Abstract
Objective
The objective of this study was to review the literature to identify and summarize strategies for evaluating responses to physical exertion after mild traumatic brain injury (mTBI) for clinical and research purposes.
Data sources
PubMed and EBSCOHost through December 31, 2016.
Study Selection
Two independent reviewers selected studies based on the following criteria: 1) inclusion of participants with mTBI/concussion, 2) use of a measurement of physiological or psychosomatic response to exertion, 3) a repeatable description of the exertion protocol was provided, 4) a sample of at least 10 participants with a mean age between 8–65 years, and 5) the article was in English. The search process yielded 2,685 articles, of which 14 studies met the eligibility requirements.
Data Extraction
A quality assessment using a checklist was conducted for each study by two independent study team members and verified by a third team member. Data were extracted by a one team member and verified by a second team member.
Data Synthesis
A qualitative synthesis of the studies revealed that most protocols employed a treadmill or cycle ergometer as the exercise modality. Protocol methods varied across studies including differences in initial intensity determination, progression parameters, and exertion duration. Common outcome measures were self-reported symptoms, heart rate, and blood pressure.
Summary/conclusions
The strongest evidence indicates that exertional assessments can provide important insight about mTBI recovery and should be administered using symptoms as a guide. Additional studies are needed to verify an optimal modes and protocols for post-mTBI exertional assessments.
Keywords: exertion testing, concussion, mild traumatic brain injury
Introduction
Mild traumatic brain injuries (mTBIs), including concussions, affect an estimated 1.6 to 3.8 million individuals annually in the United States.1, 2 Evidence increasingly indicates that mTBIs may alter the central nervous system’s functioning leading to a variety of symptoms3 and leaving the brain vulnerable to further injury for an unknown period of time.4 Due to concerns that too much exertion in the acute recovery stages could impede recovery and potentially cause more damage,5, 6 the standard of care for mTBI management has historically been rest from physical and cognitive activities.6 However, recent studies and consensus statements increasingly emphasize that rest may be associated with prolonged symptoms and delayed recovery in at least some cases.7–9 Thus, clinicians face a clinical conundrum when treating patients with mTBIs: how to determine when a patient is physiologically ready to return to higher levels of physical exertion. Exertional tolerance assessments could provide a mechanism to evaluate tolerance to increased physical exertion in a controlled and safe environment.
According to the latest American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription, clinical exercise testing can be useful for assessing a variety of medical conditions.10 However, most of the research and protocols have been designed to assess patients with ischemic heart disease.10 Treadmills and cycle ergometers (or stationary bicycles) are the two most common modes for clinical exercise testing, both of which offer advantages and disadvantages.10, 11 Treadmill assessments may allow for testing higher levels of exercise capacity because evidence suggests peak exercise capacity during cycling can be 5%–20% lower due to regional muscle fatigue.10 However, cycling may be the preferred mode of exercise testing for individuals who have orthopedic, neurologic, or other safety-related considerations (e.g., impaired standing balance or gait).10–13 Additionally, cycling often entails less movement of arms and thorax, which can make it easier to obtain better quality physiologic measures and may be critical for the objective of the test in some cases.13
Regardless of the medical condition being assessed, clinical exercise testing protocols typically employ either a continuous approach (i.e., workload is consistent throughout test) or an incremental approach (i.e., workload is progressively increased as the test proceeds).10, 13 Some incremental protocols increase workload in relatively large adjustments every few minutes (Bruce protocol), while others use what is known as a ramping method whereby workload increases occur in a more constant, steady, and continuous manner (e.g., Ball State University/Bruce ramp).10, 13 Protocols with larger increases in workload increments such as the Bruce or Ellestad may be better suited for screening individuals who are younger and more physically active, to allow for optimally challenging exercise capacity within a more manageable total testing time.13 In contrast, ramp protocols or protocols with smaller incremental increases (e.g., Balke-Ware or Naughton protocols) may be preferable for older individuals, deconditioned patients, or patients for whom large, quick increases in exertion levels may be a safety risk.13
Published exercise testing standards convey that it is important to select an exercise testing mode and protocol for each patient relative to purpose of the evaluation, the specific information desired, and the individual characteristics of the patient being tested (e.g., age, symptomatology, safety concerns).10–12 Although there are a variety of consensus statements and general calls for the assessment of post-mTBI exertional tolerance to help determine physiological readiness to return to sport or identify physiologic impairment,4, 5, 14 there is currently no gold standard protocol for activity assessments in these regards. Due to the potential multi-system effects and co-morbidities associated with mTBIs (e.g., vestibular dysfunction, autonomic dysfunction, musculoskeletal/cervicogenic dysfunction), an optimal exertional testing method for this patient population may differ from the graded exercise and cardiovascular exertion testing commonly performed in other patient populations. Likewise, unique outcome measures or interpretation of outcome measures may be necessary to adequately identify mTBI-related impairments as part of the individual’s physiological responses to exertion. Therefore, the purpose of this study was to complete a systematic review of the literature in order to identify current clinical and research strategies and rationales for evaluating post-mTBI responses to physical exertion.
