Abstract
Background
Kidney transplant (KT) recipients experience high rates of early (≤30 days) hospital readmission (EHR) after KT, and existing studies provide limited data on modifiable discharge factors that may mitigate EHR risk.
Methods
We performed a retrospective cohort study of 468 adult deceased donor KT recipients transplanted between 4/2010 and 11/2013 at five United States transplant centers. We fit multivariable mixed effects models to assess the association of two potentially modifiable discharge factors with the probability of EHR after KT: 1) weekend discharge, and 2) days to first scheduled follow-up.
Results
Among 468 KT recipients, 38% (n=178) experienced EHR after KT. In fully adjusted analyses, compared to weekday discharges, KT recipients discharged on the weekend had a 29% lower risk of EHR (adjusted odds ratio [aOR] 0.71, 95% confidence interval [CI] 0.41–0.94). Compared to follow-up within two days of discharge, KT recipients with follow-up within three to six days had a 28% higher probability of EHR (aOR 1.28, 95% CI 1.13–1.45).
Conclusions
These findings suggest that clinical decisions related to the timing of discharge and follow-up modify EHR risk after KT, independent of traditional risk factors.
Keywords: kidney, transplant, readmission, hospitalization
Introduction
Early hospital readmission (EHR) events are common following kidney transplantation (KT), a problem that has gained considerable attention given the high cost of these events, both in human health and financial terms. National studies of EHR after KT, commonly restricted in their focus to Medicare beneficiaries, have estimated that EHR rates after KT range between 18–47%,(1–3) costing Medicare approximately $10,000 per readmission.(2) Some EHR events after KT may be viewed as necessary to optimally manage the expected complications of surgery and immunosuppression, and may be cost-saving if they prevent further downstream complications,(4) while others may reflect failures in transitioning care to outpatient settings.(5, 6) This heterogeneity in the causes and consequences of EHR events may explain why there is conflicting evidence on the potential preventability of EHR after KT.(10, 12) Regardless of preventability, numerous studies have revealed that EHR after KT does signal a higher risk for poor long-term KT outcomes, including the risk of subsequent hospitalizations and mortality.(3, 7–10) Despite data that EHR rates are highly variable between transplant centers, independent of traditional risk factors,(1) little is known about the potential role of clinical decision-making as a mediator of EHR risk.
Prior studies among prevalent hemodialysis patients have identified the importance of initial hospitalization discharge factors, including weekend discharge, when predicting EHR.(13, 14) Compared to prevalent hemodialysis patients who are discharged after medical illness, KT recipients, many of whom were dialysis dependent for years before surgery, may be even more vulnerable during their initial transitions of care. These patients, with high burdens of comorbidities and functional impairments at baseline,(15–21) must recover from surgery while experiencing variable allograft function, and become accustomed to complex medication regimens with numerous side effects. Deceased donor KT (DDKT) recipients, who are more likely to have experienced long periods of dialysis exposure(22) and have fewer resources than living donor recipients,(23–26) may be particularly at risk of suboptimal transitions of care. Clinicians may exert some influence over the circumstances of this transition in their clinical decision-making. For example, they may choose to extend the patient’s initial length of stay, or select timing of discharge and follow-up based on perceived risks. However, the accuracy of clinical prediction in this setting is unknown, and may be hampered by the multifactorial reasons for EHR in this population.(10) Given the high rates of EHR after KT and the association of these events with poor long-term outcomes, studies are needed to determine the impacts of discharge decision-making on EHR risk.
Therefore, the goals of this study were to examine the association of potentially modifiable discharge decisions on the risk of EHR after KT in a multicenter cohort of DDKT recipients. We considered two discharge variables as potential predictors of EHR: 1) discharge day of the week, and 2) days to first scheduled outpatient follow-up, adjusted for traditional risk factors and variables reflective of medical condition at discharge from KT.
Materials and Methods
Study Population
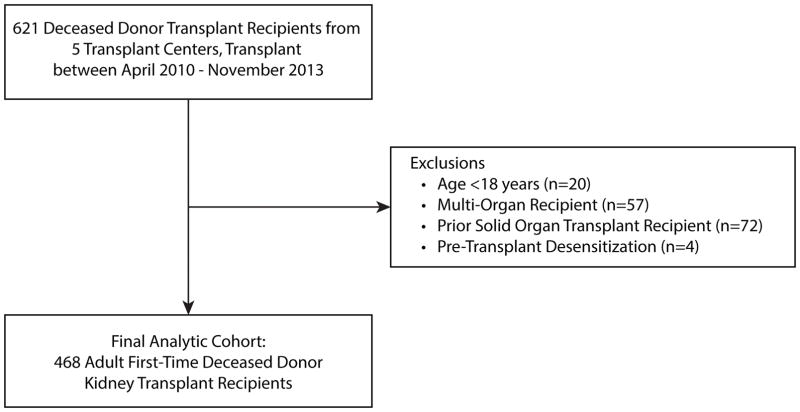
The study population was derived from the Deceased Donor Study (DDS). The DDS investigators assembled a cohort of deceased kidney donors whose surrogates consented for research, and who donated between April of 2010 and November of 2013 at five organ procurement organizations. The recipient cohort was restricted to the recipients of these kidneys at five centers: The University of Pennsylvania (Pennsylvania), Mount Sinai Hospital (New York), Harper University Hospital (Michigan), Saint Barnabas Medical Center (New Jersey), and Yale-New Haven Hospital (Connecticut; the data coordinating center). The DDS cohort does not include en bloc KT recipients, or recipients of kidneys from donors<5 years of age. For the present analysis, we included recipients who were 18 years of age or older at the time of KT. We excluded those who received a multi-organ transplant, prior solid organ transplant recipients, and those with pre-transplant desensitization (see Figure 1, Participant Inclusion Flow Diagram).
Figure 1.
