Abstract
Oil-based drill cuttings are hazardous wastes containing complex hydrocarbons, heavy metals, and brine. Their remediation is a crucial step before release to the environment. In this work, we enriched a halophilic consortium, from oil-polluted saline soil, which is capable of degrading diesel as the main pollutant of oil-based drill cuttings. The degradation ability of the consortium was evaluated in microcosms using two different diluting agents (fine sand and biologically active soil). During the bioremediation process, the bacterial community dynamics of the microcosms was surveyed using PCR amplification of a fragment of 16S rRNA gene followed by denaturing gradient gel electrophoresis (DGGE). The diesel degradation rates were monitored by total petroleum hydrocarbon (TPH) measurement and the total count of heterotrophic and diesel-degrading bacteria. After 3 months, the microcosm containing fine sand and drill cuttings with the ratio of 1:1 (initial TPH of 36,000 mg/kg) showed the highest TPH removal (40%) and its dominant bacterial isolates belonged to the genera Dietzia, Arthrobacter, and Halomonas. DGGE results also confirmed the role of these genera in drill cuttings remediation. DGGE analysis of the bacterial diversity showed that Propionibacterium, Salinimicrobium, Marinobacter, and Dietzia are dominant in active soil microcosm; whereas Bacillus, Salinibacillus, and Marinobacter are abundant in sand microcosm. Our results suggest that the bioaugmentation strategy would be more successful if the diluting agent does not contain a complex microbial community.
Keywords: Drilling fluids, Drill cuttings, DGGE, Bioremediation, Halophilic consortium
Introduction
Petroleum is the most important fossil energy resource in the industrial world. Accessing to the oil containing reservoirs requires exploration and drilling, followed by geological and geophysical surveys which are essential operations to approve the oil existence and prepare for extraction (Abdel-Aal et al. 2003; Speight 2014). To facilitate the drilling process, drilling fluids are utilized to transfer the resulted cuttings to the surface, control the pressure within the reservoir, and reduce the friction of the drill bit (Agwu et al. 2013).
Oil-based muds (OBMs) that contain diesel, generally, are the preferred drilling fluids owing to their low price, satisfactory lubricity, and applicability for drilling wells with certain conditions such as high temperature or depth (Adekunle et al. 2013; Paladino et al. 2016).
After mixing with drill cuttings, OBMs constitute one of the most hazardous wastes of the petroleum industry that contain complex hydrocarbons, heavy metals, and brine, released to the surrounding terrestrial and aquatic environment causing deleterious effects on them (Al-Ansary and Al-Tabbaa 2007).
Bioremediation is a promising alternative for the physicochemical methods to clean the drilling wastes up, due to its environmental-friendly nature and cost-effectiveness (Varjani and Upasani 2017). Bioaugmentation (addition of enriched microorganisms capable of pollutant degradation) is one of the bioremediation strategies that can be applied if ample degradation rate is not observed by the indigenous degrading microorganisms (Gojgic-Cvijovic et al. 2012). In the case of bioaugmentation, the microbial consortium is preferred to the pure culture due to the possible synergistic relations between members of the consortium and different types of enzymes produced by various members that can catalyze different steps of the removal (Cerqueira et al. 2011; Mukherjee and Bordoloi 2011).
However, the addition of high levels of petroleum hydrocarbons causes an imbalance between carbon, nitrogen, and phosphorus ratio in soil which should be restored to original ratio and optimized by addition of organic or inorganic compounds before bioaugmentation process (Sarkar et al. 2005). Since the drill cuttings are often associated with high salt concentrations, microorganisms that are used for bioaugmentation should be able to withstand and grow in high salinities (Le Borgne et al. 2008). Halophilic microorganisms are a group of extremophiles which are able to grow in the presence of high concentrations of NaCl ranging from 0.2 to 5.5 M (Zhuang et al. 2010). So far, pure or mixed cultures of halophilic microorganisms have been exploited by many researches for bioremediation of petroleum- or diesel-contaminated soils in various ranges of NaCl concentration (1–18%) (Cerqueira et al. 2011; Dastgheib et al. 2012). Representatives of the phyla Actinobacteria and Proteobacteria are among the most frequently reported genera from saline soil bioremediation, e.g., Alcaligenes (naphthalene and phenanthrene degradation) (Ashok et al. 1995), Alcanivorax (crude oil and diesel fuel), Bacillus (diesel oil) (Kebria et al. 2009), Halomonas (Phenol and p-cresol) (Haddadi and Shavandi 2013), Marinobacter (crude oil, aromatic, and aliphatic compounds) (Al-Mailem et al. 2013), Planococcus (BTEX) (Li et al. 2006), and Pseudomonas (crude oil, fuels, alkanes, and PAHs) (Kumar et al. 2008).
There are very limited reports on the bacterial remediation of drill cuttings overall. Two non-halophilic bacterial species (Bacillus subtilis and Pseudomonas aeruginosa) were used for bioremediation of aromatic compounds in oil-based drill cuttings and Pseudomonas aeruginosa shows more efficiency in degradation of aromatic compounds with more rings (Okparanma et al. 2009). However, in an isolation effort, Bacillus Thuringiensls, Bacillus oleronius, and some other halophilic bacteria (growing at 3% NaCl) have been isolated from oil-based drill cuttings (Turner 2002), but the potential of halophilic bacteria in bioremediation and bioaugmentation of drill cuttings is still untapped.
Here, we represent the isolation and characterization of a halophilic bacterial consortium able to degrade diesel oil components in the oil-based drill cuttings, and evaluate its application for bioremediation in microcosm scale.
Materials and methods
Sampling
Soil sample was collected from hydrocarbon-polluted area near an abandoned oil production facility located in Qom, Iran (N: 34°44′43.5″, E: 50°53′43.6″) (Table 1). Samples were taken using a sterile spatula and large particles and plant debris were removed by sieving. Fresh oil-based drill cuttings were collected from Lavan district of Iranian Offshore Oil Company, Lavan Island, Iran.
Table 1.
Location and characteristics of the soil sample used for enrichment
| Sampling area | Location | EC (µS/cm) | pH | TPH (mg/g) | Salinity (ppt) |
|---|---|---|---|---|---|
| Oil-contaminated soil (Beheshte Masumeh Wetland, in Qom) | N: 34°44′43.5″ E: 50°53′43.6″ |
65,250 | 8.05 | 46 | 45 |
Enrichment of diesel-degrading consortium
Diesel oil degrading consortium was enriched from oil-polluted saline soil samples. Bushnell Haas (BH) broth containing KH2PO4 1, K2HPO4 1, NH4NO3 1, MgSO4 0.2, CaCl2 0.2, FeCl3 0.05, and NaCl 70 (gram per liter), supplemented with 0.5% (v/v) autoclaved diesel oil (provided by Tehran refinery incorporation) as sole carbon source, was used as an enrichment media; and the pH was adjusted to 7–7.2 by 1 M Tris–HCl solution.
