Abstract
Rationale
Orthostatic hypotension is a common clinical problem, but the underlying mechanisms have not been fully delineated.
Objective
We describe 2 families, with four patients in total, suffering from severe life-threatening orthostatic hypotension, due to a novel cause.
Methods and Results
As in dopamine β-hydroxylase deficiency (DβH), concentrations of norepinephrine and epinephrine in the patients were very low. Plasma DβH activity, however, was normal and the DBH gene had no mutations. Molecular genetic analysis was performed to determine the underlying genetic cause. Homozygosity mapping and exome and Sanger sequencing revealed pathogenic homozygous mutations in the gene encoding cytochrome b561 (CYB561); a missense variant c.262G>A, p.Gly88Arg in exon 3 in the Dutch family and a nonsense mutation (c.131G>A, p.Trp44*) in exon 2 in the American family. Expression of CYB561 was investigated using RNA from different human adult and fetal tissues, transcription of RNA into cDNA and real-time quantitative polymerase chain reaction. The CYB561 gene was found to be expressed in many human tissues, in particular the brain. The CYB561 protein defect leads to a shortage of ascorbate inside the catecholamine secretory vesicles leading to a functional DβH deficiency. The concentration of the catecholamines and downstream metabolites was measured in brain and adrenal tissue of six CYB561 knockout mice (reporter-tagged deletion allele (post-Cre), genetic background C57BL/6NTac). The concentration of norepinephrine and normetanephrine was decreased in whole brain homogenates of the CYB561(−/−) mice compared to wild type mice (p<0.01) and the concentration of normetanephrine and metanephrine was decreased in adrenal glands (p<0.01), recapitulating the clinical phenotype. The patients responded favorably to treatment with L-dihydroxyphenylserine, which can be converted directly to norepinephrine.
Conclusions
This study is the first to implicate cytochrome b561 in disease by showing that pathogenic mutations in CYB561 cause an as yet unknown disease in neurotransmitter metabolism causing orthostatic hypotension.
Keywords: Orthostatic hypotension, catecholamines, dopamine β-hydroxylase, CYB561, sympathetic nervous system, hypotensin, low blood pressure, adrenergic regulation, dopamine, genetics
INTRODUCTION
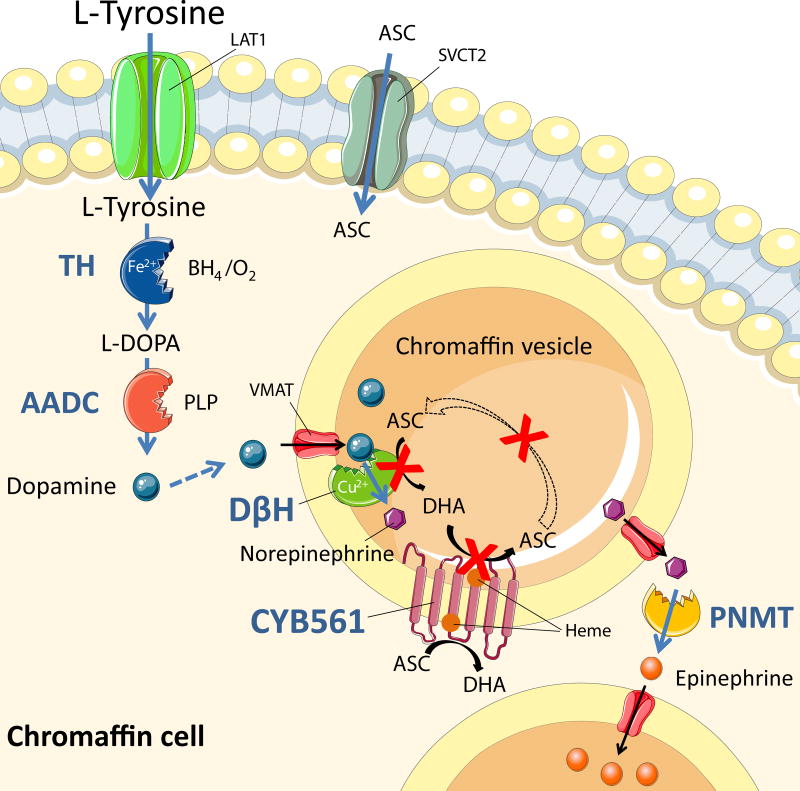
Catecholamines − dopamine, norepinephrine and epinephrine − play an essential role in many physiological processes, including regulation of vascular tone and circulation. Their synthesis from the amino acid precursor tyrosine involves several steps in which enzymes, cofactors and transporters are indispensable (Figure 1). Defects in nine enzymes in catecholamine biosynthesis and catabolism (including dopamine β-hydroxylase, DβH) and in two transporters have been described.1 Most of these lead to severe motor and/or mental dysfunction and present in early childhood. DβH deficiency presents as an orthostatic hypotension syndrome.2,3
Figure 1. Catecholamine metabolism and the function of CYB561 in this pathway.
The site of action is the chromaffin cell and vesicle. Tyrosine is taken up by the cell, where it is enzymatically converted by tyrosine hydroxylase (TH) to L-DOPA (rate-limiting reaction). L DOPA is then converted to dopamine by aromatic L-amino acid decarboxylase (AADC) and the dopamine is rapidly transported by vesicular monoamine transporter (VMAT) inside the vesicle, where it is converted by dopamine β-hydroxylase (DβH) with ascorbic acid (ASC) as cofactor and resulting in norepinephrine and dehydroascorbate (DHA). Norepinephrine is transported outside the vesicle by VMAT, where it is converted to epinephrine by cytosolic phenylethanolamine N-methyltransferase (PNMT). Dehydroascorbate inside the vesicle is reduced to ascorbic acid by CYB561, which itself becomes oxidized. CYB561 is then reduced by cytosolic ascorbic acid, completing the cycle.