Participant Inclusion Flow Diagram.
Data Sources
DDS data collection has been described in detail elsewhere.(27) Briefly, trained study coordinators at participating centers reviewed electronic medical records of recipients to ascertain data on patient demographics, comorbidities, treatments, and outcomes with verification by the principal investigator at each site and by the data coordinating center (Yale University). To ascertain discharge variables, study coordinators abstracted data from discharge summaries provided by each transplant center. Episodes of delayed graft function (DGF) were reviewed and confirmed by each study site’s lead investigator. EHR was defined as acute hospitalization within 30 days of discharge from KT, as ascertained by transplant center records. Primary and secondary reasons for EHR were ascertained by chart review. 30-day patient survival was verified by DDS study records, and by linkage of recipient and allograft transplant variables with data from the Organ Procurement and Transplantation Network (OPTN) through December 4, 2013. The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere. The Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The scientific review boards of the organ procurement organizations and the institutional review boards at each transplant hospital approved the protocols. The methods and conduct of this research study were consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Primary Exposures: Discharge Planning Decisions
We considered the discharge day of the week and the number of days to the first scheduled outpatient follow-up as primary exposures. Given the potential additive limitations on available subsequent outpatient care with Friday discharges, we categorized discharge day of the week as “weekday” if discharges occurred Monday–Thursday, “Friday,” and “weekend” if discharges occurred on Saturday or Sunday. We categorized days to first follow-up based on the cohort distribution, as ≤2 days, 3–6 days, and ≥7 days after discharge from KT.
Covariates
We considered the following potential covariates in fitting our multivariable models: 1) recipient age category at transplant, sex, race (black or non-black), body mass index (BMI, kg/m2), dialysis vintage (0, <1, ≥1 & <3, ≥3 years), history of diabetes, history of hypertension, cardiovascular disease (defined as prior myocardial infarction, coronary artery bypass grafting, coronary angioplasty, or coronary stent placement), primary insurance type (private versus public), human immunodeficiency virus (HIV) positivity, and hepatitis C seropositivity; 2) donor age, sex, race, donor type (donation after circulatory determination of death [DCDD] or not); allograft variables of primary non-function, DGF (defined as a need for dialysis therapy within one week of KT), cold ischemia time (hours), kidney donor profile index (KDPI); and variables reflecting discharge condition including length of initial transplant hospitalization (long length of stay [LOS], defined as >4 days based on cohort median LOS), discharge on warfarin, discharge on insulin, discharge with new onset diabetes after transplant (NODAT), and discharge on dialysis.
Statistical Analysis
We described categorical variables (e.g., sex, race/ethnicity) by their frequencies. We described continuous variables (e.g., age) by their mean, median, range, and standard deviation. We compared categorical variables by EHR and discharge status using the chi-square test or Fisher’s exact, as appropriate. We compared continuous variables by EHR status with Wilcoxon rank-sum tests.
Multivariable Model Building Strategy
To determine the association of discharge planning variables with the probability of EHR, we first fit a mixed effects model with a random intercept for transplant center, adjusted for recipient demographics and for traditional risk factors that were significantly associated with EHR at the 20% level on bivariate analysis (see Table 1).(28) The baseline model (Model 1) was adjusted for the following covariates: 1) recipient variables: age, race, sex, diabetes, hypertension, insurance status; 2) donor variables: age, KDPI, DGF, cold ischemia time, 3) and discharge condition variables: long transplant LOS, discharge on dialysis, discharge with new onset diabetes, and discharge on insulin. Second, we added day of the week of hospital discharge and days to follow-up in Models 2 and 3, respectively. Third, we fit a fully adjusted model (Model 4). To determine whether residual variation in the models was explained by clustering by center, we calculated intra-class correlation coefficients (ICCs) for center effects for each model. We compared our baseline and expanded models with quasi-Akaike Information Criterion (QIC), an assessment of model fit that favors parsimony (29) and also compared the models’ discriminatory abilities by calculating and comparing the c-statistics.(29–31) Also, to account for the possibility that comorbidities and discharge decisions are interrelated, we calculated variance inflation factors (VIFs) for our exposures of interest in the fully adjusted model.(32) By convention, VIFs <2 are considered to be evidence of independence between predictors.(33) All analyses were performed using SAS 9.4 statistical software for Windows (SAS Institute, Cary, NC).
Table 1.
Recipient and Donor Characteristics by Early Readmission Status.
