||
| Calculated MELD | 16.1 (9.2) | 15.9 (9.8) | 16.5 (8.0) | 0.77 |
|
| ||||
| Donor Age (years) | 45.8 (18.4) | 46.2 (17.6) | 44.8 (20.4) | 0.72 |
|
| ||||
| Cold Ischemic Time (hours) | 7.0 (5.1) | 7.3 (3.5) | 6.3 (7.5) | 0.40 |
|
| ||||
| Length of surgery (hours) | 8.5 (2.3) | 8.4 (2.2) | 8.8 (2.4) | 0.45 |
|
| ||||
| Estimated operative blood loss (liters) | 3.3 (3.7) | 3.5 (4.1) | 2.9 (2.6) | 0.52 |
H=hours, BMI=Body Mass Index, HCC=Hepatocellular Carcinoma, eGFR=Estimated GFR, DM=Diabetes Mellitus, LDLT=Living Donor Liver Transplant, LOS= Length of Surgery, EBL= Estimated Blood Loss
Unless otherwise indicated, continuous variables are expressed as mean (SD) and were compared by t-test.
One Patient with both HBV and HCV
Sixty-three (69%) patients developed stage 3 CKD post-LT at a median (range) of 180 (90 – 1080) days post-LT. Patient characteristics for those with and without CKD were generally similar between these groups. However, significant differences included a lower pre-operative MDRD eGFR, older age, higher rates of HCV infection and higher BMI among patients who developed CKD compared to those who did not (Table 1). Patients who developed CKD were more likely to have cyclosporine as their CNI (p<0.001), though this may be due to high rates of cyclosporine use in patients with HCV in this period at our center. The median follow up time was longer for those without CKD compared to patients with CKD (4.6 v. 5.1 years, respectively, p=0.01), due to increased survival time in this group.
Eleven patients experienced CKD beyond stage 3: seven patients reached stage 4 CKD (defined as MDRD eGFR < 30 mL/min/1.73m2) and 4 patients progressed to stage 5 CKD (defined as MDRD eGFR<15 mL/min/1.73m2 or renal replacement therapy). Of those with stage 5 CKD, 3 patients required chronic renal replacement therapy.
Immunosuppression
Immunosuppressive regimens are summarized in Table 2. Tacrolimus was used as the initial CNI in 56% of patients. CNI trough levels were compared between the 2 groups at 1 month, 6 months, 12 months, 2 years, 3 years, and 4 years. There was no statistical difference between the 2 groups for either cyclosporine or tacrolimus trough levels between patients with and without CKD (Table 2). Twenty-five patients (27%) with significant post-operative AKI received two-doses of basiliximab followed by delayed CNI initiation. 13 (14%) patients received an mTOR inhibitor (everolimus or sirolimus) at any time in the first 4 years post-LT as part of a renal protective strategy. Of these 13 patients, 10 developed stage 3 CKD prior to their initiation. Forty-nine patients remained on dual therapy to minimize CNI at last follow up.
Table 2.
Post-LT Immunosuppression Data
| CKD (n=63) |
Non-CKD (n=29) |
P | |
|---|---|---|---|
|
| |||
| Median Tacrolimus (ng/mL) Trough Post-LT (IQR) | |||
| 1 Month | 7.3 (6.3–9.5) | 8.8 (7.1–10.3) | 0.15 |
| 3 Months | 7.7 (6.1–10.3) | 8.2 (6.3–9.9) | 0.55 |
| 1 Year | 6.7 (4.7–7.6) | 6.6 (5.7–7.1) | 0.51 |
| 2 Years | 5.9 (4.5–7.2) | 6.5 (5.1–7.4) | 0.78 |
| 3 Years | 4.5 (3.6–7.6) | 5.6 (4.7–6.7) | 0.43 |
| 4 Years | 5.0 (4.5–6.5) | 5.2 (4.1–6.3) | 0.66 |
|
| |||
| Median Cyclosporine (ng/mL) Trough Post-LT (IQR) | |||
| 1 Month | 282 (215–373) | 243 (236–249) | 0.93 |