We describe two families, with four patients in total, suffering from severe life-threatening orthostatic hypotension, resembling DβH deficiency. As in DβH deficiency, concentrations of norepinephrine and epinephrine were very low in our patients. Plasma DβH activity, however, was normal and the DBH gene was not mutated. Homozygosity mapping, exome sequencing and subsequent Sanger sequencing revealed pathogenic homozygous mutations in the gene encoding cytochrome b561 (CYB561). As a consequence the electron shuttle necessary for the ascorbate-dehydroascorbate recycling inside catecholamine secretory vesicles is disrupted. This recycling is imperative for the synthesis of norepinephrine from dopamine within these vesicles.4 This novel genetic cause for orthostatic hypotension is an inborn error of metabolism amenable to treatment with L dihydroxyphenylserine, which can be converted directly to norepinephrine.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files. A detailed description of methods used in this study is available in the Online Supplementary Materials.
Patients
Dutch family
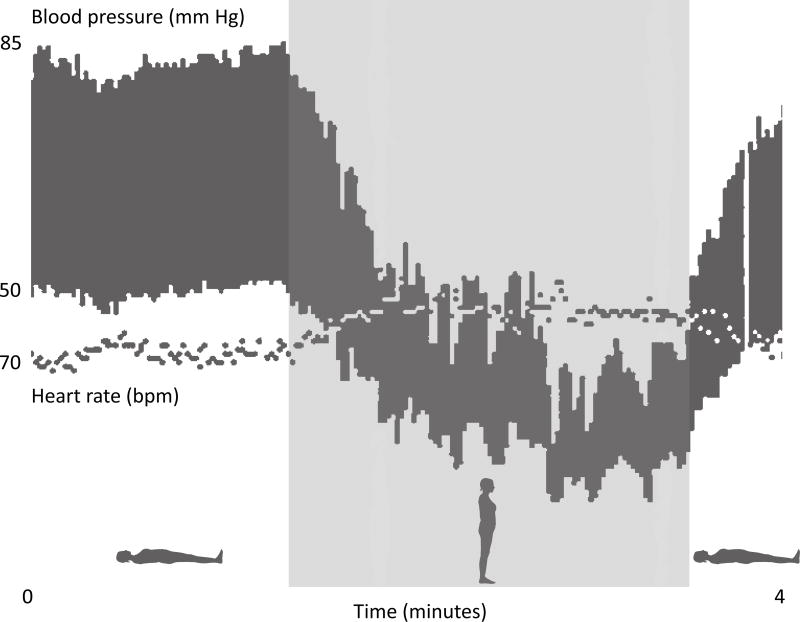
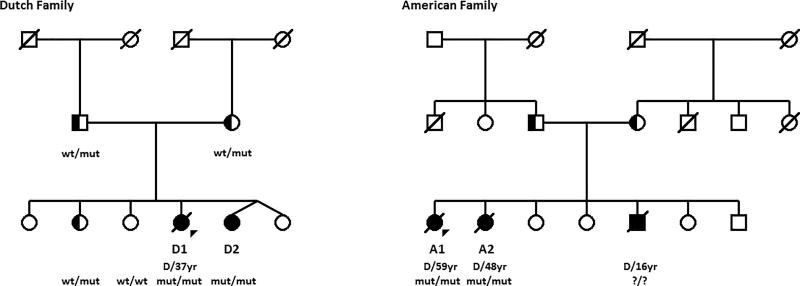
A 33-year-old female (patient D1) was referred to the University Medical Center Groningen because of severe orthostatic hypotension. From early childhood onwards, she had suffered from dizziness and occasional fainting upon standing. Her neurologic examination was within normal limits. There was no ptosis. She had normal mental and physical development and her pregnancies had been uncomplicated. Supine blood pressure was 85/50 mm Hg but after standing up it immediately dropped to 50/35 mm Hg without a significant change in heart rate (Figure 2). Because of the combination of orthostatic hypotension and lack of compensatory tachycardia, sympathetic dysfunction was suspected. Indeed, the concentration of norepinephrine, epinephrine and their downstream metabolites normetanephrine and 3 methoxy-4-hydroxyphenylglycol (MHPG) was very low in 24-hour urine and plasma. The MHPG concentration in cerebrospinal fluid was equally low, with normal homovanillic acid and 5-hydroxyindolacetic acid concentration (Table 1 and Online Table I). Sanger sequencing of DBH, the gene encoding DβH, revealed no pathogenic mutations.5 Renal function was impaired, with secondary mild anemia (Hb=7.3 mmol/L). Echography of the kidneys was unremarkable. Using 125 I iothalamate clearance, supine effective renal plasma flow was 248 ml/min and glomerular filtration rate was 56 ml/min (reference 1000–1200 and 100–120, respectively) whereas sitting effective renal plasma flow was 199 ml/min and glomerular filtration rate 38 ml/min. The patient had a 26-year-old sister (patient D2) with a comparable clinical presentation and biochemical findings (Table 1 and Online Table I). D2’s plasma DβH enzyme activity was normal.6 The DBH gene was sequenced but no pathogenic mutations were found. Neither the parents nor two other healthy siblings suffered from orthostatic hypotension and their urinary catecholamine levels were all normal (Figure 3).
Figure 2. Blood pressure and heart rate recording.