Methods
Data Sources
A literature search was conducted on July 15, 2016 using the following search terms: ((post concussion syndrome) OR (concussion) OR (mild traumatic brain injury) OR (closed head injury)) AND ((exercise test) OR (exercise assessment) OR (exercise) OR (exertion test) OR (exertion assessment) OR (exertion) OR (progressive exercise training) OR (autonomic assessment) OR (aerobic training) OR (aerobic rehabilitation) OR (active rehabilitation)). The search was performed using PubMed as well as an EBSCOHost package that included: CINAHL Plus with Full Text, MEDLINE with Full Text, Alt HealthWatch, Health Source-Consumer Edition, MEDLINE, SPORTDiscus with Full Text, Consumer Health Complete-EBSCOhost, and Academic Search Premier. The search was repeated on December 31st, 2016, and on July 20, 2017 to identify additional relevant studies that had been published since the initial search. The key word searches were also supplemented with manual searches of the bibliographies of the articles that were ultimately determined to meet the a priori inclusion and exclusion criteria and of any review articles that turned up in the initial search but were screened out during the abstract review stage. The search process was inclusive of grey literature (research disseminated outside of traditional commercial/academic channels) in order to minimize publication bias. New articles that were published or available as an electronic version ahead of print and fit the final search criteria were added to the search pool. All duplicates were removed to yield a final initial pool of articles for review.
Study Selection
Study selection was completed using two phases (a title/abstract screening phase, followed by full text review) and managed using a web-based systematic review management software (Distiller SR). The initial screening for relevancy based on the titles and abstracts was performed by two independent reviewers according to a pre-determined set of inclusion/exclusion criteria. Any disagreements were resolved by a third independent reviewer. An article was deemed qualified for the systematic review if it met the following criteria: 1) included human participants who had sustained an mTBI, 2) measured a physiological or psychosomatic response to exertion, 3) provided a detailed description of the exercise protocol, 4) included a sample of at least 10 participants with a mean age between 8–65 years, and 5) had a full-text available in English. A full-text review of the article was completed if qualification could not be determined by title and abstract alone. Any disparities were resolved by a third reviewer. Case studies, non-empirical research articles, review papers, animal tests, and experiments done only in moderate or severe TBI populations were excluded.
Data Extraction and Quality Appraisals
Data extraction was completed by a single reviewer and verified by a second reviewer. Extracted characteristics included purpose, sample, exercise modality, protocol details, relevant outcome measures, results, and limitations. Because the studies were not the same study design and no existing quality assessment tools directly met our needs for our descriptive-oriented research question, we created a unique set of items modified from other critical appraisal tools (i.e., The Cochrane Risk of Bias Tool15 and the Joanna Briggs Institute Critical Appraisal Tools Checklist for Cohort Studies16) to assess bias and relevance to our review purpose (see Table 1). The highest possible score was 10. It is important to note, that the ratings provided for each item on the appraisal tool were directly related to the purposes of the current systematic review. Therefore, a lower rating on an individual item or a lower overall score would not necessarily reflect a poorer quality study for the study’s original purpose. Rather, a lower rating on the checklist here, simply reflects less direct relevancy or lower quality of study relative to the purposes of this systematic review. The methodological quality checklist was completed for each study individually by two reviewers. Any discrepancies were resolved and verified using a consensus generation process with a third reviewer.
Table 1.
Quality Appraisal Checklist Results
| Cordingley 2016 | DeMatteo 2014 | DeMatteo 2015 | Gall 2004a | Gall 2004b | Grabowski 2017 | Hinds 2016 | Kozlowski 2013 | Kurowski 2017 | Leddy 2010 | Leddy 2011 | Manikas 2016 | Moore 2016 | Slobounov 2011 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Did the study follow a prospective design? (1=yes) | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Was a rationale regarding exertional testing mode provided by the authors? (1=yes) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Was a rationale regarding exertional testing protocol type provided by the authors? (1=yes) | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Has the protocol been shown to be reliable or valid, either directly or through previous research? (1=yes) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Were funding sources or lack thereof described? (1=yes) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Was acknowledgment of conflict of interests or lack thereof provided? (1=yes) | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Was evaluating physiologic response to exertion a primary purpose for the study (i.e. highly relevant for the purpose of the systematic review vs indirectly relevant)? (1=yes) | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Were there any significant risks of bias concerns relative to the outcomes of interest for this systematic review?* (1=no) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Were potential confounding factors discussed? (1= yes) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Were appropriate statistical analyses used? (1=yes) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| TOTAL | 6 | 6 | 8 | 5 | 7 | 5 | 7 | 6 | 5 | 7 | 7 | 6 | 8 | 5 |
This table provides the individual ratings for each included study relative to the quality appraisal form created by the authors of this systematic review. It should be noted, that this set of questions was generated for the specific purposes of the current systematic review. Therefore, a lower score does not necessarily indicate a poorer quality study in general, but rather lower direct relevancy or lower level quality of study for the purposes of this systematic review.
Criteria based on the Cochrane Risk of Bias Tool retrieved April 10, 2017 from http://handbook.cochrane.org. It should be noted, that this set of questions was generated for the specific purposes of the current systematic review. Therefore, a lower score does not necessarily indicate a poorer quality study in general, but rather lower direct relevancy or study design for the purposes of this systematic review.
Synthesis of Post-mTBI Exertional Testing for Research and Clinical Purposes
The authorship team identified key themes relative to the current post-mTBI exertional testing clinical and research strategies and rationales through a comprehensive review and comparison of the studies that met the inclusion and exclusion criteria for the systematic review. The themes were initially derived and drafted by the first author after considering the direct relevancy of each study for the systematic review purposes and evaluating the study design strengths and weaknesses, investigators’ rationales for selecting exertional testing protocols and parameters, and investigators’ discussions regarding post-mTBI exertional assessments and interventions. The other authors of this manuscript then discussed these themes both in-person and via written interactions as part of the development of the manuscript to generate a consensus on the themes, the presentation and discussion of the themes, and the analysis of the current state of the evidence. Additionally, the themes further evolved over the course of the peer review process through thoughtful critiques and feedback provided by the reviewers and editors.