One gram of the soil samples was transferred to a 250 mL flask containing 100 mL BH broth and incubated at 32 °C and 120 rpm for 1 week. Microbial growth was followed by the measurement of optical density at 620 nm and microscopic observations (Gram staining). The enrichment process was repeated seven times to obtain a stable consortium. At the final stage of the enrichment process, the total protein content of the culture was assayed by Bradford’s method using bovine serum albumin (BSA) as standard (Bradford 1976). The consortium ability to degraded diesel components was evaluated by gas chromatography analysis (7890A, Agilent, equipped with FID detector). Hydrocarbons of the enrichment media (with and without consortium) were extracted by hexane. The extract was injected into the CP-Sil8CB capillary column with the length of 30 m and an internal diameter of 0.32 mm. Helium with a purity of 99.999% was used as the carrier gas in this analysis. GC temperature program started at 70 °C and reached to 300 °C by a rate of 10 °C/min and the temperature of FID detector was also set at 300 °C.
Isolation and identification of dominant members of the consortium
The enriched consortium was serially diluted to 10−12 dilution in the modified R2A broth (Kleinsteuber et al. 2006) supplemented with 70 g/L NaCl. 100 µL of each dilution was spread on modified R2A agar plates (Buck and Cleverdon 1960). Colonies grown on the highest dilutions were considered as dominant strains of the consortium and pure cultures were obtained by repeated streaking on modified R2A agar. The genomic DNA of isolates were extracted (Marmur 1961) and 16S rRNA sequence was amplified using 27F (5′-AGAGTTTGATCMTGGGTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) universal primers (Jiang et al. 2006).
Diesel degradation by dominant members
The same cell count of each strain (1 mL of the bacterial suspensions with 1.5 × 108 cfu/mL) was transferred to the 100 mL flasks containing 30 mL of BH broth supplemented with 0.5% (w/v) sterile diesel and incubated at 32 °C, 120 rpm for 72 h, and thereafter; optical density at 620 nm and total protein content were measured as described earlier.
Microcosm preparation
Drill cutting samples were collected from an offshore drilling rig near Lavan Island, Persian Gulf (see Table 2 for sample characteristics). The petroleum hydrocarbon content of the drill cuttings was extracted and the carbon distribution of the extract was determined using Simulated Distillation (GC-SimDis) method based on ASTM D7169 and ASTM D2887 standards (Boczkaj et al. 2011; Riazi 2005; Vendeuvre et al. 2005).
Table 2.
Characteristics of Lavan drill cuttings
| Parameter | Value |
|---|---|
| Salinity (PPT) | 6.4 |
| Total petroleum hydrocarbon (mg/kg) | 60,000 |
| Nickel (µg/g) | 47 |
| Cadmium (µg/g) | 3.5 |
| Chromium (µg/g) | 92.5 |
| Lead (µg/g) | 36.5 |
| Barium (µg/L) | 12 |
For microcosm preparation, drill cutting samples were mixed by 1:1 w/w ratio with fine sands (S) or biologically active soil (B) as diluting agents. In this research, a soil sample without oil pollution, which has not been treated with chemical fertilizers in the last 10 years and had adequate moisture to support the microbial activity, has been considered as the biologically active soil. Diluting agents were used to adjust the diesel concentration of drill cuttings to proper levels and facilitate the aeration and nutrients diffusion. The ratio was selected based on the previous study that shows the ratio of 1:1 w/w has the highest degradation rate of hydrocarbons (Rastegarzadeh et al. 2006). There were three treatments for each diluting agent, including: abiotic control (containing 0.5% sodium azide as bactericide agent) named S1 and B1 for fine sand and biologically active soil, respectively, control microcosm without consortium inoculation (S2 and B2), and bioaugmentation microcosm with consortium inoculation (S3 and B3, respectively). The final weight of cuttings amended with diluting agents in each microcosm was 400 g. NH4NO3 and K2HPO4 salts were used as nitrogen and phosphorous sources, at the ratio of 100:10:1 for C:N:P. The suspension of consortium (containing approximately 106–107 cfu/mL initial cell density) was added to the B3 and S3 microcosms with the ratio of 1 mL/kg.
Microcosms were prepared in uniform plastic containers (30 × 21 × 14 cm) and equal numbers of holes (0.5 cm in diameter) were created on their lids to facilitate the air flow, and were kept at room temperature for 3 months. During this period, the humidity of each microcosm was kept constant at 20% W/W by weekly controlling weight loss and addition of deionized sterile water for compensation.
Microcosm sampling
The content of each microcosm was mixed thoroughly once every 2 weeks (using separate steal spoons sterilized by autoclaving) to homogenize the content and the required amount of the soil was transferred to sterile vials for subsequent analysis.
TPH measurement by solvent extraction
Equal weight of dried Na2SO4 (to absorb the residual moisture) and 10 mL n-hexane as an organic solvent were added to the microcosm soil samples to extract diesel. After vigorous mixing, samples were centrifuged at 4500 rpm, 15 °C for 10 mins. The supernatant was transferred to a dry pre-weighted glass plate and placed under the chemical fume hood for hexane evaporation. This procedure was repeated three times to ensure that diesel has been completely extracted from soil. Eventually, the difference between initial and final weights of the plate was used to calculate the residual TPH (Schwab et al. 1999).
Enumeration of heterotrophic and diesel-degrading bacteria
Cell density of heterotrophic and diesel-degrading bacteria of each microcosm was estimated by the spread plate method (as mentioned earlier) every 2 weeks. R2A agar medium supplemented with 7% NaCl was used for heterotrophic bacteria and BH agar medium containing 7% NaCl and coated with a thin layer of sprayed diesel (which has been sterilized by autoclave at 121 °C for 15 min) was used for enumeration of diesel-degrading bacteria. Plates were incubated for 7 days at 30 °C. The initial cell density of heterotrophic and diesel-degrading bacteria of fine sand, drill cuttings, and biologically active soil samples was also measured as well using the same method.
PCR-DGGE analysis
Sampling for this analysis was performed at the beginning, middle, and final stages of the investigation. The DNA of each sample was extracted by FastDNA® kit for soil (MP Biomedical). Genomic DNA of the consortium grown on BH medium was extracted using modified Marmur method. To increase the DNA extraction efficiency and for complete cell lysis, lysozyme treatment step was raised to 2 h rather than 30 min, and a freeze–thaw step was added after that. GC clamp-357F (5′-GC-clamp CCTACGGGAGGCAGCAG-3′) and 907R (5′-CCGTCAATTCMTTTGAGTTT) primers were used for the amplification of v3 to v5 region of 16S rRNA gene (Sánchez et al. 2007).
The polymerase chain reaction was conducted in iCycler Bio-Rad Hercules using the following reaction conditions: initial denaturation at 94 °C for 4 min, 30 replication cycles which include denaturation at 94 °C, annealing at 50 °C and extension step at 72 °C all for 60 s, and final extension at 72 °C for 7 min. Polyacrylamide gel (7.5%) with the 40–60% continuous gradient of DNA denaturants (100% denaturant gel composed of 7 M urea and 40% v/v deionized formamide) was prepared and placed inside the DGGE tank containing 7 liters of 1X TAE buffer. Approximately 350 ng of PCR product was loaded in each well and electrophoresis was done at 60 °C in DCode™ system (Bio-Rad, Hercules, CA) for 16 h at constant voltage of 80 V. After staining with ethidium bromide (15 min) and destaining, the gel was inspected under UV illumination. Desired DNA bands were excised from the gel under UV trans-illuminator, transferred to separate tubes containing 20 µL of sterile distilled water and kept at 4 °C overnight. Two milliliters of the eluted DNA were used as template for re-amplification of DGGE band by 357F (without GC clamp) and 907R primers. Single bands without smears were selected for sequencing (Chong et al. 2009; Kurola et al. 2005).