| All (N=468) | No Early Readmission (N=290) | Early Readmission (N=178) | P* | |
|---|---|---|---|---|
| Recipient Characteristics | ||||
| Age at Transplant (Years) | 57 (48, 63.5) | 57 (48, 63) | 57.5 (48, 64) | 0.42 |
| Black Race | 223 (48%) | 126 (43%) | 97 (54%) | 0.02 |
| Male Sex | 307 (66%) | 188 (65%) | 119 (67%) | 0.65 |
| Dialysis Vintage (Years) | ||||
| 0 | 51 (11%) | 34 (12%) | 17 (10%) | 0.26 |
| <1 year | 38 (8%) | 26 (9%) | 12 (7%) | |
| >=1 and <3 years | 82 (18%) | 56 (19%) | 26 (15%) | |
| >=3 years | 297 (63%) | 174 (60%) | 123 (69%) | |
| History of Hepatitis C | 31 (7%) | 19 (7%) | 12 (7%) | 0.94 |
| History of HIV | 16 (3%) | 7 (2%) | 9 (5%) | 0.23 |
| Diabetes | 173 (37%) | 95 (33%) | 78 (44%) | 0.02 |
| Hypertension | 446 (95%) | 272 (94%) | 174 (98%) | 0.05 |
| History of myocardial infarction, coronary bypass, or angioplasty/stent | 80 (17%) | 45 (16%) | 35 (20%) | 0.29 |
| Primary Insurance Type | ||||
| Medicaid & Medicare | 328 (70%) | 193 (67%) | 135 (76%) | 0.03 |
| Private Insurance | 140 (30%) | 97 (33%) | 43 (24%) | |
| Body Mass Index (kg/m2) | 28.32 (24.53, 32.01) | 28.49 (24.81, 32.58) | 27.95 (24.21, 31.6) | 0.25 |
| Donor/Allograft Characteristics | ||||
| Age (Years) | 45.5 (29, 54) | 43 (28, 53) | 49 (32, 57) | 0.001 |
| Donor Race | ||||
| White | 325 (69%) | 210 (72%) | 115 (65%) | 0.51 |
| Black | 76 (16%) | 42 (14%) | 34 (19%) | |
| Asian | 13 (3%) | 7 (2%) | 6 (3%) | |
| Other | 2 (0%) | 1 (0%) | 1 (1%) | |
| Unknown | 52 (11%) | 30 (10%) | 22 (12%) | |
| Donor Male Sex | 286 (61%) | 179 (62%) | 107 (60%) | 0.73 |
| Kidney Donor Profile Index | 54 (31, 73) | 46.5 (26, 70) | 62 (41, 77) | <.001 |
| Delayed Graft Function | 180 (38%) | 95 (33%) | 85 (48%) | 0.001 |
| DCDD | 90 (19%) | 56 (19%) | 34 (19%) | 0.96 |
| Cold Ischemia Time (Hours) | 15.01 (11.55, 20.51) | 14.21 (11.08, 19.24) | 16.08 (12.48, 22) | 0.002 |
| Discharge Condition | ||||
| NODAT | 58 (12%) | 42 (14%) | 16 (9%) | 0.08 |
| Length of Stay > 4 Days | 206 (44%) | 113 (39%) | 93 (52%) | 0.005 |
| Discharge on Dialysis | 77 (16%) | 39 (13%) | 38 (21%) | 0.03 |
| Discharge on Insulin | 189 (42%) | 117 (42%) | 72 (43%) | 0.87 |
Abbreviations: HIV—human immunodeficiency virus; kg—kilogram; m—meters; DCDD—donation after circulatory determination of death; NODAT—new onset diabetes after transplant
comparing recipients with and without early readmission
Results presented as median (interquartile range) or n(column%)
Missing data
No more than 5% of the cohort had missing data on any covariates. Variables with missing observations included discharge on insulin (missing in 5%), discharge on heparin (missing in 5%), and cold ischemia time (missing in 0.02%). Individuals with missing data were not included in regression models for primary analyses. Data on the timing of follow-up visits were unavailable from discharge summaries among 32% of the cohort. In our primary analyses, we grouped these observations into an “unavailable from discharge summary” category. We also performed sensitivity analyses in which individuals with unavailable data on follow-up were first assigned to the “≤2 days” category, and then to the “≥7 days” category, respectively.
Results
Study Population
A total of 468 DDKT recipients were included in this analysis (see Figure 1). As shown in Table 1, the median age was 57 years (IQR 48–64 years); 48% were black, 66% were male, and 37% were diabetic. The majority of recipients (n=294, 63%) were discharged between Monday and Thursday, whereas 14% of recipients were discharged on a Friday and 23% of recipients were discharged on a weekend day. Further, 21% of recipients had scheduled follow-up within two days of discharge, 33% of recipients were scheduled for outpatient appointments within one week, and 14% were scheduled ≥7 days from discharge. Recipient and allograft characteristics, stratified by discharge day and follow-up time, respectively, are displayed in Table 2. Recipients discharged on weekends were less likely to have had a long LOS (35% vs 49%, p=0.03) or DGF (28% vs 41%, p=0.03) than those discharged on Monday–Thursday. Compared to recipients with follow-up within two days, recipients discharged with follow-up scheduled for 3–6 days were more likely to have been transplanted before starting dialysis (18% vs 6%, p=0.005) and have had white donors (76% vs 61%, p=0.002). One recipient died on the day of planned discharge, and one recipient died within 30 days of discharge; these recipients were excluded from the primary analysis.
Table 2.
