Abstract
Rationale and objectives
Although delta/mu receptor interactions vary as a function of behavioral endpoint, there have been no assessments of these interactions using assays of pain-depressed responding. This is the first report of delta/mu interactions using an assay of pain depressed behavior.
Methods
A mult-cycle FR10 operant schedule was utilized in the presence of (nociception) and in the absence of (rate suppression) a lactic acid inflammatory pain-like manipulation. SNC80 and methadone were used as selective/high efficacy delta and mu agonists, respectively. Both SNC80 and methadone alone produced dose-dependent restoration of pain-depressed responding and dose-dependent response rate suppression. Three fixed ratio mixtures, based on the relative potencies of the drugs in the nociception assay, also produced dose-dependent antinociception and sedation. Isobolographic analysis indicated that all three mixtures produced supra-additive antinociceptive effects and simply additive sedation effects.
Conclusions
The therapeutic index (TI) inversely varied as a function of amount of SNC80 in the mixture, such that lower amounts of SNC80 produced a higher TI, and larger amounts produced a lower TI. Compared to literature using standard pain-elicited assays, the orderly relationship between SNC80 and TI reported here, may be a unique function of assessing pain-depressed behavior.
Keywords: opioid receptor interactions, pain-depressed behaviors, operant conditioning, SNC80, rats
Introduction
Mu opioid receptor agonists possess analgesic efficacy to treat a range of acute and chronic pain conditions, however these drugs are not uniformly effective and possess a multitude of deleterious side effects including addiction potential, respiratory depression, GI paralysis, and profound sedation/mental clouding (Shalmi 2004). Delta opioid agonists also possess analgesic efficacy, but produce potentially dangerous side effects including seizure activity (reviewed in Narita and Suzuki 2003). The opioid literature also suggests that single molecule, dual-action mu/delta opioid receptor drugs, under some conditions, possess a higher therapeutic index than mu agonists alone (Mosberg et al. 2014; Schiller et al. 1995; Wells et al. 2001). One method of determining and refining the optimum ratio for delta and mu receptor activation has been the characterization of delta/mu receptor interactions in vivo. This method can provide a way to quantitatively determine the optimum proportion of delta vs. mu activation in order to produce enhanced therapeutic effects and/or attenuated side effects (Adams et al. 1993; Stevenson et al. 2003, 2005; Tallarida 2000).
To date, a wide range of receptor interaction studies including combinations of delta + mu agonists, or combinations of adrenergic + mu agonists, in rodents have utilized nociception assays of pain-stimulated behavior such as withdrawal responses, writhes, vocalizations (Adams et al. 1993; Porreca et al. 1990; Siemian et al. 2016; Stone et al. 2014; Su et al. 1998). These pain-stimulated behaviors have several limitations, including evidence that candidate analgesics may produce motor disruption resulting in a false positive analgesic outcome, and that clinical pain conditions do not consistently manifest with symptom topography of pain-stimulated behavior, and this is especially true in chronic pain populations (Reviewed in Negus et al. 2006). In contrast, our laboratory, and others, have been developing and refining assays of pain-depressed behavior by characterizing the effects of acute and chronic pain states on conditioned and unconditioned behavior, using endpoints such as schedule-controlled responding for food under progressive ratio reinforcement schedules, intracranial self-stimulation, open field locomotor activity, voluntary wheel running, nesting, and free feeding (Altarifi et al. 2015; Leitl et al. 2014; Negus et al. 2015; Stevenson et al. 2006, 2009, 2011; Warner et al. 2015). The advantages of studying pain-depressed behavioral endpoints are that they correlate well with human and veterinary clinical pain assessment tests (IOM 2011), and are not subject to false positive analgesic drug effects of pain-stimulated assays. These data suggest that pain-depressed behavioral outcomes may have some clinical and translational relevance.
In vivo delta/mu opioid receptor interactions have been shown to vary as a function of delta or mu agonist efficacy, the ratio of compounds in the mixture, the species tested, and the behavioral endpoint (Adams et al. 1993; Stevenson et al. 2003; Su et al. 1998). To date, delta/mu mixtures have been tested only in assays that measure pain-elicited behaviors, such as withdrawal responses from a cold or hot surface. The majority of these studies report that the addition of a delta agonist such as DPDPE, or SNC80 enhances the antinociceptive effects of mu opioid agonists, with no effect on or attenuated side effects. However, there have been no assessments of in vivo receptor interactions on pain-depressed behaviors in rodents. Given the potential translational validity of pain-depressed measures, the putative differential circuitry that may underlie both sets of pain-related behaviors, and the divergent effects of pain manipulations and analgesics on pain-stimulated vs. pain-depressed behaviors, the characterization of delta/mu interactions on pain-depressed behaviors is warranted.