16S rRNA gene sequence analysis was conducted using the BLAST (Altschul et al. 1997) and EzTaxon online database (http://www.ezbiocloud.net/eztaxon). Isolated strains were deposited in the microbial bank of Iranian Biological Resource Center (IBRC) under assigned reference numbers (IBRC-M 10561, IBRC-M 10562, and IBRC-M 10965). The partial 16S rRNA gene sequences of selected DGGE bands and isolates have been submitted to the GenBank and accession numbers are provided in the phylogenetic trees.
A workflow of the main steps of the study is summarized and depicted in Fig. 1.
Fig. 1.
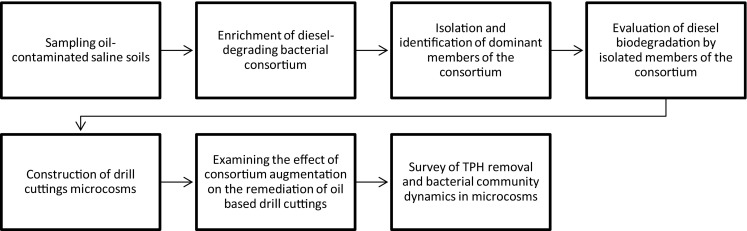
Summarized workflow of the main steps of this study
Results
Consortium preparation and characterization
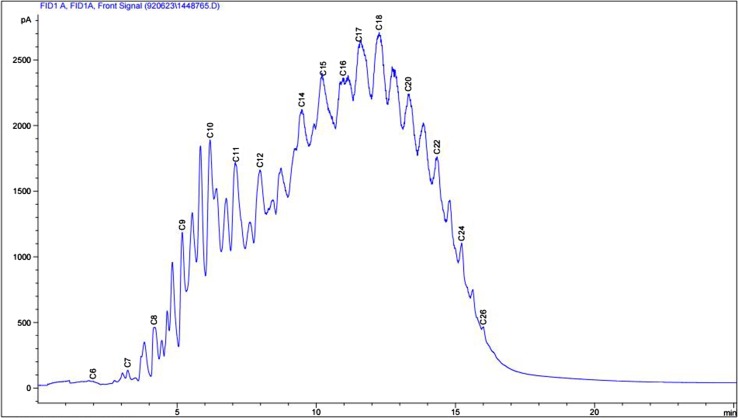
After seven rounds of enrichment on diesel oil in saline media, a consortium with OD620 equal to 3.1 and total protein concentration of 0.192 mg/mL was obtained. According to GC analysis results, the consortium was able to reduce diesel hydrocarbon content of the medium significantly (Fig. 2). Three dominant cultivable members of the consortium with different morphological properties were isolated and identified by 16S rRNA gene sequence analysis. According to the results, the three strains isolated from saline soil sample were identified as Dietzia sp. Q1 (IBRC-M 10965) with 100% similarity to Dietzia maris (Rainey et al. 1995), Halomonas sp. Q2 (IBRC-M 10961) with 97.1% similarity to Halomonas nitrilicus (Chmura et al. 2008), and Arthrobacter sp. Q3 (IBRC-M10562) with 98.4% similarity to Arthrobacter crystallopoietes (Ensign and Rittenberg 1963). The phylogenetic relationship of dominant strains of the consortium is shown in Fig. 3.
Fig. 2.
GC analysis graphs of the diesel oil utilized by enriched consortium. Blue and red graphs represent the culture media before and after the growth of the consortium respectively
Fig. 3.
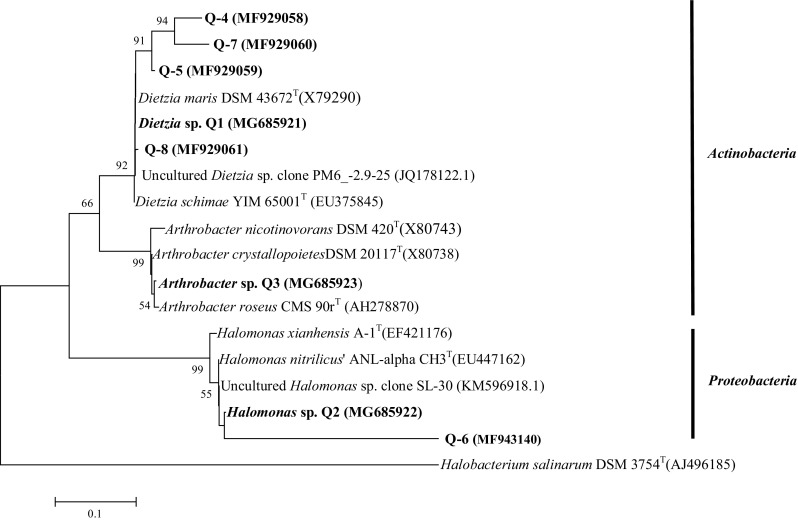
Phylogenetic relationship of the dominant strains of enriched consortium based on 16S rRNA gene sequences (culture-based and DGGE method) using neighbor-joining algorithm. The accession number of each strain is shown in parentheses. The numbers noted in the branches represent Bootstrap values (%) based on 1000 replicates. The scale bar indicates 0.05 substitutions per nucleotide position. Halobacterium salinarum DSM 3754 (T) was considered as the out-group
Among isolated strains, only strain Dietzia sp. Q1 was able to utilize diesel as the sole source of carbon without the presence of the other members of the microbial consortium and reach the OD620 of 0.7 and protein concentration of 0.05 mg/mL.
Characteristics of drill cuttings and other components of microcosms
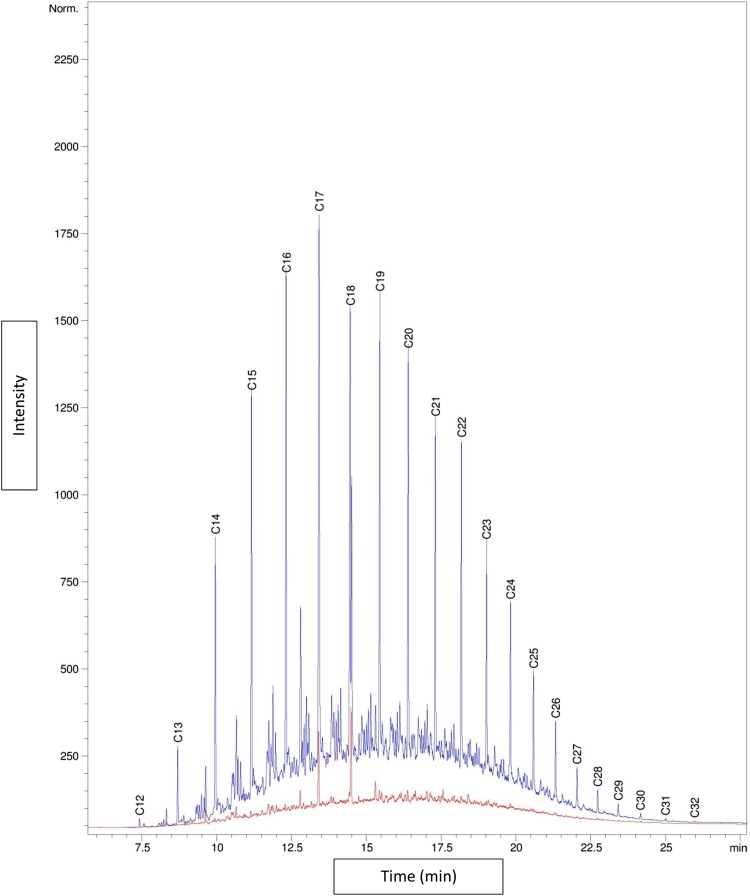
GC-SimDis analysis of carbon distribution of the hydrocarbon extracted from Lavan drill cutting suggests that the hydrocarbon phase is mainly composed of hydrocarbons ranging from C10 to C20 (Fig. 4) which is very close to diesel fuel composition.
