Abstract
Notch is a crucial cell signaling pathway in metazoan development. By means of cell-cell interactions, Notch signaling regulates cellular identity, proliferation, differentiation and apoptosis. Within the last decade, numerous studies have demonstrated an important role for this pathway in the development and homeostasis of mammalian stem cell populations. Hematopoietic stem cells are a well-defined population that display self-renewal and multi-lineage differentiation potential with the clinically relevant capacity to repopulate the hematopoietic system of an adult organism. Here, we review the emergence, development and maintenance of hematopoietic stem cells (HSCs) during mammalian embryogenesis and adulthood with respect to the role of Notch signaling in hematopoietic biology.
The hematopoietic system is one of the most well defined tissues for understanding and studying stem cells. Numerous studies and reviews have dealt in depth with its development, differentiation and involvement in multiple activities ranging from oncogenesis to therapy (1–5). Blood cells rapidly turn over, especially when responding to stress; thus, successful hematopoiesis requires the ability to continually replenish itself. This is accomplished by maintaining a pool of hematopoietic stem cells (HSCs) that have the ability to both self-renew and give rise to all differentiated blood lineages. Defects, either inborn or acquired, that inhibit HSC function can have disastrous consequences for the adult organism. HSCs have been successfully used in the clinic in diverse applications that include bone marrow transplant and gene therapy, and these successes have spurred investigations to understand the mechanisms that control HSC self-renewal and differentiation. Recent interest has focused on several evolutionarily conserved pathways that include Notch, Wnt and Hedgehog, which regulate self-renewal and differentiation in organisms ranging from Drosophila to humans. This review will focus on the role of Notch signaling in HSCs. Although it is known that Notch is required to establish the earliest embryonic HSCs, other functions for Notch in HSC biology are less well understood and controversial. This review will summarize what is known about the functions of Notch signaling in mammalian HSCs and attempt to shed some light on the areas of controversy.
Notch signaling
In its most simplistic view, Notch signaling delivers paracrine signals between neighboring cells. The components of the Notch signaling network, which are highly conserved from Drosophila to mammals, consist of four classes of molecules: Notch receptors, ligands, positive and negative regulators, and transcription factors. However, as is the case with the majority of highly conserved signaling pathways, simply cataloguing the pathway components tells a very small part of the story. Widespread expression in numerous tissues at diverse developmental times renders any broad functional generalizations for Notch signaling moot as the specific outcomes of Notch signaling depend on context, timing and dose. Thus, in some contexts Notch promotes differentiation, proliferation or survival, whereas in other contexts, Notch inhibits these processes. As a result, attempts to surmise a single authoritative role for Notch in mammalian hematopoietic development and maturation, at best, has created a lot of food for thought, and at worst, ample fodder for controversy.
Notch was discovered and named in 1917 for its famous phenotype in Drosophila (6, 7), and the earliest paradigms of Notch signaling were laid out in invertebrates. These studies suggest that the interplay between receptors and ligands influences lineage determination by two mechanisms: lateral inhibition, in which Notch signaling in one cell inhibits Notch signaling in its neighbor leading to diverse cell fates, and boundary induction, in which Notch signaling in one layer of cells induces signaling in its neighbor layer leading to similar cell fates as the signaling cell, such as in the Drosophila wing primordium where signaling is detected at the boundary between stromal Serrate-expressing cells (8, 9). Vertebrate Notch homologs were first described in Xenopus (10) and then in humans (11). In mammals, four Notch receptors (Notch1-4) have been characterized and exhibit both unique and overlapping patterns of expression. The receptors are comprised of a series of iterated structural elements. In the case of Notch1, these include the epidermal growth factor like repeats (EGFR), Lin12 Notch repeats (LNR), a RBP-Jκ-associated module (RAM) domain, seven Ankyrin repeats (ANK), two nuclear localization signals (NLS), a transactivation domain (TAD) and PEST sequences, which are important in protein turnover. Notch is most divergent in its C-terminus, where Notch3 and 4 lack the strong TAD that is present in Notch1 and 2. More detailed information on Notch receptor structure can be found in other reviews on the topic (12).
