Abstract
Background
The effects of increases in maxillary sinus (MS) airflow following functional endoscopic sinus surgery (FESS) are unknown. The goal of this study was to quantify the effects of FESS on airflow into the MS in a cohort of patients with chronic rhinosinusitis, and compare MS flow rate with patient-reported outcome measures.
Methods
A pilot study was conducted in which preoperative and postoperative computed tomography scans of 4 patients undergoing bilateral or unilateral FESS were used to create 3-dimensional (3D) reconstructions of the nasal airway and paranasal sinuses using Mimics™ (Materialise, Inc.). The size of the maxillary antrostomies post-FESS ranged from 107 to 160 mm2. Computational meshes were generated from the 3D reconstructions, and steady-state, laminar, inspiratory airflow was simulated in each mesh using the computational fluid dynamics (CFD) software Fluent™ (ANSYS, Inc.) under physiologic, pressure-driven conditions. Airflow into the MS was estimated from the simulations and was compared preoperatively and postoperatively. In addition, patients completed preoperative and postoperative Rhinosinusitis OutcomeMeasure-31 (RSOM-31) questionnaires and scores were compared with MS airflow rates.
Results
CFD simulations predicted that average airflow rate into post-FESS MS increased by 18.5 mL/second, and that average flow velocity into the MS more than quadrupled. Simulation results also showed that MS flow rate trended with total RSOM-31 and all domain scores.
Conclusion
CFD simulations showed that the healed maxillary antrostomy after FESS can greatly enhance airflow into the MS. Our pilot study suggests that to some extent, increasing airflow into the MS may potentially improve chronic rhinosinusitis patients’ quality of life pre-FESS and post-FESS.
Keywords: computational fluid dynamics, chronic rhinosinusitis, FESS, nasal airflow, maxillary sinus
Chronic rhinosinusitis (CRS) is a complex disease process that results in inflammation of the mucosa of the nose and sinuses.1 These inflamed tissues may result in obstruction of the anatomic drainage pathway of the paranasal sinuses, collectively termed the ostiomeatal complex (OMC), thereby hindering ventilation and clearance. Defined as sinonasal inflammation lasting longer than 12 weeks, CRS is known to affect approximately 13% and 15% of the population of the United States and United Kingdom, respectively. CRS has a tremendous economic impact in the United States, where it costs an estimated $8.6 billion annually; it was the 9th most expensive condition for employers in 1999.2–4
The mainstays of medical management of CRS include courses of culture-directed systemic/topical antibiotics and topical corticosteroid nasal sprays, as well as saline irrigations, systemic steroids, or other adjunct therapies.5 Surgery is indicated when disease persists despite maximal medical therapy. Functional endoscopic sinus surgery (FESS) is the treatment of choice for medically recalcitrant CRS, and has been shown to have a well-established safety and efficacy profile.1,5–7
The principal goal of FESS is to break the infection-inflammation cycle that defines CRS. The procedure removes diseased tissue, eliminates anatomic obstructions, and facilitates sinonasal ventilation, drainage, and mucociliary clearance through the opened natural ostia.1, 2,8 One of the basic tenets of FESS is to restore normal function of the sinuses and preserve healthy sinus mucosa in keeping with Messerklinger’s descriptions of intranasal pathophysiology and mucociliary transport.9 Despite a reported 90% success rate in primary cases,10,11 the quantitative effects of FESS on sinus ventilation and physiology remain unknown. Since this surgical intervention is known to significantly alter the anatomic structure of the sinonasal cavity, it is important to understand the changes that may occur in nasal airflow and aerodynamics as a result of the structural changes.2,8
There has been growing interest in the use of computational fluid dynamics (CFD) techniques to simulate 3-dimensional (3D) nasal physiology in order to collect objective data resulting from interindividual nasal anatomy and presurgical and postsurgical changes. A number of studies have used CFD to study airflow, heat transport, and water transport, as well as deposition of particulates in the nasal cavity2, 8,12–21; however, no CFD studies to date have evaluated the airflow effects of FESS in a preoperative and postoperative clinical trial.