Fig. 4.
Carbon distribution of petroleum compounds extracted from the drill cutting waste by GC-SimDis method
The initial abundance of heterotrophic and diesel-degrading bacteria was 1.42 × 107 cfu/g soil and 2.2 × 106 cfu/g soil for biologically active soil treatment and 104 CFU/gram and 5 × 104 CFU/g sand for fine sand treatment. Drill cuttings did not show any growth on R2A and BH agar media after 14 days of incubation, indicating that no bacterial activity exists neither for heterotrophic nor diesel-degrading bacteria. The salinity of B and S microcosms was about 0.26 and 0.29% respectively.
Microcosm studies
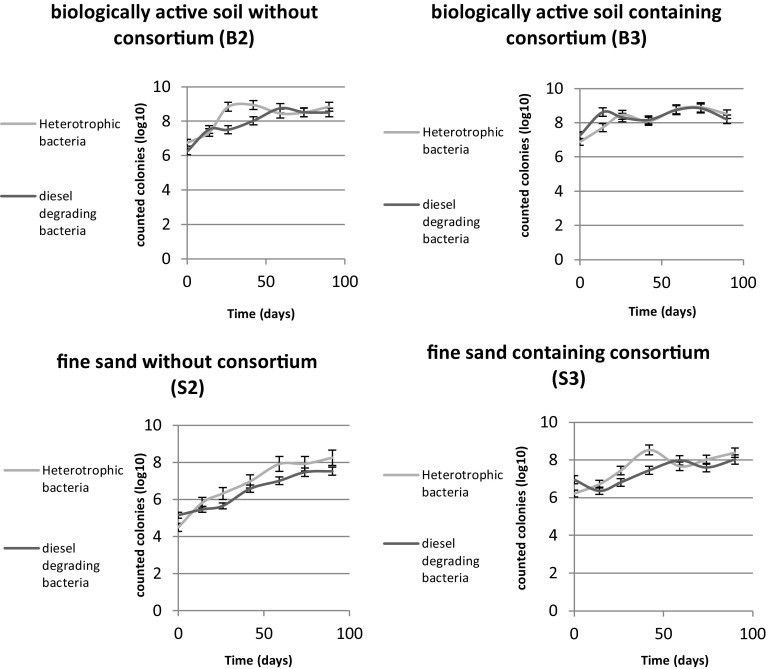
During the 3-month study, total counts of heterotrophic and diesel-degrading bacteria were surveyed for each microcosm in 2-week intervals (Fig. 5). The initial cell density of either heterotrophic or diesel-degrading bacteria of B2 and S2 microcosms increased after a short lag phase (probably due to the adaptation of native bacterial communities to diesel as the sole source of carbon). While in the bioaugmented microcosms, increasing of bacterial cell density was observed after a very short lag phase in S3 and almost with no lag phase in B3 microcosm, which could be expected due to inoculation of adapted consortium with the ability to degrade diesel.
Fig. 5.
Number of heterotrophic and diesel-degrading bacteria of each microcosm (except abiotic controls) during the 3 months. Results represent the means of three separate experiments, and bars indicate standard deviation. The absence of bars indicates that errors were smaller than symbols
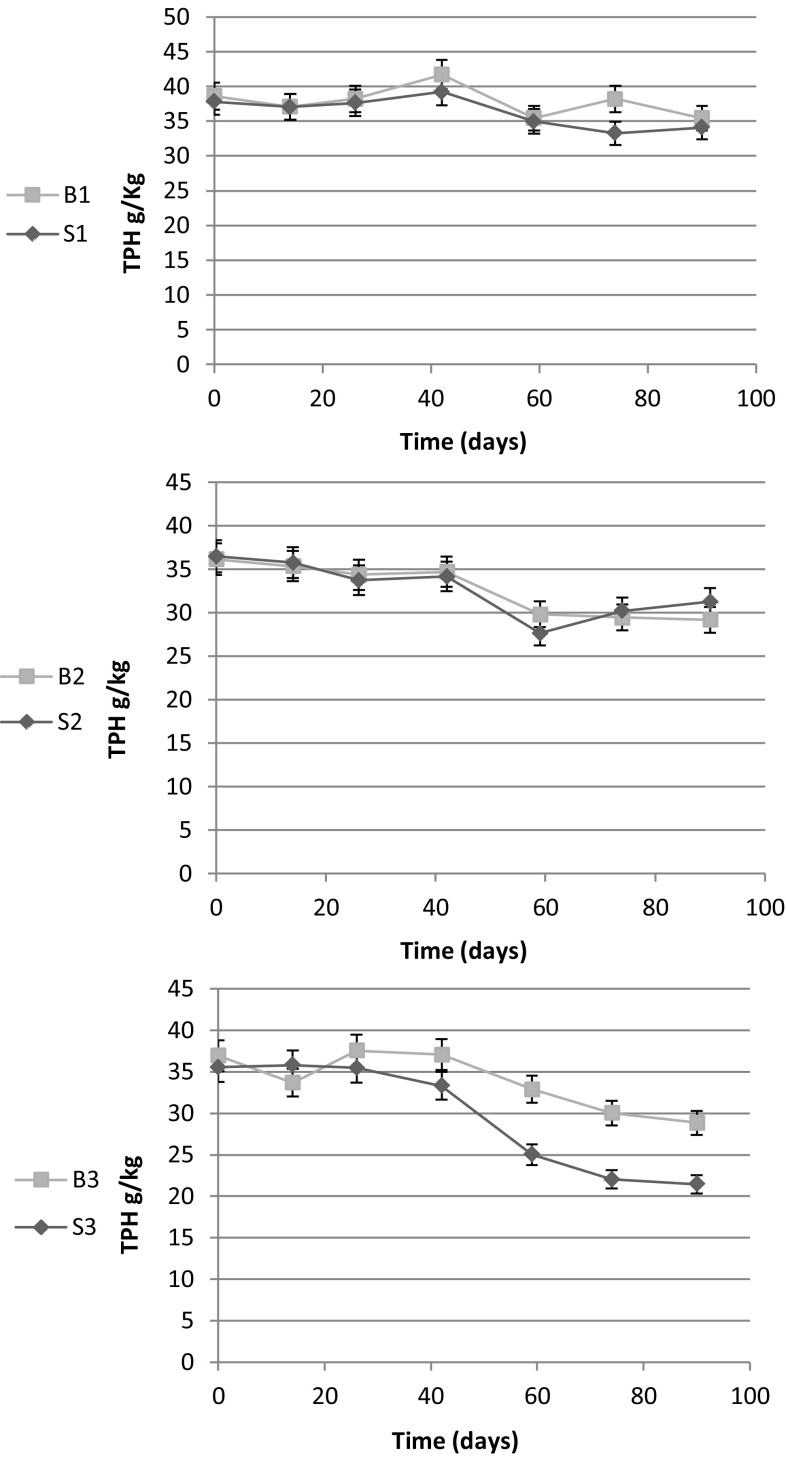
Both bioaugmented microcosms showed higher degradation rates in comparison with corresponding non-augmented microcosms based on analysis of the amount of residual TPH (g/kg soil) (Fig. 6). The highest degradation rate was measured for the S3 microcosm with approximately 40% TPH removal (reducing from the initial value of 36–22 g/kg) during the study period.