| 3 Months | 242 (184–305) | 259 (255–263) | 0.48 |
| 1 Year | 189 (152–241) | 421 (133–708) | 0.71 |
| 2 Years | 108 (86–138) | 97 (68–125) | 0.48 |
| 3 Years | 100 (66–171) | 48* | 0.18 |
| 4 Years | 94.5 (56–125) | 47* | 0.19 |
|
| |||
| Tacrolimus as initial CNI (%) | 26 (41.3) | 26 (89.7) | <0.01 |
|
| |||
| CNI Conversion (%) | 9 (14.3) | 1 (3.4) | 0.12 |
|
| |||
| mTOR Conversion (%) | 10 (15.9) | 3 (10.3) | 0.48 |
|
| |||
| Dual Therapy at Last Follow Up (%) | 33 (52.4) | 16 (55.2) | 0.80 |
|
| |||
| Basiliximab and Delayed CNI (%) | 20 (31.7) | 5 (17.2) | 0.15 |
CNI= Calcineurin Inhibitor
mTOR= mTOR Inhibitor
There was only a single patient without CKD that remained on cyclosporine
Biomarkers of Peri-LT Renal Function
Median uNGAL levels pre-LT, immediately post-LT, 3 hours post-LT, 18 hours post-LT and 24 hours post-LT were compared between those who did and did not develop stage 3 CKD post-LT (Table 3). Overall, peak uNGAL levels were seen at 3 hours post-reperfusion; however, prolonged uNGAL level elevation at 24 hr post-reperfusion (uNGAL-24h) had the strongest association with long-term progression to stage 3 CKD (35.3 ng/mL vs. 18.0 ng/mL, p=0.04).
Table 3.
Perioperative kidney indices in patients who did and did not develop CKD
| Pre-LT eGFR < 60 (mL/min/1.73m2) | Pre-LT eGFR > 60 (mL/min/1.73m2) | P-value | |
|
| |||
| Median uNGAL (ng/mL) (IQR) | |||
| Pre-LT | 21.5 (13.7 – 57.1) | 7.9 (3.2 – 17.8) | <0.01 |
| Post-LT | 26.3 (13.2 – 90.0) | 21.5 (6.3 – 55.8) | 0.41 |
| 3 Hours Post | 44.4 (23.1 – 116.7) | 24.7 (6.2 – 93.5) | 0.21 |
| 18 Hours Post | 56.0 (13.1 – 147.5) | 28.1 (12.6 – 62.9) | 0.06 |
| 24 Hours Post | 48.8 (11.5 – 142.5) | 28.3 (11.6 – 56.3) | 0.23 |
|
| |||
| CKD (n=63) | Non-CKD (n=29) | P-value | |
|
| |||
| Median uNGAL (ng/mL) (IQR) | |||
| Pre-LT | 11.7 (4.6 – 23.4) | 8.1 (3.4 – 14.8) | 0.31 |
| Post-LT | 23.0 (7.1 – 50.0) | 21.7 (6.6 – 99.0) | 0.79 |
| 3 Hours Post | 37.9 (10.2 – 115.4) | 21.3 (5.4 – 104.7) | 0.38 |
| 18 Hours Post | 28.1 (11.5 – 56.0) | 36.3 (16.6 – 84.3) | 0.09 |
| 24 Hours Post | 35.3 (14.0 – 113.0) | 18.0 (11.5 – 38.0) | 0.04 |
|
| |||
| Median MDRD eGFR (mL/min/1.73m2) (IQR) | |||
| Pre-LT | 83.4 (59.1 – 107.9) | 109.4 (94.0 – 125.1) | <0.01 |
| Post-LT | 72.5 (53.8 – 91.4) | 93.2 (77.2 – 120.5) | <0.01 |
| 24 Hours Post | 59.8 (40.3 – 80.7) | 90.8 (71.4 – 130.9) | <0.01 |
|
| |||
| AKI by AKIN Criteria (%) | 30 (47.6) | 8 (27.6) | 0.07 |
|
| |||
| AKI by RIFLE-R (%) | 26 (41.3) | 11 (37.9) | 0.76 |
|
| |||
| AKI by RIFLE-I (%) | 10 (15.9) | 4 (13.8) | 0.80 |
In ROC analysis, the AUC for uNGAL-24h for the prediction of CKD at 4 years post-LT was 0.65 (0.52 – 0.78). The AUCs for CKD at 4 years post-LT for MDRD eGFR pre-LT, post-LT and 24h post-LT were 0.72 (0.58 – 0.85), 0.73 (0.59 – 0.86), and 0.75 (0.62 – 0.88), respectively. These were not significantly different from the AUC of uNGAL-24h (p=0.48, p=0.42, p=0.26, respectively). There uNGAL-24h > 93 ng/mL was highly specific for the development of CKD with a positive predictive value (PPV) of 100%. uNGAL-24h > 93 ng/mL significantly predicted differences in mean eGFR by 6 months post-LT (Figure 1), as well as the development of CKD in Kaplan-Meier survival analysis (p<0.01, Figure 2).