In this study, we used CFD techniques to investigate the extent of increases in maxillary sinus (MS) airflow following FESS as well as compare MS flow rate with patient-reported quality of life (QOL) outcomes using a standardized, disease-specific QOL survey. The data presented in this study is part of an ongoing prospective trial using CFD to objectively analyze nasal physiology and drug delivery after FESS.
Patients and methods
Patient recruitment and treatment
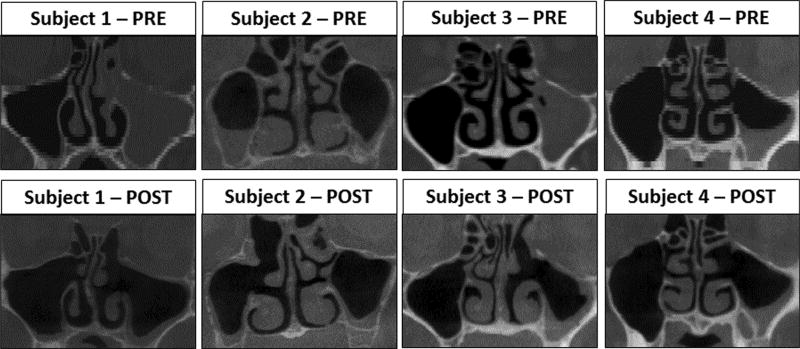
As part of a prospective study approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, 4 patients undergoing primary FESS (Table 1) for persistent CRS despite maximal medical management were enrolled after providing written informed consent. To ensure a homogenous study population, only patients with sinus disease limited to the OMC region were included. Each of the subjects underwent a fine-cut (0.6 mm) preoperative computed tomography (CT) scan of the paranasal sinuses as part of their standard of care. One of the subjects (subject 4) was noted to have accessory maxillary ostia bilaterally (Fig. 1). Surgical intervention (FESS) for unilateral disease was performed in 3 subjects, while 1 subject (subject 1) underwent bilateral FESS (Table 1, Fig. 1). The size of the maxillary antrostomies post-FESS ranged from 107 to 160 mm2 (Table 2). Axial CT scans were performed at least 12 weeks postoperatively using a cone-beam CT scanner following the “as low as reasonably achievable” (ALARA) protocol to limit exposure to ionizing radiation. At the time of the postoperative CT scan, it was observed that subject 2 had developed clinical and radiographic evidence of sinusitis on the contralateral (left), unoperated MS.
TABLE 1.
Patients’ demographic data and surgical intervention
| Patient | Race | Gender | Age (years) | Weight (kg) | Surgical intervention performed |
|---|---|---|---|---|---|
| Subject 1 | Caucasian | M | 39 | 94.5 | Left total ethmoidectomy; left frontal sinusotomy; bilateral maxillary antrostomy |
| Subject 2 | African American | F | 62 | 94.5 | Right anterior ethmoidectomy; right frontal sinusotomy; right maxillary antrostomy |
| Subject 3 | Caucasian | M | 66 | 76.2 | Left anterior ethmoidectomy; Left maxillary antrostomy |
| Subject 4 | Caucasian | F | 51 | 61.7 | Left maxillary antrostomy |
FIGURE 1.
Coronal views from pre-FESS and post-FESS CT scans of all patients. CT = computed tomography; FESS = functional endoscopic sinus surgery.
TABLE 2.
Surface area, airflow rate, and average flow velocity in the maxillary ostium
| Patient: side on which FESS was done | Surface area of maxillary ostium (mm2) | Inflow rate (mL/second) | Inflow velocity (mm/second) | |||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | |
| Subject 1: left | 0.00 | 143.99 | 0.000 | 19.033 | 0.00 | 482.39 |
| Subject 1: right | 5.68 | 107.62 | 0.025 | 5.184 | 7.04 | 142.31 |
| Subject 2: right | 8.68 | 159.88 | 0.005 | 28.232 | 0.96 | 453.36 |
| Subject 3: left | 2.40 | 116.01 | 0.002 | 17.922 | 10.16 | 418.73 |
| Subject 4: left | 45.97 | 149.75 | 5.588 | 27.603 | 454.86 | 566.36 |
FESS = functional endoscopic sinus surgery; POST = post-FESS; PRE = pre-FESS.
