Abstract
Background
In cross-sectional studies, triclosan and parabens, ubiquitous ingredients in personal care and other products, are associated with allergic disease.
Objectives
We investigated the association between prenatal and early life triclosan and parabens exposure and childhood allergic disease in a prospective, longitudinal study.
Methods
Subjects were enrollees in VDAART, the Vitamin D Antenatal Asthma Reduction Trial. Triclosan, methyl paraben and propyl paraben concentrations were quantified in maternal plasma samples pooled from first and third trimesters and urine samples from children at age 3 or 4 years. Outcomes were parental report of physician-diagnosed asthma or recurrent wheezing, and allergic sensitization to food or environmental antigens based on serum specific IgE levels at age 3 in high-risk children.
Results
Analysis included 467 mother-child pairs. Overall, there were no statistically significant associations of maternal plasma or child urine triclosan or parabens concentrations with asthma or recurrent wheeze, food or environmental sensitization at age 3. A trend toward an inverse association between triclosan and parabens exposure and allergic sensitization was observed. There was evidence of effect measure modification by sex, with higher odds of environmental sensitization associated with increasing concentrations of parabens in males compared to females.
Conclusions
We did not identify a consistent association between prenatal and early life triclosan or parabens concentrations and childhood asthma, recurrent wheeze or allergic sensitization in the overall study population. The differential effects of triclosan or parabens exposure on allergic sensitization by sex observed in this study warrants further exploration.
Keywords: triclosan, paraben, prenatal sensitization asthma
INTRODUCTION
Triclosan and parabens, including methyl-, propyl-, ethyl- and butyl paraben, are present in a wide variety of personal care and other products.1,2 Triclosan and parabens have antibacterial properties3,4 and endocrine-disrupting characteristics.2,5–10 Exposure to these chemicals is ubiquitous and occurs via multiple routes including ingestion and absorption from dermal or mucosal applications.2,11 In the National Health and Nutrition Examination Survey (NHANES), administered in the United States by the Centers for Disease Control and Prevention (CDC), triclosan was detectable in urine in almost 75% of participants1, and detection frequencies for the parabens were 99.1%, 92.7%, 47%, and 42.4% for urinary methyl, propyl, butyl and ethyl parabens, respectively12.
Cross-sectional evidence suggests that triclosan and paraben exposure may increase the risk of allergic disease. Studies utilizing NHANES data have found a positive association between urinary triclosan concentrations and diagnosis with hay fever13 and allergic sensitization14,15 in children ages 6 to 18 years, and with recent asthma exacerbations among those with asthma aged 6 years and older16. A study of 10 year-old Norwegian children also found an association between urinary triclosan and allergic sensitization and rhinitis17. The evidence with regards to parabens exposure has been less consistent. In NHANES participants age 6 to 18 years, propyl paraben, butyl paraben, and methyl paraben urinary concentrations were positively associated with aeroallergen sensitization14,15. However, methyl paraben was negatively associated with nonatopic asthma or wheeze and propyl paraben exposure was not associated with food sensitization or atopic asthma or wheeze14.
In this study, we aimed to clarify the relationship between prenatal and early-life triclosan and parabens exposure and risk of food sensitization, environmental sensitization, and asthma or recurrent wheeze at age 3 years in a post-hoc analysis of a relatively large and ethnically diverse clinical trial population with high risk of allergic disease. We hypothesized that higher maternal plasma concentrations of triclosan and parabens during pregnancy or higher child urinary concentrations at age 3 or 4 years are associated with increased risk of allergic disease at age 3 years. To our knowledge, this is the first study utilizing prospective longitudinal data to evaluate an effect of prenatal and early life triclosan and parabens exposure on risk of allergic disease.