In mammals, there are five Notch ligands of the Delta (Dll1, Dll3 and Dll4) and Jagged (Jag1 and Jag2) families, which interact with Notch receptors on neighboring cells. Similar to the receptors, Notch ligands also contain multiple EGF-like repeats as well as DSL domains, which together are responsible for ligand-receptor binding and the subsequent initiation of signaling. While both Jagged and Delta initiate signaling via their DSL domains, they differ in the number of EGF repeats (13); however, the mechanism by which EGF repeat number influences function is poorly understood. As discussed below, different Notch ligands appear to have specificity for different Notch receptors. For example, Dll1 is the critical ligand for Notch2 in murine marginal zone B cell development and Dll4 binds Notch1 to efficiently initiate T cell development (14). Diversity in Notch signaling is not only restricted to the expression of different ligands in neighboring cells; post-translational modification of the Notch receptor by glycosyltransferases of the Fringe family also influences ligand specificity (15). In addition, protein turnover, specifically ubiquitination of the Delta ligand, regulates its endocytosis, thus affecting signaling near cells where Delta is presented (16). These examples illustrate the complexity of Notch receptor-ligand interactions, which are discussed in detail in recent reviews (17, 18)
Initiation of Notch signaling occurs when the Notch receptor is activated by ligand-induced proteolysis. Two successive proteolytic events occur subsequent to binding of the ligand DSL domain to the EGF-repeats of the receptor: 1) ADAM10, a metalloprotease, first cleaves the extracellular domain of the Notch receptor, then 2) the intracellular notch (ICN) domain is released from the membrane by cleavage via the intramembrane aspartyl protease complex γ–secretase (19, 20). γ–secretase cleavage releases ICN, permitting it to translocate into the nucleus where it associates with CSL (acronym for RBP-J; CBF, Suppressor of Hairless, Lag1) (21, 22). Nuclear translocation of ICN leads to the formation of a transcriptionally active complex comprised of ICN, CSL, and a member of the Mastermind-like (MamL) family, which are large scaffolding proteins that recruit co-activators with histone acetyl transferase activity, including p300 (23–25). While there are many Notch target genes, some of which will be further discussed below in the hematopoietic context, the Hes and Hrt proteins are two of the best characterized targets with roles in multiple developmental programs including neurogenesis, myogenesis, and intestinal differentiation (13, 26). Hes genes generally function to repress transcription of other genes by forming complexes with co-repressors, such as TLE/Groucho. It is important to note that many Notch transcriptional targets, including Hes, are not exclusively activated by Notch; thus, target gene expression, by itself, is not indicative of Notch signaling. The Notch transcriptional activation complex is short-lived. MamL likely recruits kinases, such as Cdk8, that lead to ICN phosphorylation and ubiquitination (27). An important E3 ligase in this process is FBW7 (27, 28). The physiological rate of ICN turnover is of great importance, and is best exhibited by the pro-oncogenic consequences of PEST mutations, which decrease turnover (29, 30)
Notch and angiogenic development
The initial functions of Notch in embryonic HSC development are intimately tied to its activities in vasculogenesis; thus before discussing Notch and HSCs, it is important to review the role of Notch in vascular development and angiogenesis. The first embryonic HSCs are observed in the dorsal aorta, vitelline artery and umbilical artery (31, 32) but not in venous vessels, suggesting that arterial specification is an important prerequisite for HSC emergence. Notch is essential for mammalian vascular morphogenesis and artery specification; Notch1-deficient embryos have widespread vascular defects leading to death at embryonic day (e) 9.5. Although Notch4 deficiency by itself does not perturb embryonic development, it exacerbates Notch1 deficiency by producing embryos with more severe defects in angiogenic organization (33). Targeted deletions of other components of the Notch signaling pathway, such as CSL (33, 34) Mib1 (35), or Hey1 and Hey2 (36), also caused loss of arterial specification. Loss of just a single copy of the ligand, Delta-like-4 (Dll4), led to aortic constriction with reduced vitelline circulation and an increased pericardial space (37). The severe arterial phenotype caused by decreased Dll4 copy number suggests that this is the most important Notch ligand in vascular development. Although some defects are observed in Jagged1 mutants, additional work is needed to determine its role in angiogenesis.
In terms of the angiogenic signal transduction pathways, Notch lies downstream of VEGF and upstream of EphrinB2. Targeted mutagenesis of VegfA identified an essential role in vascular development as mouse embryos heterozygous for a VegfA mutation have constricted blood vessels (38, 39). Conversely, expression of individual VegfA isoforms in transgenic mouse models demonstrated that VegfA regulates arterial endothelial cell fates by allowing different populations of endothelial cells to migrate, proliferate and form new blood vessels in microenvironments of various tissues (40). When mammalian cell cultures are supplemented with VEGFA, they induce Notch1 and Dll4 expression in arterial endothelial cells, but not in venous endothelial cells (41). Furthermore, the VegfA-Notch signaling axis is also prominent during the emergence of tip cells and formation of angiogenic sprouts. Tip cells and the stalk cells that follow them are responsible for the generation of new blood vessels while maintaining contact with the original vessel from which the sprout formed. Tip cells are induced by a VEGF gradient, and express Dll4, thus activating the Notch1 receptor on the stalk cells (42). The interplay between tip and stalk cells allows Notch signaling to function in the growth and maturation of newly formed arterial vessels. COUP-TFII inhibits Notch signaling in endothelial cells, allowing veins to form instead of arteries (43). EphrinB2, which produces a ligand for the Eph receptor, was identified as a direct target of Notch signaling in human endothelial cell lines (44). Downstream of Notch, EphrinB2 maintains arterial cell identity, prevents arteriovenous shunt formation and allows for remodeling of the vascular plexus into individual vessels and capillary beds (45). When Notch signaling is impaired, EphrinB2 expression is lost (33, 34, 36, 37, 46, 47).