All subjects completed a Rhinosinusitis Outcome Measure-31 (RSOM-31) quality of life (QOL) survey of symptoms before and at 12 weeks after surgery. The RSOM-31 is a validated 31-item rhinosinusitis-specific questionnaire developed by Piccirillo et al.,22 which asks patients about 7 symptom domains: nasal, eye, sleep, ear, general, practical, and emotional. Patients are asked to evaluate their symptoms on 2 scales: “magnitude” and “importance.” The 6-item “magnitude” scale (0–5) is as follows: 0 = indicating no problem; 1 = very mild problem; 2 = mild or slight problem; 3 = moderate problem; 4 = severe problem; and 5 = problem is as bad as it can be. The 4-item “importance” scale (1–4) is as follows: 1 = not important; 2 = somewhat important; 3 = moderately important; and 4 = extremely important. Each domain score was calculated from the sum of the products of the magnitude and importance for each item in that specific subset. In this study, an increase in RSOM-31 is considered a worsening of the patient’s symptoms.
3D nasal reconstruction
3D reconstructions of the nasal airspace and paranasal sinuses were created from CT scan images imported into a medical imaging software package (Mimics™ 14.01; Materialise, Plymouth, MI) in Digital Imaging and Communications in Medicine (DICOM) file format. Preoperative and postoperative models of the nasal airspace, including the MS and frontal, ethmoid, and sphenoid sinuses, were created. Co-registration of pre-FESS and post-FESS models for each subject was performed using Mimics™ with 3D reconstructions of the facial bones from both scans. The co-registered nasal models were exported from Mimics™ in stereolithography (STL) file format into the computer-aided design (CAD) and mesh generating software package ICEM-CFD™ 12.1 (ANSYS, Canonsburg, PA). Planar nostril and outlet surfaces, as well as regions around the MS for simulated airflow analysis, were created.
Airflow simulation
In order to solve the equations that govern fluid flow, computational meshes of the nasal and sinus cavities were created in ICEM-CFD™ using approximately 4million unstructured, graded tetrahedral elements. Steady-state, laminar inspiratory airflow was simulated using the CFD software package Fluent™ 12.1.4 (ANSYS, Inc., Canonsburg, PA) under pressure-driven conditions. Although nasal airflow can become turbulent at high flow rates (eg, when sniffing), laminar flow has been shown to suffice for the simulation of resting breathing.23,24 Simulation at steady-state assumed that time-dependent variables were constant and all derivatives with respect to time were zero. Fluent™ uses the finite volume method to solve the Navier-Stokes equations numerically. Airflow rates for steady-state inspiration were calculated by assuming that the duration times of inspiration and expiration were the same, so that the airflow rate for inspiration alone was twice the minute-volume (the amount of air exhaled in 1 minute), which was estimated from body weight using gender-specific allometric curves.17 Analysis and visualization of airflow simulations were conducted using the postprocessing software package Fieldview™ 12 (Intelligent Light, Lyndhurst, PA). Maxillary inflow rates were plotted against changes in total RSOM-31 and each of the 7 symptom domains scores to determine if a trend existed between airflow into the MS and the patient’s QOL assessments.
Results
CFD-derived measures
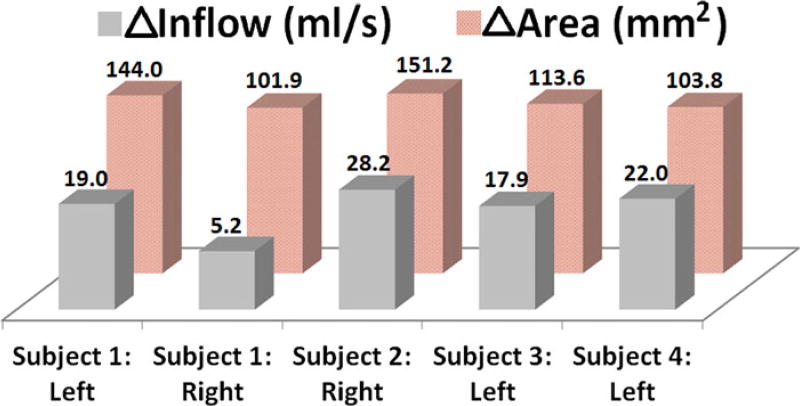
Predictions of the difference in airflow rates into the MS (post-FESS minus pre-FESS) showed increased flow ranging from 5.2 mL/second to 28.2 mL/second in all patients (Fig. 2), and on average, airflow into the post-FESS MS increased by 18.5 mL/second. Preoperative airflow into the MS was noted to be the highest in subject 4 at 5.6 mL/second (Table 2), which may be attributed to the presence of an accessory ostium. In addition, average flow velocity in the entrance to the post-FESS MS was about 4.4 times higher than pre-FESS MS entrance velocity.