METHODS
Study Design
The Vitamin D Antenatal Asthma Reduction Trial (VDAART) has been previously described (NCT00920621)18,19. Briefly, VDAART is a randomized, double-blind, placebo-controlled trial of Vitamin D supplementation during pregnancy to determine whether higher prenatal maternal vitamin D levels prevent asthma and other allergic disease in childhood. Pregnant women age 18 to 39 who presented between estimated gestational ages of 10 and 18 weeks were recruited from clinical sites in Boston, St. Louis and San Diego, United States, between October 2009 and July 2011. All participants either had a history of asthma, eczema or allergic rhinitis, or the biologic father of the child had a history of asthma, eczema or allergic rhinitis. All participants were non-smokers. Participants were randomized to daily 4,400 IU (treatment arm) or 400 IU vitamin D (placebo or usual care arm); a subset of participants in both arms of the trial are included in the current analysis. The study protocol was approved by the institutional review boards at each participating institution and at the Brigham and Women’s Hospital. All women provided written informed consent.
Participating mothers provided blood samples at enrollment during the 1st trimester (10–18 weeks gestation) and again in the 3rd trimester (32–38 weeks gestation). After delivery, children were monitored by telephone every 3 months and in-person annually for 3 years and provided a blood sample at age 3 years for measurement of total and serum specific IgE concentrations.
Allergic Outcome Ascertainment
The outcome of asthma or recurrent wheeze was based on parental report of physician diagnosis of asthma or occurrence of recurrent wheeze in the child’s first 3 years of life as previously reported19. Serum total and specific IgE was measured from plasma collected at the 3rd year visit in a subset of VDAART study participants by ThermoFisher PIRL lab (Phadia Immunology Reference Laboratory, Portage, MI). Of all participants in the current analysis (that is, who had available triclosan and parabens concentrations), a subset also had available serum specific IgE concentrations and this subset included both children with and without asthma or recurrent wheeze. Food allergens tested were: egg white, walnut, milk, peanut, soybean, and wheat. Environmental allergens tested were: Alternaria alternata, Dermatophagoides farinae, Dermatophagoides pteronyssinus, German cockroach, cat dander, dog dander, grass pollen mix, and tree pollen mix. Food or environmental sensitization was determined to be present if specific IgE levels to at least one of the tested foods or environmental allergens, respectively, was ≥ 0.35 kU/l. Food or environmental sensitization was considered missing if specific IgE levels were not available to all food or environmental allergens, respectively.
Evaluation of Biomarkers Concentrations
Triclosan and parabens concentrations were measured in a subset of the VDAART study population. Child urine collections at age 3 years were initiated after approximately one third of participants had already completed their year 3 visits, so urine samples were not obtained for the entire cohort and in many children urine collection was deferred until age 4 years when they no longer required diapers. Urine collection kits were provided to participants for home collection to be followed by storage in a refrigerator for less than 24 hours before delivery to the study site. Maternal plasma samples were collected in EDTA tubes and child urinary samples were collected in Starplex Scientific™ LeakBuster™ 3 specimen containers (Starplex Scientific, Etobicoke, Ontario, Canada). At study sites, specimens were frozen until shipment to the Channing Division of Network Medicine, Brigham and Women’s Hospital. There, samples were divided into aliquots and frozen at −80 °C until shipped overnight to the Centers for Disease Control and Prevention (CDC). Plasma samples from the 1st and 3rd trimester visits were pooled for each participating mother. All samples were analyzed at the National Center for Environmental Health of the CDC for triclosan, methyl paraben, propyl paraben, butyl paraben and ethyl paraben by online solid phase extraction-high-performance liquid chromatography-isotope dilution tandem (mass spectrometry; the limits of detection (LODs) were 0.1 ng/mL for propyl paraben and butyl paraben, and 1 ng/mL for ethyl paraben, methyl paraben and triclosan.20–22 A subset of plasma samples were analyzed for free (e.g., unconjugated) concentrations of the biomarkers. The major species of triclosan and parabens were conjugates (data not shown), suggesting that plasma concentrations of these biomarkers reflect true exposures and are not the result of external contamination23–25. Four children had two aliquots from the same urinary collection date; triclosan and parabens concentrations were similar and were in the same tertile of chemical concentration for each subject with the exception of one subject who had discrepant triclosan concentrations: one in the second tertile (1.8 ng/mL) and another in the third tertile (10.6 ng/mL). For these four children, the average of the two concentrations for each chemical was used in analysis. One mother had biomarker concentrations available from two aliquots, and the average of the two concentrations for each biomarker was used in analysis. For analyte concentrations less than the LOD, a value equal to the LOD divided by the square root of 2 was used for analysis as previously described26. The involvement of the CDC laboratory did not constitute engagement in human subjects research.