Definitive hematopoiesis initiates in several anatomical sites from hemogenic endothelium. (48–53). The dorsal aorta in the vicinity of the developing urogenital ridges (aorta/gonad/mesonephros, or AGM region), is one of the best characterized regions of hematopoietic stem cell (HSC) formation (54). In the AGM, expression of the Notch receptors, Notch1 and Notch4, and ligands, Dll4, Jagged1 and Jagged2, persists following arterial specification through the time when hematopoietic cells are forming via an endothelial to hematopoietic cell transition (55–57). Multiple cell surface markers, such as PECAM-1 (58), the angiopoietin receptor Tie-2 (59), CD34 (60), VE-cadherin and Flk1 (61, 62) are shared between endothelial and hematopoietic cells (63, 64). The close relationship between endothelial cells and HSCs is strengthened by functional studies showing that endothelial cells can generate hematopoietic cells in vivo, and that endothelial progenitors derived from embryonic stem cells can differentiate first into endothelial cells, which in turn give rise to hematopoietic cells (29, 48, 49, 51–53, 65–67). Notch is thought to function both in establishing arterial identity and generating the definitive HSC (55, 68–70). Nevertheless, it has not yet been possible to determine whether Notch is responsible for the architectural organization of the sites of HSC emergence or whether it plays a direct mechanistic function in the formation of the definitive hematopoietic stem cell.
HSC emergence
Embryonic hematopoiesis is accomplished by the collective and overlapping specification of progenitor cells into blood cells at various embryonic sites that fluctuate during development (Fig. 1). The first organ with hematopoietic potential is the yolk sac, starting at e7.25 in the mouse (71, 72). At this time of embryonic development, a functional vascular network has not yet emerged, resulting in the in situ generation of hematopoietic cells in the yolk sac. The yolk sac initially produces macrophages, megakaryocytes, and nucleated red blood cells, the latter of which aid in the oxygenation of the rapidly developing embryo (72, 73). This initial wave of hematopoiesis is deemed “primitive”, as the most predominant cells produced are primitive erythrocytes, which can be distinguished from adult “definitive” erythrocytes by virtue of their large size, the presence of a nucleus in circulating cells, and the expression of embryonic hemoglobin (74).
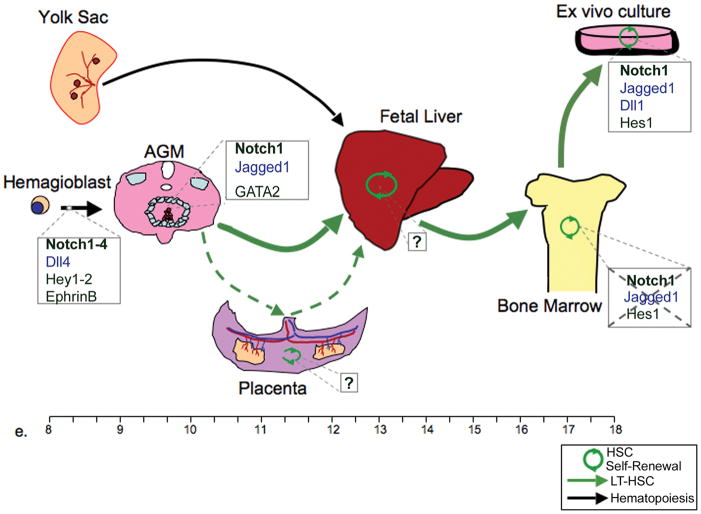
Figure 1. The role of Notch in generation and maturation of hematopoietic stem cells.
Diagram shows the progression of hematopoietic stem cells (HSCs) in fetal development from the emergence of the LT-HCSs in the AGM at day e.9 and the migration to sites of self-renewal and expansion in the placenta and fetal liver (e.11) as well as final prenatal localization of HSCs in the bone marrow at e.17. Known involvement of Notch receptors (bold), ligands (in blue) and targets at various stages of HSC development are marked in boxes next to each fetal organ. Likewise, specific lack of function for Notch is displayed by strikethrough.
Definitive hematopoiesis begins in the yolk sac, but then shifts to the embryo proper, the vitelline and umbilical vasculature, and the placenta. (54). The first definitive cells produced are committed progenitors for definitive erythroid and myeloid lineage cells but not lymphoid lineage cells, and appear in the yolk sac starting at e8.5, and slightly later in the caudal region of the embryo itself (75). At e9.5, HSCs capable of engrafting the myeloid and erythroid lineages of busulfan-conditioned neonatal mice (neonatal repopulating HSCs), but not adult mice can be found in the yolk sac (76). Later, beginning at e10.5, HSCs that can engraft an adult mouse and give rise to all of the blood lineages, including lymphocytes, appear. (32, 77). The best-characterized location for the generation of HSCs is the para-aortic splanchnopleura aorta-gonad-mesonephros (PsP/AGM) region, which includes the dorsal aorta (32, 77). Multiple systematic approaches involving several model systems, such as quail-chick chimeras, diploid-triploid Xenopus embryos, and transplantation experiments with mice showed that the AGM-like region harbored HSCs (78, 79). The dorsal aortae in the AGM region are formed at e8 by the medial migration of a sheet of lateral mesoderm, which touches a plate of endodermal cells. The paired aortae later fuse to form a single aortic tube (80). Visible clusters of hematopoietic cells are observed to form beginning at e9.5 in the vitelline and umbilical arteries, and slightly afterwards in the ventral wall of the dorsal aorta (64, 81). The combination of HSC activity and the visualization of hematopoietic clusters makes these major arteries (dorsal aorta, vitelline and umbilical) the most credible sites for the emergence of the first definitive mammalian HSC. Recent evidence suggests that the placenta is also a site of early HSC emergence or at least a niche for HSC expansion (82–84). The placental HSCs are clearly functional, however, the onset of placental LT-HSC activity is not earlier than in the AGM, and given its high vascular content and location downstream of the dorsal aorta in the fetal circulation, it may be that the HSCs, which emerged in the AGM, migrated and colonized the placenta before moving on to their eventual embryonic depository in the fetal liver. An inherent challenge in defining the anatomic origins of HSCs is that blood is a mobile organ, thus distinguishing sites of emergence from those of colonization can be difficult. For example HSCs are found in the yolk sac at e11.5, but they are also detectable in circulating blood, raising the possibility that they may have migrated to the yolk sac through the circulation (85, 86).