Beat-to-beat recordings of blood pressure and heart rate in the Dutch proband (patient D1) when she was supine and standing. After standing up, her blood pressure dropped profoundly without a significant change in heart rate.
Table 1.
Clinical Signs and Symptoms, and Genetic and Biochemical Hallmarks of Patients with a CYB561 Defect
| A. Clinical signs and symptoms | American patients (n=2) | Dutch patients (n=2) |
|---|---|---|
| Severe orthostatic hypotension | + | + |
| Episodes of hypoglycemia in childhood | + | ND |
| Disturbed renal function (mild-severe) | ND | + |
| Mild anemia | ND | + |
| Early death | + | + |
| B. Genetic characteristics | ||
| Mutation cDNA: NM_001915.3(CYB561) | c.131G>A | c.262G>A |
| Mutation gDNA: Chr17(GRCh37) | g.61514778C>T | g.61513454C>T |
| Protein change | p.Trp44* | p.Gly88Arg |
| C. Biochemical hallmarks | ||
| Very low norepinephrine in urine and plasma | ||
| Very low normetanephrine in urine and plasma | ||
| Very low MHPG in urine | ||
| Very low CSF MHPG with normal CSF HVA and 5-HIAA | ||
| Normal dopamine and metabolites in urine and plasma | ||
| Normal dopamine beta hydroxylase enzymatic activity in plasma | ||
CSF indicates cerebrospinal fluid; 5-HIAA, hydroxyindolacetic acid; HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; ND not documented.
Figure 3. Pedigrees of the Dutch and the American family.
Patients diagnosed with orthostatic hypotension are labelled with D1/D2 and A1/A2, respectively. Squares indicate males, circles indicate females. Diagonal line indicates deceased, “D/XXyr” the age at death. Filled symbols indicate affected individuals. Half-filled symbols indicate unaffected carriers. CYB561 mutation status is shown by wt: wildtype; mut: mutation; ?: unknown. Triangles indicate the probands of each family. No consanguinity was known and therefore not shown by a double line between the parents of the affected individuals.
American family
A 39 year-old female (patient A1) and her 38-year-old sister (patient A2) presented at the Vanderbilt University School of Medicine because of severe orthostatic hypotension since infancy. They had episodes of symptomatic hypoglycemia during infancy. Patient A1 underwent a partial pancreatectomy at age three years for this reason but she showed no improvement in these episodes. Both sisters had a normal mental and physical development and uncomplicated pregnancies. They had a brother with comparable complaints but he died at age 16 of an unknown cause. Both parents and four other sibs had no complaints (Figure 3). Plasma norepinephrine, epinephrine and the intraneuronal norepinephrine metabolite dihydroxyphenylglycol were below the limit of detection (<0.01 nmol/L) (Online Table I). Plasma DβH activity was normal.
Molecular genetic analysis
To determine the genetic basis of the phenotypes genomic DNA from the two affected Dutch sisters (D1, D2) and their relatives and from the two affected American sisters (A1, A2) was extracted from peripheral blood using standard protocols (PUREGENE; QIAGEN, Venlo, the Netherlands). Homozygosity mapping and exome and Sanger sequencing was then performed (Online Data Supplement). The analyses conformed to principles defined in the Helsinki Declaration and the medical ethics committees of the two centers. Written informed consent was obtained from the patients or their relatives.
RESULTS
Mutation identification
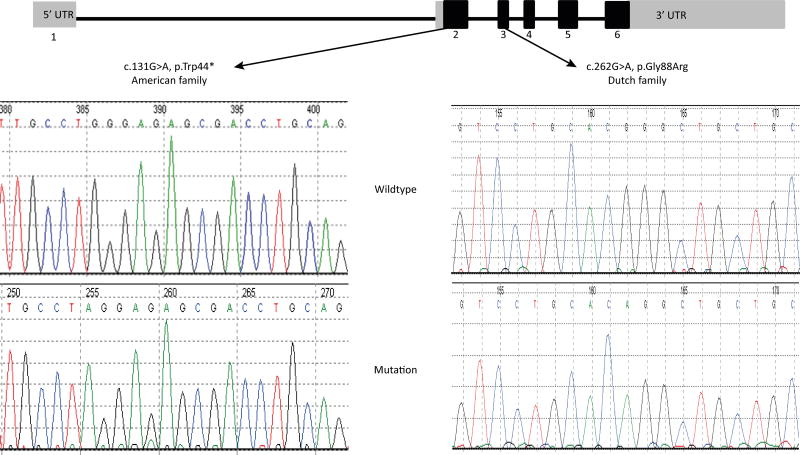
Homozygosity mapping in patients D1 and D2 revealed 11 shared homozygous regions larger than 2.0 cM (Online Figure I). The largest region of homozygosity was located on chromosome 17, 17q22-17q24.2, between rs11657462 and rs4968819 (g.55,463,146-g.66,742,160; UCSC Genome Browser, build hg19), spanning 550 SNPs and 12.26 cM, and containing 220 genes. Using different data analysis curing programs, whole exome sequencing identified 22,689 variants in patient D1. After removal of all known variants with >1% frequency from the exome sequencing data of patient D1 we selected all the stop-gain, stop-loss, missense, splice site, frame shift and in-frame coding indel variants that were concordant with autosomal recessive inheritance. Fifteen variants in 10 different genes fulfilled these