Recipient and Donor Characteristics, Stratified by Discharge Timing and Scheduled Follow-Up from the Initial Transplant Admission
| Monday–Thursday N=294 |
Friday N=65 |
Weekend N=109 |
Pa | Follow Up in ≤2 days N=99 |
3–6 days N=153 |
7+ days N=67 |
Missing N=149 |
Pb | |
|---|---|---|---|---|---|---|---|---|---|
| Recipient Characteristics | |||||||||
| Age at Transplant (Years) | 58 (48, 64) | 56 (46, 63) | 54 (46, 62) | 0.23 | 54 (46, 64) | 59 (48, 64) | 57 (51, 62) | 57 (47, 62) | 0.23 |
| Black Race | 139 (47%) | 36 (55%) | 168 (47%) | 0.34 | 48 (48%) | 76 (50%) | 30 (45%) | 69 (46%) | 0.90 |
| Male Sex | 201 (68%) | 37 (57%) | 69 (63%) | 0.18 | 67 (68%) | 97 (63%) | 43 (64%) | 100 (67%) | 0.87 |
| Dialysis Vintage (Years) | 0.32 | 0.005 | |||||||
| 0 | 36 (12%) | 7 (11%) | 8 (7%) | 6 (6%) | 28 (18%) | 6 (9%) | 11 (7%) | ||
| <1 year | 24 (8%) | 5 (8%) | 9 (8%) | 8 (8%) | 15 (10%) | 8 (12%) | 7 (5%) | ||
| >=1 and <3 years | 42 (14%) | 14 (22%) | 26 (24%) | 20 (20%) | 28 (18%) | 14 (21%) | 20 (13%) | ||
| >=3 years | 192 (65%) | 39 (60%) | 66 (61%) | 65 (66%) | 82 (54%) | 39 (58%) | 111 (74%) | ||
| Hepatitis C | 19 (6%) | 4 (6%) | 8 (7%) | 0.86 | 7 (7%) | 10 (7%) | 4 (6%) | 10 (7%) | 0.62 |
| HIV | 8 (3%) | 3 (5%) | 5 (5%) | 0.78 | 6 (6%) | 1 (1%) | 1 (1%) | 8 (5%) | 0.11 |
| Diabetes | 107 (36%) | 24 (37%) | 42 (39%) | 0.93 | 38 (38%) | 61 (40%) | 23 (34%) | 51 (34%) | 0.73 |
| Hypertension | 278 (95%) | 64 (98%) | 104 (95%) | 0.40 | 92 (93%) | 146 (95%) | 61 (91%) | 147 (99%) | 0.05 |
| CAD | 52 (18%) | 9 (14%) | 19 (17%) | 0.81 | 19 (19%) | 17 (11%) | 9 (13%) | 35 (23%) | 0.14 |
| Primary Insurance Type | 0.76 | 0.16 | |||||||
| Medicaid & Medicare | 208 (71%) | 43 (66%) | 77 (71%) | 70 (71%) | 100 (65%) | 44 (66%) | 114 (77%) | ||
| Private Insurance | 86 (29%) | 22 (34%) | 32 (29%) | 29 (29%) | 53 (35%) | 23 (34%) | 35 (23%) | ||
| BMI (kg/m2) | 28 (24, 32) | 30 (25, 33) | 29 (26, 32) | 0.13 | 29 (25, 33) | 28 (24, 32) | 28 (25, 31) | 28 (25, 33) | 0.67 |
| Donor/Allograft Characteristics | |||||||||
| Donor Age (Years) | 46 (30, 54) | 38 (26, 55) | 44 (28, 54) | 0.31 | 43 (31, 54) | 44 (27, 53) | 49 (30, 55) | 48 (29, 54) | 0.43 |
| Donor Race | 0.76 | 0.002 | |||||||
| White | 202 (69%) | 43 (66%) | 80 (73%) | 60 (61%) | 116 (76%) | 42 (63%) | 107 (72%) | ||
| Black | 46 (16%) | 12 (18%) | 18 (17%) | 19 (19%) | 19 (12%) | 19 (28%) | 19 (13%) | ||
| Asian | 9 (3%) | 3 (5%) | 1 (1%) | 1 (1%) | 3 (2%) | 3 (4%) | 6 (4%) | ||
| Unknown | 36 (12%) | 7 (11%) | 9 (8%) | 18 (18%) | 14 (9%) | 3 (4%) | 17 (11%) | ||
| Donor Male Sex | 175 (60%) | 35 (58%) | 73 (67%) | 0.35 | 66 (67%) | 83 (54%) | 42 (63%) | 95 (64%) | 0.19 |
| KDPI | 54 (31, 73) | 54 (33, 74) | 51 (32, 74) | 0.36 | 47 (27, 68) | 52 (27, 72) | 62 (39, 76) | 55 (36, 74) | 0.31 |
| Delayed Graft Function | 122 (41%) | 28 (43%) | 30 (28%) | 0.03 | 37 (37%) | 65 (42%) | 27 (40%) | 51 (34%) | 0.51 |
| DCDD | 56 (19%) | 12 (18%) | 22 (20%) | 0.95 | 16 (16%) | 24 (16%) | 15 (22%) | 35 (23%) | 0.26 |
| Cold Ischemia Time (Hours) | 15 (12, 21) | 16 (12, 19) | 14 (11, 23) | 0.98 | 15 (12, 18) | 14 (11, 18) | 15 (11, 21) | 16 (13, 22) | 0.02 |
| Discharge Condition | |||||||||
| NODAT | 36 (12%) | 7 (11%) | 15 (14%) | 0.36 | 11 (11%) | 21 (14%) | 9 (13%) | 17 (11%) | 0.90 |
| Length of Stay > 4 Days | 143 (49%) | 25 (38%) | 38 (35%) | 0.03 | 41 (41%) | 57 (37%) | 32 (48%) | 76 (51%) | 0.09 |
| Discharge on Dialysis | 48 (16%) | 12 (18%) | 17 (16%) | 0.88 | 14 (14%) | 30 (20%) | 10 (15%) | 23 (15%) | 0.63 |
| Discharge on Insulin | 114 (42%) | 27 (42%) | 48 (44%) | 0.92 | 41 (43%) | 72 (49%) | 27 (40%) | 49 (36%) | 0.14 |
Abbreviations: HIV—Human Immunodeficiency Virus; CAD—Coronary Artery Disease (defined as history of myocardial infarction, coronary bypass, or angioplasty/stent); BMI—Body Mass Index; k—kilograms; m—meters; KDPI—Kidney Donor Profile Index; DCDD—Donation after Circulatory Determination of Death; NODAT—New Onset Diabetes after Transplantation
—Comparing weekday and weekend discharge
—Comparing durations to outpatient follow-up
A total of 178 recipients (38%) experienced the primary outcome of EHR after KT. Median time to first EHR event was 8 days (IQR 5–15 days). Compared to recipients without EHR, recipients with EHR were more likely to be black (54% vs 43%, p=0.02) and diabetic (44% vs 33%, p=0.02), and less likely to have private insurance (24% vs 33%, p=0.03). Further, those with EHR were more likely to have received higher KDPI kidneys (median KDPI 62 vs 47, P<0.001), have had a long KT LOS (>4 days, 52% vs 39%, p=0.005), and have been discharged on dialysis (21% vs 13%, p=0.03). The majority of participants with EHR (92%, n=163) were readmitted after their first outpatient follow-up appointment.