Despite its robust potency to generate de novo LT-HSCs, the AGM is not a site for HSC expansion (87). This may be due to its anatomical location in a high flow area of the circulation, however, this may not be the full explanation as large clusters are present in the umbilical artery, which connects the dorsal aorta to the placenta (64, 81). HSCs are detected in the fetal liver circa. e11 and reside there until near birth at e18. It is during their time in the fetal liver that the fetal HSCs expand and acquire the surface markers that ultimately define them in the adult bone marrow (BM) (88, 89). The fetal liver supports HSC differentiation into all of the hematopoietic lineages, with the exception of T cells, whose specification occurs in the thymus (72).
The fetal liver is the site of greatest HSC expansion during ontogeny (90–92). While it would seem that this unique feature of the fetal liver environment would be of high interest for its potential clinical relevance in understanding and harnessing HSC expansion, an absence of markers that would allow better identification of the HSC niche in the fetal liver, has made direct visualization and subsequent study of the interaction between the hematopoietic and stromal compartments in the fetal liver challenging. To date, it is not fully understood how HSCs can expand so rapidly in the fetal liver, and yet maintain their long-term reconstituting potential.
Notch and Definitive Hematopoiesis
The intimate connection between embryonic arterial and HSC development suggested that Notch signaling may have a role in this process (69, 70). The connection between Notch and definitive hematopoiesis was made by Kumano et al., who showed that Notch1 signaling was required to generate neonatal-repopulating HSCs in the AGM region (55). When analyzing embryos of either Notch1-deficient or Notch2-deficient mice, they observed that neonatal repopulating HSC development and angiogenesis were severely impaired in the Notch1-null embryos but were unaffected in Notch2-null embryos. Furthermore, they found that eliminating Notch signaling had no effect on either primitive or definitive hematopoiesis in the yolk sac. To establish the timing of Notch involvement, they explanted e9 or e10 wild-type AGM regions in the presence of a γ-secretase inhibitor, and observed that elimination of Notch signaling impaired the generation of hematopoietic progenitors at e9. However, when γ-secretase inhibitor was added at e10, it did not affect the ability of hematopoietic progenitors to proliferate or differentiate in colony assays (55). These data argued that Notch signaling was required for the generation, but not for the proliferation or maintenance of hematopoietic cells.
The essential role of Notch1 signaling in generating HSCs has been confirmed by multiple approaches. Like the Notch1-deficient mice, RBP-J/CSL and mindbomb (Mib1)-deficient embryos lacked AGM hematopoiesis, but contained primitive progenitors in the yolk sac (56, 93) some of which, such as the erythroid linneage, showed an increase in cell number (94). A cell autonomous function for Notch1 was demonstrated in studies by Hadland et al., who investigated the developmental potential of Notch1-deficient embryonic stem cells using in vitro differentiation and in vivo chimera generation (68). They found that despite the capacity to maintain a progenitor pool in the yolk sac, the Notch1-deficient embryonic stem cells contributed poorly to the fetal liver and failed to contribute to bone marrow. In contrast, Notch2-4 deficiency does not inhibit hematopoiesis, even though Notch2 and Notch3-deficient mice exhibit defects in vasculogenesis (33, 93, 95, 96). It is not clear whether the essential function for Notch1 in embryonic HSC generation results from the unique expression of this receptor in the HSC progenitor cells, expression of ligands which favor Notch1 signaling or specific aspects of the Notch1 receptor itself. With regards to the latter, Notch1 is the most potent of the four receptors in activating transcription. Studies using knockin mice containing chimeric Notch receptors will help to elucidate the role of specific Notch receptors in HSC generation.
From the ligand perspective, animals deficient in Dll4 die at e9.5 from severe vascular defects primarily due to the lack of arterial organization (37). In contrast, loss of Jagged1 allows for the establishment of the arterial program and expression of EphrinB2, but impairs hematopoietic potential of the AGM (57); whereas, Jagged2 knockout mice exhibit no differences in vasculogenesis or hematopoiesis when compared to wildtype mice. Taken together, these results begin to attribute certain developmental roles to specific ligands, with Dll4 being responsible for the initiation of the Notch-mediated vascular program and Jagged1 coupling with Notch1 to initiate definitive hematopoiesis. As the HSC defect is not absolute in the Jagged1-deficient mice (57), other ligands are likely to be involved. The precise way that Notch ligands orchestrate vasculogenesis and hematopoiesis is currently unknown.