Urine specific gravity was determined using a digital handheld refractometer (Atago Co, Model PAL-10S, Tokyo, Japan). For analyses utilizing specific gravity-corrected chemical concentrations, the following formula was used: Pc = P[(1.021-1)/(SG-1)] where Pc is the specific gravity-adjusted urinary concentration (ng/mL), P is the measured urinary concentration, and SG is the specific gravity of the urine sample. A specific gravity of 1.021 was the median specific gravity for this group of urine samples. Four children for whom urinary triclosan and parabens concentrations were measured, but for whom specific gravity could not be determined were omitted from analysis.
Evaluation of Personal Care Product Use
Questionnaires were completed by participating parents with regard to child use of a variety of personal care products either at age 3 years or within a few days of urine collection. Parents were first asked if their child had used the personal care product in the past month. If the answer was yes, parents were then asked if their child had used the personal care product daily, not daily but at least once weekly, or less than once weekly. Questionnaire responses from within 100 days of the date of child urine collection for measurement of triclosan and parabens were analyzed for frequency of personal care product use and differences in frequencies of personal care product use by sex and by race/ethnicity.
Covariates
Additional characteristics included in analyses were maternal and child race and ethnicity (Hispanic, Non-Hispanic Black, White, and Other), maternal education (less than high school, high school or technical school, some college, and college graduate or higher), treatment group assignment, study center, maternal age and child age at urine collection. Household income was reported but not included in analyses because over 20% of subjects did not provide household income (see Table 1). Caretakers who completed questionnaires when children were 3 years old were asked whether they or other household members currently smoke cigarettes, pipes, cigars or cigarillos, and those results were tabulated and reported with other baseline characteristics.
Table 1.
Baseline characteristics of mothers
| Mothers in Present Study (n=467) | Mothers in VDAART cohort (n=876)a | |
|---|---|---|
| Age (years) | 27.3 (5.6) | 27.5 (5.6) |
| Treatment assignment | ||
| 4400 IU/day Vitamin D | 246 (53) | 440 (50) |
| 400 IU/day Vitamin D | 221 (47) | 436 (50) |
| Study center | ||
| Boston | 109 (23) | 262 (30) |
| St. Louis | 221 (47) | 314 (36) |
| San Diego | 137 (29) | 300 (34) |
| Maternal comorbidities | ||
| Asthma | 185 (40) | 358 (41) |
| Allergic rhinitis | 306 (66) | 558 (64) |
| Eczema | 132 (28) | 278 (32) |
| Maternal partner comorbidities | ||
| Asthma | 117 (25) | 202 (23) |
| Allergic rhinitis | 195 (42) | 365 (42) |
| Eczema | 86 (18) | 144 (16) |
| Smoker in home at age 3 years | 47 (10) | 63 (9) |
| Maternal race/ethnicity | ||
| Black, non-Hispanic | 202 (43) | 337 (38) |
| White, non-Hispanic | 120 (26) | 230 (26) |
| Hispanic | 109 (23) | 244 (28) |
| Other | 36 (8) | 65 (7) |
| Maternal education status | ||
| Less than high school | 61 (13) | 108 (12) |
| High school or technical school | 141 (30) | 265 (30) |
| Some college | 107 (23) | 213 (24) |
| College graduate or higher | 158 (34) | 290 (33) |
| Household income (US dollars) | ||
| <30,000 | 155 (33) | 260 (30) |
| 30,000–49,999 | 50 (11) | 120 (14) |
| 50,000–74,999 | 58 (12) | 101 (12) |
| 75,000–99,999 | 40 (9) | 81 (9) |
| 100,000–149,999 | 40 (9) | 71 (8) |
| >150,000 | 21 (4) | 32 (4) |
| Refused to say or unknown | 103 (22) | 211 (24) |
Mean (standard deviation) is given for age; number (%) of participants are given for all other variables.