Differences in EHR between Transplant Centers
The overall EHR rate was 38%, and was not significantly different between transplant centers (p=0.66). Transplant centers differed significantly in their distributions of recipient and donor age, cold ischemia time, and warm ischemia time. Transplant centers populations also differed by recipient KT LOS, recipient and donor race, recipient dialysis vintage, recipients discharged on insulin, and recipients with new onset diabetes. (p<0.01 for all comparisons).
The Association of Discharge Decisions on EHR risk
In Table 3, we display the results of our iterative modeling approach. In the fully adjusted model (Model 4), both discharge planning variables were independently associated with EHR (see Figure 2). Specifically, adjusted for traditional covariates and center effects, compared to discharge on Monday–Thursday, discharge on the weekend was associated with a 29% lower probability of EHR (adjusted Odds Ratio [aOR] 0.71, 95% Confidence Interval [CI] 0.41–0.94). Compared to follow-up within two days of discharge, KT recipients with follow-up within three to six days had a 28% higher probability of EHR (aOR 1.28, 95% CI 1.13–1.45). There was no statistically significant difference in the odds of EHR when comparing discharges with follow-up ≥7 days to those with follow-up ≤2 days (aOR 0.91, 95% CI 0.55, 1.51). In the fully adjusted model, black race, diabetes status, private insurance, longer cold ischemia time, and discharge on dialysis were also independently associated with higher EHR risk (Supplemental Table 1).
Table 3.
Comparison of Multivariable Mixed Effects Regression Models for the Outcome of Early Readmission.
| Model 1* N=437 |
Model 2 N=437 |
Model 3 N=437 |
Model 4 N=437 |
VIF** | ||
|---|---|---|---|---|---|---|
| Discharge Day of the Week | Weekend | 0.67 (0.52, 0.87) | 0.71 (0.57, 0.88) | 1.07 | ||
| Friday | 1.02 (0.78, 1.34) | 0.99 (0.75, 1.31) | ||||
| Monday–Thursday | 1.00(Reference) | 1.00 (Reference) | ||||
| Days to first scheduled follow-up | Unavailable on Discharge Summary | 1.46(0.95, 2.26) | 1.34 (0.98, 1.93) | 1.12 | ||
| ≥7 Days | 0.97 (0.50, 1.88) | 0.91 (0.55, 1.51) | ||||
| 3–6 Days | 1.37 (1.07, 1.83) | 1.25 (1.10, 1.42) | ||||
| ≤ 2 Days | 1.00(Reference) | 1.00 (Reference) | ||||
| QIC | 984.64 | 981.44 | 987.42 | 985.49 | ||
| AUC | 0.68(0.03) | 0.69 (0.04) | 0.69 (0.03) | 0.70 (0.03) | ||
| ICC | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Likelihood Ratio | 39.52 | 48.52 |
Abbreviations: VIF—variance inflation factor; QIC—Quasi-Akaike Information Criterion; AUC—Area under the Receiver Operating Curve; ICC—Intraclass Correlation Coefficient
Models adjusted for recipient variables: age, race, sex, diabetes, hypertension, and insurance coverage; donor/allograft variables: age, kidney donor profile index, delayed graft function, and cold ischemia time; discharge variables: discharge on dialysis, discharge with new onset diabetes, discharge on insulin, long length of stay.
Figure 2. Associations of Discharge Decision-Making on Early Readmission Risk after Kidney Transplantation.
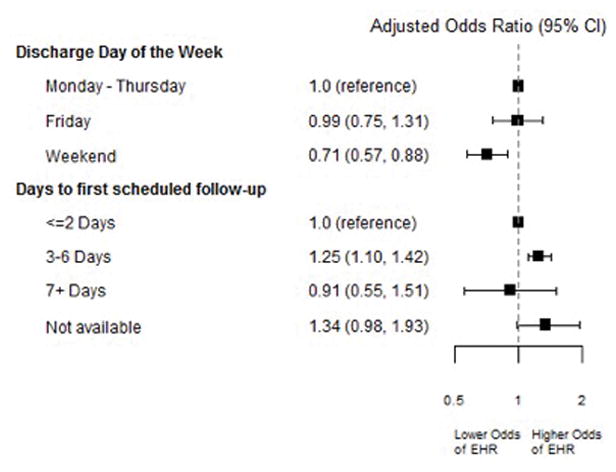
Figure displays results of mixed effects model also adjusted for recipient variables: age, race, sex, diabetes, hypertension, and insurance coverage; donor/allograft variables: age, kidney donor profile index, delayed graft function, and cold ischemia time; and discharge condition variables: discharge on dialysis, discharge with new onset diabetes, discharge on insulin, long length of stay.
Model Comparisons
In the fully adjusted model, VIFs for the exploratory variables were <2, consistent with adequate levels of independence.(33) The optimal model by QIC criterion (i.e., lowest QIC) was Model 2, which included the baseline covariates and discharge day of the week. Addition of the discharge decision-making variables did not significantly improve predictive ability between Models 1 and 4 (C-statistic 0.68 vs 0.70, p=0.16). Intra-class correlation coefficients were zero for all models, indicating that there was no residual confounding in the models that could be explained by clustering by center.
Reasons for EHR
The three most common primary reasons for EHR were electrolyte and volume disorders (n=40, 22%), infection (n=32, 17%), and surgical site complications (n=32, 17%). Other reasons were rejection, cardiac complications, acute kidney injury, drug toxicity, and gastrointestinal complications. There were no statistically significant differences in primary and secondary reasons for EHR between weekday versus weekend discharges, or between those with shorter or longer durations to follow-up after discharge from DDKT (Table 4).
Table 4.