Fig. 6.
TPH concentration of each microcosm during the 3 months. Results represent the means of three separate experiments and bars indicate standard deviation. The absence of bars indicates that errors were smaller than symbols
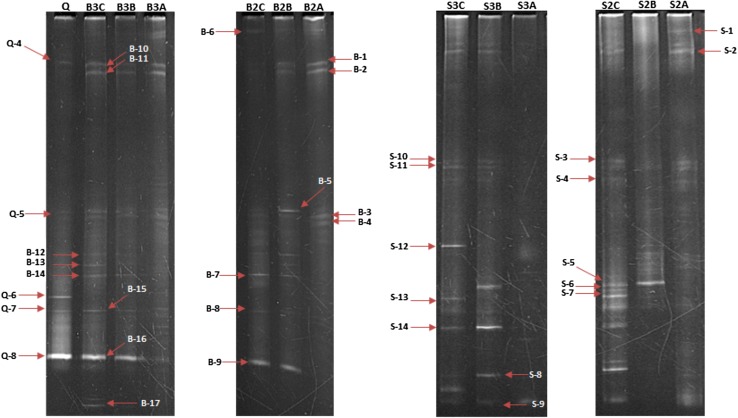
The DGGE profiles of each microcosm during first (first day of microcosm preparation), middle (day 45), and end stages (day 90) shows that the number and intensity of the bands in B2 and B3 microcosms is not significantly different from the beginning to the end point, while, in S2 and S3 treatments, the patterns of DGGE bands obviously change after addition of the consortium (Fig. 7). The phylogenetic relationships of re-amplified and sequenced DGGE bands of S and B microcosms are presented in Figs. 8 and 9 respectively.
Fig. 7.
DGGE profiles of B2, B3, S2, and S3 treatments of lab scale microcosms. A, B, and C Letters after the name of each treatment, respectively, refer to the first, day 45, and day 60 of sampling of each treatment. Q is referred to the inoculated consortium in B3 and S3 treatments. In each lane, bands pointed to arrows were re-amplified by non GC clamp primer and sequenced
Fig. 8.
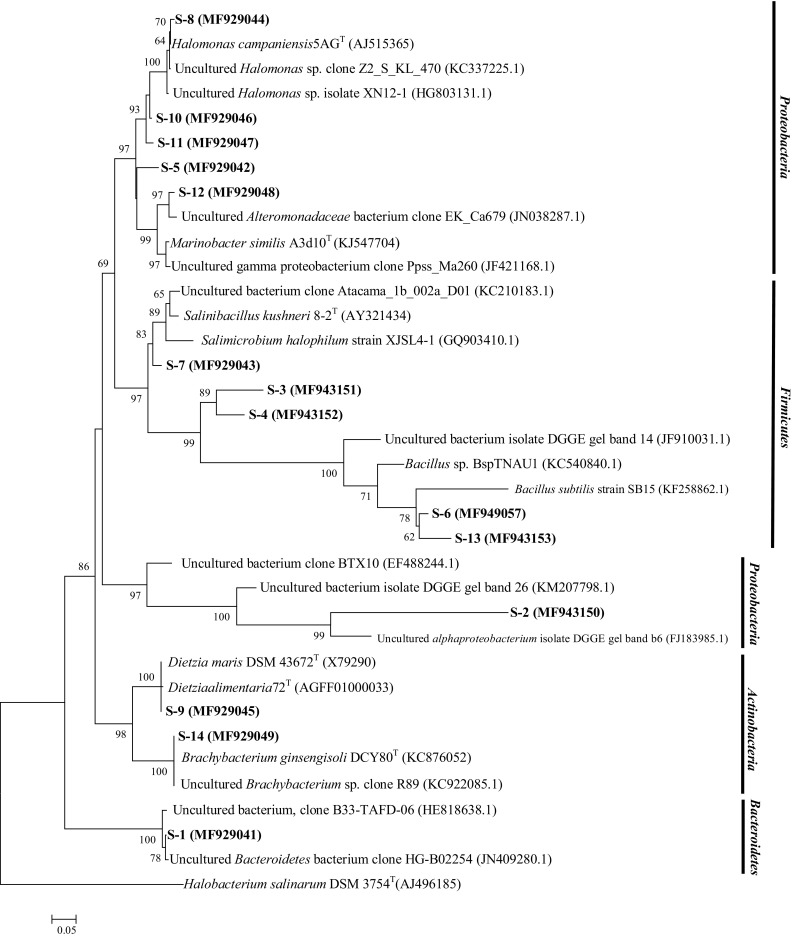
Phylogenetic relationships of sequenced bands of S2 and S3 microcosm treatments based on neighbor-joining algorithm. The accession number is shown in parentheses. Halobacterium salinarum DSM 3754 (T) is used as out-group
Fig. 9.
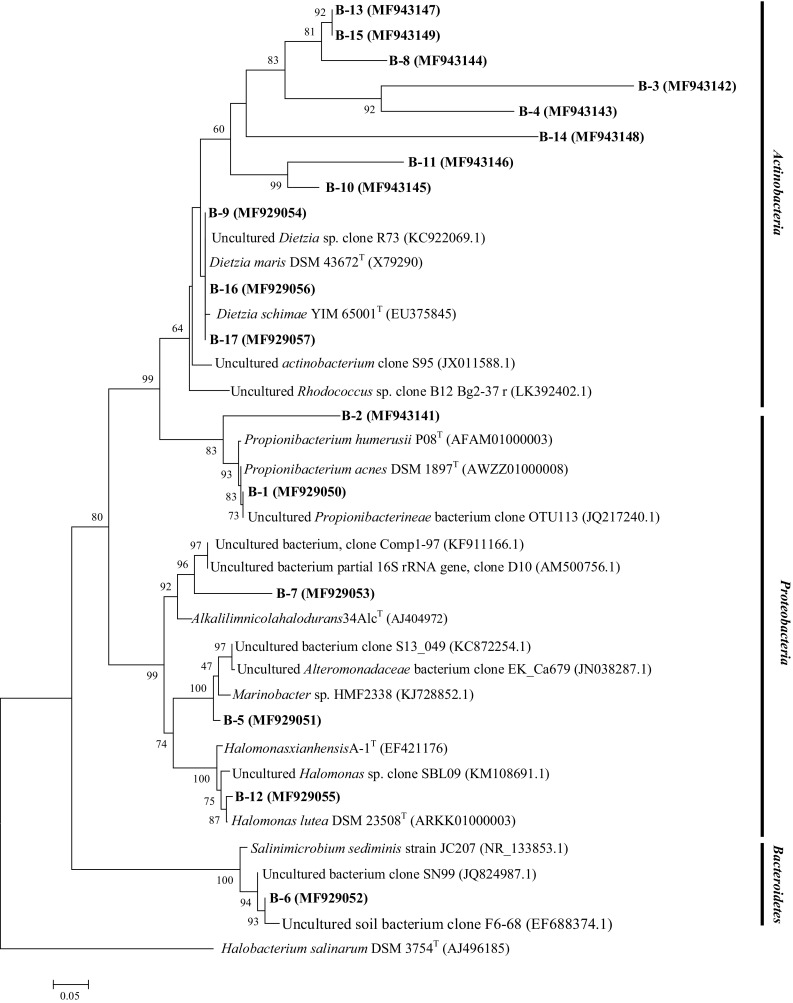
Phylogenetic relationships of sequenced bands of B2 and B3 microcosm trials based on neighbor-joining algorithm. The accession number is shown in parentheses. Halobacterium salinarum DSM 3754 (T) is used as out-group
Discussion
Consortium characteristics
In the bioremediation process, exploiting bacterial consortia for degradation of petroleum hydrocarbons is more preferable to pure cultures and its efficiency has been reported in different studies (Bento et al. 2005); however, the application of halophilic consortia for biodegradation of oil-polluted drill cuttings is not well studied.