Figure 1.
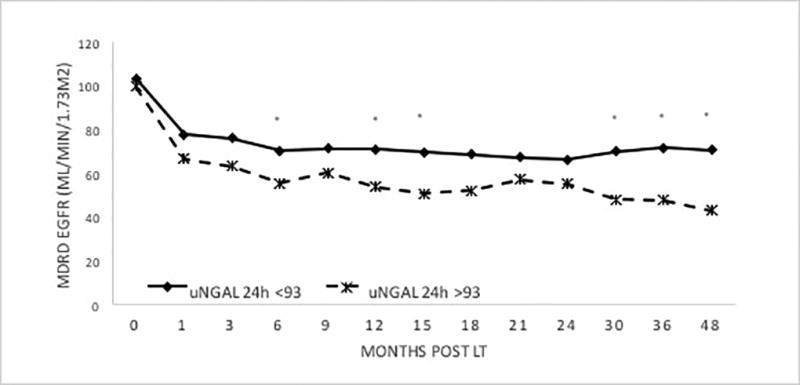
GFR Trend over time based on uNGAL 24h Levels
Asterisk = p<0.05, uNGAL24h-PR= uNGAL level 24 hours post-reperfusion
Figure 2.
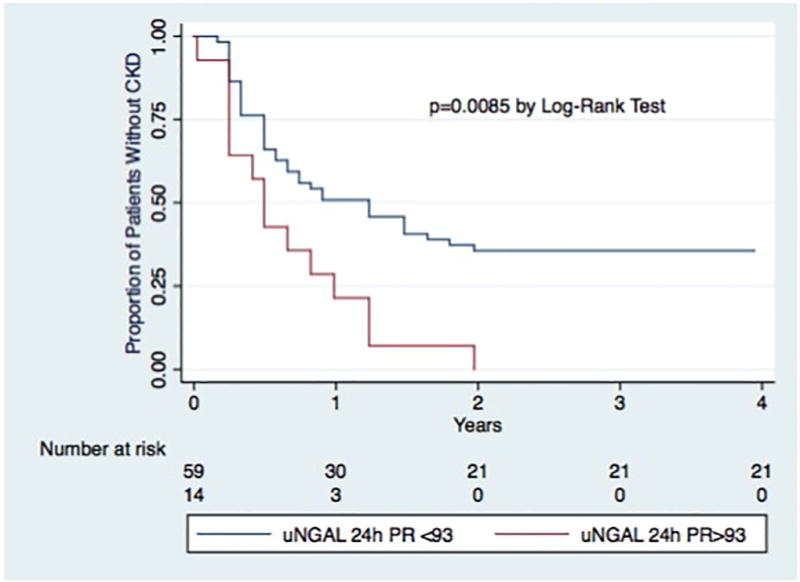
Kaplan-Meier Curve for time to CKD by uNGAL 24h Levels
uNGAL24h-PR= uNGAL level 24 hours post-reperfusion
Perioperative GFR was significantly lower in the group that developed CKD at all time points reviewed (pre-LT, post-LT, and 24h post-LT). Conversely, there was no statistically significant difference in the frequency of post-operative AKI as defined by AKIN, RIFLE-R, or RIFLE-I between those who did and did not develop CKD (Table 3).
Models to Predict the Development of CKD
In univariable analysis, uNGAL-24h (HR 1.10 per 100 ng/mL, p<0.01), pre-LT MDRD eGFR (HR 0.89 per 10 mL/min/1.73m2, p<0.01), post-LT MDRD eGFR (HR 0.87 per 10 mL/min/1.73m2, p<0.01), 24h-Post-LT MDRD eGFR (HR 0.85 per 10 10 mL/min/1.73m2, p<0.01), age (HR 1.43 per decade, p=0.01), BMI (HR 1.05, p=0.01), HCV infection (HR 3.04, p<0.01), HCC (HR 2.17, p<0.01), pre-LT HTN (HR 1.72, p=0.03), and cyclosporine as initial CNI (HR 3.14, p<0.01) were associated with the development of CKD (Table 4).