FIGURE 2.
Changes (post-FESS minus pre-FESS) in maxillary ostium surface area and flow rate into the maxillary sinus before and after FESS. FESS = functional endoscopic sinus surgery.
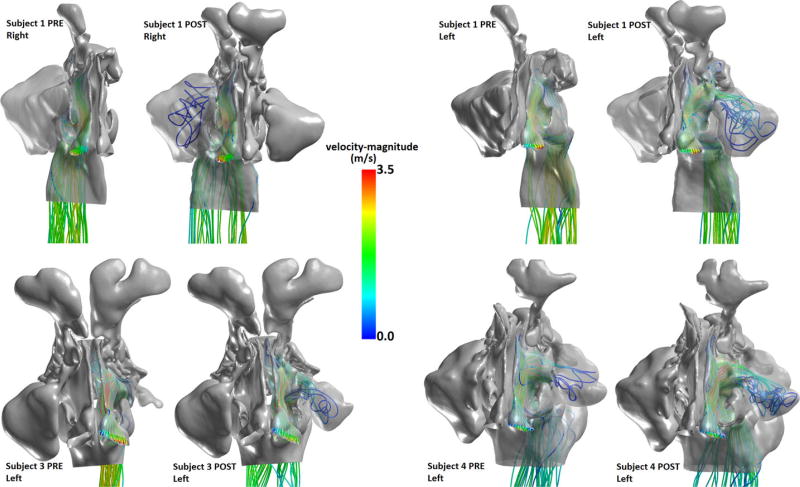
After surgical antrostomy, the size of the maxillary ostium was, on average, larger by 122.9 mm2 (Table 2, Fig. 2), with a minimum change in ostium size of about 101.9 mm2 and a maximum change of 151.2 mm2. The effect of these enlargements on airflow streamline patterns showed increased ventilation in the MS post-FESS (Fig. 3). Based on the nostril-surface reference points selected to compute flow patterns, preoperatively, little or no airflow could be visualized circulating through the MSs in subjects 1, 3, and 4. However, post-FESS, airflow could be visualized circulating through the operated MSs (Fig. 3).
FIGURE 3.
Airflow streamlines on the surgically altered side pre-FESS and post-FESS for select patients. Color indicates airflow velocity magnitude. FESS = functional endoscopic sinus surgery.
Patient-reported measures
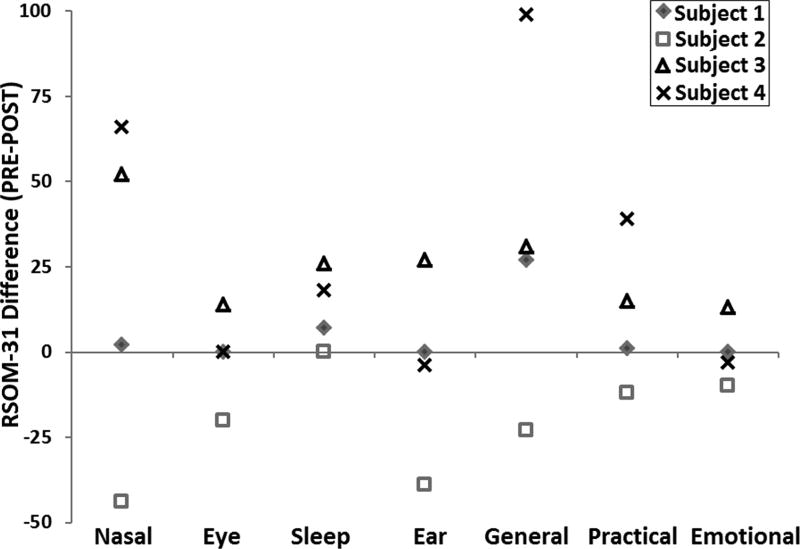
Pre-FESS minus post-FESS differences in RSOM-31 survey of all patients stratified by symptoms domain (Fig. 4) revealed an improvement in nasal, sleep, and general symptoms for subjects 1, 3, and 4. Subject 2 shows worsening in every domain except sleep, suggesting that the occurrence of symptomatic disease on the contralateral side confounded this patient’s post-FESS QOL assessments. The “emotional consequences” domain showed the least change in RSOM-31 scores.