criteria. The respective genes and variants were computationally analyzed and classified using OMIM (https://www.omim.org), GeneCards (http://www.genecards.org) and UniProt (http://www.uniprot.org) (gene classification) and the Alamut software (Interactive biosoftware) (Online Table II). Based on that analysis only the variant in the CYB561 gene was prioritized as possible candidate disease gene. Moreover, the variant in that gene was the only homozygous variant located in the longest shared homozygous region (chromosome 17q22-17q24.2) seen in patients D1 and D2, as identified by homozygosity mapping. In this chromosomal region a homozygous missense variant c.262G>A, p.Gly88Arg in exon 3 of the Cytochrome b561 gene (CYB561, NM_001915.3) (Figure 4) was found as the most likely causative variant. Analysis of the CYB561 closest homologs in other species showed that the nucleotide c.262 and the glycine 88 both are highly evolutionary conserved (Figure 5). The Gly88 residue is located in a crucial functional protein domain (Cytochrome b561/ferric reductase transmembrane) (Figure 6). The in silico pathogenicity prediction programs Mutation taster and PolyPhen-2 both suggest a pathogenic effect. Sanger sequencing confirmed the presence of the c.262G>A mutation in homozygous form in the two affected Dutch sisters while it was heterozygous in the parents and in an unaffected sister and wild type in the other sister (Table 1 and Figure 4). Further evidence for the relation between CYB561 gene variants and orthostatic hypotension was obtained from the American family. Both affected sisters (A1 and A2) had a homozygous nonsense mutation (c.131G>A, p.Trp44*) in exon 2 of the gene (Table 1 and Figure 4). This variant leads to reading frame interruption by a premature stop codon. We have assessed the frequencies of the CYB561 variants in the Genome Aggregation Database (http://gnomad.broadinstitute.org). The missense variant c.262G>A, p.Gly88Arg identified in the Dutch family was absent in over 100,000 Non-Finnish European control alleles. Another missense variant resulting in the same amino acid change (c.262G>C, p.Gly88Arg) was found in heterozygous state two times in 126,628 Non-Finnish European control alleles. The nonsense mutation (c.131G>A, p.Trp44*) identified in the American family was found once in 110,060 Non-Finnish European control alleles.
Figure 4. CYB561 mutations.
The upper part shows the structure of the CYB561 gene, with the positions of the identified mutations indicated. Grey boxes indicate 5’ and 3’ UTRs (untranslated regions), black boxes indicate coding exon sequences. The lower part depicts spherograms showing the Sanger sequencing data of the patients (mutation) and controls (wild type).
Figure 5. Evolutionary conservation of the cytochrome b561 amino acid sequence.
Part of the protein sequence of human CYB651 and the corresponding sequence in the Dutch patients (D1 and D2) with the glycine to arginine replacement at position 88 (in red) is shown. Corresponding sequences across different organisms are aligned. TM denotes trans-membrane.
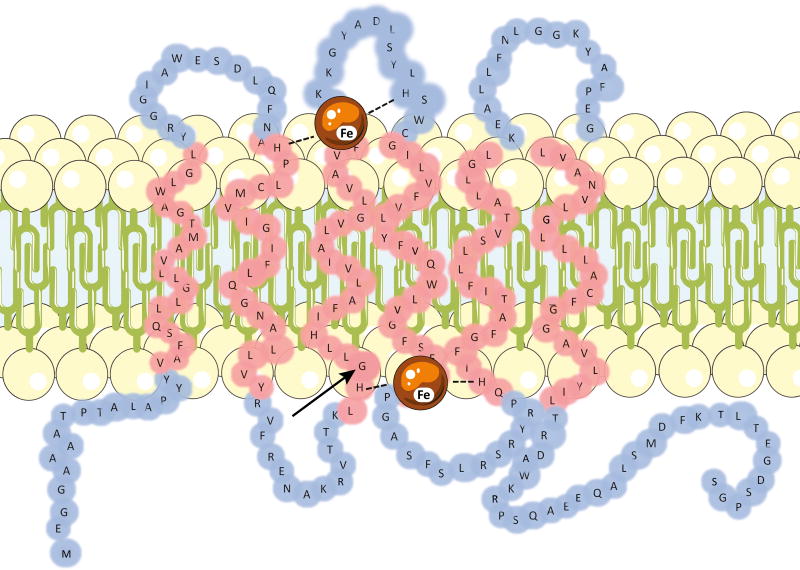
Figure 6. Cytochrome b561 protein conformation.
The upper part shows the CYB561 protein conformation in the chromaffin vesicle membrane. The protein contains two heme goups positioned on either side of the vesicular membrane. The arrow indicates the mutated glycine in the Dutch patients (D1 and D2). The lower part shows the full protein sequence of CYB561. In yellow the 6 transmembrane parts of the protein; in green the positions where the mutations in the two families occur; in red letters (“H”) the four Histine residues coordinating the two heme groups; the two stretches NALLVYRVF and SLHSW are the putative substrate reducing sites.
CYB561 expression in human tissues
After identification of the mutations in expression of CYB561 was investigated using RNA from different human adult and fetal tissues, transcription of RNA into cDNA and real-time quantitative polymerase chain reaction. (Online Data Supplement). The CYB561 gene was found to be expressed in many human tissues. Expression is ubiquitous in the brain and very strong, especially in the cortex and hippocampus (Online Figure II).