Reasons for Readmission, Stratified by Discharge Timing and Scheduled Follow-Up from the Initial Transplant Admission
| Discharge Monday–Thursday (N=147) | Friday | Weekend (N=31) | Pa | Follow Up ≤2 days (N=31) | 3–6 days (N=63) | 7+ days (N=19) | Pb | ||
|---|---|---|---|---|---|---|---|---|---|
| Primary reason | Rejection | 5 (4%) | 5 (19%) | 2 (6%) | 0.71 | 2 (6%) | 3 (5%) | 1 (5%) | 0.54 |
| Infection | 22 (18%) | 1 (4%) | 5 (16%) | 6 (19%) | 13 (20%) | 2 (11%) | |||
| Cardiac | 6 (5%) | 1 (4%) | 1 (3%) | 3 (5%) | 1 (5%) | ||||
| Surgical Site Complication | 22 (18%) | 1 (4%) | 7 (22%) | 5 (16%) | 7 (11%) | 3 (16%) | |||
| Acute Kidney Injury | 4 (3%) | 2 (6%) | 1 (3%) | 4 (6%) | |||||
| Drug Toxicity | 2 (2%) | 3 (11%) | 1 (2%) | ||||||
| Gastrointestinal Complication | 9 (8%) | 7 (26%) | 5 (16%) | 3 (10%) | 4 (6%) | 2 (11%) | |||
| Electrolyte or Volume Disorder | 26 (22%) | 9 (33%) | 6 (19%) | 8 (26%) | 10 (15%) | 4 (21%) | |||
| Other | 24 (20%) | 5 (19%) | 5 (16%) | 5 (16%) | 21 (32%) | 6 (32%) | |||
| Secondary reason | Infection | 5 (17%) | 2 (29%) | 1 (11%) | 0.96 | 1 (9%) | 4 (18%) | 2 (50%) | 0.23 |
| Cardiac | 1 (3%) | ||||||||
| Surgical Site Complication | 1 (3%) | 1 (11%) | 1 (25%) | ||||||
| Acute Kidney Injury | 1 (3%) | 1 (14%) | 1 (11%) | 2 (9%) | |||||
| Drug Toxicity | 1 (3%) | ||||||||
| Gastrointestinal Complication | 2 (7%) | 1 (9%) | |||||||
| Electrolyte or Volume Disorder | 1 (3%) | 1 (5%) | |||||||
| None | 28 (61%) | 18 (60%) | 4 (57%) | 9 (82%) | 15 (68%) | 1 (25%) |
Comparison of readmission reasons by weekday (Monday–Friday) or weekend discharge
Comparison of readmission reasons by duration to scheduled follow-up
Sensitivity Analysis
In analyses in which we assigned the unavailable data on days to first post-discharge follow-up to the extreme values (i.e., ≤2 days and ≥7 days, Supplemental Table 2), weekend discharge retained an independent and inverse association with the probability of EHR after DDKT.
Discussion
In this multicenter, retrospective study of DDKT recipients, we found that two decisions made during discharge planning were independently associated with the probability of EHR after DDKT. Specifically, compared to those that were discharged from transplant on weekdays, DDKT recipients discharged on the weekend had a lower probability of EHR. Conversely, we found that when compared to recipients who were scheduled for follow-up with two days of discharge, DDKT recipients with longer scheduled times to follow-up had a higher risk of EHR. These findings were robust to adjustment for traditional clinical risk factors and center effects, and raise compelling questions about the as-yet unmeasured factors that drive clinical risk-stratification and discharge decision-making.
While no studies to date have directly measured clinician prediction of EHR risk after KT, our discharge planning factors may reasonably represent proxies of such clinical risk stratification. For example, clinicians may reserve weekend discharge and longer follow-up for patients who they clinically assess to be less vulnerable. These decisions are likely based on a combination of traditional measured risk factors (e.g., older age, higher comorbidity burden) and unmeasured risk factors (e.g., perceived frailty). However, our results suggest that if such risk stratification took place when making these discharge decisions, it may have been more successful in one case (weekend discharge) and less successful in another (close follow-up). It is possible that clinicians are more in tune to certain patient vulnerabilities than others; for example, clinicians may recognize that traditionally measured risk factors (e.g., clinical stability, burden of comorbidities) make some patients more vulnerable to weekend discharge, and avoid it appropriately in those cases. Conversely, important factors that are often unmeasured, such as poor cognitive function or low health literacy,(34, 35) may escape clinical assessment and also render some KT recipients more vulnerable to longer durations without follow-up. However, the addition of discharge planning variables did not improve the ability of our models to predict EHR, suggesting that further work is needed to help clinicians predict EHR more accurately. In this respect, our findings are consistent with many prior studies that sought to predict EHR events with traditional and nontraditional risk factors,(36–40) highlighting the challenge of predicting events for which there are multifactorial causes.(10)
Our study explored the potential for a “weekend effect” on EHR risk after DDKT. In general medicine and surgery populations, admissions or elective surgery on the weekend, compared to weekdays, have been linked to poorer patient outcomes.(41, 42) Studies of maintenance hemodialysis patients have also identified weekend discharge as a risk factor for EHR.(13, 14) In contrast, in transplantation, data have been conflicting. A recent study by Anderson et al. examined outcomes for DDKT recipients in the United Kingdom who received KT on the weekend, and found that KT performed on the weekend was not associated with excess risk of adverse outcomes.(43) However, at least one prior study has observed that weekend discharge was a risk factor for higher rates of EHR after KT. This single center study by our authorship group was performed at the University of Pennsylvania,(10) also a center in the current study, and included an older cohort of both living donor and DDKT recipients (transplanted between 2003–2007). There are numerous potential explanations for the observed discrepancy in the findings between the two studies, including temporal trends in staffing at the University of Pennsylvania to facilitate transitions of care for KT recipients, such as the hiring of nurse practitioners to support the work of transplant teams on the weekends. Further, greater awareness of the “weekend effect” in recent years and avoidance of high-risk weekend discharges may explain why weekend discharge was associated with lower EHR risk in the present analysis. For example, a lower proportion of weekend discharges in our cohort had experienced DGF and prolonged LOS than discharges on Mondays-Thursdays, suggesting that these patients were appropriately selected for weekend discharge due to less marginal graft function and lower overall medical complexity.