In this study, the diesel degradation ability of the enriched consortium was confirmed by gas chromatograph. Subsequently, the bacterial community composition of the consortium was assessed using cultivation method and PCR-DGGE analysis of 16S rRNA gene sequences. The obtained results revealed that dominant members of the consortium belonged to the phyla Actinobacteria and Proteobacteria. Figure 3 indicates that abundant bacterial members of the microbial consortium belonged to the genera Dietzia and Halomonas which are well-known halophilic/halotolerant bacteria whose hydrocarbon biodegradation ability, especially under extreme conditions, has been reported in several studies (Duran and Cravo-Laureau 2016; Gharibzahedi et al. 2014; Koshlaf and Ball 2016). Another dominant member of the consortium belongs to the genus Arthrobacter whose ability to degrade aliphatic (Al-Awadhi et al. 2012) and aromatic hydrocarbons (Al Disi et al. 2017; Lacatusu et al. 2017) and biosurfactant production (Wijesekara et al. 2017) has been reported in the previous studies.
Among culture-dependent isolated strains, only Dietzia sp. Q1 show the ability to utilize diesel as the sole source of carbon without the presence of other members of the microbial consortium. It appears that the presence of all members of the consortium even though not able to utilize hydrocarbon as sole carbon source is necessary for efficient diesel degradation and removal and each member may have its own role in the degradation pathway of diesel.
Microcosm preparation
To study the applicability of the consortium in diesel degradation of drill cuttings, lab scale microcosms were designed. Drill cutting waste has very low permeability and porosity, which makes them unfitting for microbial growth and bioremediation. In the previous studies, researchers had blended drill cuttings with different bulking and diluting agents including soil, compost, sawdust, or wood chips as a source of degrading microorganisms or to improve the microbial activity (Ayotamuno et al. 2007; Rebekka 2002). Okparanma et al. evaluated the ability of a mixed culture of non-halophilic bacteria in degradation of aromatic hydrocarbons present in oil-based drill cuttings. They applied soil as diluting agent with the ratio of 6–1 (Okparanma et al. 2009). In another study conducted by Rastegarzadeh et al., synthetic drill cuttings blended with different ratios of soil as a source of microorganisms and the highest degradation have occurred at the ratio 1:1 (Rastegarzadeh et al. 2006). The drill cutting used in this study was a highly viscous sludge-like waste material with relatively high TPH (Table 2). Two different bulking agents (soil and fine sand) by the ratio of 1:1 [according to the previous studies (Rastegarzadeh et al. 2006)] were used to adjust the TPH content and increase the efficiency of bioremediation in microcosm studies. Because of the geographical location of Lavan Island, where drill cutting samples provided from, and its proximity to the coastal region, fine sand was considered as a readily available and less expensive diluting agent.
TPH measurement of microcosms
The highest degradation rate of diesel was seen in the microcosm containing fine sand amended by halophilic diesel-degrading consortium, which showed about 40% diesel removal (Fig. 6). Fine sand increases oxygen transfer to the various parts of the microcosm by improving the porosity and aeration of the medium and also enhancing nutrient availability, and, thus, provides more suitable conditions for growth and activity of microbial communities (Zazoua et al. 2012). Similarly, in a survey conducted by inoculating a mixed culture with high cell density of 1.5 × 1012 CFU/mL, the TPH content of the microcosm decreased from 223.52 mg/kg to below the detection limit after 6 weeks (Okparanma et al. 2009).
Reduced amount of initial TPH observed in the B1 and S1 microcosms during the study was probably due to physical removal mechanisms such as evaporation of volatile compounds.
Comparison of B2 and S2 microcosms (Fig. 6) indicated that the degradation extent in B2 (19.3%) was more than S2 (14.3%) which may be in consequence of higher initial cell density of B2 microcosm compared to S2 due to the presence of different micro-environments in the soil texture, which could harbor different microorganisms. Hydrocarbon-degrading bacteria are important part of microbial community even in clean soils. Prior to contamination with petroleum pollutants, natural processes in water and soil can produce trace amount of hydrocarbons. Hence, diesel degradation by bacterial communities of B2 trial containing soil sample without pollution history is not surprising (Marchal et al. 2003; Prince et al. 2010).
Heterotrophic and diesel-degrading Bacteria of microcosms
In the soil-amended microcosms (Fig. 5), by inoculating diesel-degrading consortium (in B3 microcosm), no significant changes occurred in the population of heterotrophic and diesel-degrading bacteria in comparison with control microcosm (B2). However, the addition of consortium to S3 microcosm resulted in a significant increase in the number of both heterotrophic and diesel-degrading bacteria that were approximately 100 times more than S2 microcosm.
The variation pattern in the number of heterotrophic and diesel-degrading bacteria was almost identical in all microcosms’ treatments especially in B3 and S2. This observation suggests that, over the time, the initial heterotrophic microorganisms, which are present in microcosms, will be replaced by diesel-degrading types and will constitute the dominant population. Moreover, in support of this statement, sequence analysis revealed that at the beginning, B2 microcosm showed relatively high diversity at the genus level (e.g., containing Propionibacterium, Salinimicrobium, Marinobacter, and Dietzia sp.) However, with the addition of consortium, especially to the B3 microcosm, few genera with hydrocarbon-degrading capability at moderate salinities [0.5–2.5 M concentrations of NaCl (Zhuang et al. 2010)] originated from the inoculated consortium, such as Dietzia, became dominant. As it is shown in Fig. 5, the final bacterial density of all four microcosms is almost the same after 3 months. Each environment has a certain and definite capacity for bacteria (Ueno et al. 2007) that might be the same in the mentioned microcosms. In this study, the initial microbial load and diversity of active soil, which has been added to drill cuttings, was higher than fine sand. In situations where most niches of an environment are occupied by indigenous bacteria, augmented microorganisms are unable to successfully reside and propagate; and, consequently, the degradation rate does not increase as much as expected.
DGGE analysis
The pattern of DGGE bands did not show considerable difference between B2 and B3 microcosms. However, by the addition of consortium, remarkable difference between DGGE profile of S2 and S3 microcosms occurred. In DGGE pattern of S3 microcosm, the number and diversity of bands increased from the beginning to the end of the experiment (S3B–S3C lane).