Results
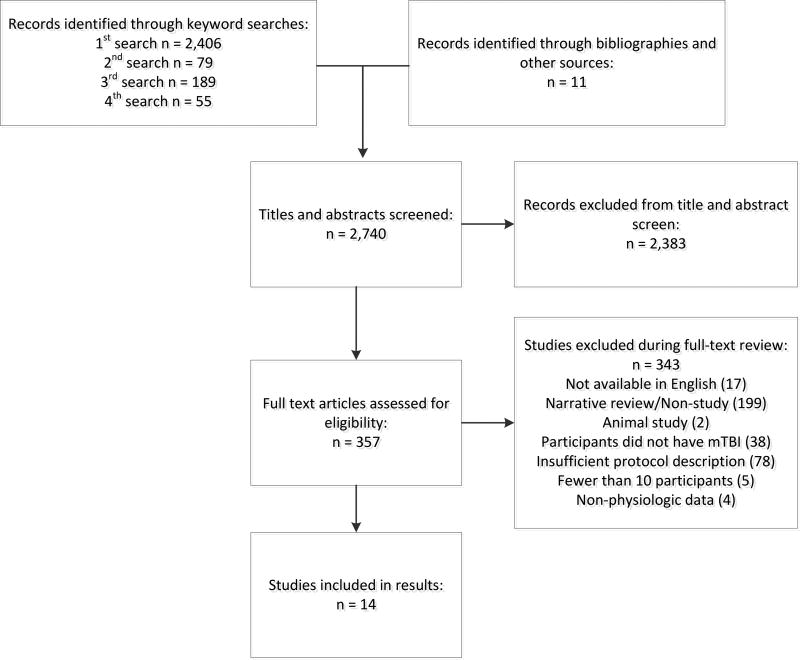
A total of 2,406 potential records were identified through the initial key word search (Figure 1). The second search yielded an additional 79 potential records and the third search identified 189 potential records. Eleven additional sources were found through a review of bibliographies for a total of 2,685 potential records. After the full text review relative to the eligibility criteria, 14 studies were identified as appropriate for the intended purposes of this review. Quality appraisals for each included study are provided in Table 1 and detailed study descriptions as they relate to the systematic review study question are provided in Table 2.
Figure 1.
PRISMA Diagram of Search and Record Selection Process
Table 2.
Exertion Assessment Protocols
| Author | Purpose and Study Design |
Sample | Protocol Exertion Mode, Type, and Rationale |
Protocol Intensity and Progression Parameters |
Relevant Observed Measures |
Relevant Results | Pertinent Study Limitations |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cordingley, 2016 | Retrospective case series evaluating safety, clinical use, and patient outcomes | 106 patients (46 M, 60 F); Mean age 15.1 years, range of 11–19 years SD 1.5 years; Median days from injury until initial testing 8 days (IQR 5–22 days) | Mode and Type: Treadmill with incremental progressions in treadmill grade (modified Balke protocol/Buffalo Concussion Treadmill Test) |
|
|
|
|
| Rationale: No specific rationale provided for mode, but protocol choice was noted as selected based on prior studies | |||||||
|
| |||||||
| DeMatteo, 2014 | Cross-sectional case series study evaluating the Nintendo Wii games for assessing return to activity readiness | 24 participants (14 M, 10 F); Mean age 14.9 years, range of 9–18 years, time since injury range of 1–12 months with a mean of 5.5 months and SD of 3.68 months | Mode and Type: 6 Wii Games of progressive intensity |
|
|
|
|
| Rationale: A rationale for Wii games for assessment was described as a way to assess functional mobility and exertion. No specific rationale was provided for the games selected or protocol specifically | |||||||
|
| |||||||
| DeMatteo, 2015 | Case series of a single exercise testing bout with repeated measures of response to exertion and recovery from exertion | 54 participants with recent mTBI (32 M, 22 F) Mean age of 14.8 years, SD of 2.3. Time from most recent injury range of .7–35.3 months with median of 4.1 months | Mode and Type: Cycle ergometer with incremental progressions in work rate (McMaster All-Out Progressive Continuous Cycling Test) |
|
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
|
| |||||||
| Gall, 2004a | Repeated measure cohort comparison study of 2 exercise testing bouts within 72 hours of being asymptomatic at rest and again 5 days later | 14 hockey players with mTBI and 14 players without mTBI matched 1:1 by investigators, no report of sex of participants in sample, mean age of participants reported by cohorts | Mode and Type: Cycle ergometer with 10 minutes of steady-state cycling, followed by high intensity intervals | Low-moderate steady-state exercise at 80–90 rpm against a constant load of 1.5W/kg of body weight for 10 minutes. High intensity bouts: 40 seconds at 4.7 W/kg with a pedaling frequency of 90–100 rpm, followed by a 20 second free pedal (30 W), and then 20 second rest period - Test continued until the participant could no longer maintain the workload |
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
|
| |||||||
| Gall, 2004b | Repeated measure cohort comparison study of 2 exercise testing bouts within 72 hours of being asymptomatic at rest and again 5 days later | 14 hockey players with mTBI and 14 players without mTBI matched 1:1 by investigators, no report of sex of participants in sample, mean age of participants reported by cohorts | Mode and Type: Cycle ergometer with 10 minutes of steady-state cycling, followed by high intensity intervals | Low-moderate steady-state exercise at 80–90 rpm against a constant load of 1.5W/kg of body weight for 10 minutes. -High intensity bouts: 40 seconds at 4.7 W/kg with a pedaling frequency of 90–100 rpm, followed by a 20 second free pedal (30 W), and then 20 second rest period - Test continued until the participant could no longer maintain the workload |
|
|
|
| Rationale: The authors noted that the protocol was designed to reflect high intensity intervals that often occur while playing ice hockey. | |||||||
|
| |||||||
| Grabowski, 2017 | Retrospective case series evaluating the implementation, safety, and feasibility of multimodal impairment-based physical therapy (including vestibular therapy, manual therapy and exercise therapy for cervical and thoracic spine, and aerobic training) | 25 patients (11 M/14 F) mean age of 15 years (range 12–20 years) following sport-related concussion with a mean of 41 days post-injury (range 21–228 days) prior to first visit | Mode and Type: Patient specific treadmill (modified Balke protocol/Buffalo Concussion Treadmill Test) vs cycle ergometer incremental increases in bike resistance |
|
|
|
|
| Rationale: The treadmill protocol was the default, with the cycle ergometer used according to patient preference and/or in the presence of significant vestibular symptoms in order to minimize head movement | |||||||
|
| |||||||
| Hinds, 2016 | Case series and case-control comparisons using a repeated measure design investigating exercise assessment while symptomatic and after deemed recovered | 40 athletes (23 M, 17 F) ages 12–18 years (mean 15.5 years) and comparison of 30 athletes without a history of recent concussion (18 M, 12 F) | Mode and Type: Treadmill: Incremental progressions in treadmill grade (modified Balke protocol/Buffalo Concussion Treadmill Test) |
|
|