Catecholamine measurements in CYB61 knockout mouse tissue
In order to obtain additional functional evidence tissue from CYB561 knockout mice (reporter-tagged deletion allele (post-Cre), genetic background C57BL/6NTac) was obtained through the International Mouse Phenotyping Consortium (Release 6.1.).7 The mouse studies in the International Mouse Phenotyping Consortium are approved by the appropriate review boards. Using liquid chromatography in combination with isotope dilution tandem mass spectrometry, we measured the concentration of the catecholamines and downstream metabolites in brain and adrenal tissue of six CYB561(−/−) mice and six wild type mice. Mouse brain and adrenal gland tissue homogenates were prepared as previously described.8 In short: tissue homogenates (10 % weight:volume (w/v)) were prepared by sonification at 11–12 W for 30 seconds on ice in 0.08 M acetic acid with 0.8 % (w/v) reduced glutathione. After homogenization, samples were centrifuged and supernatant was used for analysis by isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described. Isotope labeled standards were used for each respective analyte.9,10 The concentration of norepinephrine and normetanephrine was profoundly and significantly decreased in whole brain homogenates of six CYB561(−/−) mice compared to six wild type (median and range in nmol/g wet weight for norepinephrine: 0.126 (0.10–0.19) vs 2.88 (2.26–3.84); p<0.01) (Table 2). This nicely illustrates the consequence of the CYB561 defect for the norepineprine presence in the brain. The concentration of the norepinephrine and epinephrine catabolites normetanephrine and metanephrine was significantly decreased in adrenal glands (p<0.01). Dopamine is the direct precursor of norepinephrine. Its concentration was not significantly different between wild type and CYB561(−/−) mice in the adrenals and the brain. The concentration of the dopamine catabolite 3-methoxytyramine, however, was significantly increased in adrenal glands, but not in the brain of CYB561(−/−) mice (Table 2). The normal dopamine concentration reflects that dopamine biosynthesis is fully normal in CYB561(−/−) mice. The defect affects the catecholamine biosynthesis downstream of dopamine at the level of dopamine beta synthase. Low DβH enzyme activity is due to the interruption of the ascorbate cycle caused by the defect in CYB561 (Figure 1).
Table 2.
Concentrations of Catecholamines and Metanephrines for Brain- and Adrenal Gland Homogenates of Wild Type and CYB561−/− Mice (n = 6 per Group)
| Wild type | CYB561−/− | P* | |
|---|---|---|---|
| Adrenal glands | |||
| 3-Methoxytyramine | 0.018 (0.010–0.020) | 0.078 (0.05–0.36) | p < 0.01 |
| Normetanephrine | 1.53 (0.96–1.89) | 0.703 (0.61–0.97) | p < 0.01 |
| Metanephrine | 1.48 (0.75–1.63) | 0.530 (0.19–1.34) | p < 0.01 |
| Dopamine | 0.431 (0.12–3.79) | 1.94 (0.16–13.2) | n.s. |
| Norepinephrine | 10.4 (2.24–139) | 2.35 (0.18–35.6) | n.s. |
| Epinephrine | 2.67 (0.84–57.9) | 0.68 (0.03–37.5) | n.s. |
| Brain | |||
| Normetanephrine | 0.503 (0.32–0.71) | 0.027 (0.02–0.05) | p < 0.01 |
| Norepinephrine | 2.88 (2.26–3.84) | 0.126 (0.10–0.19) | p < 0.01 |
| 3-Methoxytyramine | 0.898 (0.76–1.06) | 1.02 (0.83–1.45) | n.s. |
| Dopamine | 7.46 (6.51–8.12) | 6.79 (4.52–8.17) | n.s. |
Concentrations are in nmol/g wet weight as median values (min-max values)
Mann-Whitney U test (two-tailed).
Consequences at the level of the Cytochrome b561 protein
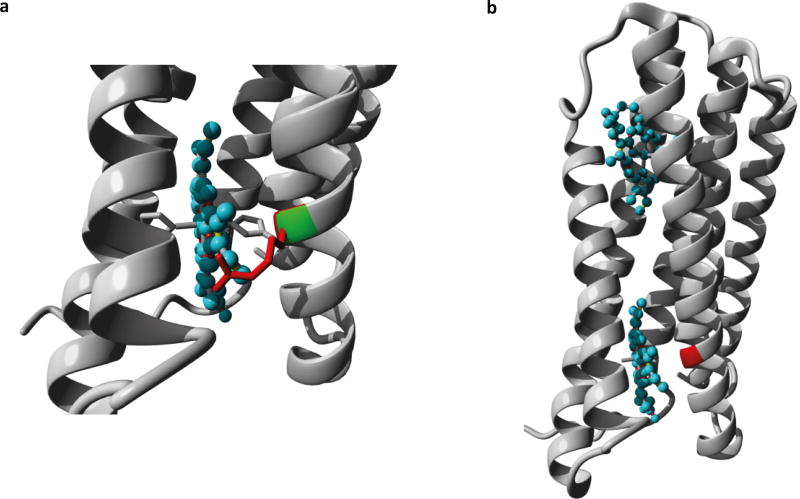
It is generally accepted that CYB561 has six transmembrane alpha helix domains4. It has four His-residues coordinating two heme-b molecules, one on the cytoplasmic side and one on the luminal side of the membrane (Figure 6). The heme groups accept electrons from ascorbate. The p.Gly88Arg change found in the Dutch family occurs in the third transmembrane domain while the c.131G>A mutation in the American family truncates the protein at Trp44 in between the first two transmembrane segments (Figure 6). No experimentally solved structure of the human cytochrome b561 is known. We have therefore built a homology model using the known cytochrome b561 structure of A. Thaliana as a template.11 The model suggests that the introduction of a hydrophilic and positively charged arginine in the third transmembrane region might affect the interactions between the hydrophobic lipids and can alter transmembrane anchoring of the protein (Figure 7). In addition, a smaller effect on ligand binding can be expected as the mutation occurs next to the central histidine residue which is detrimental for heme-Fe binding.