Table 4.
Cox proportional hazards model to predict the development of stage 3 CKD
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
|
| ||||||
| Age per decade | 1.43 | 1.10 – 1.85 | 0.01 | 1.44 | 1.02 – 2.04 | 0.04 |
|
| ||||||
| Male Gender | 0.93 | 0.56 – 1.54 | 0.77 | |||
|
| ||||||
| BMI (Kg/m2) | 1.05 | 1.01 – 1.09 | 0.01 | |||
|
| ||||||
| HCV | 2.72 | 1.62 – 4.58 | <0.01 | |||
|
| ||||||
| HCC | 2.17 | 1.32 – 3.58 | <0.01 | |||
|
| ||||||
| DM | ||||||
| Before LT | 1.69 | 0.68 – 2.00 | 0.57 | |||
| After LT | 1.16 | 0.71 – 1.90 | 0.56 | |||
|
| ||||||
| Hypertension | ||||||
| Before LT | 1.72 | 1.05 – 2.83 | 0.03 | |||
| After LT | 1.16 | 0.69 – 1.96 | 0.58 | |||
|
| ||||||
| LDLT | 0.40 | 0.16 – 1.00 | 0.05 | |||
|
| ||||||
| Calculated MELD | 1.00 | 0.97 – 1.03 | 0.97 | |||
|
| ||||||
| Cyclosporine as initial CNI | 3.14 | 1.87 – 5.25 | <0.001 | 2.07 | 1.17 – 3.69 | 0.01 |
|
| ||||||
| AKI by AKIN Criteria | 1.56 | 0.95 – 2.56 | 0.08 | |||
|
| ||||||
| Pre-LT MDRD eGFR (per 10 mL/min/1.73m2) | 0.89 | 0.83 – 0.96 | 0.002 | |||
|
| ||||||
| Post-LT MDRD eGFR (per 10 mL/min/1.73m2) | 0.87 | 0.80 – 0.94 | <0.01 | |||
|
| ||||||
| 24h Post-LT MDRD eGFR (per 10 mL/min/1.72m2) | 0.85 | 0.79 – 0.92 | <0.01 | 0.89 | 0.80 – 0.98 | 0.01 |
|
| ||||||
| uNGAL at 24h PR (per 100 ng/ml) | 1.10 | 1.03 – 1.19 | <0.01 | 1.09 | 1.01 – 1.18 | 0.04 |
In multivariable models only uNGAL-24h (HR 1.09 per 100 ng/mL, p=0.04), 24h post-LT MDRD eGFR (HR 0.89 per 10 mL/min/1.73m2, p=0.01), cyclosporine as initial CNI (2.07, p=0.01), and age per year (1.04, p=0.04) remained significant predictors of CKD (Table 4). This model was highly predictive for the development of CKD with an AUC of 0.84 (0.74 – 0.95) at 4 years post-LT. There was a strong correlation between pre-LT MDRD eGFR and post-LT MDRD eGFR (r=0.77, p<0.001) and 24h post-LT (r=0.67, p<0.001). It is likely because of this correlation, that the addition of pre-LT MDRD eGFR or post-LT MDRD eGFR did not improve the accuracy of the multivariable model.
Models to Predict the Development of CKD Among Subjects with Normal Pre-LT Renal Function
50 patients with preserved renal function at LT were then evaluated in a subgroup analysis. Preserved pre-LT renal function was defined as pre-operative GFR > 90 mL/min/1.73m2. In this subgroup, 28 patients (56%) developed post-LT CKD. Median NGAL levels at 24h PR were statistically higher in the group that developed CKD (35.3 ng/mL v. 16.0 ng/mL, p=0.02). Additionally, in univariable modeling an uNGAL level > 93 ng/mL was significantly associated with the development of CKD (HR 4.39, p=0.01). Similarly, a multivariable model was completed in this subgroup that demonstrated uNGAL-24h (HR 1.37 per 100 ng/mL, p<0.01), HCV (HR 4.75, p=0.04), and age (HR 2.24 per decade, p=0.02) were all significant independent predictors of CKD. These findings are supported by Kaplan-Meier Survival analysis that demonstrates that uNGAL-24h>93 ng/mL is associated with increased rates of CKD (p<0.01). Conversely, pre-LT GFR was not significantly associated with the development of CKD in this population (HR 0.98, p=0.11).