The present manuscript aimed to characterize delta/mu interactions using a multiple-cycle FR10 operant conditioning assay of pain-depressed behavior, in which nociception was defined by the degree to which response rates were decreased relative to baseline responding, and antinociception (therapeutic effect) was defined by dose-dependent restoration of pain-depressed response rates. Delta/mu interactions were also characterized in the same mult-cycle FR10 operant conditioning assay, but in the absence of the pain stimulus, as a way to assess overall drug-induced rate suppression or sedation (side effect, loosely defined). Thus, drugs alone and drug mixtures were tested in both the presence of (antinociception) and absence of (sedation) a pain-like manipulation. The pain-like manipulation chosen was i.p. delivery of dilute lactic acid because it mimics a mild-to-moderate form of peritonitis, and use of a chemical inflammatory agent allows for the characterization of a range of concentrations and thus a range of nociception intensities (Altarifi et al. 2015; Do Carmo et al. 2009). Further, these studies will provide proof-of-concept for subsequent chronic pain manipulations. SNC80 and methadone were chosen as test drugs because SNC80 represents a high-efficacy and highly selective delta agonist (Bilsky et al. 1995), and methadone represents a high-efficacy and highly selective mu agonist (eg: ~1,000-fold more selective for opioid mu vs. NMDA receptor: Gorman et al. 1997; Peckham and Traynor 2006), and the determination of the nature of delta/mu interactions requires selective ligands at delta and mu sites (Tallarida 2000).
Methods
Animals
Male Sprague Dawley rats (200–250g, Charles River Laboratories) were used for all experiments. Rats were housed singly in standard Plexiglas containers with water available ad libitum. All animals were maintained at 85% free-feeding weight for the duration of the experiment, and were maintained in a temperature and humidity controlled colony on a 12-h light/dark cycle (lights on at 0700 and off at 1900). Animals were acclimated in the animal facility for at least 5 days prior to use. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and procedures were approved by the University of New England Institutional Animal Care and Use Committee (IACUC). The health of the animals was assessed daily by laboratory technicians and/or animal care staff.
Operant Conditioning Experiments
Apparatus
All studies were conducted in operant conditioning chambers (Med Associates, model MED-008-B1) placed within sound-attenuating cubicles equipped with a house light and exhaust fan. Each chamber contained two response levers situated on the front wall of the chamber. A shallow steel cup situated between the two levers, and just above the floor, contained a reservoir for consumption of food pellets. A pellet dispenser delivered 45 mg grain-based food pellets (see detailed methods below). Stimulus lights were situated above each response lever and were programmed to signal the availability of food.
Food training
Lever pressing was initially shaped during daily training sessions (30 min day 1; 15 min days 2–8). Food training involved reinforcement of successive approximations of lever-press behavior with delivery of a food pellet. Once shaping was complete, 45 mg food pellets (Noyes brand) were available under a Fixed Ratio (FR) 1 schedule of reinforcement for a 30-min session. In a fixed ratio schedule the subject emits a fixed number of responses for each reinforcer. Thus, an FR1 schedule indicates that 1 response is required to obtain each reinforcer. Illumination of the stimulus light above the active lever served as a discriminative stimulus that the lever response-reinforcer contingency was in place. Responding on the other inactive lever was counted but had no programmed consequences (animals were counterbalanced such that the left lever was active for half the rats and the right lever was active for the other half). A maximum of 50 food reinforcers was available during each daily training session. Once responding stabilized under the FR1 schedule (three consecutive days in which response rates varied by no more than 20% and at least 70% of responses emitted on the active lever), the FR requirement was raised to FR10 (typically over the course of 3–5 days), such that 10 responses were required to obtain each reinforcer. Response rate during training and for all subsequent schedules was calculated as the number of responses on the active lever in the 5 min response period ÷ 5 min.
Terminal schedule Mult-Cycle FR10
After responding stabilized under the FR10 schedule, using the same criteria as above, rats were moved onto a Mult-Cycle FR10 schedule. Each of four cycles was 15 min in length and consisted of two components: a 10-min time-out period followed by a 5-min response period. During the time out period, no stimulus lights were illuminated, and lever responding had no scheduled consequences. During the response period, the stimulus light was illuminated, and the rats could respond for up to 10 food pellets under an FR10 schedule of reinforcement. If all 10 pellets were earned before the 5 min had elapsed, the stimulus light was turned off, and responding had no scheduled consequences for the remainder of that response period. Sessions were conducted 5 days a week. Test sessions were conducted on Wednesdays, and only if rats responded at rates greater than 30 responses/min for all 4 cycles on the preceding day. During test sessions, test compounds were administered either in the presence or in the absence of the pain stimulus lactic acid (see below). Only one dose of test drug was administered on each test day, and at the beginning of the first time-out period.
Lactic acid pain manipulation
A complete concentration-effect curve for lactic acid (0.1 – 1.8%) was determined in the mult-cycle operant conditioning assay. The 1.8% concentration reliably and robustly depressed response rates and this concentration was used, in combination with drugs alone or drug mixtures, for all subsequent antinociception studies. Each drug or mixture was tested in the presence of 1.8% lactic acid, and also in the absence of lactic acid. The former condition served as an assay of pain-depressed behavior, and the latter condition served as an assay of rate suppression, or sedation.