The interaction occurring on the surface of two neighboring cells between the Notch receptor and its ligand is just the initial step in a process that ultimately leads to the transcriptional regulation of Notch target genes and definitive HSC emergence. Tissue specific regulation of Notch targets is a common theme in Notch signaling; some targets, such as Hes and Hrt, play roles in numerous tissues ranging from the nervous system to muscle (26), while other targets such as GATA3, CD25 and pre-Tα have specific and temporally regulated functions in T-cell development (97–99). In the hematopoietic program, identifying the pertinent target genes has not been easy, mainly due to the challenge of distinguishing between a Notch target involved in determination of the aortic cell fate as opposed to a target specifically required for HSC generation. To date, the list of proposed candidates is neither long nor definitive. GATA2 expression is lost in the aortic endothelium of Jagged1-null embryos, where it is directly regulated by Notch1 (57). Evidence of the role of Hes1 in maintenance of HSC self-renewal has been observed in vitro (100) yet its importance in HSCs emergence from the AGM is not known. Runx1 is required for HSC emergence (62), where its function is primarily restricted to the endothelial to hematopoietic cell fate conversion and does not extend to other aspects of HSC biology, although it loss contributes to leukemia (101). Direct regulation of Runx1 by Notch has not yet been described, however, the observation that ectopic Runx expression partially rescues the hematopoietic defects observed in the Notch1-deficient AGM suggests that these two pathways are linked (102). In addition, mutations that affect Notch signaling have been shown to decrease Runx1 expression in both mice and zebrafish (55, 57, 93, 103). In zebrafish, BMP4 is required for Runx1-dependent HSC generation (104), and its expression has been reported in the mesenchyme tissue below the dorsal aorta, adjacent to Runx1 expressing cells (105, 106). Synergy between Notch and BMP signaling may be required for GATA2 and Runx1 expression, which in turn, drives the endothelial to hematopoietic cell fate conversion and ultimately the generation of HSCs.
Notch in adult hematopoietic stem cells
Although it is clear that Notch1 is essential to produce definitive HSCs in the embryo, the requirement for Notch signaling at subsequent stages of mammalian HSC development has generated significant controversy. Some of this controversy is driven by gain of function studies in which Notch signaling was induced in vitro by various experimental techniques; whereas other aspects of the controversy are driven by disparate results utilizing different reagents.
Multiple gain-of-function studies have suggested that Notch induction can expand hematopoietic progenitors, including stem cells. Adding soluble Jagged1 ligand to ex vivo cultures of human CD34+CD38−Lin− cord blood cells (107) or incubating murine BM Lin−Sca1+cKit+ (LSK) cells with a fusion protein containing the Dll1 ligand (108) caused in vitro expansion of these progenitor populations and allowed for short-term hematopoietic reconstitution in mice following transplantation. In another study, osteoblastic cells engineered to express high levels of Jagged1 by constitutive expression of parathyroid hormone regulators, led to an increase in the number of hematopoietic cells in the BM of mice (109). Although this study showed that Jagged1 upregulation led to concomitant intracellular Notch1 production, the ultimate effect of constitutive parathyroid hormone activation in osteoblasts may account for alternative means of HSC expansion as parathyroid hormone activates bone remodeling and the osteoblastic secretion of multiple growth factors, including IGF-1 and BMPs, all of which could influence HSC homeostasis (110, 111). Constitutive Notch signaling via expression of the Notch1 intracellular domain expanded hematopoietic progenitors both in vitro and in vivo, however, these Notch1-expanded progenitors either showed extra-thymic T-cell development or were unable to provide serial reconstitution of lethally irradiated mice, suggesting that they were not bone fide LT-HSCs (103, 112, 113). Hes1 may be an important mediator of progenitor expansion as retroviral Hes1 expression prolongs ex vivo self-renewal of murine HSCs (100) Employing this well-established ex vivo system, recent findings have uncovered diverse roles carried out by Notch receptors, particularly Notch2, in the generation and maintenance of primitive progenitors (sca1+c-kit+) at the expense of myeloid differentiation (114). Perhaps the most compelling evidence for Notch-induced expansion of LT-HSCs comes from recent ex vivo co-culture experiments that rely on endothelial cell expression of Jagged1, Jagged2, Dll1 and Dll4 to stimulate Notch signaling in HSCs. These studies showed that Notch expanded the CD34−Flt3−LSK long-term repopulating fraction, which was able to provide durable, multi-lineage hematopoietic reconstitution in lethally irradiated mice. The self-renewal capacity observed in this study required both Notch1 and Notch2 expression in the co-cultured HSCs (115)
Together, these gain-of-function studies show that Notch induction has the potential to expand multipotent hematopoietic progenitors that can generate all of the hematopoietic lineages, and in some circumstances, produce bone fide LT-HSCs. These findings have already led to promising applications in the clinics. For example, Notch ligands were used to expand cord blood progenitors that shortened the time to engraftment in several patients that received the ex vivo expanded cells (116). As shortening the time to engraftment decreases the complications of bone marrow transplant, these results suggest that Notch-expanded HSCs have the potential for tremendous benefit in the post-BMT setting.