FIGURE 4.
PRE minus POST differences in RSOM-31 survey for all subjects. A positive difference indicates improvement in symptom domain after surgery. POST = post-FESS; PRE = pre-FESS; RSOM-31 = Rhinosinusitis Outcome Measure-31.
Comparison of patient-reported and CFD-derived measures
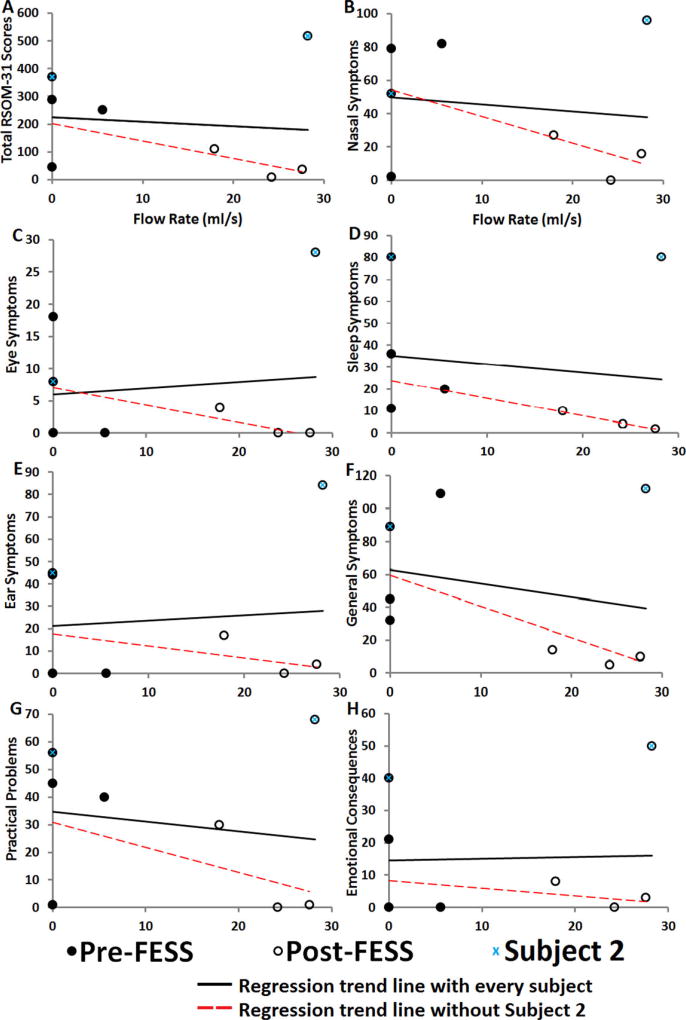
Preliminary results with all patients as indicated by regression trend lines showed a weak negative (shallow slope) trend between total RSOM-31 scores and MS flow rate (Fig. 5A). This suggests that as flow rate increases post-FESS into the MS, patients report marginal improvement in their QOL outcome. Similarly, we detected weak trend in every symptom domain score and MS flow rate (Fig. 5B–H). Nasal symptoms, sleep symptoms, general symptoms, practical problems, and emotional consequences demonstrated evidence of weak negative trend with increase flow rate; while eye and ear symptoms domains showed weak positive trend with MS flow rate, reflecting patients’ perception of worsening eye and ear symptoms as flow in the MS increases. Without subject 2 (patient who developed sinusitis on the unoperated MS), the trend became much stronger, implying that as more flow circulated the MS, RSOM-31 scores improved in every domain.
FIGURE 5.