Drug alone and mixture testing
Complete dose-effect curves were determined for each drug alone, and test days occurred one day per week, such that drug was administered a maximum of one day per week. Following determination of dose-effect curves for SNC80 alone and methadone alone, three (3) fixed-ratio mixtures of SNC80 + methadone were tested, and the proportions of each drug in the mixtures were based on the relative potencies of the two drugs alone in the nociception assay (see Data Analysis for details). Each drug alone and mixture was tested according to Latin Square sequence with one dose or combination tested each week, and both ascending and descending order of doses occurred across all rats. Each mixture was tested 2 times, and 1 week separated each test. Mixture values were calculated as test1 + test2 ÷ 2.
Assay of pain-depressed responding
Lactic acid (LA) or saline was administered at −5 min, half-way through the first time-out period. Pretreatments of saline, or drug, or mixture were administered at −20 min prior to the first time-out period, and prior to LA. Thus, groups were defined as: Saline + LA; Drug + LA; Mix + LA. Drugs and mixtures were all administered s.c., and LA was administered i.p. route. Antinociception was defined by the degree to which a drug or mixture restored LA-depressed responding, and was quantified as % Maximum Possible Effect (%MPE) by the following formula:
Assay of drug-induced rate suppression
Saline was administered at −5 min, half-way through the first time-out period. Pretreatments of saline, or drug, or mixture were administered at −20 min before the first time-out period, and prior to saline. Thus, groups were defined as: Drug + Saline; Mix + Saline. Drugs and mixtures were all administered s.c., and saline was administered i.p. route. Rate suppression was defined by the degree to which a drug alone or mixture alone suppressed operant response rates, and was quantified as % control by the following formula:
Studies with and without lactic acid were conducted in the same subjects.
Data Analysis
For the assay of pain-depressed responding, response rates from each cycle were converted to % MPE. The ED50 for each drug alone was defined as the dose of drug that produced a 50% Maximum Possible Effect. Individual ED50s were calculated by interpolation if only 2 data points were available (one above and one below 50%) or by linear regression when at least 3 data points were available. For drug mixtures, the quantity, Zmix was also calculated for each rat, and was defined as the total drug dose (eg: dose SNC80 + dose methadone) that produced a 50% MPE.
For the assay of rate suppression, response rates from each cycle were converted to % control, using the average rates from the preceding two days as control. The ED50 for each drug alone was defined as the dose of drug that produced a 50% decrease in the percent of control rate of responding. Individual ED50s were calculated by interpolation if only 2 data points were available (one above and one below 50%) or by linear regression when at least 3 data points were available. For drug mixtures, the quantity, Zmix was also calculated for each rat, and was defined as the total drug dose (eg: dose SNC80 + dose methadone) that produced a 50% decrease in responding.
Interactions between SNC80 and methadone were assessed using both graphical and statistical approaches (Prism 5; Tallarida 2000). Graphically, mean ED50 values (+/− confidence limits) for SNC80 alone, methadone alone and all three (3) SNC80 + methadone mixtures, were plotted in an isobologram that contains a line connecting the ED50 values for each drug alone. This line shows predicted ED50 values for drug mixtures assuming additivity. Points that fall below the line (toward the origin) are suggestive of supra-additivity, whereas points that fall above the line (away from the origin) are suggestive of sub-additivity.
Statistical evaluation of drug interactions was accomplished by comparing the experimentally determined Zmix (experimentally determined ED50 value of mixture) with the predicted additive Zadd, (predicted additive ED50 value of mixture) as described by Tallarida (2000). Zmix values were determined empirically as described above. Zadd values were calculated individually for each rat from the following equation: Zadd = fA + (1−f)B, where A is the ED50 for SNC80 alone, B is the ED50 for methadone alone, and f is the fractional multiplier of A in the computation of the additive total dose. The choice for f is related to the proportion of drug A (pA) in a mixture according to the following equation: pA = fA/Zadd. The present study examined effects produced by mixtures in which f = 0.25, 0.5, 0.75. Thus, f = 0.25 leads to pA = A/(A/3 + B), and the ratio of the SNC80 concentration to the methadone concentration is [(A/B) ÷ 3]:1; for f = 0.5, the ratio of SNC80 to methadone is A/B:1; for f = 0.75, the ratio of SNC80 to methadone is [(A/B) × 3]:1.
Drugs
SNC80 (free base) was synthesized by Dr. Kenner C. Rice (National Institutes of Health, Bethesda, MD). Methadone hydrochloride (racemate) was donated by Dr. Edward Bilsky. All drugs and mixtures were dissolved in distilled water.
Results
Effects of lactic acid concentration on operant responding
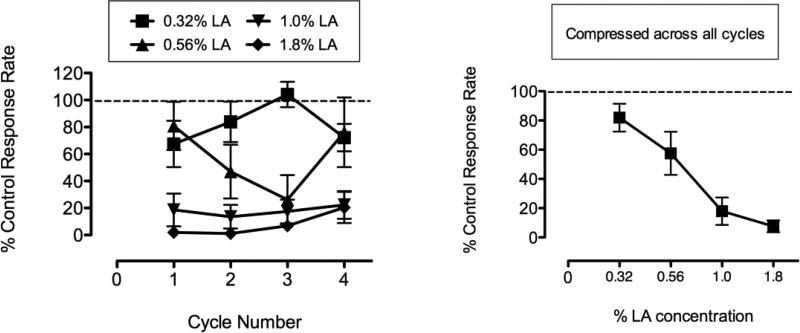
Figure 1 shows the effects of a range of concentrations of lactic acid (0.32 – 1.8%, i.p.) in the Mult-cycle FR10 operant conditioning assay, across all 4 cycles (Left Panel), and the same data compressed across all 4 cycles (Right Panel). Lactic acid (LA) produced a concentration-dependent decrease in response rates. The highest concentration of 1.8% produced robust and sustained depression of behavior to ~7% of control. Control rates of responding for individual rats (averaged across all 4 cycles) ranged from 36.55 to 119.06 responses/min with a mean of 76.62 responses/min. Response rates returned to baseline levels for all rats on the next day (day following lactic acid administration; data not shown).