Biomarkers of Peri-LT Renal Function to Predict Post-LT Mortality
Twenty-two patients (23.9%) died in follow up with a median time to death of 1.4 years post-LT. All patients who died in this study developed CKD prior to their death (p<0.01). The causes of death included sepsis (5), HCV recurrence (3), malignancy other than HCC (3), HCC recurrence (2), cardiovascular disease (1) and other (8). There was no statistically significant difference in median peri-operative uNGAL levels between the groups.
In ROC analysis, the AUC for uNGAL-24h for the prediction of death at 4 years post-LT was 0.62 (0.43 – 0.76). uNGAL-24h > 30 ng/mL significantly predicted death in Kaplan-Meier survival analysis (p=0.04, Figure 3).
Figure 3.
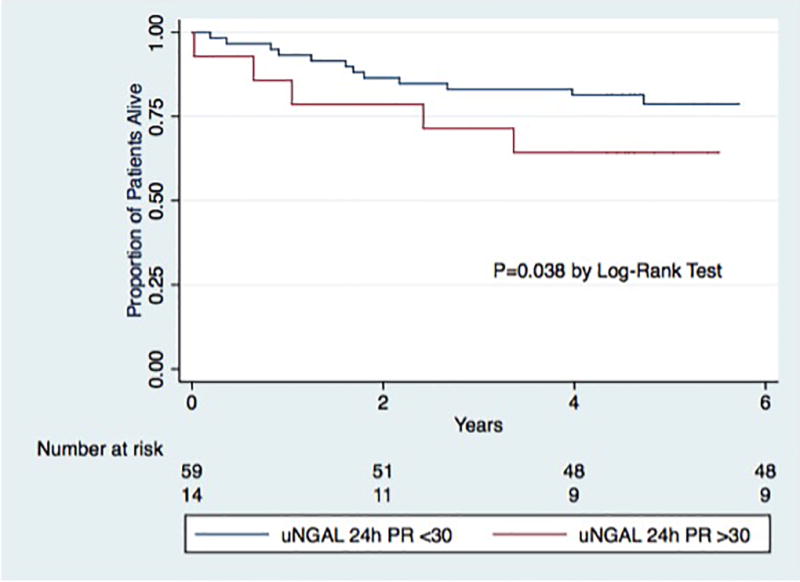
Kaplan-Meier Curve for time to Death by uNGAL 24h Levels
uNGAL24h-PR= uNGAL level 24 hours post-reperfusion
MDRD eGFR pre-LT, post-LT, or 24h post-LT was not statistically different in patients who did and did not survive at a minimum of 4 years of follow up (p=0.55, p=0.83 and p=0.13, respectively). There was no statistically significant difference in the frequency of post-operative AKI as defined by AKIN, RIFLE-R, or RIFLE-I (p=0.35, p=0.12 and p=0.82, respectively) between these 2 groups.
Multivariable Models to Predict Post-LT Mortality
The overall estimated 4-year survival was 79% (95% confidence interval 70 – 86%). In univariable analysis, age at LT (HR 1.78 per decade, p=0.03), BMI (HR 1.07, p=0.03), HCV (HR 5.08, p<0.01), HCC (HR 2.45, p=0.04), DM after LT (HR 3.00, p=0.02), and HTN before LT (HR 2.41, p=0.04) were predictive of post-LT mortality (Table 5). Cox regression was used to estimate the effect of a post-LT CKD on mortality. Time dependent post-LT CKD was highly predictive of post-LT mortality (HR 36.83, p<0.01). In the final multivariable model, DM post-LT (HR 2.97, p=0.02) and time dependent post-LT CKD (HR 30.25, p=<0.01) remained significantly predictive of mortality. uNGAL-24h (HR 1.01 per 100 ng/mL, p=0.85) was not significant in either univariable or multivariable analysis.
Table 5.