Comparison of patients’ reported QOL survey and flow rate into the maxillary sinus. (A) Total RSOM-31 scores plotted against maxillary sinus inflow rate. (B) Nasal symptoms domain scores plotted against maxillary sinus inflow rate. (C) Eye symptoms domain scores plotted against maxillary sinus inflow rate. (D) Sleep symptoms domain scores plotted against maxillary sinus inflow rate. (E) Ear symptoms domain scores plotted against maxillary sinus inflow rate. (F) General symptoms domain scores plotted against maxillary sinus inflow rate. (G) Practical problems domain scores plotted against maxillary sinus inflow rate. (H) Emotional consequences domain scores plotted against maxillary sinus inflow rate. QOL = quality of life; RSOM-31 = Rhinosinusitis Outcome Measure-31.
Discussion
In this preliminary study, computational models of the nasal and sinus cavities before and after FESS were created to compare airflow into the MS. To our knowledge, the sample size (n = 4) of this report makes it the largest CFD study to investigate changes in MS flow magnitude in pre-FESS and post-FESS patients, as well as attempt to compare patients’ reported outcome measures with objective data. Due to the high computational intensity needed to generate results for each patient, and because it takes several months to have a complete result from patient recruitment to surgical intervention to CFD analysis, we are unable to include additional patients in this pilot study.
Our CFD simulations predicted that on average, airflow into the MSs increased by 18.5 mL/second after FESS, suggesting that there is a relationship between the size of the post-FESS maxillary ostium and amount of airflow into the MS (Table 2, Fig. 2). In addition, our results showed that FESS increased the average velocity of flow into the MS in these subjects. Our findings are consistent with previously reported results in the literature,2, 8,18 which report significant increases in simulated airflow distribution in the post-FESS paranasal sinuses.8 In their article, Hood et al.2 found that sinus ventilation through a single natural ostium is very slow, whereas air velocity for wider or shorter ostia is much faster and capable of a much higher transport rate. They also showed that the estimated convective exchange time for a 6-mm-diameter ostium was 5.2 minutes, and 5040 minutes for a 3-mm-diameter ostium.
While increased airflow into the MS may be associated with better QOL outcomes, as is the case in the present study, this relationship may not be causal. In fact, there is evidence in the literature to suggest that increased airflow and higher flow velocity into the MS could lead to more mucosal drying, enhancing the likelihood of developing crusts in the MS. As noted by Hood et al.,2 the size and location of the natural ostia may help prevent the sinus mucosa from excessive drying as well as sterilize its environment with significantly high nitric oxide concentrations, thereby reducing access to pathogens. Since the density of mucus-producing glands and serous cells in the sinus mucosa is not indicative of a capacity to handle excess ventilation and drying, excessive drying may be a causative factor for persistent disease following FESS. Furthermore, Garcia et al.15 suggested that the effect of drying due to airflow increases mucus viscosity, thereby stimulating the formation of crusts which may in turn impact mucociliary transport, leading to additional crust formation and chronic rhinitis.
Our CFD results suggest that the presence of an accessory ostium increases both airflow circulation in the MS and flow speed over patients with natural ostia. As the natural ostium in subject 4 was completely occluded pre-FESS, it is not clear how the presence of both natural and accessory ostia would impact MS ventilation in this patient. However, studies have shown that the existence of both natural and accessory ostia can significantly increase air circulation in the sinus.2,16 Hood et al.2 suggested that the reason for such increased circulation of airflow is due to an alternative access for cross ventilation. Similarly, Zhu et al.16 suggested that the velocity magnitude around areas where accessory ostia are usually connected to the nasal passage (inferiorly in the middle meatus) is higher and thus enhances air ventilation.