|
|
| Rationale: No specific rationale provided for mode, but protocol choice was noted as selected based on prior studies | |||||||
|
| |||||||
| Kozlowski, 2013 | Case control comparisons for a single exercise bout comparing exercise tolerance for individuals with persistent postconcussion symptoms compared to a healthy control cohort | 59 participants 34 injured (17 M, 17 F; mean age = 25.9 SD 10.9 years), 22 controls (11 M, 11 F; mean age = 23.3 SD 6.2 years) | Mode and Type: Treadmill Walking: Incremental progressions in treadmill grade (modified Balke protocol/Buffalo Concussion Treadmill Test) | Constant speed of 3.3 miles per hour, starting at 0% incline. After 1 minute, fixed 2% grade increase; after 2 minutes, fixed 1% grade increase each minute until speed could not be maintained, post-mTBI symptoms returned, or max of 21 minutes was reached |
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
|
| |||||||
| Kurowski, 2017 | Randomized clinical trial investigating aerobic exercise training versus a stretching control group for a 6 week program | 30 adolescents (13 M, 17 F ages 12–17 years between 4 – 16 weeks post-injury with 87% reporting regular participation in an organized sport; randomization was performed within stratified age and gender blocks | Mode and Type: Cycle ergometer with incremental progressions in intensity | Self-selected speed consistent with their personal Borg rate of perceived exertion of level 11 (fairly light pace) with fixed resistance at level 2, progressions of intensity occurred every five minutes with participants increasing workload by a Borg rating of 1 level until they started to experience an increase in symptoms or for a maximum of 30 minutes |
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
|
| |||||||
| Leddy, 2010 | Case series of exercise training with pre- and post- intervention exertional testing | 13 participants (7M, 5F) 6 – 52 weeks post-injury, with age range of 16 – 53 years (mean = 27.9, SD 14.3 years); 6 of 12 participants were athletes | Mode and Type: Treadmill Walking: Incremental progressions in treadmill grade (modified Balke protocol/Buffalo Concussion Treadmill Test) |
|
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
|
| |||||||
| Leddy, 2011 | Case series with participants who had a recent concussion to assess retest reliability on effort and physiologic measures and a set of actors without concussion to evaluate interrater reliability to determine symptom exacerbation | 21 participants with concussion (11 M, 10 F), age range of 1554 (mean = 29.8, SD 14.8 years) who were an average of 33.2 weeks postinjury, 11 were athletes; 10 healthy, sedentary participants (4 M, 6 F) age range of 18 – 45 years (mean = 26.5, SD 8.2 years) | Mode and Type: Treadmill Walking: Incremental progressions in treadmill grade (modified Balke protocol/Buffalo Concussion Treadmill Test) |
|
|
|
|
| Rationale: No specific rationale provided for mode, but protocol choice was noted as selected based on prior studies | |||||||
|
| |||||||
| Manikas, 2016 | Experimental study investigating the impact of exercise on symptom exacerbation and neurocognition at 2 time points (Days 2 and 10 post selfreport of symptom resolution | 30 patients (25 M, 5 F) between the ages of 10 and 17 years of age; participants were tested two days and ten days after they reported resolution of symptoms; mean of 5.4 (range of 0–24) days until self-report of resolution of symptoms | Mode and Type: Cycle ergometer-modified version of the McMaster All-Out Progressive Continuous Cycling Test |
|
|
|
|
| Rationale “shown to be suitable for children” | |||||||
|
| |||||||
| Moore, 2016 | Case series describing functional changes in adults with persistent post mTBI symptoms and disability after completing a supervised home exercise program with combination of vestibular and aerobic training | 14 patients (6 M, 8 F) median age of 43 years (range 18–72) referred for vestibular rehabilitation; median time between injury and initial evaluation was 107 days (range 14–992) | Mode and Type: Cycle ergometer using a modified Balke protocol/Buffalo Concussion Test |
|
|
|
|
| Rationale: A cycle ergometer was used instead of a treadmill due to the likelihood of head excursion that occurs while walking or running on a treadmill and to minimize conflicting sensory stimulation; no rationale for Balke protocol was specifically cited though prior mTBI studies were cited | |||||||
|
| |||||||
| Slobounov, 2011 | Observational cohort comparison study of brain connectivity patterns at rest and in response to a physical exertion test | 17 college athletes with a recent concussion and 17 college athletes with no history of concussion | Mode and Type: Cycle Ergometer: 4 stages of increasing resistance | Resistance determined by YMCA stress test, 4 stages of increasing resistance, 3 min per stage, progression to next stage was determined by heart rate |
|
|
|
| Rationale: No specific rationale provided for mode or protocol selection | |||||||
M = male, F = female; HR = heart rate; RPE = Ratings of perceived exertion
Sample Characteristics
Seven studies included samples with a mean age or age range of less than 18 years,17–22 while the other seven utilized samples with a mean age of 18 years or older.23–29 Four studies used a sample consisting of only athletes,23–25, 30 three studies reported using a combination of athletes and non-athletes,27, 28, 30 and seven studies were unclear in their reporting of athletes versus non-athletes within their samples.17–20, 22, 26, 29