Cox proportional hazards model to predict Death
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
|
| ||||||
| Age per decade | 1.78 | 1.06 – 2.97 | 0.03 | |||
|
| ||||||
| Male Gender | 1.24 | 0.51 – 3.05 | 0.64 | |||
|
| ||||||
| BMI (Kg/m2) | 1.07 | 1.01 – 1.13 | 0.03 | |||
|
| ||||||
| Diagnosis | ||||||
| Hepatitis C | 5.08 | 1.72 – 15.02 | <0.01 | |||
| Hepatitis B | 0.66 | 0.09 – 4.94 | 0.69 | |||
| Alcohol | 0.31 | 0.04 – 2.33 | 0.26 | |||
| Other | 0.19 | 0.04 – 0.82 | 0.03 | |||
|
| ||||||
| Time dependent post-LT CKD | 36.83 | 4.86 – 267.15 | <0.01 | 30.25 | 4.73–193.48 | <0.01 |
|
| ||||||
| DM | ||||||
| Before LT | 2.000 | 0.85 – 4.69 | 0.11 | |||
| After LT | 2.995 | 1.22 – 7.35 | 0.02 | 2.97 | 1.20–7.31 | 0.02 |
|
| ||||||
| Hypertension | ||||||
| Before LT | 2.407 | 1.03 – 5.63 | 0.04 | |||
| After LT | 1.863 | 0.69 – 5.05 | 0.22 | |||
|
| ||||||
| LDLT | 0.600 | 0.14 – 2.57 | 0.49 | |||
|
| ||||||
| HCC | 2.45 | 1.05 – 5.74 | 0.04 | |||
|
| ||||||
| Calculated MELD | 1.000 | 0.96 – 1.05 | 1.00 | |||
|
| ||||||
| Cyclosporine as initial CNI (%) | 5.536 | 2.04 – 15.02 | <0.01 | |||
|
| ||||||
| Cold Ischemic Time (per hour) | 1.09 | 0.99 – 1.10 | 0.13 | |||
|
| ||||||
| Pre-LT MDRD eGFR (per 10 mL/min/1.73m2) | 0.97 | 0.87 – 1.08 | 0.59 | |||
|
| ||||||
| uNGAL at 24h PR (per 100 ng/ml) | 1.01 | 0.88—1.17 | 0.85 | |||
Discussion
AKI is a common and significant complication of LT22–24. Urine NGAL is an established predictor of early post-LT AKI, both in the immediately post-operative setting and at 3 months15,18. However, to date no study has followed patients beyond 3 months or studied serial NGAL measurements to determine the optimal time to measure NGAL for predictive models. In this study, we expand upon these prior observations and demonstrate the importance of prolonged urine NGAL elevation up to 24 hours post-operatively, as a marker of long-term CKD risk, especially in patients with preserved kidney function at the time of LT.
The majority of LT recipients in this cohort (69%) developed stage 3 CKD by up to 5 years post-LT, a number similar to rates previously published1. Despite this high rate of CKD, a minority of patients had significant AKI or CKD prior to transplant, as noted by median pre-LT MDRD eGFR of 83.4 and 109.4, for those who did and did not develop CKD, respectively. By excluding pre-LT AKI, uNGAL associated post-LT AKI in this study likely represents a combination of intra-operative renal injury, post-operative complications, and medication side effects.
Post-LT CKD is a strong predictor of clinical outcomes including mortality2 and thus the identification of patients at risk for CKD is a priority. To date, a number of studies have identified predictors of post-LT CKD, including pre-LT eGFR, post-LT HTN, calcineurin inhibitor-based immunosuppression, age, HCV status, and immediately post-LT eGFR5,7. However, it is clear that better biomarkers of CKD risk are urgently needed to monitor patients in real time. Both creatinine and eGFR are known to be inaccurate in the setting of cirrhosis as well as with potentially dynamic kidney function in the early post-LT period. In addition, serum creatinine levels lag behind hemodynamic changes in renal function25. A number of biomarkers have been investigated in AKI in patients with cirrhosis, including NGAL, interleukin-18, kidney injury molecule-1, liver-type fatty acid binding protein and albumin26. However, there is little data investigating these biomarkers in predicting long-term outcomes after LT. NGAL was investigated in this study because it increases rapidly after and in proportion to the degree of renal injury, and may thus be ideal for the dynamic period immediately post-LT27.
uNGAL-24h and perioperative MDRD eGFR were the only perioperative variables in this cohort that could significantly differentiate between those who did and did not develop. Further, once a specific uNGAL-24h threshold was reached it could accurately predict the development of CKD. We hypothesize that uNGAL at the 24-hour time point was significant because it demonstrates the presence of persistent renal injury in the perioperative LT setting. Based on multivariable modeling, it appears that uNGAL-24h can predict the development of CKD independent of baseline renal function; again, highlighting that uNGAL-24h may serve as a marker of perioperative injury, rather than pre-existing injury. Additionally, when those patients with preserved pre-LT renal function (MDRD eGFR > 90 mL/min/1.73m2) were analyzed uNGAL-24h levels were highly associated with the development of CKD (HR 4.39, p=0.01). This is a critical finding, as renal dysfunction in patients with preserved pre-LT renal function is difficult to predict, and uNGAL may serve as a key biomarker in this population.