Fig. 1.
Figure 1 shows concentration-effect curves for lactic acid to depress operant response rates in a multiple-cycle FR10 schedule of reinforcement. Left panel shows data across all 4 cycles of the schedule and right panel shows data collapsed across all 4 cycles.
Effects of methadone alone or SNC80 alone in the presence or absence of lactic acid
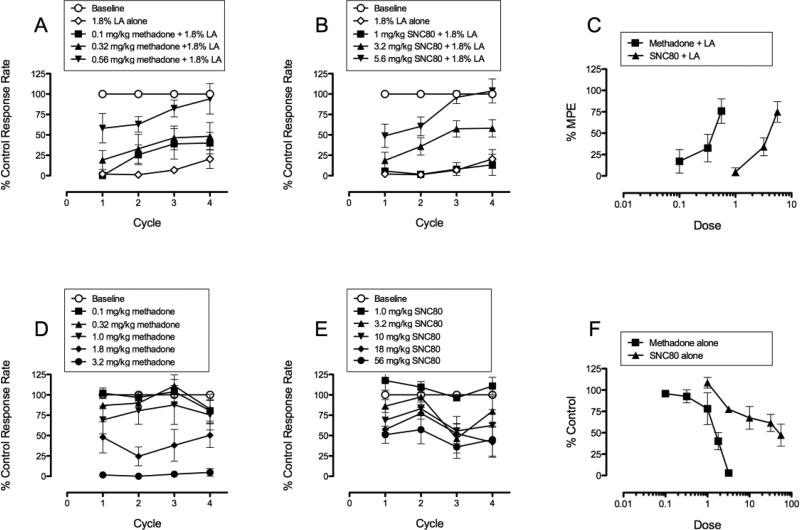
Figure 2 shows the effects of methadone or SNC80 in the presence of (top panels) or absence of (bottom panels) the lactic acid pain manipulation. Figures 2A and 2B show the effects of a range of doses of methadone alone (0.1 – 0.56 mg/kg, s.c.) and a range of doses of SNC80 alone (1 – 5.6 mg/kg, s.c.) on 1.8% LA-depressed responding in the Mult-cycle FR10 operant conditioning assay, across all 4 cycles. Figure 2C is a transformation of Figures 2A and 2B, such that dose effect curves in Figure 2C are collapsed across all 4 cycles. Both methadone and SNC80 produced dose-dependent restoration of LA-depressed responding. The slopes of methadone and SNC80 for antinociception were 73.03 +/− 27.68 and 88.10 +/− 18.83 and were not significantly different from each other (F = 0.201, p = 0.657). A dose of 0.56 mg/kg methadone produced ~77% restoration of LA-depressed responding, whereas a dose of 5.6 mg/kg SNC80 produced ~75% restoration of LA-depressed responding. Figures 2D and 2E show the effects of a range of doses of methadone alone (0.1 – 3.2 mg/kg, s.c.) and a range of doses of SNC80 alone (1 – 56 mg/kg, s.c.) on responding in the absence of LA, across all 4 cycles. Figure 2F is a transformation of Figures 2D and 2E, such that dose effect curves in Figure 2F are collapsed across all 4 cycles. Both methadone and SNC80 produced dose-dependent decreases in % control response rate. The slopes of methadone and SNC80 for rate suppression were −56.26 +/− 10.34 and −30.71 +/− 6.73 and were significantly different from each other (F = 4.519, p = 0.038). A dose of 3.2 mg/kg methadone produced near complete suppression of rates, whereas a dose of 56 mg/kg SNC80 produced rate suppression to ~45% of control response rates. Higher doses of SNC80 were not tested in order to decrease the likelihood that animals would experience toxic effects, such as seizures, reported in rodents with higher doses (Chung et al. 2015; Jutkiewicz et al. 2006). ED50 values for all dose-effect curves in 2C and 2F are reported in Table 2.
Fig. 2.
Figure 2 shows effects of methadone alone and SNC80 alone in presence of (top panels) and absence of (bottom panels) lactic acid. Panels A, B, D, E, show data across all 4 cycles. Panels C and F show data collapsed across the 4 cycles.
Table 2.