Results displayed for the 876 eligible mothers who underwent randomization in the VDAART cohort.
Note: Paternal asthma, allergic rhinitis and eczema status was unknown for 1, 2 and 4 subjects, respectively. Whether there were smokers in the home was unknown for 138 subjects.
Statistical Analyses
Statistical analyses were conducted using R version 3.3.1 (R Foundation for Statistical Computing; packages “stats”, “forestplot”). Chi square tests were used to determine associations between child demographic characteristics (race/ethnicity and sex) and allergic outcomes. Kruskal-Wallis and Mann Whitney U tests were used to determine associations between demographic characteristics (race/ethnicity, child sex, maternal education, study center, and treatment assignment) and biomarker concentrations (analyzed as a continuous variable). Chi square tests were used to evaluate differences in the frequency of personal care product use among children by sex and by race/ethnicity, and Wilcoxin rank sum tests were used to evaluate differences in biomarker concentrations by personal care product use. Spearman correlation analyses were performed to evaluate the association between maternal plasma concentrations during pregnancy and child urinary concentrations at age 3 to 4 years for each chemical.
Plasma and urinary triclosan and parabens concentrations had non-normal distributions, and were therefore divided into tertiles or were dichotomized when fewer than 40% of subjects had detectable concentrations, as was the case for maternal plasma butyl paraben and ethyl paraben and child urinary butyl paraben. For child urinary ethyl paraben, the 35% of subjects with non-detectable concentrations were categorized as the 1st tertile, subjects with the highest 1/3 of values categorized as the 3rd tertile, and the remainder were in the 2nd tertile.
Logistic regression and multiple linear regression were used to determine the association between biomarker concentrations (divided into tertiles and analyzed as categorical variables of factor class) and asthma or recurrent wheeze, food sensitization, environmental sensitization and log-transformed total IgE. Tests for trend were performed by repeating regression analyses with urinary concentrations divided into tertiles and analyzed as continuous variables of numeric class. All models of the effect of child urinary concentrations on allergic outcomes included urine specific gravity as a covariate. Additional covariates in adjusted models were selected based on a priori knowledge regarding associations with triclosan or parabens concentrations with allergic outcomes. Analyses of maternal plasma biomarkers were adjusted for maternal race/ethnicity, maternal education, maternal age, Vitamin D supplement treatment assignment, and study center. Analyses of child urinary biomarkers were adjusted for child sex, child race/ethnicity, child age at urine collection, maternal education, Vitamin D supplement treatment assignment, and study center.
Because prior studies have shown effect measure modification by sex of the association of triclosan and parabens concentrations with allergic sensitization14, we stratified adjusted logistic regression analyses of associations between biomarker tertiles and allergic outcomes by child sex. Adjusted logistic regression analyses were also performed with the inclusion of sex x chemical tertile interaction terms. All tests were 2-sided and the significance level was pre-specified at p < .05, including for analyses of interactions, despite limited power to detect interactions in our sample. Given the exploratory nature of this study, adjustments were not made for multiple comparisons.
RESULTS
Subject Characteristics
Of the 810 mother-child pairs who participated in VDAART, triclosan and parabens concentrations were available either from maternal plasma or child urine for a total of 467 mother-child pairs; 459 pairs had both maternal and child concentrations, seven pairs had maternal concentrations only and one pair had child concentrations only. The median child age on the day of urine collection was 3.2 years (interquartile range, 3.0–4.0). Of mother-child pairs in whom triclosan and parabens concentrations were available from maternal plasma or child urine or both, subsets of 391 and 390 children had available serum specific IgE concentrations to at least one food or at least one environmental antigen, respectively. Demographic characteristics of the mothers are provided in Table 1; based on these characteristics, there is no evidence that the mothers included in the present analysis differ systematically from the mothers in the full VDAART study population.