It is clear from prior studies that the development of CKD is associated with increased mortality after LT28. This cohort demonstrated a clear association between the development of CKD and mortality that was independent of other risk factors. Every patient that died during follow up developed CKD prior to his or her death. Lastly, it appears that an elevated uNGAL-24h level is associated with a decreased survival; however, this association was not independent based on Cox Hazard analyses and should be validated in a larger cohort.
There are limitations of this study. Primarily, the relatively small number of patients from a single-center may impact the generalizability of these findings as well as the statistical power to identify independent predictors of clinical outcomes. However, the strong consistent effect size and predictive value even in those with normal renal function support the biologic effect. In addition, creatinine based calculations are known to be inaccurate in cirrhosis, and future studies should consider inulin clearance or cystatin C to better calculate GFR29. The peri-operative setting is a dynamic time, making it difficult to control for all possibly confounding variables. The dataset is missing some variables that are associated with the development of CKD, namely proteinuria. It is unknown if uNGAL will outperform this metric; we hypothesize given its kinetics uNGAL will better capture transient renal injury than proteinuria, but given the missing data this remains hypthetical. Lastly, this study is retrospective and ideally would be repeated in a prospective setting.
In conclusion, uNGAL measured 24 hours post-LT appears to be an independent marker of CKD risk in the perioperative LT setting, especially in patients with preserved eGFR at the time of transplant. By accurately identifying perioperative LT renal injury, uNGAL could serve to identify patients at higher risk for CKD and trigger preemptive modifications in immunosuppressive regimen to limit nephrotoxicity or other renal sparing strategies.
Acknowledgments
Funding
This work was funded by the intramural grant support from the Department of Anesthesiology, Columbia University College of Physicians and Surgeons, New York, NY, USA. The project was supported by Grant Number (UL1 RR024156) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website.
Abbreviations
- uNGAL
urinary neutrophil gelatinase-associated lipocalin
- CKD
chronic kidney disease
- LT
liver transplantation
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- HCV
hepatitis C
- AKI
acute kidney injury
- HBV
hepatitis B
- DM
diabetes mellitus
- HTN
hypertension
- CNI
calcineurin inhibitor
Footnotes
Authors Contribution:
Giuseppe Cullaro: Data Acquisition, Statistical Analysis, Interpretation of data, Preparation of Manuscript, including final approval for publication; No conflict of interests.
Joseph F. Pisa: Data Acquisition, Interpretation of data, Preparation of Manuscript, including final approval for publication; No conflict of interests.
Robert S. Brown, Jr.: Interpretation of data, Preparation of Manuscript, including final approval for publication; No conflict of interests.
Gebhard Wagener: Data Acquisition, Statistical Analysis, Interpretation of data, Preparation of Manuscript, including final approval for publication; No conflict of interests.
Elizabeth C. Verna: Data Acquisition, Statistical Analysis, Interpretation of data, Preparation of Manuscript, including final approval for publication; No conflict of interests.
Disclosures:
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Elizabeth C. Verna – Advisory Committees or Review Panels: Gilead; Grant/Research Support: Salix, Merck. Robert S. Brown – Advisory Committees or Review Panels: Vital Therapies; Consulting: BMS, Gilead, Merck, Abbvie, Janssen; Grant/Research Support: Gilead, Merck, Vertex, Abbvie, Salix, Janssen, BMS, Vital Therapies. The Following people have nothing to disclose: Giuseppe Cullaro, Joseph F. Pisa, and Gebhard Wagener.