There is substantial evidence demonstrating an improvement in QOL in patients undergoing FESS for management of CRS.25 While FESS appears to yield significant subjective symptom improvement,11 the objective data on FESS is less clear. Our pilot study suggests a weak relationship (as indicated by trend lines in Fig. 5) between airflow into the MS with patients’ QOL assessments before and after FESS, with the data showing a stronger trend and potential ability of CFD to predict surgical outcomes with the exclusion of subject 2 (Fig. 5). Similar efforts to objectively measure post-FESS improvements have failed to correlate with subjective outcomes when using modalities such as radiographic findings, endoscopic examination, acoustic rhinometry, and rhinomanometry. Furthermore, previous CFD studies on FESS and how this form of surgical intervention alters airflow characteristics did not attempt to compare effects of ostium size on simulated MS airflow rate with QOL data.8, 18,26 Xiong et al.8 performed virtual FESS on a healthy patient and demonstrated increased airflow to the MS, ethmoid sinus, and sphenoid sinus after surgically opening the middle meatus. Similarly, Chen et al.18 characterized the airflow in a subject after unilateral FESS and were able to demonstrate increased airflow distribution (13% vs 1.6%) to the MS on the operated side compared to the unaffected side. The continued systematic collection of controlled and paired (preoperative and postoperative) clinical trial outcomes combined with CFD analysis will allow for a more complete understanding of postoperative physiology. Additionally, once enough power is introduced into this type of clinical trial, CFD outcomes may help understand and predict post-FESS CRS failures.
Conclusion
In conclusion, we used a CFD technique in this pilot study to examine the effect of FESS on MS aeration in 4 patients with CRS. Simulations predicted that FESS greatly enhances both airflow and flow velocity in the MS by several-fold. Maxillary antrostomy size significantly contributed to the magnitude of airflow in the subjects studied. In addition, the existence of accessory ostia in the MS increased the magnitude of sinus ventilation compared to natural ostia. Our preliminary findings show that subject 2 confounded the relationship between airflow in the MS and patients’ QOL assessments, as well as highlighted how the occurrence of disease on the previously asymptomatic side can skew postsurgery QOL survey on the side surgery was done. More information is needed on the effects of FESS on nasal airflow turbulence, water flux, and drying; and on their potential relationship with antrostomy sizes and positions. Future directions include continued further recruitment of a much larger cohort of CRS patients to increase the statistical power of our analyses. Finally, we plan to expand our inclusion criteria to disease beyond the OMC region, to broaden the generalizability of our findings.
Acknowledgments
We acknowledge the residents, nurses, technicians, and administrative staff who helped with patient recruitment, survey administration, and follow-up visits.
Footnotes
Potential conflict of interest: None provided.
References
- 1.Anselmo-Lima WT, Ferreira MD, Valera FC, et al. Histological evaluation of maxillary sinus mucosa after functional endoscopic sinus surgery. Am J Rhinol. 2007;21:719–724. doi: 10.2500/ajr.2007.21.3102. [DOI] [PubMed] [Google Scholar]
- 2.Hood CM, Schroter RC, Doorly DJ, et al. Computational modeling of flow and gas exchange in models of the human maxillary sinus. J Appl Physiol. 2009;107:1195–1203. doi: 10.1152/japplphysiol.91615.2008. [DOI] [PubMed] [Google Scholar]
- 3.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 10. 2010;(249):1–207. [PubMed] [Google Scholar]
- 4.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 5.Welch KC, Stankiewicz JA. A contemporary review of endoscopic sinus surgery: techniques, tools, and outcomes. Laryngoscope. 2009;119:2258–2268. doi: 10.1002/lary.20618. [DOI] [PubMed] [Google Scholar]
- 6.Dubin MG, Liu C, Lin SY, Senior BA. American Rhinologic Society member survey on “maximal medical therapy” for chronic rhinosinusitis. Am J Rhinol. 2007;21:483–488. doi: 10.2500/ajr.2007.21.3047. [DOI] [PubMed] [Google Scholar]