Although these studies demonstrate an important role for Notch in expanding multipotent progenitors, they do not show that Notch is essential for HSCs subsequent to their emergence in the early embryo. Although one study suggested that Notch signaling was high in murine HSCs and played an important role in self-renewal (117), multiple studies using different loss-of-function approaches have failed to identify an essential role for Notch signaling in adult HSCs in the mouse. Deletion of Notch1, Notch1 and Notch2 or RBP-J had no effect on adult HSCs in the mouse (118–122). Likewise, a combination of Notch1 and Jagged1 inactivation in BM progenitors or BM stromal cells produced similar results (119). In addition, transgenic expression of DNMAML, which prevents transcriptional activation by all four Notch receptors failed to influence LT-HSCs in mice (122). All four of these approaches eliminated Notch1-dependent T cell development, showing that Notch signaling had been blocked. Not only did these studies provide immunophenotypic and functional evidence that Notch did not influence adult HSC homeostasis under steady-state conditions, but they also failed to identify an important function for Notch when HSCs were exposed to proliferative stress. For example, neither 5-FU treatment or a competitive secondary transplant of DNMAML-expressing HSCs resulted in a significant difference in the reconstitution of the hematopoietic system (122). Even though both Notch1 and Notch2 were expressed on murine HSCs, the expression of several Notch transcriptional targets, such as Hes1 and Dtx1 was very low (122). One caveat to the latter point is that the identity of the direct Notch targets in HSCs is unknown. Furthermore, even though Hes1 may have a role in HSC expansion, conditional genetic inactivation of Hes1 in adult mice, had no effect on the LT-HCS and progenitor frequency in the BM (123). Together, these multiple lines of genetic data provide strong evidence that Notch signaling does not have an essential role in many aspects of adult murine HSC biology.
Concluding Remarks
While the debate over the role of Notch in post-natal hematopoiesis has persisted over a decade, the wealth of data generated by both sides of the argument has increased our understanding of Notch signaling and of the potential that this important signaling pathway could provide if properly harnessed. Though a direct role of Notch signaling in adult HCSs residing in the bone marrow microenvironment cannot be established, the capacity to expand cord blood or bone marrow stem cells and progenitors by the careful administration of Notch ligands in regulated culture conditions may prove to be of great clinical importance. Future investigation on the mechanism by which these quiescent, Notch irresponsive post-natal HCSs, are capable of self-renewal in the presence of Notch ligand should provide important insights into the developmental biology of hematopoiesis. For example, the spatiotemporal regulation of Notch signaling in hematopoiesis may not be a rigid one-way street, but may be visualized as a parabolic swing of the developmental pendulum where high expression of Notch in HCSs indicates an earlier developmental stage, such as HCS emergence from the AGM or expansion without exhaustion exhibited in the fetal liver, low expression represents the quiescent state of HSCs in the bone marrow niche, and once again, high levels are required for T cell development in adult hematopoiesis. Though this is a very simplified view of what is likely a very complex molecular mechanism, it is possible that the gain-of-function studies have rekindled an aspect of HSC developmental memory during which hematopoietic cells were actively expanding under the influence of Notch. These speculations, when placed in the overall context of Notch function in adult hematopoiesis, are likely to be most important in ex vivo experimental conditions, as extraphysiological levels of Notch in BM HSCs leads to abnormal hematopoiesis and ectopic T-cell development (120, 124). Nevertheless, the possibility of Notch to expand multipotent progenitors will not only impact patient care but may also be useful in human ES and iPS technologies, where it may be possible to use these strategies to expand developing HSCs.
References
- 1.Czechowicz A, Weissman IL. Purified hematopoietic stem cell transplantation: the next generation of blood and immune replacement. Immunol Allergy Clin North Am. 2010 May;30(2):159–171. doi: 10.1016/j.iac.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000 Oct;1(1):57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- 3.Sell S. Leukemia: stem cells, maturation arrest, and differentiation therapy. Stem Cell Rev. 2005;1(3):197–205. doi: 10.1385/SCR:1:3:197. [DOI] [PubMed] [Google Scholar]
- 4.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 5.Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther. 2002 May;9(10):606–612. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 6.Mohr OL. Character Changes Caused by Mutation of an Entire Region of a Chromosome in Drosophila. Genetics. 1919 May;4(3):275–282. doi: 10.1093/genetics/4.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan TH. The theory of the gene. Am Nat. 1917;(51):513–544. [Google Scholar]
- 8.Simpson P. Notch signalling in development: on equivalence groups and asymmetric developmental potential. Curr Opin Genet Dev. 1997 Aug;7(4):537–542. doi: 10.1016/s0959-437x(97)80083-4. [DOI] [PubMed] [Google Scholar]
- 9.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006 Sep;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 10.Coffman C, Harris W, Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990 Sep 21;249(4975):1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- 11.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991 Aug 23;66(4):649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 12.Allman D, Punt JA, Izon DJ, Aster JC, Pear WS. An invitation to T and more: notch signaling in lymphopoiesis. Cell. 2002 Apr;109( Suppl):S1–11. doi: 10.1016/s0092-8674(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 13.Ohishi K, Katayama N, Shiku H, Varnum-Finney B, Bernstein ID. Notch signalling in hematopoiesis. Semin Cell Dev Biol. 2003 Apr;14(2):143–150. doi: 10.1016/s1084-9521(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 14.Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, Zuniga-Pflucker JC. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010 Jul 15;185(2):867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 15.Irvine KD. Fringe, Notch, and making developmental boundaries. Curr Opin Genet Dev. 1999 Aug;9(4):434–441. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 16.Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001 Dec;1(6):783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- 17.Axelrod JD. Delivering the lateral inhibition punchline: it’s all about the timing. Sci Signal. 2010;3(145):pe38. doi: 10.1126/scisignal.3145pe38. [DOI] [PubMed] [Google Scholar]