ED50s (95% CL) for SNC80 alone, methadone alone, and 3 mixtures of SNC80+methadone (expressed as methadone dose), in assays of pain-depressed behavior (Antinociception), and rate suppression (Sedation). Therapeutic index (TI = ED50 sedation ÷ ED50 antinociception) also listed.
| Drug or Mixture |
ED50 Antinociception (95% CL) |
ED50 Sedation (95% CL) |
TI |
|---|---|---|---|
| SNC80 | 3.19 (2.33, 4.38) | 32.94 (18.36, 59.11) | 10.33 |
| Methadone | 0.33 (0.19, 0.59) | 1.39 (1.01, 1.93) | 4.21 |
| 3.33:1 SNC80/Meth | 0.01 (.006, .022) | 0.99 (.894, 1.10) | 99 |
| 10:1 SNC80/Meth | 0.04 (.023, .077) | 1.04 (.719, 1.51) | 26 |
| 30:1 SNC80/Meth | 0.03 (.016, .050) | 0.42 (.319, .557) | 14 |
Effects of delta/mu mixtures in the presence or absence of lactic acid
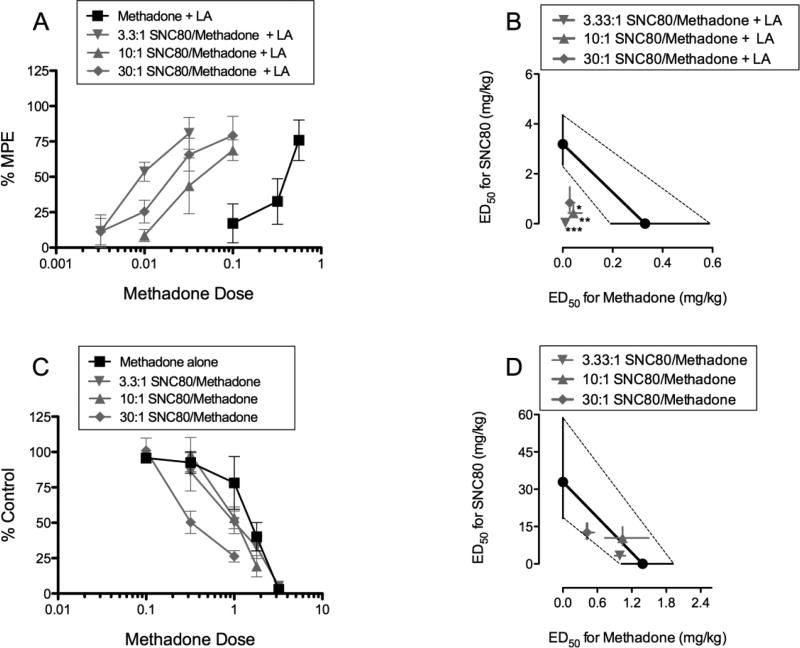
Figure 3 shows dose effect curves and isobolograms for all three (3) mixtures in the presence of (top panels), or absence of (bottom panels) lactic acid. The mixtures in 3A and 3C are graphed as a function of methadone dose. Addition of SNC80 to methadone produced pronounced left-ward shifts in the antinociception assay (3A) and less pronounced left-ward shifts in the sedation assay (3C). Figures 3B and 3D show isobolograms for all three (3) mixtures of SNC80/methadone in the antinociception assay (presence of LA) and sedation assay (absence of LA), respectively. All three mixture ED50s for antinociception fell below the theoretical line of additivity (3B), suggestive of supra-additivity. In contrast, all three mixture ED50s for sedation fell near or on top of the theoretical line of additivity (3D), suggestive of simple additivity. Reporting of Zadd and Zmix values can be found in Table 1.
Fig. 3.
Figure 3 shows effects of all three fixed-dose delta + mu mixtures as dose-effect curves (left) and isobolograms (right), in the presence of lactic acid (top panels) and in the absence of lactic acid (bottom panels). For panel B: *, **, *** = significantly less than predicted additive at p ≤ 0.05, .01, .001.
Table 1.
Experimentally determined Zmix and expected additive Zadd (95% CL) for 3 mixtures of SNC80+methadone, in assays of pain-depressed behavior (Antinociception), and rate suppression (Sedation).
| Mixture | Zmix (95% CL) | Zadd (95% CL) |
|---|---|---|
| Antinociception | ||
| 3.33:1 SNC80/Meth | 0.048 (.024, .096)a | 1.13 (.899, 1.43) |
| 10:1 SNC80/Meth | 0.467 (.257, .852)a | 1.83 (1.35, 2.49) |
| 30:1 SNC80/Meth | 0.868 (.487, 1.55)a | 2.52 (1.78, 3.58) |
| Sedation | ||
| 3.33:1 SNC80/Meth | 4.30 (3.87, 4.78) | 9.47 (5.60, 16.04) |
| 10:1 SNC80/Meth | 11.47 (7.91, 16.63) | 17.31 (9.86, 30.37) |
| 30:1 SNC80/Meth | 13.07 (9.89, 17.26) | 25.12 (14.10, 44.73) |
= Zmix significantly lower than Zadd (supra-additivity).
Experimentally determined Zmix and expected additive Zadd values
Table 1 shows the experimentally determined Zmix and expected additive Zadd values (95%CL) for all three SNC80/methadone mixtures, in the assays of pain-depressed behavior (antinociception) and rate suppression (sedation). In the antinociception assay, all three SNC80/methadone mixtures produced supra-additive effects, whereas in the sedation assay the same three mixtures produced simply additive effects.