References
- 1.Charlton MR, Wall WJ, Ojo AO, et al. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15(11):S1–S34. doi: 10.1002/lt.21877. [DOI] [PubMed] [Google Scholar]
- 2.Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15(Suppl 2(S2)):S70–S74. doi: 10.1002/lt.21900. [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Held PJ, Port FK, et al. Chronic Renal Failure after Transplantation of a Nonrenal Organ. 2009;349(10):931–940. doi: 10.1056/NEJMoa021744. http://dxdoiorg/101056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 4.Cantarovich M, Tchervenkov J, Paraskevas S, et al. Early changes in kidney function predict long-term chronic kidney disease and mortality in patients after liver transplantation. Transplantation. 2011;92(12):1358–1363. doi: 10.1097/TP.0b013e3182384aff. [DOI] [PubMed] [Google Scholar]
- 5.Giusto M, Berenguer M, Merkel C, et al. Chronic kidney disease after liver transplantation: pretransplantation risk factors and predictors during follow-up. Transplantation. 2013;95(9):1148–1153. doi: 10.1097/TP.0b013e3182884890. [DOI] [PubMed] [Google Scholar]
- 6.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9(7):741–747. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 7.Burra P, Senzolo M, Masier A, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41(5):350–356. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Velidedeoglu E, Bloom RD, Crawford MD, et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77(4):553–556. doi: 10.1097/01.tp.0000114609.99558.41. [DOI] [PubMed] [Google Scholar]
- 9.Barri YM, Sanchez EQ, Jennings LW, et al. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009;15(5):475–483. doi: 10.1002/lt.21682. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez EQ, Melton LB, Chinnakotla S, et al. Predicting Renal Failure After Liver Transplantation From Measured Glomerular Filtration Rate: Review of up to 15 Years of Follow-Up. Transplantation. 2010;89(2):232–235. doi: 10.1097/TP.0b013e3181c42ff9. [DOI] [PubMed] [Google Scholar]
- 11.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am J Kidney Dis. 2003;41(2):269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 12.Mishra J. Identification of Neutrophil Gelatinase-Associated Lipocalin as a Novel Early Urinary Biomarker for Ischemic Renal Injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 13.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil Gelatinase-Associated Lipocalin: A Novel Early Urinary Biomarker for Cisplatin Nephrotoxicity. Am J Nephrol. 2004;24(3):307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 14.Verna EC, Brown RS, Farrand E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57(9):2362–2370. doi: 10.1007/s10620-012-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26(5):1717–1723. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagundes C, Pépin M-N, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57(2):267–273. doi: 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Niemann CU, Walia A, Waldman J, et al. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl. 2009;15(12):1852–1860. doi: 10.1002/lt.21938. [DOI] [PubMed] [Google Scholar]
- 18.Aberg F, Lempinen M, Hollmén M, Nordin A, Mäkisalo H, Isoniemi H. Neutrophil gelatinase-associated lipocalin associated with irreversibility of pre-liver transplant kidney dysfunction. Clin Transpl. 2014;28(8):869–876. doi: 10.1111/ctr.12394. [DOI] [PubMed] [Google Scholar]
- 19.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative W. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Vol 8. BioMed Central. 2004:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ. Acute Renal Disease, as Defined by the RIFLE Criteria, Post-Liver Transplantation. Am J Transplant. 2007;7(1):168–176. doi: 10.1111/j.1600-6143.2006.01602.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Singhapricha T, Hu K-Q, et al. Postliver Transplant Acute Renal Injury and Failure by the RIFLE Criteria in Patients With Normal Pretransplant Serum Creatinine Concentrations: A Matched Study. Transplantation. 2011;91(3):348–353. doi: 10.1097/TP.0b013e31820437da. [DOI] [PubMed] [Google Scholar]
- 24.Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. In: Hemmings HC, editor. Br J Anaesth. 6. Vol. 114. 2015. pp. aeu556–aeu926. [DOI] [PubMed] [Google Scholar]
- 25.Razonable RR, Findlay JY, O'Riordan A, et al. Critical care issues in patients after liver transplantation. Liver Transpl. 2011;17(5):511–527. doi: 10.1002/lt.22291. [DOI] [PubMed] [Google Scholar]
- 26.Belcher JM, Garcia Tsao G, Sanyal AJ, et al. Association of AKI With mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57(2):753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50(9):1505–1517. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 28.Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation – A time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61(2):286–292. doi: 10.1016/j.jhep.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caregaro L, Menon F, Angeli P, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154(2):201–205. [PubMed] [Google Scholar]