Our study is consistent with prior work in identifying that black recipients and those with diabetes are at greater risk for EHR after KT.(1, 11, 44) However, some of our findings are distinct from results of prior studies regarding predictors of EHR, possibly due to our inclusion of novel discharge metrics. For example, we included initial transplant LOS in our multivariable models, which has been shown to be associated with EHR risk in prior studies.(45, 46) However, we found that initial LOS did not retain an independent association with EHR risk after adjustment for metrics of discharge condition and decision-making. Further, DGF, often defined as the requirement for dialysis within one week of KT,(47) did not retain an independent association with EHR risk in our models, in contrast to discharge on dialysis. These findings suggest that inclusion of metrics of discharge condition may add important insight to EHR risk after KT. Finally, our study identified that those with private insurance, potentially a proxy for higher socioeconomic status, are at lower risk for EHR after KT. This finding has not been previously described in multicenter studies on EHR after KT, as these have been historically restricted to Medicare beneficiaries.(1, 3)
Strengths of our study include its granular data on discharge condition and decision-making, detailed data on potential confounders, and a multicenter population that was not limited to Medicare beneficiaries, unlike prior work. However, as a retrospective study of medical records, our study was limited in its ability to adjudicate the preventability of EHR events and to ascertain plausible risk factors for EHR that are not reliably found in hospital records including poor social support,(13, 48) prior hospitalization frequency,(3, 14) frailty,(11, 49) and functional status.(3) We also limited ascertainment of EHR events to transplant hospital medical records, but we are confident that few EHR episodes would have resulted in hospitalization at other institutions because of how closely transplant programs manage transplant recipients in the early post-surgical period. In support of this assertion, the EHR rates observed in this study are similar to those reported in national studies of Medicare claims.(1, 3)
In conclusion, modifiable discharge decisions are associated with the risk of EHR after DDKT. Weekend discharge is associated with lower EHR risk, possibly reflecting accurate clinical decision-making. Future prospective studies are needed to determine factors that promote improved clinical prediction of EHR events and tailor discharge strategies to facilitate optimal transitions of care after KT.
Supplementary Material
Supplemental Table 1. Results of Fully Adjusted Mixed Effects Model for Early Readmission.
Supplemental Table 2. Results of Sensitivity Analysis Replacing Unavailable Data on Days to Follow-Up as ≤2 days and as ≥7 days in Multivariable Mixed Effects Regression for the Outcome of Early Readmission.
Acknowledgments
M.N.H. is supported by K23DK105207 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
This work was supported by National Institutes of Health Grants R01DK-93770 and K24DK090203, a Roche Organ Transplantation Research Foundation Award (to C.R.P.), an award from the American Heart Association (to I.E.H.), and Health Resources and Services Administration Contract 234-2005-37011C.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Abbreviations
- EHR
Early Hospital Readmission
- QIC
Quasi-Akaike Information Criterion
- CI
Confidence Interval
- CAD
Coronary Artery Disease
- DGF
Delayed Graft Function
- DCDD
Donation after Circulatory Determination of Death
- ESRD
End Stage Renal Disease
- OR
Odds Ratio
- DDKT
Deceased donor kidney transplantation
- HIV
Human Immunodeficiency Virus
- KT
Kidney transplantation
- LOS
Length of Stay
- NODAT
New Onset Diabetes after Transplantation
- SRTR
Scientific Registry of Transplant Recipients
- US
United States
Footnotes
Disclosures
None.
Author Contributions:
Concept/Design: MNH, YJ, HTP, BB, RG, FW, IEH, MD, BS, CRP, PPR
Data Collection: BB, RG
Data Analysis/Interpretation: MNH, YJ, HTP
Drafting Article: MNH, YJ, HTP, BB, RG, FW, IEH, MD, BS, CRP, PPR
Approval of Article: MNH, YJ, HTP, BB, RG, FW, IEH, MD, BS, CRP, PPR
Funding secured by: CRP
References
- 1.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12(12):3283–3288. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 2.Englesbe MJ, Dimick JB, Fan Z, Baser O, Birkmeyer JD. Case mix, quality and high-cost kidney transplant patients. Am J Transplant. 2009;9(5):1108–1114. doi: 10.1111/j.1600-6143.2009.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harhay MN, Hill AS, Wang W, Even-Shoshan O, Mussell AS, Bloom RD, et al. Measures of Global Health Status on Dialysis Signal Early Rehospitalization Risk after Kidney Transplantation. PloS one. 2016;11(6):e0156532. doi: 10.1371/journal.pone.0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med. 2010;363(3):297–298. doi: 10.1056/NEJMc1001882. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AM. Revisiting readmissions--changing the incentives for shared accountability. N Engl J Med. 2009;360(14):1457–1459. doi: 10.1056/NEJMe0901006. [DOI] [PubMed] [Google Scholar]
- 6.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 7.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL. Sequelae of early hospital readmission after kidney transplantation. Am J Transplant. 2014;14(2):397–403. doi: 10.1111/ajt.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghani Lankarani M, Noorbala MH, Assari S. Causes of re-hospitalization in different post kidney transplantation periods. Ann Transplant. 2009;14(4):14–19. [PubMed] [Google Scholar]