Figure 7. CYB561 homology modeling.
a. Close-up of mutation G88R in CYB561. The protein is shown in ribbon-presentation in grey, the heme ligand is shown in ball-stick presentation and coloured cyan. The side-chains of the mutant residue, wild type residue, and the histidine that interacts with the Fe-atom in the heme group are all shown in stick presentation. The wild type and mutant residues are coloured green and red respectively. b. Overview of the Cyb561 model shown in ribbon presentation. The two heme-ligands are show in ball-stick presentation and coloured cyan. The position of the G88R mutant is indicated in red.
The biochemical phenotype
Norepinephrine and its downstream metabolites normetanephrine and MHPG were profoundly decreased in body fluids of the patients (plasma: norepinephrine <0.05 nmol/L, normetanephrine <0.01 nmol/L; urine: norepinephrine <2 µmol/mmol creatinine). Other downstream metabolites from the catecholamine pathway were also lower than normal (Online Data Supplement). The concentration of MHPG, the principal downstream metabolite of norepinephrine in the brain, was extremely low in CSF (<3 nmol/L; reference 26–64). The downstream metabolites of dopamine and serotonin, HVA and 5-HIAA respectively, had a normal concentration in CSF. This underpins the central role of CYB561 in the brain, the normal biosynthesis and availability of dopamine and serotonin in the patients and the profound consequences of pathogenic mutations in the CYB561 gene for the norepinephrine availability in the brain of the patients. Plasma DβH enzymatic activity was normal in all patients. This parameter helps to discriminate between defects in CYB561 and DBH. The overall biomarker profile allows the biochemical diagnosis of the CYB561 defect in plasma or urine in other patients.
Treatment
The four patients were treated with L-dihydroxyphenylserine (L-DOPS), a synthetic precursor of norepinephrine, with dosages ranging from 200 mg once to three times daily. This treatment led a rise blood pressure in all 4 patients: supine blood pressure invariably exceeded 100/70 mmHg and blood pressure in the upright position usually rose to at least 90/60 mmHg. More importantly, treatment with L-DOPS ameliorated the symptoms of orthostatic hypotension (dizziness).These beneficial effects were supported by the urinary norepinephrine levels which we measured in the Dutch patients (D1 and D2) after initiating L-DOPS treatment; levels were markedly higher and even beyond the upper limit of normal. However, L-DOPS tolerability was hampered by nausea, headache and pain in the lumbar region. Four years after presentation, patient D1 suddenly lost consciousness with a prolonged circulatory arrest (while she was off L-DOPS treatment). After resuscitation measures and administration of a low-dose epinephrine, her blood pressure quickly recovered, but she had incurred severe brain damage and died after being in coma for five days. Her sister (D2) is still alive albeit with progressive renal insufficiency (glomerular filtration rate 26 ml/min). Both American patients (A1 and A2) were lost to follow up and died of unknown cause at age 59 and 48, respectively.
DISCUSSION
Here we report a novel autosomal recessive orthostatic hypotension syndrome caused by mutations in the CYB561 gene. These mutations interfere with norepinephrine biosynthesis, a situation that resembles DβH deficiency in terms of clinical signs and symptoms and biochemical hallmarks.2,3 DβH and CYB561 deficiency are both characterized by orthostatic hypotension, recurrent hypoglycemia and low norepinephrine levels. Ptosis and skeletal muscle hypotonia have been described in DβH patients but were not observed in our CYB561-patients.2,3 Instead, the syndrome in our patients appears to be more malignant in terms of renal insufficiency and reduced life expectancy, either due to hypotensive crises or profound hypoglycemia. Fortunately, treatment with L-DOPS affords alleviation of symptoms, although tolerability has been an issue.
Data on the role of CYB561 gene in humans are very limited. In a study in healthy volunteers, a SNP (rs_20582013) in CYB561 was shown to be associated with lower norepinephrine levels.12 A study in healthy twin pairs showed an association between another SNP (A+1485G, rs_3087776) in the microRNA motif in the 3’UTR of CYB561 and (stress-augmented) heart rate.13 These findings concur with our data, implicating CYB561 in the sympathetic control of heart rate and blood pressure12,13. Our report is the first to actually implicate CYB561 in disease.
CYB561 encodes the protein CYB561, named as such because of its optical absorbance at 561nm. The protein is attached to the membrane of catecholamine and neuropeptide secretory vesicles in neuroendocrine tissues (Figure 1 and Figure 6). CYB561 is a heme-containing enzyme that is necessary for the continuous regeneration of semidehydroascorbate to ascorbate inside the chromaffin granules and neuropeptide secretory vesicles. It is widely expressed in human tissues. In the brain it is strongly expressed in the cortex and hippocampus and to a lesser extent in other parts (Online Figure II). Its presence was demonstrated in various neuroendocrine tissues.14 CYB561 functions as a trans-membrane electron carrier, maintaining the redox state between cytosolic and intra-vesicular ascorbic acid (Figure 1 and Figure 6).15 Intravesicular ascorbic acid is required by DβH and peptidylglycine alpha-amidating monooxygenase for their enzymatic activity. DβH uses ascorbic acid as a cofactor to convert dopamine to norepinephrine, and peptidylglycine alpha-amidating monooxygenase uses ascorbic acid to amidate neuropeptides to prolong and increase activity (e.g. α-melanocyte-stimulating hormone, oxytocin, vasopressin, gastrin). In the CYB561 catalyzed reaction, ascorbic acid is converted into dehydroascorbic acid. Vesicles cannot take up ascorbic acid, only semidehydroascorbate, so that semidehydroascorbate needs to be recycled inside the vesicle.16,17 CYB561 reduces dehydroascorbate back to ascorbic acid, thereby oxidizing itself. Cytoplasmic ascorbic acid is then required to reduce CYB561, and so the cycle proceeds when enough ascorbic acid is available in the cytosol. Without CYB561 activity, norepinephrine synthesis comes to a halt, due to insufficient vesicular ascorbate levels for the conversion of dopamine to norepinephrine by DβH.