- 18.D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008 Sep 1;27(38):5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002 Sep;3(9):673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 20.Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004 Feb 3;14(3):R129–138. [PubMed] [Google Scholar]
- 21.Hamaguchi Y, Mastunami N, Yamamoto Y, Kuze K, Kangawa K, Matsuo H, et al. Cloning and characterization of a protein binding to the J kappa recombination signal sequence of immunoglobulin genes. Adv Exp Med Biol. 1991;292:177–186. doi: 10.1007/978-1-4684-5943-2_20. [DOI] [PubMed] [Google Scholar]
- 22.Kovall RA, Hendrickson WA. Crystal structure of the nuclear effector of Notch signaling, CSL, bound to DNA. Embo J. 2004 Sep 1;23(17):3441–3451. doi: 10.1038/sj.emboj.7600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer CJ, Lamar E, Turbachova I, Kintner C, Jones KA. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 2002 Jun 1;16(11):1397–1411. doi: 10.1101/gad.991602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallberg AE, Pedersen K, Lendahl U, Roeder RG. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol Cell Biol. 2002 Nov;22(22):7812–7819. doi: 10.1128/MCB.22.22.7812-7819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Sun T, Kobayashi K, Gao P, Griffin JD. Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol. 2002 Nov;22(21):7688–7700. doi: 10.1128/MCB.22.21.7688-7700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003 Mar;194(3):237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 27.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004 Nov 19;16(4):509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Tsunematsu R, Nakayama K, Oike Y, Nishiyama M, Ishida N, Hatakeyama S, et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004 Mar 5;279(10):9417–9423. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004 Jul;21(1):31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004 Feb;14(1):48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 31.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000 Jun 1;19(11):2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996 Sep 20;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 33.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000 Jun 1;14(11):1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004 Oct 15;18(20):2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005 Aug;132(15):3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 36.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004 Apr 15;18(8):901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004 Oct 15;18(20):2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996 Apr 4;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996 Apr 4;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 40.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002 Feb;109(3):327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003 Jan;23(1):14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003 Jun 23;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005 May 5;435(7038):98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 44.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007 Mar;12(3):415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998 May 29;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 46.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001 Oct;128(19):3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 47.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001 Nov 8;414(6860):216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 48.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009 Feb 12;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008 Dec 4;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaffredo T, Bollerot K, Sugiyama D, Gautier R, Drevon C. Tracing the hemangioblast during embryogenesis: developmental relationships between endothelial and hematopoietic cells. Int J Dev Biol. 2005;49(2–3):269–277. doi: 10.1387/ijdb.041948tj. [DOI] [PubMed] [Google Scholar]
- 51.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010 Mar 4;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 52.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010 Mar 4;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 53.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010 Mar 4;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008 Feb;9(2):129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003 May;18(5):699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 56.Yoon MJ, Koo BK, Song R, Jeong HW, Shin J, Kim YW, et al. Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches. Mol Cell Biol. 2008 Aug;28(15):4794–4804. doi: 10.1128/MCB.00436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. Embo J. 2008 Jul 9;27(13):1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamaguchi I, Huang XL, Takakura N, Tada J, Yamaguchi Y, Kodama H, et al. In vitro hematopoietic and endothelial cell development from cells expressing TEK receptor in murine aorta-gonad-mesonephros region. Blood. 1999 Mar 1;93(5):1549–1556. [PubMed] [Google Scholar]
- 59.Hsu HC, Ema H, Osawa M, Nakamura Y, Suda T, Nakauchi H. Hematopoietic stem cells express Tie-2 receptor in the murine fetal liver. Blood. 2000 Dec 1;96(12):3757–3762. [PubMed] [Google Scholar]
- 60.Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995 Jan 1;85(1):96–105. [PubMed] [Google Scholar]
- 61.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004 Dec 2;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 62.North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999 Jun;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 63.Bollerot K, Romero S, Dunon D, Jaffredo T. Core binding factor in the early avian embryo: cloning of Cbfbeta and combinatorial expression patterns with Runx1. Gene Expr Patterns. 2005 Dec;6(1):29–39. doi: 10.1016/j.modgep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010 Nov;137(21):3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009 Feb 12;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 67.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009 Feb 12;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004 Nov 15;104(10):3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigas A, Robert-Moreno A, Espinosa L. The Notch pathway in the developing hematopoietic system. Int J Dev Biol. 2010;54(6–7):1175–1188. doi: 10.1387/ijdb.093049ab. [DOI] [PubMed] [Google Scholar]
- 70.Gering M, Patient R. Notch signalling and haematopoietic stem cell formation during embryogenesis. J Cell Physiol. 2010 Jan;222(1):11–16. doi: 10.1002/jcp.21905. [DOI] [PubMed] [Google Scholar]
- 71.Evans T. Hematopoiesis: A Developmental Approach(LI Zon) Oxford: Oxford University Press; 2001. Blood formation during Xenopus embryogenesis; pp. 154–161. [Google Scholar]