- 7.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142:55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong G, Zhan J, Zuo K, et al. Numerical flow simulation in the post-endoscopic sinus surgery nasal cavity. Med Biol Eng Comput. 2008;46:1161–1167. doi: 10.1007/s11517-008-0384-1. [DOI] [PubMed] [Google Scholar]
- 9.Govindaraj S, Adappa ND, Kennedy DW. Endoscopic sinus surgery: evolution and technical innovations. J Laryngol Otol. 2010;124:242–250. doi: 10.1017/S0022215109991368. [DOI] [PubMed] [Google Scholar]
- 10.Bassiouni A, Naidoo Y, Wormald PJ. When FESS fails: the inflammatory load hypothesis in refractory chronic rhinosinusitis. Laryngoscope. 2012;122:460–466. doi: 10.1002/lary.22461. [DOI] [PubMed] [Google Scholar]
- 11.Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Frank DO, Kimbell JS, Pawar S, Rhee JS. Effects of anatomy and particle size on nasal sprays and nebulizers. Otolaryngol-Head Neck Surg. 2011;146:313–319. doi: 10.1177/0194599811427519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimbell JS, Garcia GJ, Frank DO, et al. Computed nasal resistance compared with patient-reported symptoms in surgically treated nasal airway passages: a preliminary report. Am J Rhinol Allergy. 2012;26:94–98. doi: 10.2500/ajra.2012.26.3766. [DOI] [PubMed] [Google Scholar]
- 14.Rhee JS, Cannon DE, Frank DO, Kimbell JS. Role of virtual surgery in preoperative planning: assessing the individual components of functional nasal airway surgery. Arch Facial Plast Surg. 2012;14:354–359. doi: 10.1001/archfacial.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia GJM, Bailie N, Martins DA, Kimbell JS. Atrophic rhinitis: a CFD study of air conditioning in the nasal cavity. J Appl Physiol. 2007;103:1082–1092. doi: 10.1152/japplphysiol.01118.2006. [DOI] [PubMed] [Google Scholar]
- 16.Zhu JH, Lee HP, Lim KM, et al. Effect of accessory ostia on maxillary sinus ventilation: a computational fluid dynamics (CFD) study. Respir Physiol Neurobiol. 2012;183:91–99. doi: 10.1016/j.resp.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Garcia GJM, Schroeter JD, Segal RA, et al. Dosimetry of nasal uptake of water-soluble and reactive gases: a first study of interhuman variability. Inhal Toxicol. 2009;21:607–618. doi: 10.1080/08958370802320186. [DOI] [PubMed] [Google Scholar]
- 18.Chen XB, Lee HP, Chong VF, Wang de Y. Aerodynamic characteristics inside the rhino-sinonasal cavity after functional endoscopic sinus surgery. Am J Rhinol Allergy. 2011;25:388–392. doi: 10.2500/ajra.2011.25.3669. [DOI] [PubMed] [Google Scholar]
- 19.Frank DO, Kimbell JS, Cannon D, et al. Deviated nasal septum hinders intranasal sprays: a computer simulation study. Rhinology. 2012;50:311–318. doi: 10.4193/Rhin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank DO, Kimbell JS, Cannon D, Rhee JS. Computed intranasal spray penetration: comparisons before and after nasal surgery. Int Forum Allergy Rhinol. 2013;3:48–55. doi: 10.1002/alr.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon DE, Frank DO, Kimbell JS, et al. Modeling nasal physiology changes due to septal perforations. Otolaryngol Head Neck Surg. 2013;148:513–518. doi: 10.1177/0194599812472881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Edwards D, Haiduk A, et al. Psychometric and clinimetric validity of the 31-item Rhinosinusitis Outcome Measure (RSOM-31) Am J Rhinol. 1995;9:297–306. [Google Scholar]
- 23.Garlapati RR, Lee HP, Chong FH, Wang DY. Indicators for the correct usage of intranasal medications: a computational fluid dynamics study. Laryngoscope. 2009;119:1975–1982. doi: 10.1002/lary.20660. [DOI] [PubMed] [Google Scholar]
- 24.Shanley KT, Zamankhan P, Ahmadi G, et al. Numerical simulations investigating the regional and overall deposition efficiency of the human nasal cavity. Inhal Toxicol. 2008;20:1093–1100. doi: 10.1080/08958370802130379. [DOI] [PubMed] [Google Scholar]
- 25.Smith TL, Batra PS, Seiden AM, Hannley M. Evidence supporting endoscopic sinus surgery in the management of adult chronic rhinosinusitis: a systematic review. Am J Rhinol. 2005;19:537–543. [PubMed] [Google Scholar]
- 26.Zhao K, Pribitkin EA, Cowart BJ, et al. Numerical modeling of nasal obstruction and endoscopic surgical intervention: outcome to airflow and olfaction. Am J Rhinol. 2006;20:308–316. doi: 10.2500/ajr.2006.20.2848. [DOI] [PubMed] [Google Scholar]