Five studies performed exertional assessments with participants who were in the relatively acute post-concussion phases (1–3 weeks post injury).18, 22–25 Seven studies used a sample of participants who were in a more sub-acute to chronic phase of recovery (3 weeks or more post injury).17, 26–31 One study was a retrospective chart review that included exertion testing examples in both acute and more chronic patients.20 The remaining study appeared to have an initial test that was performed in the acute phase and a second test when the participants were asymptomatic, but the timing of the second test was unclear.19
Exertion Protocols
Five of the 10 studies utilized a modified Balke progressive treadmill test referred to as the Buffalo Concussion Treadmill Test.19, 20, 26–28 Seven of the studies used various forms of a cycle ergometer, or stationary bicycling test.17, 22–25, 29, 31 One study utilized either a treadmill or cycle, depending on patient presentation and preference.30 One study evaluated Nintendo Wii games.18 All 14 of the studies entailed some form of intensity progression in the protocol. See Tables 1 and 2 for further details about study design and exertion testing protocols relative to the purposes of this review.
Outcome Measures
As the nature of the study questions varied among studies, a variety of outcome measures were used for the overall body of included studies. Multiple physiological and psychosomatic measures were used to assess response to exertion. All of the studies assessed and evaluated heart rate in some capacity, however one study also specifically considered heart rate variability metrics.23 Eight of the studies included a post-concussion symptom checklist or assessment,17, 24–26, 28–31 five studies evaluated blood pressure,20, 26–29 and six evaluated rate of perceived exertion.17, 19, 22, 26, 28, 29 Additional outcome measures (e.g., blood lactate levels, exercise duration, oxygen saturation and V02 estimates) were also measured in several studies.17, 23, 24, 28, 29 }
Quality Appraisal Results
All but two of the studies used a prospective study design.20, 30 Only three of the studies provided specific rationales for the mode used for exertion testing.18, 29, 30 Five studies provided a rationale for exertional testing protocol type,19, 20, 24, 29, 30 however, most of the rationales simply described that prior research on mTBI had used similar protocols. Seven of the studies investigated specific aspects of the body’s response to an exertional test,17, 19, 20, 23, 24, 32,26–28 while this was an indirect purpose for the other studies.
Only the Buffalo Concussion Treadmill Test had available data associated with reliability relative to mTBI,19, 20, 26–28, 30 while the studies that used the cycle ergometer and Nintendo Wii had no established data on the reliability or validity relative to samples with mTBI. One study specifically evaluated the retest reliability and interrater reliability of the Buffalo Concussion Treadmill Test.27 Specifically, the study found that the Buffalo Concussion Treadmill Test had good test retest reliability for assessment of maximum heart rate in participants with a concussion and healthy controls and that raters achieved relatively high sensitivity (99%) and specificity (89%) for identifying participants with and without mTBI symptom exacerbation. It is important to note, however, that the study only had 11 participants with mTBI who were an average of 33.2 weeks post-injury.
Synthesis of Key Findings from Included Studies
Collectively, the body of evidence identified through the systematic search process was relatively limited with a variety of study designs, most of which were case series or case-control comparison studies. Given the wide variability in study designs, sample characteristics, and exertion testing protocols, a quantitative synthesis of the results of the studies was deemed inappropriate. Instead, a qualitative synthesis was used to identify key themes relative to the current clinical and research strategies found in the body of evidence for clinical evaluation and studying post-mTBI responses to physical exertion.
In general, the studies identified for this review provided foundational evidence that post-mTBI exertional testing can be safe and useful for identifying residual impairments including impaired physiologic responses to exertion (e.g., symptom emergence/exacerbation, altered heart rate variability with exertion, and blunted heart rate response to exertion). Cordingley et al. specifically found that the Buffalo Concussion Treadmill Test can be well-tolerated and safe for many youth ages 11–19 years even in the relatively acute stages of recovery.20 A general expectation should be that exertional testing may exacerbate symptoms in many patients, however, this should be temporary. As DeMatteo et al’s findings suggest, many patients may initially experience a worsening of symptoms with exertion, but within 24 hours many report an improvement in symptoms that exceeds the level of baseline symptoms reported prior to the exertional test.17 Moreover, if certain metrics are utilized (e.g., heart rate variability), physiologic impairment may be discernable during exertion in ways that are unable to be captured through clinical assessments performed at rest.19, 23, 24
Overall, optimal testing mode and protocols for assessing and studying post-mTBI exertional responses remains unclear based on the current body of evidence. The studies identified for this systematic review primarily utilized either treadmill testing or cycle ergometer testing, with a modified Balke (Buffalo Concussion Treadmill Test) being the most frequently used in the literature. The rationales investigators used for selecting these modes and protocols were limited and lacking specific empirical and theoretical justifications. Additional, very little reliability and validity testing has been performed with the protocols. These are critical areas to clarify in order to improve the utility of exertional testing for clinical and research purposes.