ED50s and Therapeutic Indices for delta and mu agonists alone and delta/mu mixtures
Table 2 shows the ED50 values (95% CL) for SNC80 alone, methadone alone, and all three mixtures of SNC80/methadone, in both assays of pain-depressed responding (antinociception) and rate suppression (sedation). Drugs and mixtures were more potent in producing antinociception than in producing rate suppression. The resulting dose ratios (therapeutic indices) are reported in the right-most column. SNC80 alone and methadone alone possessed relatively narrow TIs of 10.33 and 4.21, respectively. Addition of SNC80 increased the TI, and the amount of SNC80 in the mix was indirectly proportional to the resulting TI, such that greater amounts of SNC80 were associated with a smaller TI (e.g.: 30:1 SNC80/methadone mixture TI = 14). The inverse is also true that smaller amounts of SNC80 were associated with a larger TI (e.g.: 3.33:1 SNC80/methadone mixture TI = 99).
Discussion
The present paper is the first report of delta/mu opioid receptor interactions using an assay of pain-depressed behavior. Delta/mu interactions were assessed using the selective, high efficacy delta agonist SNC80, and the selective, high efficacy mu agonist methadone in two assays: a multiple-cycle FR10 operant conditioning procedure in the presence of, or in the absence of an acute inflammatory pain manipulation. The former procedure represented an assay of pain-depressed behavior, whereas, the latter procedure represented an assay of drug-induced rate suppression, loosely defined as sedation, and thus allowed for calculation of therapeutic index (TI). The main finding was that all three fixed-ratio delta + mu mixtures produced supra-additive antinociceptive effects in the assay of pain-depressed behavior, and only additive effects in the assay of sedation. A second, and unique finding was that, relative to delta and mu drugs alone, the therapeutic indices for delta + mu mixtures were not only larger but the amount of SNC80 in each mixture was inversely proportional to the magnitude of TI. Specifically, compared to TI estimates extrapolated from the literature (see below) in which antinociception was evaluated with traditional pain-stimulated assays (eg: tail withdrawal), the TIs in the present study using pain-depressed behaviors appear comparatively larger and the amount of SNC80 in the mixture produced an orderly effect on TI: more SNC80 yielded a narrow TI; less SNC80 yielded a larger TI. These data suggest the potential for a greater therapeutic index in assays of pain-depressed behavior than pain-stimulated behavior.
The present findings of supra-additive antinociceptive effects and simply additive sedative effects are consistent with the delta/mu opioid interaction literature (Jiang et al. 1990; Porreca et al. 1990; Stevenson et al. 2003). Both SNC80 and methadone produced dose-dependent antinociception in the assay of pain-depressed behavior. Fixed-ratio mixtures also produced dose-dependent antinociception that was more potent than drugs alone. Early reports in rodents have also demonstrated that the addition of selective or non-selective delta ligands DPDPE or LEU-enkephalin produced synergistic antinociception in combination with the non-selective opioid agonist morphine (Porreca 1990), but not with the highly selective mu agonist PL017 (Adams et al. 1993). In contrast, use of different delta ligands such as DPDPE, deltorphin, or BW373U86 had no effect on antinociception, but did reverse mu-induced side effect of hypercapnia (Su et al. 1998).
Consistent with the present rodent data, studies using non-human primates (Dykstra et al. 2002; Stevenson et al. 2003) demonstrate that addition of SNC80 to mu agonists produced enhanced or synergistic antinociceptive effects and additive sedation (also measured by operant rate suppression). Comparing across species, SNC80 was much more potent in decreasing food-maintained responding in monkeys than rats, and both SNC80 and methadone were less potent and/or effective in producing antinociception in monkeys (Stevenson et al. 2003) than in pain-depressed responding in rats. Although both studies agree that mixtures are synergistic and produce larger TI than drugs alone, the magnitude of therapeutic index was much greater, up to a factor of 50, in the present study (maximum TI = ~100) than in the 2003 study (maximum extrapolated TI = ~2). As a final point, although lower amounts of SNC80 in the present study produced successively larger TI when combined with methadone, it is highly likely that if the dose of methadone were continuously decreased, its ability to enhance methadone’s antinociceptive effects would begin to wane, and thus, the supra-additive effects may be a function of an inverted U-shape function.