- 9.Naderi M, Aslani J, Hashemi M, Assari S, Amini M, Pourfarziani V. Prolonged rehospitalizations following renal transplantation: causes, risk factors, and outcomes. Transplant Proc. 2007;39(4):978–980. doi: 10.1016/j.transproceed.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 10.Harhay M, Lin E, Pai A, Harhay MO, Huverserian A, Mussell A, et al. Early Rehospitalization After Kidney Transplantation: Assessing Preventability and Prognosis. Am J Transplant. 2013 doi: 10.1111/ajt.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAdams-Demarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfield NI, McCullough EC, Hughes JS, Tang AM, Eastman B, Rawlins LK, et al. Identifying potentially preventable readmissions. Health care financing review. 2008;30(1):75–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Hilbert J, Kshirsagar AV, Gilet CA. Psychosocial Factors and 30-Day Hospital Readmission among Individuals Receiving Maintenance Dialysis: A Prospective Study. American journal of nephrology. 2017;45(5):400–408. doi: 10.1159/000470917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flythe JE, Katsanos SL, Hu Y, Kshirsagar AV, Falk RJ, Moore CR. Predictors of 30-Day Hospital Readmission among Maintenance Hemodialysis Patients: A Hospital’s Perspective. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(6):1005–1014. doi: 10.2215/CJN.11611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9(3):506–516. doi: 10.1111/j.1600-6143.2008.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant. 2010;10(4 Pt 2):987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29(6):621–635. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand S, Johansen KL, Grimes B, Kaysen GA, Dalrymple LS, Kutner NG, et al. Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: the comprehensive dialysis study. Hemodialysis international International Symposium on Home Hemodialysis. 2013;17(1):50–58. doi: 10.1111/j.1542-4758.2012.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brogan DJ, Haber M, Kutner NG. Functional decline among older adults: comparing a chronic disease cohort and controls when mortality rates are markedly different. Journal of clinical epidemiology. 2000;53(8):847–851. doi: 10.1016/s0895-4356(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 20.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney international. 2008;73(11):1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 21.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 22.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. Kidney. Am J Transplant. 2016;16(Suppl 2):11–46. doi: 10.1111/ajt.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi S, Gaynor JJ, Bayers S, Guerra G, Eldefrawy A, Chediak Z, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95(2):309–318. doi: 10.1097/TP.0b013e31827191d4. [DOI] [PubMed] [Google Scholar]
- 24.Patzer RE, Perryman JP, Schrager JD, Pastan S, Amaral S, Gazmararian JA, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall EC, James NT, Garonzik Wang JM, Berger JC, Montgomery RA, Dagher NN, et al. Center-level factors and racial disparities in living donor kidney transplantation. Am J Kidney Dis. 2012;59(6):849–857. doi: 10.1053/j.ajkd.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. Journal of the American Society of Nephrology : JASN. 2011;22(4):743–751. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potluri VS, Parikh CR, Hall IE, Ficek J, Doshi MD, Butrymowicz I, et al. Validating Early Post-Transplant Outcomes Reported for Recipients of Deceased Donor Kidney Transplants. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(2):324–331. doi: 10.2215/CJN.06950615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DLS. Applied Logistic Regression. 2. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 30.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 32.Wooldridge JM. Introductory Econometrics: A Modern Approach. 5. South Western: 2012. [Google Scholar]
- 33.Kendall MGaJDG. Rank Correlation Methods. 5. London: Griffin; 1990. [Google Scholar]
- 34.Taylor DM, Fraser SDS, Bradley JA, Bradley C, Draper H, Metcalfe W, et al. A Systematic Review of the Prevalence and Associations of Limited Health Literacy in CKD. Clinical journal of the American Society of Nephrology : CJASN. 2017 doi: 10.2215/CJN.12921216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, et al. Vascular risk factors and cognitive impairment in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(2):248–256. doi: 10.2215/CJN.02660310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan O, Meltzer DO, Shaykevich SA, Bell CM, Kaboli PJ, Auerbach AD, et al. Hospital readmission in general medicine patients: a prediction model. Journal of general internal medicine. 2010;25(3):211–219. doi: 10.1007/s11606-009-1196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shadmi E, Flaks-Manov N, Hoshen M, Goldman O, Bitterman H, Balicer RD. Predicting 30-day readmissions with preadmission electronic health record data. Medical care. 2015;53(3):283–289. doi: 10.1097/MLR.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 38.Hummel SL, Katrapati P, Gillespie BW, Defranco AC, Koelling TM. Impact of prior admissions on 30-day readmissions in medicare heart failure inpatients. Mayo Clinic proceedings. 2014;89(5):623–630. doi: 10.1016/j.mayocp.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donze J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA internal medicine. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 40.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. Jama. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668. doi: 10.1056/NEJMsa003376. [DOI] [PubMed] [Google Scholar]
- 42.Aylin P, Alexandrescu R, Jen MH, Mayer EK, Bottle A. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ. 2013;346:f2424. doi: 10.1136/bmj.f2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson BM, Mytton JL, Evison F, Ferro CJ, Sharif A. Outcomes after weekend admission for deceased donor kidney transplantation: a population cohort study. Transplantation. 2016 doi: 10.1097/TP.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 44.DeMarco MAG ME, Hall E, Segev DL. Predictors of Early Hospital Readmission after Kidney Transplantation. Am J Transplant. 2012;12(Supplement s3):27–542. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 45.Zapatero A, Barba R, Marco J, Hinojosa J, Plaza S, Losa JE, et al. Predictive model of readmission to internal medicine wards. European journal of internal medicine. 2012;23(5):451–456. doi: 10.1016/j.ejim.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan TA. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. The American journal of medicine. 1999;107(1):13–17. doi: 10.1016/s0002-9343(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 47.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amarasingham R, Moore BJ, Tabak YP, Drazner MH, Clark CA, Zhang S, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Medical care. 2010;48(11):981–988. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 49.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Archives of internal medicine. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Results of Fully Adjusted Mixed Effects Model for Early Readmission.
Supplemental Table 2. Results of Sensitivity Analysis Replacing Unavailable Data on Days to Follow-Up as ≤2 days and as ≥7 days in Multivariable Mixed Effects Regression for the Outcome of Early Readmission.