Discussion
The purpose of this study was to review the literature to identify and summarize strategies for evaluating responses to physical exertion after mild traumatic brain injury (mTBI) for clinical and research purposes. Protocol characteristics for mode, workload, intensity, progression procedures, and test cessation determinants varied greatly across studies. The outcome measures used were inconsistent and often unique to each study’s specific questions and design. The sample characteristics also varied widely across studies in terms of sex, age, time since injury, and pre-injury physical activity levels. Collectively, the large differences in protocols, sample compositions, and application of outcome measures greatly constrain the ability to draw strong conclusions about the best strategies to use for post-mTBI exertional tests. Further research is needed to provide more clarity around specific protocols and measures that can best identify post-mTBI exertion-related impairments. The benefits and limitations for the protocols and metrics identified through the systematic search process are discussed below to provide suggestions for refinement and optimization of post-mTBI exertional testing in future studies.
Exertional Testing Mode
A concern that may be particularly unique to exertional testing with patients with mTBI as compared to other patient populations who are frequently assessed with graded exercise tests (e.g., patients with cardiovascular disease) is the potential for a protocol to trigger symptoms that may confound the ability to specifically identify exertional intolerances.33 For example, walking or running may trigger vestibular or oculomotor symptoms due to the visual and movement cues the body must process while on the treadmill. They may also trigger musculoskeletal or cervicogenic-related symptoms because of the forces that may go through the cervical spine or added stress on the neck and scapular postural muscles. This suggests that it may be important to perform impairment specific assessments (e.g., oculomotor, vestibular, and cervicogenic/musculoskeletal) in conjunction with exertional tolerance assessments.
Several investigators in the current review postulated that treadmill testing may be difficult or inappropriate for some patients.29, 30 Additionally, since the symptoms associated with vestibular, oculomotor, and cervical spine related impairments are very similar to exertional intolerance impairments (e.g., headache, dizziness, nausea) and are also common after concussion, assessments with a high probability of producing vestibular, oculomotor, and cervical spine impairments may make it challenging to distinguish exertional-related impairments from these other impairments after concussion. There is potential that stationary biking may provide specific insight into physiological exertional tolerance by reducing the risk for confounding symptoms, however, no studies specific to post-concussion assessments have directly evaluated this contention. Additionally, stationary biking assessments may be less functional and transferable to everyday skills, particularly for those who engage in activities that require higher fitness levels.33 This logically calls into question whether a single, gold-standard exertional testing mode can be identified for this patient population or if there is a need to demarcate certain types of testing modes relative to specific clinical and/or research questions.34, 35
Another important future research question to investigate is whether certain patient attributes lead to distinctive responses to different modes of testing. For example, patients who have previously used treadmills or stationary bicycles may tolerate these testing modes better than those who have not.36 Other types of questions along these lines include: 1) do older patients respond differently to different modes of exertion testing compared to younger patients, 2) do post-mTBI athletes respond differently than non-athletes to different modes of exertion testing, and 3) does time since injury influence feasibility and effectiveness of different modes of exertion testing? All of these questions speak directly to the validity of post-mTBI exertional assessments and underlie justifications for why a clinician or researcher may choose to use one mode of exertion testing over another. Therefore, future studies investigating post-mTBI exertional testing modes in these regards would be beneficial.
Exertional Testing Protocols
All of the identified studies followed various levels of workload intensity. However, determinants for starting workloads, progressions of the workload, and the duration of the testing varied significantly across the studies. Additional studies comparing these parameters may help inform the determination of optimal testing protocols for use post-mTBI. In addition, all of the studies utilized staged progressions, whereby intensity was increased incrementally instead of continuously. Consequently, it may be hard to determine uniformity of responses and expected responses. For researchers interested in characterizing the mechanistic impairments that may be present post-mTBI, a ramped protocol, which increases work rate in a constant and continuous manner (as compared to staged progressions which introduce larger jumps in workload) may allow for more accurate estimates of exercise capacity, ventilatory threshold, and autonomic responsiveness to exertion.13, 36 Regardless, it is clear that future studies that more comprehensively consider the theoretical foundations for protocol selection and empirically compare protocols for patients with mTBI would be beneficial.
Outcome Measures
Collectively, the studies indicated that mTBI may lead to changes in physiologic responses to exertion. However, the metrics used to capture altered physiologic responses may differ in their capacity to capture mTBI impairments as well as their clinical and research utilities. For example, many of the studies evaluated symptom responses to exertion, with mTBI-related symptom onset or exacerbation serving as test termination criteria. Symptom monitoring is a clear and useful way to help screen for readiness to return to higher levels of exertional activities as well as help identify patients who may benefit from progressive, active rehabilitation protocols.3, 5, 26–28, 31, 34, 37 Nonetheless, symptom assessments are subjective and highly variable within and across patients. Additionally, there is insufficient evidence to clearly determine that physiologic impairments are not present even though a patient may be asymptomatic. For these reasons, there is a great need for further studies to identify more objective means to evaluate atypical physiologic responses to exertion as well as clarify implications for impaired physiologic responses to exertion relative to function and further injury risk.