Sequencing of DGGE bands revealed that the bacterial diversity in sand/sand containing microcosm was similar at the end of investigation, so that most of dominant bacterial population belonged to the genera Dietzia and Halomonas. These findings suggest that, despite the initial differences in the composition of microbial community in the soil and fine sand microcosms, they finally end up with similar bacterial diversity. It was also reported previously that long-term exposure to diesel, especially at elevated salinities, resulted in population shift to halophilic genera, including Halomonas, Dietzia, and Alcanivorax (Kleinsteuber et al. 2006).
The presence of S-9 (belonging to Dietzia genus), S-11, and S12 bands (affiliated to Halomonas genus) in the DGGE profile of S3 microcosm (from the beginning of consortium addition to the end of treatment) confirmed the role of consortium members in the degradation process. The role of consortium addition also confirmed in the B3 DGGE picture by the presence of B-15, 16, and B-12 bands that belong to Dietzia and Halomonas genera, respectively. As it is evident from DGGE results, entrance of pollutants to a new environment will reduce the diversity by selecting the adapted bacteria to survive in harsh conditions (LaMontagne et al. 2004). Soil and fine sand have their own indigenous bacterial populations, which expose to high concentration of hydrocarbons after combining with drill cuttings. By the addition of consortium, in a competition between indigenous and exogenous bacteria, species with better degradation capabilities become dominant, in both fine sand and soil containing microcosms.
Conclusions
From the results of the microcosms study, it could be concluded that each environment has a specified capacity for harboring microorganisms. Therefore, applying bioaugmentation is effective if only the polluted soil has poor initial microbial flora in which augmentation of bacterial population improves degradation rate. Therefore, inoculating halophilic consortium to sand-amended drill waste was an effective strategy of bioremediation in the current study, due to low initial microbial density of the fine sand.
Acknowledgements
This research was supported by the Research Institute of Petroleum Industry (RIPI).
References
- Abdel-Aal HK, Aggour MA, Fahim MA. Petroleum and gas field processing. Boca Raton: CRC; 2003. [Google Scholar]
- Adekunle IM, Igbuku AOO, Oguns O, Shekwolo PD (2013) Emerging trend in natural resource utilization for bioremediation of oil—based drilling wastes in Nigeria. In Biodegradation-Engineering and Technology, 1st edn. InTechopen, London. pp 389–432
- Agwu OA, Ilori MO, Nwachukwu SU. Utilization of drilling fluid base oil hydrocarbons by microorganisms isolated from diesel-polluted soil soil and sediment contamination. Int J. 2013;22:817–828. [Google Scholar]
- Al Disi Z, Jaoua S, Al-Thani D, Al-Meer S, Zouari N. Considering the specific impact of harsh conditions and oil weathering on diversity, adaptation, and activity of hydrocarbon-degrading bacteria in strategies of bioremediation of harsh oily-polluted soils. BioMed Res Int. 2017;2017:8649350. doi: 10.1155/2017/8649350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ansary MS, Al-Tabbaa A. Stabilisation/solidification of synthetic petroleum drill cuttings. J Hazard Mater. 2007;141:410–421. doi: 10.1016/j.jhazmat.2006.05.079. [DOI] [PubMed] [Google Scholar]
- Al-Awadhi H, Al-Mailem D, Dashti N, Khanafer M, Radwan S. Indigenous hydrocarbon-utilizing bacterioflora in oil-polluted habitats in Kuwait, two decades after the greatest man-made oil spill. Arch Microbiol. 2012;194:689–705. doi: 10.1007/s00203-012-0800-7. [DOI] [PubMed] [Google Scholar]
- Al-Mailem D, Eliyas M, Radwan S. Oil-bioremediation potential of two hydrocarbonoclastic, diazotrophic Marinobacter strains from hypersaline areas along the Arabian Gulf Coasts. Extremophiles. 2013;17:463–470. doi: 10.1007/s00792-013-0530-z. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST : a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok B, Saxena S, Musarrat J. Isolation and characterization of four polycyclic aromatic hydrocarbon degrading bacteria from soil near an oil refinery. Lett Appl Microbiol. 1995;21:246–248. doi: 10.1111/j.1472-765X.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Ayotamuno M, Okparanma R, Nweneka E, Ogaji S, Probert S. Bio-remediation of a sludge containing hydrocarbons. Appl Energy. 2007;84:936–943. doi: 10.1016/j.apenergy.2007.02.007. [DOI] [Google Scholar]
- Bento FM, Camargo FA, Okeke BC, Frankenberger WT. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation biostimulation bioaugmentation. Bioresour Technol. 2005;96:1049–1055. doi: 10.1016/j.biortech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Boczkaj G, Przyjazny A, Kamiński M. A new procedure for the determination of distillation temperature distribution of high-boiling petroleum products and fractions. Anal Bioanal Chem. 2011;399:3253–3260. doi: 10.1007/s00216-010-4427-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck JD, Cleverdon RC. The spread plate as a method for the enumeration of marine bacteria. Limnol Oceanogr. 1960;5:78–80. doi: 10.4319/lo.1960.5.1.0078. [DOI] [Google Scholar]
- Cerqueira VS, Hollenbach EB, Maboni F, Vainstein MH, Camargo FA, Maria do Carmo RP, Bento FM. Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol. 2011;102:11003–11010. doi: 10.1016/j.biortech.2011.09.074. [DOI] [PubMed] [Google Scholar]
- Chmura A, Shapovalova A, Van Pelt S, Van Rantwijk F, Tourova T, Muyzer G, Sorokin DY. Utilization of arylaliphatic nitriles by haloalkaliphilic Halomonas nitrilicus sp. nov. isolated from soda soils. Appl Microbiol Biotechnol. 2008;81:371–378. doi: 10.1007/s00253-008-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C, Tan GA, Wong RC, Riddle MJ, Tan IK. DGGE fingerprinting of bacteria in soils from eight ecologically different sites around Casey Station, Antarctica Polar. Biology. 2009;32:853–860. [Google Scholar]
- Dastgheib SMM, Amoozegar MA, Khajeh K, Shavandi M, Ventosa A. Biodegradation of polycyclic aromatic hydrocarbons by a halophilic microbial consortium. Appl Microbiol Biotechnol. 2012;95:789–798. doi: 10.1007/s00253-011-3706-4. [DOI] [PubMed] [Google Scholar]
- Duran R, Cravo-Laureau C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol Rev. 2016;40:814–830. doi: 10.1093/femsre/fuw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign JC, Rittenberg SC. A crystalline pigment produced from 2-hydroxypyridine by Arthrobacter crystallopoietes n. sp. Archiv für Mikrobiologie. 1963;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Razavi SH, Mousavi M. Potential applications and emerging trends of species of the genus Dietzia: a review. Ann Microbiol. 2014;64:421–429. doi: 10.1007/s13213-013-0699-5. [DOI] [Google Scholar]
- Gojgic-Cvijovic G, et al. Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation. 2012;23:1–14. doi: 10.1007/s10532-011-9481-1. [DOI] [PubMed] [Google Scholar]