- 72.McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005 Sep;33(9):1021–1028. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007 Feb 15;109(4):1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K, et al. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. Embo J. 1988 May;7(5):1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 76.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994 Jul;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 78.Cormier F, Dieterlen-Lievre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. 1988 Feb;102(2):279–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- 79.Kau CL, Turpen JB. Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hemopoietic cells in Xenopus laevis. J Immunol. 1983 Nov;131(5):2262–2266. [PubMed] [Google Scholar]
- 80.Galloway JL, Zon LI. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr Top Dev Biol. 2003;53:139–158. doi: 10.1016/s0070-2153(03)53004-6. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Porrero JA, Godin IE, Dieterlen-Lievre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 1995 Nov;192(5):425–435. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- 82.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005 Mar;8(3):365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 83.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005 Mar;8(3):377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008 Mar 6;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002 Nov;129(21):4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 86.Yokota T, Huang J, Tavian M, Nagai Y, Hirose J, Zuniga-Pflucker JC, et al. Tracing the first waves of lymphopoiesis in mice. Development. 2006 May;133(10):2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- 87.Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J Exp Med. 1999 Jul 5;190(1):43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005 Aug 1;106(3):903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rebel VI, Miller CL, Eaves CJ, Lansdorp PM. The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their liver adult bone marrow counterparts. Blood. 1996 Apr 15;87(8):3500–3507. [PubMed] [Google Scholar]
- 91.Rebel VI, Miller CL, Thornbury GR, Dragowska WH, Eaves CJ, Lansdorp PM. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Exp Hematol. 1996 Apr;24(5):638–648. [PubMed] [Google Scholar]
- 92.Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000 Apr 1;95(7):2284–2288. [PubMed] [Google Scholar]
- 93.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005 Mar;132(5):1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 94.Robert-Moreno A, Espinosa L, Sanchez MJ, de la Pompa JL, Bigas A. The notch pathway positively regulates programmed cell death during erythroid differentiation. Leukemia. 2007 Jul;21(7):1496–1503. doi: 10.1038/sj.leu.2404705. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010 Oct 1;107(7):860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001 Feb;128(4):491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 97.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004 May 14;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 98.Pear WS, Aster JC. T cell acute lymphoblastic leukemia/lymphoma: a human cancer commonly associated with aberrant NOTCH1 signaling. Curr Opin Hematol. 2004 Nov;11(6):426–433. doi: 10.1097/01.moh.0000143965.90813.70. [DOI] [PubMed] [Google Scholar]
- 99.Rothenberg EV. T-lineage specification and commitment: a gene regulation perspective. Semin Immunol. 2002 Dec;14(6):431–440. doi: 10.1016/s1044532302000787. [DOI] [PubMed] [Google Scholar]
- 100.Kunisato A, Chiba S, Nakagami-Yamaguchi E, Kumano K, Saito T, Masuda S, et al. HES-1 preserves purified hematopoietic stem cells ex vivo and accumulates side population cells in vivo. Blood. 2003 Mar 1;101(5):1777–1783. doi: 10.1182/blood-2002-07-2051. [DOI] [PubMed] [Google Scholar]
- 101.Link KA, Chou FS, Mulloy JC. Core binding factor at the crossroads: determining the fate of the HSC. J Cell Physiol. 2010 Jan;222(1):50–56. doi: 10.1002/jcp.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakagawa M, Ichikawa M, Kumano K, Goyama S, Kawazu M, Asai T, et al. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006 Nov 15;108(10):3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 103.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005 Oct 1;19(19):2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, Kimelman D, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009 Jun;16(6):909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000 Aug 15;96(4):1591–1593. [PubMed] [Google Scholar]
- 106.Pimanda JE, Donaldson IJ, de Bruijn MF, Kinston S, Knezevic K, Huckle L, et al. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000 Nov 6;192(9):1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003 Mar 1;101(5):1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 109.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 110.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006 Dec;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 111.Whitfield JF, Morley P, Willick GE. The control of bone growth by parathyroid hormone, leptin, & statins. Crit Rev Eukaryot Gene Expr. 2002;12(1):23–51. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.20. [DOI] [PubMed] [Google Scholar]
- 112.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002 Apr 1;99(7):2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 113.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000 Nov;6(11):1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 114.Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. Feb 1; doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. Mar 5;6(3):251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. Feb;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005 Mar;6(3):314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 118.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002 Jun;14(6):637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 119.Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005 Mar 15;105(6):2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 120.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999 May;10(5):547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 121.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002 May;3(5):443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 122.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008 Apr 10;2(4):356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wendorff AA, Koch U, Wunderlich FT, Wirth S, Dubey C, Bruning JC, et al. Hes1 is a critical but context-dependent mediator of canonical Notch signaling in lymphocyte development and transformation. Immunity. 2010 Nov 24;33(5):671–684. doi: 10.1016/j.immuni.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 124.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999 Sep;11(3):299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]