The studies identified in this review highlighted some potential options for more comprehensive studies into metrics that may and may not be as useful for evaluating post-mTBI physiologic impairments. It has been theorized that mTBI can lead to a disruption of the autonomic nervous system and autoregulatory functions of the body3. Patients with persistent post-mTBI symptoms may have autonomic systems that are in a state of disequilibrium,3 which could help explain the variety of impairments that emerge when they are confronted with physical and cognitive challenges.40, 41, 43 Effectively capturing this phenomenon, however, may not be as simple as measuring heart rate and blood pressure as is done with conventional clinical screening techniques and exercise testing metrics used for other patient populations (e.g., cardiovascular exercise testing) because heart rate and blood pressure may not appear outside of what is expected as a normal response. However, protocols and metrics that specifically seek to consider autonomic responsiveness may reveal important differences between healthy and impaired post-mTBI states (e.g., heart rate variability).23 Systematic studies specifically evaluating typical and atypical responses to exertion with a robust set of physiologic metrics are needed to provide a more comprehensive understanding of which metrics are most useful, relative to different protocols, and for which specific purposes.
Overall Considerations
As specified in the American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription, the exercise modality, protocol, and outcome measures employed should be based on the purpose of the testing, the specific information desired, and the characteristics of the individual being tested.33 There is a robust literature on exercise testing for cardiologic and respiratory-related diagnoses.33, 36 However, the transferability and utility of the specific modalities, protocols, and outcome measures relative to patients with mTBI are currently unclear. Early work in this patient population suggests that exertional testing may be a useful clinical screening tool and a valuable line of inquiry to pursue to further understanding of the mechanisms and recovery trajectories associated with mTBI. Additional studies are needed to refine exertional testing protocols for this patient population and optimize recommendations and guidelines for specific clinical and research purposes.
Limitations
This systematic review has several notable limitations. First, although a comprehensive and thorough search strategy was used, there remains a potential for publication bias because the search was limited to peer-reviewed journal outlets in the English language. Therefore, it is possible that relevant studies may have been missed. Likewise, mTBI research is a rapidly evolving field of study. Consequently, the potential for relevant grey literature and new published works in the window of time that has passed since the search ended is relatively high. In addition, as with all systematic searches and critical appraisal processes, there is a certain degree of subjectivity involved. In particular, the quality appraisal process was completed through a self-generated form specifically targeting the purposes for this systematic review. We attempted to minimize the risk for this by using multiple screening rounds and multiple independent reviewers during all stages of the process. Nonetheless, it is possible that a different set of reviewers may have interpreted the studies differently. Finally, the small amount of available studies and the variable study purposes and study designs included in this review limit the conclusions that can be drawn from this research. It is clear that future research is needed to refine, extend, and validate the points discussed in this manuscript.
Conclusion
The current body of evidence provides a variety of post-mTBI exertional assessment options and protocols. The strongest evidence indicates that exertional assessments can provide important insight into recovery status after mTBI and should be administered using symptom presence and exacerbations as a guide. The synthesis and critical appraisal of the protocols identified through the systematic search process suggest that there is a great need for further research to evaluate optimal testing modes and protocols that can meet the unique and specific needs for clinically assessing and studying this patient population.
Acknowledgments
The authors would also like to acknowledge the Cincinnati Children's Hospital and University of Cincinnati Summer Undergraduate Research Fellowship Program. Resources were made available in part through Cincinnati Children’s Hospital Research Foundation Trustees Award, Cincinnati Children’s Hospital Research in Patient Services PS2 Award, and the National Institutes of Health KL2TR001426-01 and 1K23HD074683-01A1.
Abbreviations
- mTBI
Mild Traumatic Brain Injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest:
The authors have disclosures to report relevant to this work.
Compliance with Ethical Standards
No funding was received for this review. The authors have no conflicts of interest.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged</=19 years--United States, 2001–2009. MMWR Morbidity and mortality weekly report. 2011;60(39):1337–42. [PubMed] [Google Scholar]
- 3.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: Implications for treatment. NeuroRehabilitation. 2007;22(3):199–205. [PubMed] [Google Scholar]
- 4.McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvorak J, Echemendia R, et al. Consensus statement on Concussion in Sport - The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Physical Therapy in Sport. 2013;14(2):e1–e13. doi: 10.1016/j.ptsp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of Concussion and Post-concussion Syndrome. Sports health. 2012;4(2):147–54. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus Statement on Concussion in Sport-the 4th International Conference on Concussion in Sport Held in Zurich, November 2012. Clin J Sport Med. 2013;23(2):89–117. doi: 10.1097/JSM.0b013e31828b67cf. [DOI] [PubMed] [Google Scholar]
- 7.McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017 doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 8.Buckley TA, Munkasy BA, Clouse BP. Acute Cognitive and Physical Rest May Not Improve Concussion Recovery Time. J Head Trauma Rehabil. 2016;31(4):233–41. doi: 10.1097/HTR.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213–23. doi: 10.1542/peds.2014-0966. [DOI] [PubMed] [Google Scholar]
- 10.Medicine ACoS, editor. ACSM's Guidelines for Exercise Testing and Prescription. Tenth. Philadelphia, PA: Wolters Kluwer; 2018. Tenth ed. [Google Scholar]
- 11.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–74. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medicine ACoS. ACSM'S guidelines for exercise testing and prescription. Ninth. Philadelphia, PA: Lippencott Williams & Wilkins; 2014. 9th ed. [Google Scholar]
- 14.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current sports medicine reports. 2013;12(6):370–6. doi: 10.1249/JSR.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute JB. [cited 2016 4/13/2017];The Joanna Briggs Institute. 2016 Available from: URL: http://joannabriggs.org/research/critical-appraisal-tools.html.