The mutation in the Dutch patients introduces a change in a transmembrane domain of CYB561. Such changes are known to alter the structure and function of transmembrane proteins in general.18 The introduction of a hydrophilic and positively charged arginine in the transmembrane region may affect the interactions between the hydrophobic lipids and can alter transmembrane anchoring of the protein. Besides that, a smaller effect on ligand binding can be expected as the mutation occurs next to the central histidine residue which is detrimental for heme-Fe binding (Figure 6 and Figure 7). Sequence alignment shows that Gly88 is highly conserved throughout various species. This is another indication that its substitution is likely to be detrimental for the function of CYB561 (Figure 4). Loss of CYB561 function is also likely in the American patients as their CYB561 mutation leads to a premature stop codon.
The catecholamine measurements in tissues of CYB561 knockout mice neatly recapitulated the clinical findings. An important consideration when using mice in ascorbic acid studies is that mice, unlike humans, can produce ascorbic acid de novo. Our biochemical findings in CYB561 −/− mice are comparable to two studies in which mice lack the vitamin C transporter SVCT2 and/or the enzyme gluconolactonase in the initial steps of ascorbic acid synthesis.19,20 Both studies demonstrated a decreased concentration of norepinephrine while the dopamine concentration was not abnormal as was also shown in our patients and in the CYB561(−/−) mice. These results support our findings that CYB561 mutations lead to disrupted ascorbate recycling and subsequently disturbed norepinephrine synthesis. Of note, as part of the International Mouse Phenotyping Consortium pipeline all mice are routinely culled at 16 weeks, including the CYB561 knock out mice, and phenotyping is limited. In particular, no data on blood pressure are available which is a limitation of our study.
In conclusion, our findings define biallelic mutations in CYB561 as a novel cause for orthostatic hypotension in human. This novel inborn error of metabolism is amenable to treatment. Further studies are required to find an optimal medication dosing regime.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Catecholamines play an essential role in regulation of vascular tone and blood pressure.
Diseases affecting catecholamine biosynthesis and catabolism can cause orthostatic hypotension, which are often hereditary.
What New Information Does This Article Contribute?
We report a novel autosomal recessive orthostatic hypotension syndrome in 2 families, characterized by incapacitating orthostatic hypotension, renal insufficiency, hypoglycemia, and reduced life expectancy.
The underlying genetic defect was identified to be homozygous mutations in the gene encoding cytochrome b561 (CYB561). The mutations disrupt the electron shuttle necessary for the ascorbate-dehydroascorbate recycling inside catecholamine secretory vesicles and halts production of norepinephrine.
This novel genetic cause for orthostatic hypotension is an inborn error of metabolism amenable to treatment with L dihydroxyphenylserine, which can be converted directly to norepinephrine.
Catecholamines play an essential role in regulation of vascular tone and blood pressure. Diseases affecting catecholamine biosynthesis and catabolism can cause orthostatic hypotension. We describe two families, with four patients in total, suffering from severe life-threatening orthostatic hypotension, renal insufficiency, hypoglycemia, and reduced life expectancy. As in dopamine β-hydroxylase deficiency (DβH) deficiency, concentrations of norepinephrine and epinephrine were very low in our patients. Plasma DβH activity, however, was normal and the DBH gene did not carry a mutation. Homozygosity mapping, exome sequencing and subsequent Sanger sequencing led to identification of pathogenic homozygous mutations in the gene encoding cytochrome b561 (CYB561). The mutations disrupted the electron shuttle necessary for the ascorbate-dehydroascorbate recycling inside catecholamine secretory vesicles. This recycling is imperative for the synthesis of norepinephrine from dopamine within these vesicles. The CYB561 gene was found to be expressed in several human tissues, in particular the brain. The concentrations of the catecholamines and downstream metabolites were measured in brain and adrenal tissue of six Cyb561 knockout mice, which recapitulated the clinical phenotype. This study is the first to implicate cytochrome b561 in human disease by showing that pathogenic mutations in CYB561 cause a novel form of orthostatic hypotension caused by abnormalities in neurotransmitter metabolism. This novel inborn error of metabolism is amenable to treatment with L dihydroxyphenylserine, which can be converted directly to norepinephrine.
Acknowledgments
We thank Jackie Senior for editing the manuscript.
SOURCES OF FUNDING
The work was supported by the following grants: Rowida Almomani (Netherlands Heart Foundation,grant 2010B164 to J.D.H.J.), Italo Biagionni (NIH grants RR024975 and NS065736), Ron Wevers (E.C. Noyons foundation).
Nonstandard Abbreviations and Acronyms
- CYB561
cytochrome b561
- DβH
dopamine β-hydroxylase
- L-DOPS
L dihydroxyphenylserine
Footnotes
DISCLOSURES
None.