The reason for an orderly, inverse correlation between amount of SNC80 in the mixture and magnitude of TI is unknown. Single molecule, mixed-action delta/mu agonists have been tested in a variety of assays, including nociception, conditioned place preference, drug self-administration, tolerance and dependence (Anand et al. 2016; Lowery et al. 2011; Matsumoto 2014; Stevenson et al. 2015), and there is evidence for therapeutic utility with compounds that have varying selectivities for delta vs. mu receptors. For example, Matsumoto reported that the mixed-action delta/mu agonist MGM-16 showed much higher anti-allodynic potency than morphine alone using a neuropathic pain manipulation. Although no side effect endpoints were measured, the enhanced antinociceptive potency may also be suggestive of a higher TI. Dual profile compounds have also been developed that contain mu agonist and delta antagonist profiles (Schiller et al. 1995). As an example of this, a mixed action mu agonist / delta antagonist VRP26 produced comparable antinociception to fentanyl but did not produce tolerance or dependence after repeated administration, relative to the high efficacy mu agonist fentanyl alone (Anand et al. 2016). Again, these data suggest that the therapeutic index for mixed-action delta/mu compounds is likely larger than that of mu agonists alone, and thus represents an enhanced safety profile. However, caution should be exercised when interpreting drug combination studies as differences in drug time course may impact interaction outcomes. Although the literature on antinociception for methadone and SNC80 show overlapping peak effect times of 15–30 min in a variety of nociception assays (Bilsky et al. 1995; Lemberg et al. 2006; Pradhan and Clarke 2005), the duration of action for both compounds are overlapping but not identical, with SNC80 efficacy lasting 60–90 minutes, and methadone efficacy lasting up to 120 min. In contrast, in assays of locomotor activity, low dose SNC80 effects were present up to 120 min whereas methadone effects were no longer present by 90 min (although later time points were not tested for methadone) (Jutkiewicz et al. 2005; Taracha et al. 2009).
The potential neural mechanisms that underlie in vivo delta/mu interactions are unknown, but represent an exciting and parallel area of discovery. Initial work in this area by the laboratories of Devi, Rothman and others, suggested the existence of mu-delta receptor complexes and ultimately, discovery of co-immunoprecipitation of mu and delta receptors into functional heteromers and oligomers (reviewed in Costatino et al. 2012). More recently, Margolis and colleagues (2017) have shown evidence for two distinct delta receptors and that delta and mu receptors interact in VTA neurons to potentially alter downstream signaling. The task of mapping a mu-delta receptor brain atlas has also been accomplished (Massotte 2015) and this interactive data set suggests neuronal co-localization in sub-cortical areas that mediate, in part, perception, and aversive stimuli, and possibly pain perception.
A related concept is the possibility that the neural mechanisms underlying antinociception in pain-stimulated vs. pain-depressed behaviors are distinct. For example, it is likely that analgesic-induced suppression of pain-stimulated behavior may recruit descending and ascending pain pathways (Basbaum and Fields 1984; Bee and Dickenson 2007; Bernard et al. 1996; Ossipov et al. 2000). In contrast, restoration of pain-depressed behavior may recruit overlapping but distinct pathways to include not only descending and ascending pain pathways but also regions that mediate the organization and planning of movement including motor, visual, and association cortex, as well as medial reticular formation, mesencephalic locomotor region, and cerebellum (Kandel 2013). Thus, articulation of delta/mu interactions in assays of pain-depressed responding may facilitate discovery of translationally relevant and optimum receptor activation. Finally, use of operant conditioning procedures may allow for assessment of the affective dimensions of pain-like behavior, for at least three reasons. First, utilization of FR and progressive ratio schedules of reinforcement in rodents may indicate the degree of motivation in a subject (Martin et al. 2004; Okun et al. 2016; Schwartz et al. 2014; Warner et al. 2015) which represents a salient parameter in affective domains of behavior. Second, the pain-induced behavioral depression in pain-depressed assays is consistent with behavioral depression seen in cases of pain + comorbid depressive disorder (Turk and Okifuji 2004). Third, the subsequent analgesic reversal of pain-induced behavioral depression in these kinds of assays is consistent with how pain, co-morbid depression, and analgesia are quantified in the clinic (Turk and Okifuji 2004). Future studies would benefit from characterization of delta/mu interactions using chronic pain manipulations as well as incorporation of more clinically meaningful side effect measures, including respiratory depression, GI slowing, and addiction liability.
Acknowledgments
This research was supported by an NIH COBRE grant (P20GM103643) that supports an animal behavior core facility, and a National Institutes of Health (NIAMS) R15 AREA grant (AR054975-02A1) to G.W.S. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcoholism and Alcohol Abuse.