- 17.Dematteo C, Volterman KA, Breithaupt PG, Claridge EA, Adamich J, Timmons BW. Exertion Testing in Youth with Mild Traumatic Brain Injury/Concussion. Medicine and science in sports and exercise. 2015;47(11):2283–90. doi: 10.1249/MSS.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 18.DeMatteo C, Greenspoon D, Levac D, Harper JA, Rubinoff M. Evaluating the Nintendo Wii for assessing return to activity readiness in youth with mild traumatic brain injury. Phys Occup Ther Pediatr. 2014;34(3):229–44. doi: 10.3109/01942638.2014.885103. [DOI] [PubMed] [Google Scholar]
- 19.Hinds A, Leddy J, Freitas M, Czuczman N, Willer B. The Effect of Exertion on Heart Rate and Rating of Perceived Exertion in Acutely Concussed Individuals. J Neurol Neurophysiol. 2016;7(4) doi: 10.4172/2155-9562.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordingley D, Girardin R, Reimer K, Ritchie L, Leiter J, Russell K, et al. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. Journal of neurosurgery Pediatrics. 2016;25(6):693–702. doi: 10.3171/2016.5.PEDS16139. [DOI] [PubMed] [Google Scholar]
- 21.Bassani R, Rosazza C, Ghirardin L, Caldiera V, Banco E, Casati C, et al. Crying spells triggered by thumb-index rubbing after thalamic stroke: a case report. BMC research notes. 2017;10(1):109. doi: 10.1186/s13104-017-2425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manikas V, Babl FE, Hearps S, Dooley J, Anderson V. Impact of Exercise on Clinical Symptom Report and Neurocognition after Concussion in Children and Adolescents. Journal of neurotrauma. 2017;34(11):1932–8. doi: 10.1089/neu.2016.4762. [DOI] [PubMed] [Google Scholar]
- 23.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Medicine and science in sports and exercise. 2004;36(8):1269–74. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 24.Gall B, Parkhouse WS, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004;38(6):773–7. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, et al. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55(4):1716–27. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. Journal Of Athletic Training. 2013;48(5):627–35. doi: 10.4085/1062-6050-48.5.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clinical Journal Of Sport Medicine: Official Journal Of The Canadian Academy Of Sport Medicine. 2011;21(2):89–94. doi: 10.1097/JSM.0b013e3181fdc721. [DOI] [PubMed] [Google Scholar]
- 28.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clinical Journal Of Sport Medicine: Official Journal Of The Canadian Academy Of Sport Medicine. 2010;20(1):21–7. doi: 10.1097/JSM.0b013e3181c6c22c. [DOI] [PubMed] [Google Scholar]
- 29.Moore BM, Adams JT, Barakatt E. Outcomes Following a Vestibular Rehabilitation and Aerobic Training Program to Address Persistent Post-Concussion Symptoms. Journal Of Allied Health. 2016;45(4):e59–e68. [PubMed] [Google Scholar]
- 30.Grabowski P, Wilson J, Walker A, Enz D, Wang S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: A retrospective analysis on safety and feasibility. Physical therapy in sport : official journal of the Association of Chartered Physiotherapists in Sports Medicine. 2017;23:22–30. doi: 10.1016/j.ptsp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Kurowski BG, Hugentobler J, Quatman-Yates C, Taylor J, Gubanich PJ, Altaye M, et al. Aerobic Exercise for Adolescents With Prolonged Symptoms After Mild Traumatic Brain Injury: An Exploratory Randomized Clinical Trial. J Head Trauma Rehabil. 2017;32(2):79–89. doi: 10.1097/HTR.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli A, Lombardi F. Heart rate variability regression and risk of sudden unexpected death in epilepsy. Medical hypotheses. 2017;99:49–52. doi: 10.1016/j.mehy.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Medicine ACoS, editor. ACSM'S Guidelines for Exercise Testing and Prescription. 10. Philadelphia, PA: Wolters Kluwer; 2017. [Google Scholar]
- 34.Ellis MLJWB. Physiological, vestibulo-ocular and cervicogenic postu-concussion disorders: an evidence-based classification system with directions for treatment. Brain Injury. 2015;29(2) doi: 10.3109/02699052.2014.965207. [DOI] [PubMed] [Google Scholar]
- 35.Leddy J, Baker JG, Haider MN, Hinds A, Willer B. A Physiological Approach to Prolonged Recovery From Sport-Related Concussion. Journal of athletic training. 2017;52(3):299–308. doi: 10.4085/1062-6050-51.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation. 2016;133(24):e694–711. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 37.Leddy JJ, Cox JL, Baker JG, Wack DS, Pendergast DR, Zivadinov R, et al. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. The Journal Of Head Trauma Rehabilitation. 2013;28(4):241–9. doi: 10.1097/HTR.0b013e31826da964. [DOI] [PubMed] [Google Scholar]
- 38.Robertson DBI, Burnstock G, Low P, Paton J, editors. Primer on the Autonomic Nervous System. Third. 2012. [Google Scholar]
- 39.Goldberger AL. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet. 1996;347(9011):1312–4. doi: 10.1016/s0140-6736(96)90948-4. [DOI] [PubMed] [Google Scholar]
- 40.Goldberger AL. Fractal variability versus pathologic periodicity: complexity loss and stereotypy in disease. Perspect Biol Med. 1997;40(4):543–61. doi: 10.1353/pbm.1997.0063. [DOI] [PubMed] [Google Scholar]
- 41.Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99(Suppl 1):2466–72. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldberger JJ, Ahmed MW, Parker MA, Kadish AH. Dissociation of heart rate variability from parasympathetic tone. Am J Physiol. 1994;266(5 Pt 2):H2152–7. doi: 10.1152/ajpheart.1994.266.5.H2152. [DOI] [PubMed] [Google Scholar]
- 43.Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Relationship of heart rate variability to parasympathetic effect. Circulation. 2001;103(15):1977–83. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]