References
- 1.Ng J, Papandreou A, Heales SJ, Kurian MA. Monoamine neurotransmitter disorders – clinical advances and future perspectives. Nat Rev Neurol. 2015;11:567–84. doi: 10.1038/nrneurol.2015.172. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D, Goldberg MR, Onrot J, Hollister AS, Wiley R, Thompson JG, Jr, Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission. Evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–7. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 3.Man in 't Veld AJ, Boomsma F, Moleman P, Schalekamp MA. Congenital dopamine-beta-hydroxylase deficiency. A novel orthostatic syndrome. Lancet. 1987;1(8526):183–8. doi: 10.1016/s0140-6736(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 4.Asard H, Barbaro R, Trost P, Bérczi A. Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid Redox Signal. 2013;19(9):1026–35. doi: 10.1089/ars.2012.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deinum J, Steenbergen-Spanjers GC, Jansen M, Boomsma F, Lenders JW, van Ittersum FJ, Hück N, van den Heuvel LP, Wevers RA. DBH gene variants that cause low plasma dopamine beta hydroxylase with or without a severe orthostatic syndrome. J Med Genet. 2004;41:e38. doi: 10.1136/jmg.2003.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui H, Kato N, Yamamoto C, Fujita K, Sakai H, Nagatsu T. A sensitive fluorometric assay for dopamine-beta-hydroxylase activity by high-performance liquid chromatography. Biochem Med. 1984;31(2):140–6. doi: 10.1016/0006-2944(84)90019-x. [DOI] [PubMed] [Google Scholar]
- 7.Koscielny G, Yaikhom G, Iyer V, Meehan TF, Morgan H, Atienza-Herrero J, Blake A, Chen CK, Easty R, Di Fenza A, Fiegel T, Grifiths M, Horne A, Karp NA, Kurbatova N, Mason JC, Matthews P, Oakley DJ, Qazi A, Regnart J, Retha A, Santos LA, Sneddon DJ, Warren J, Westerberg H, Wilson RJ, Melvin DG, Smedley D, Brown SD, Flicek P, Skarnes WC, Mallon AM, Parkinson H. The International Mouse Phenotyping Consortium Web Portal, a unified point of access for knockout mice and related phenotyping data. Nucleic Acids Res. 2014;42:D802–9. doi: 10.1093/nar/gkt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Vliet D, Bruinenberg VM, Mazzola PN, van Faassen MH, de Blaauw P, Kema IP, Heiner-Fokkema MR, van Anholt RD, van der Zee EA, van Spronsen FJ. Large neutral amino acid supplementation exerts its effect through three synergistic mechanisms: proof of principle in phenylketonuria mice. PloS One. 2015;10(12):e0143833. doi: 10.1371/journal.pone.0143833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, de Vries EG, Kema IP. Plasma free metaneprhine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin Chem. 2007;53:1684–93. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- 10.van de Merbel NC, Hendriks G, Imbos R, Tuunainen J, Rouru J, Nikkanen H. Quantitative determination of free and total dopamine in human plasma by LC-MS/MS: the importance of sample preparation. Bioanalysis. 2011;3(17):1949–61. doi: 10.4155/bio.11.170. [DOI] [PubMed] [Google Scholar]
- 11.Lu P, Ma D, Yan C, Gong X, Du M, Shi Y. Structure and mechanism of a eukaryotic transmembrane ascorbate-dependent oxidoreductase. Proc Natl Acad Sci. 2014;111:1813–8. doi: 10.1073/pnas.1323931111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghimire LV, Kohli U, Li C, Sofowora GG, Muszkat M, Friedman EA, Solus JF, Wood AJJ, Stein CM, Kurnik D. Catecholamine pathway gene is associated with norepinephrine and epinephrine concentrations at rest and exercise. Pharmacogenet Genomics. 2012;22:254–60. doi: 10.1097/FPC.0b013e328350a274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Deacon DC, Rao F, Schork AJ, Fung MM, Waalen J, Schork NJ, Nievergelt CM, Chi NC, O’Connor DT. Human heart rate. Heritability of resting and stress values in twin pairs, and influence of genetic variation in the adrenergic pathway at a microribonucleic acid microRNA motif in the 3’-UTR of cytochrome b561. J Am Coll Cardiol. 2014;63:358–68. doi: 10.1016/j.jacc.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruss RM, Shepard EA. Cytochrome b561 can be detected in many neuroendocrine tissues using a specific monoclonal antibody. Neuroscience. 1987;22:149–57. doi: 10.1016/0306-4522(87)90205-3. [DOI] [PubMed] [Google Scholar]
- 15.Njus D, Kelley PM, Harnadek GJ, Pacquing YV. Mechanism of ascorbic acid regeneration mediated by cytochrome b561. Ann N Y Acad Sci. 1987;493:108–19. doi: 10.1111/j.1749-6632.1987.tb27188.x. [DOI] [PubMed] [Google Scholar]
- 16.Tirrell JG, Westhead EW. The uptake of ascorbic acid and dehydroascorbic acid by chromaffin granules of the adrenal medulla. Neuroscience. 1979;4:181–6. doi: 10.1016/0306-4522(79)90227-6. [DOI] [PubMed] [Google Scholar]
- 17.Dhariwal KR, Washko P, Hartzell WO, Levine M. Ascorbic acid within chromaffin granules. In situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989;264:15404–9. [PubMed] [Google Scholar]
- 18.Molnár J, Szakács G, Tusnády GE. Characterization of disease-associated mutations in human transmembrane proteins. PLoS One. 2016;17:e0151760. doi: 10.1371/journal.pone.0151760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano A, Tsunoda M, Aigaki T, Maruyama N, Ishigami A. Effect of ascorbic acid deficiency on catecholamine synthesis in adrenal glands of SMP30/GNL knockout mice. Eur J Nutr. 2014;53:177–85. doi: 10.1007/s00394-013-0515-9. [DOI] [PubMed] [Google Scholar]
- 20.Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, Eisenhofer G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2) FASEB. 2003;13:1928–30. doi: 10.1096/fj.02-1167fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.