- Haddadi A, Shavandi M. Biodegradation of phenol in hypersaline conditions by Halomonas sp. strain PH2-2 isolated from saline soil. Int Biodeterior Biodegrad. 2013;85:29–34. doi: 10.1016/j.ibiod.2013.06.005. [DOI] [Google Scholar]
- Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW. Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl Environ Microbiol. 2006;72:3832–3845. doi: 10.1128/AEM.02869-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebria DY, Khodadadi A, Ganjidoust H, Badkoubi A, Amoozegar M. Isolation and characterization of a novel native Bacillus strain capable of degrading diesel fuel. Int J Environ Sci Technol. 2009;6:435–442. doi: 10.1007/BF03326082. [DOI] [Google Scholar]
- Kleinsteuber S, Riis V, Fetzer I, Harms H, Müller S. Population dynamics within a microbial consortium during growth on diesel fuel in saline environments. Appl Environ Microbiol. 2006;72:3531–3542. doi: 10.1128/AEM.72.5.3531-3542.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshlaf E, Ball AS. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2016;3:25–49. doi: 10.3934/microbiol.2017.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, León V, Materano ADS, Ilzins OA, Luis L. Biosurfactant production and hydrocarbon-degradation by halotolerant and thermotolerant Pseudomonas sp. World. J Microbiol Biotechnol. 2008;24:1047–1057. doi: 10.1007/s11274-007-9574-5. [DOI] [Google Scholar]
- Kurola J, Salkinoja-Salonen M, Aarnio T, Hultman J, Romantschuk M. Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiol Lett. 2005;250:33–38. doi: 10.1016/j.femsle.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Lacatusu AR, Lacatusu R, Dumitru M, Moraru IR, Vrinceanu A, Balaceanu C, Burtan L. Decontamination of a petroleum hydrocarbons polluted soil by different bioremediation strategies. Ann Univ Craiova Agric Montanol Cadastre Ser. 2017;46:326–334. [Google Scholar]
- LaMontagne MG, Leifer I, Bergmann S, Van De Werfhorst LC, Holden PA. Bacterial diversity in marine hydrocarbon seep sediments. Environ Microbiol. 2004;6:799–808. doi: 10.1111/j.1462-2920.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- Le Borgne S, Paniagua D, Vazquez-Duhalt R. Biodegradation of organic pollutants by halophilic bacteria and archaea. J Mol Microbiol Biotechnol. 2008;15:74–92. doi: 10.1159/000121323. [DOI] [PubMed] [Google Scholar]
- Li H, et al. Biodegradation of benzene and its derivatives by a psychrotolerant and moderately haloalkaliphilic Planococcus sp. strain ZD22. Res Microbiol. 2006;157:629–636. doi: 10.1016/j.resmic.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Marchal R, Penet S, Solano-Serena F, Vandecasteele J. Gasoline and diesel oil biodegradation. Oil Gas Sci Technol. 2003;58:441–448. doi: 10.2516/ogst:2003027. [DOI] [Google Scholar]
- Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208-IN201. [Google Scholar]
- Mukherjee AK, Bordoloi NK. Bioremediation and reclamation of soil contaminated with petroleum oil hydrocarbons by exogenously seeded bacterial consortium: a pilot-scale study. Environ Sci Pollut Res. 2011;18:471–478. doi: 10.1007/s11356-010-0391-2. [DOI] [PubMed] [Google Scholar]
- Okparanma RN, Ayotamuno JM, Araka PP. Bioremediation of hydrocarbon contaminated-oil field drill-cuttings with bacterial isolates African. J Environ Sci Technol. 2009;3:131–140. [Google Scholar]
- Paladino G, Arrigoni J, Satti P, Morelli I, Mora V, Laos F. Bioremediation of heavily hydrocarbon-contaminated drilling wastes by composting. Int J Environ Sci Technol. 2016;13:2227–2238. doi: 10.1007/s13762-016-1057-5. [DOI] [Google Scholar]
- Prince R, Gramain A, McGenity T. Handbook of hydrocarbon and lipid microbiology. Berlin: Springer; 2010. Prokaryotic hydrocarbon degraders; pp. 1669–1692. [Google Scholar]
- Rainey F, Klatte S, Kroppenstedt R, Stackebrandt E. Dietzia, new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. Int J Syst Evol Microbiol. 1995;45:32–36. doi: 10.1099/00207713-45-1-32. [DOI] [PubMed] [Google Scholar]
- Rastegarzadeh L, Nelson Y, Ririe TG (2006) Biotreatment of synthetic drill-cutting waste in soil. In: Sass BM (ed) Proceedings of the International Conference on Remediation of Chlorinated Recalcitrant Compounds: Monterey. Battelle Press, Colombus, OH. ISBN 1-57477-157-4
- Rebekka R. The potential for anaerobic mineralisation of hydrocarbon constituents of oily drill cuttings from the North Sea seabed. J Environ Monit. 2002;4:553–557. doi: 10.1039/b203991p. [DOI] [PubMed] [Google Scholar]
- Riazi M. Characterization and properties of petroleum fractions. West Conshohocken: ASTM International; 2005. [Google Scholar]
- Sánchez O, Gasol JM, Massana R, Mas J, Pedrós-Alió C. Comparison of different denaturing gradient gel electrophoresis primer sets for the study of marine bacterioplankton communities. Appl Environ Microbiol. 2007;73:5962–5967. doi: 10.1128/AEM.00817-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Ferguson M, Datta R, Birnbaum S. Bioremediation of petroleum hydrocarbons in contaminated soils: comparison of biosolids addition, carbon supplementation, and monitored natural attenuation. Environ Pollut. 2005;136:187–195. doi: 10.1016/j.envpol.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schwab A, Su J, Wetzel S, Pekarek S, Banks M. Extraction of petroleum hydrocarbons from soil by mechanical shaking. Environ Sci Technol. 1999;33:1940–1945. doi: 10.1021/es9809758. [DOI] [Google Scholar]
- Speight JG. The chemistry and technology of petroleum. Boca Raton: CRC; 2014. [Google Scholar]
- Turner KP. Bioremediation of drill cuttings from oil based muds. Nottingham: University of Nottingham; 2002. [Google Scholar]
- Ueno A, Ito Y, Yumoto I, Okuyama H. Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J Microbiol Biotechnol. 2007;23:1739–1745. doi: 10.1007/s11274-007-9423-6. [DOI] [PubMed] [Google Scholar]
- Varjani SJ, Upasani VN. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad. 2017;120:71–83. doi: 10.1016/j.ibiod.2017.02.006. [DOI] [Google Scholar]
- Vendeuvre C, Ruiz-Guerrero R, Bertoncini F, Duval L, Thiébaut D, Hennion M-C. Characterisation of middle-distillates by comprehensive two-dimensional gas chromatography (GC × GC): a powerful alternative for performing various standard analysis of middle-distillates. J Chromatogr A. 2005;1086:21–28. doi: 10.1016/j.chroma.2005.05.106. [DOI] [PubMed] [Google Scholar]
- Wijesekara S, Seneviratne M, Vithanage M. Agro-environmental sustainability. Berlin: Springer; 2017. Role of biosurfactants on microbial degradation of oil-contaminated soils; pp. 165–181. [Google Scholar]
- Zazoua A, Zazoua A, Taleb A, Jaffrezic-Renault N (2012) Potential of selected microbial strains to degrade the gasoil of hydrocarbon polluted soil. In: Proceedings of world academy of science, engineering and technology, vol 64. World Academy of Science, Engineering and Technology
- Zhuang X, Han Z, Bai Z, Zhuang G, Shim H. Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ Pollut. 2010;158:1119–1126. doi: 10.1016/j.envpol.2010.01.007. [DOI] [PubMed] [Google Scholar]