References
- Adams JU, Tallarida RJ, Geller EB, Adler MW. Isobolographic superadditivity between delta and mu opioid agonists in the rat depends on the ratio of compounds, the mu agonist and the analgesic assay used. J Pharmacol Exp Ther. 1993;266:1261–1267. [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Effects of u-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of μ-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther. 2015;352:208–217. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Boyer BT, Mosberg HI, Jutkiewicz EM. The behavioral effects of a mixed efficacy antinociceptive peptide, VRP26, following chronic administration in mice. Psychopharm. 2015;233:2479–2487. doi: 10.1007/s00213-016-4296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphine circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neuroscience. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Bernard JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cog Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Chung PC, Boehrer A, Stephan A, Matifas A, Scherrer G, Darcq E, Befort K, Kieffer BL. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res. 2015;278:429–434. doi: 10.1016/j.bbr.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia (2012) Exp Rev Molec Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: Further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Granger AL, Allen RM, Zhang X, Rice KC. Antinociceptive effects of the selective delta opioid agonist SNC80 alone and in combination with mu opioids in the squirrel monkey titration procedure. Psychopharm. 2002;163:420–429. doi: 10.1007/s00213-002-1100-8. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, Koch M, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2015;220:677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AL, Elliott KJ, Inturrisi CE. The d- and l- isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Relieving pain in America: A blueprint for transforming prevention, care, education, and research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Jiang Q, Mosberg HI, Porreca F. Selective modulation of morphine antinociception, but not development of tolerance, by ∂ receptor agonists. Eur J Pharmacol. 1990;186:137–141. doi: 10.1016/0014-2999(90)94071-5. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the δ-opioid agonist SNC80 [(+)-4-[(αR)-α-[(2S,5R)-2,5-Dimethyl-4-(2-propenyl)-1-peperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice JC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmcol Exp Ther. 2006;317(3):1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5. McGraw Hill; NY: 2013. [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers. Molec Pain. 2014;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg K, Kontinen VK, Viljakka K, Kylanlahti I, Yli-Kauhaluoma J, Kalso E. Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg. 2006;102:1768–1774. doi: 10.1213/01.ane.0000205751.88422.41. [DOI] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In vivo characterization of MMP-2200, a mixed ∂/μ opioid agonist, in mice. J Pharmacol Exp Ther. 2011;336:767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fujita W, Devi LA, Fields HL. Two delta opioid receptors subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor. Neuropharm. 2017;123:420–432. doi: 10.1016/j.neuropharm.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat. Anesth. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, Tashima K, Devi L, Suzuki T, Takayama H, Horie S. Orally active opioid μ/∂ dual agonist MGM-16, a derivative of the indole alkaloid mitragynine, exhibits potent antiallodynic effect on neuropathic pain in mice. J Pharmacol Exp Ther. 2014;348:383–392. doi: 10.1124/jpet.113.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM. Development of a bioavabilable μ opioid receptor (MOPr) agonist, ∂ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J Med Chem. 2014;57:3148–3153. doi: 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future directions. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Suzuki T. Delta opioid receptor-mediated antinociception/analgesia. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. 1. CRC Press; Boca Raton, FL: 2003. pp. 331–354. [Google Scholar]
- Okun A, Mckinzie DL, Witkin JM, Remeniuk B, Husein O, Gleason SD, Oyarzo J, Navratilova E, McElroy B, Cowen S, Kennedy JD, Porreca F. Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain. 2016;157(12):2731–2738. doi: 10.1097/j.pain.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann NY Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR. Morphine-like compounds in male and female Sprague-dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- Porreca F, Jiang Q, Tallarida RJ. Modulation of morphine antinociception by peripheral [Leu5] encephalin: a synergistic interaction. Eur J Pharmacol. 1990;179:463–468. doi: 10.1016/0014-2999(90)90190-h. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Clarke P. Pharmacologically selective block of mu opioid antinociception by peptide nucleic acid antisense in absence of detectable ex vivo knockdown. Eur J Pharmacol. 2005;506:229–236. doi: 10.1016/j.ejphar.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Schiller PW, Weltrowska G, Schmidt R, Nguyen TMD, Berezowska I, Lemieux C, Chunb NN, Carpenter KA, Wilkes BC. Four different kinds of opioid peptides with mixed μ agonist/∂ antagonist properties. Analgesia. 1995;1(4–6):703–706. [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalmi CL. Opioids for non-malignant pain – issues and controversy. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. McGraw-Hill; 2004. pp. 601–611. [Google Scholar]
- Siemian JN, Obeng S, Zhang Y, Zhang Y, Jun-Xu L. Agonist 2-BFI and opioid in rats: role of efficacy at the μ-opioid receptor. J Pharmacol Exp Ther. 2016;357:509–519. doi: 10.1124/jpet.116.232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: Effects of ∂ + μ and ∂ + k agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2003;307:1054–1064. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 and heroin. J Pharmacol Exp Ther. 2005;314:221–231. doi: 10.1124/jpet.104.082685. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Luginbuhl A, Dunbar C, LaVigne J, Dutra J, Atherton P, Bell B, Cone K, Giuvelis D, Polt R, Streicher JM, Bilsky EJ. The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol Biochem Behav. 2015;132:49–55. doi: 10.1016/j.pbb.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL. Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLOS One. 2014;9(10):e109903. doi: 10.1371/journal.pone.0109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-F, McNutt RW, Chang K-J. Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmcol Exp Ther. 1998;287:815–823. [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. CRC Press; Boca Raton, FL: 2000. [Google Scholar]
- Taracha E, Chrapusta SJ, Lehner M, Skorzewska A, Plaznik A. Methadone is substantially less effective than morphine in modifying locomotor and brain Fos responses to subsequent methadone challenge in rats. Prog Neuro-Psychopharm Biol Psychia. 2009;33:1032–1039. doi: 10.1016/j.pnpbp.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Turk DC, Okifuji A. Psychological aspects of pain. In: Warfield CA, Bajwa ZH, editors. Principles and practice of pain medicine. 3. McGraw-Hill; 2004. pp. 139–147. [Google Scholar]
- Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid μ-agonist/∂-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J Pharmacol Exp Ther. 2001;297(2):597–605. [PubMed] [Google Scholar]
- Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neuro & Biobeh Rev. 1986;10:103–113. doi: 10.1016/0149-7634(86)90021-7. [DOI] [PubMed] [Google Scholar]