Prevalence of Allergic Outcomes
The prevalence of asthma or recurrent wheeze, food sensitization, and environmental sensitization at age 3 years is shown in Table 2 by child sex and race/ethnicity. Food sensitization was significantly associated with child race/ethnicity with higher prevalence of food sensitization seen in black children (50%) than in white (34%) or Hispanic (29%) children (p = 0.003). There was a trend toward higher prevalence of the composite asthma and/or recurrent wheeze outcome in males (31%) than in females (24%), though this association was not statistically significant (p = 0.10). Of the 131 children with asthma or recurrent wheeze, 117 had recurrent wheeze, 71 had physician-diagnosed asthma and 57 had both recurrent wheeze and physician-diagnosed asthma.
Table 2.
Clinical outcomes in children by sex and race/ethnicity
| Total | Asthma/Wheeze | Food Sensitization | Environmental Sensitization | ||||
|---|---|---|---|---|---|---|---|
| No (%) | No (%) | p value | No (%) | p value | No (%) | p value | |
| All subjects | 467 (100) | 131 (28) | 156 (40) | 113 (29) | |||
| Sex | 0.10 | 0.76 | 1.0 | ||||
| Boy | 255 (55) | 80 (31) | 83 (39) | 62 (29) | |||
| Girl | 212 (45) | 51 (24) | 73 (41) | 51 (29) | |||
| Race/ethnicitya | 0.14 | 0.003 | 0.14 | ||||
| Black, non-Hispanic | 203 (44) | 67 (33) | 87 (50) | 57 (33) | |||
| White, non-Hispanic | 93 (20) | 25 (27) | 27 (34) | 19 (23) | |||
| Hispanic | 139 (30) | 33 (24) | 31 (29) | 26 (24) | |||
| Other | 29 (6) | 5 (17) | 11 (42) | 11 (42) | |||
Child race/ethnicity data were missing for 3 subjects.
P values are for Chi square tests.
Note: Results for sensitization outcomes shown for the 391 and 390 children with available serum specific IgE concentrations to food and environmental antigens, respectively.
Triclosan and Parabens Concentrations
Maternal plasma triclosan and parabens concentrations are displayed in Table 3 and child urinary triclosan and parabens concentrations are displayed in Table 4. For all biomarkers, concentrations were lower in maternal plasma than in child urine. Among mothers, 3.9, 2.6, 81.1, 69.1, and 23.0 percent of subjects had plasma concentrations below the LOD for methyl paraben, propyl paraben, butyl paraben, ethyl paraben and triclosan, respectively. Among children, 1.3, 62.6, 35.0, and 12.6 percent of subjects had urinary concentrations below the LOD for propyl paraben, butyl paraben, ethyl paraben and triclosan, respectively. Methyl paraben was detectable in all child urinary specimens. Because of the high percentage of butyl paraben and ethyl paraben concentrations that were below the LOD for both maternal and child samples, these biomarkers were not included in subsequent analyses.
Table 3.
Maternal plasma triclosan and parabens concentrations (ng/mL) by maternal race/ethnicitya
| All subjects (n=466) | Black, Non-Hispanic (n=202) | Hispanic (n=108) | White, Non-Hispanic (n=120) | Other Race/Ethnicity (n=36) | p valueb | |
|---|---|---|---|---|---|---|
| Methyl paraben | 12.5 (5.3, 26.0) | 15.1 (7.1, 25.0) | 10.2 (5.2, 22.2) | 7.2 (3.2, 15.3) | 24.2 (7.3, 46.7) | <0.01 |
| Propyl paraben | 2.0 (0.7, 4.7) | 2.6 (1.0, 5.3) | 1.7 (0.6, 4.3) | 1.2 (0.5, 2.7) | 4.1 (1.3, 7.3) | <0.01 |
| Triclosan | 2.2 (1.1, 6.7) | 1.7 (<LOD, 3.4) | 2.8 (1.2, 9.5) | 2.8 (1.5, 8.7) | 3.8 (1.9, 7.9) | <0.01 |
Medians (25th, 75th percentiles) are displayed. Triclosan limit of detection (LOD) = 1 ng/mL
For Kruskal-Wallis test statistic
Table 4.
Child urinary triclosan and parabens concentrations (ng/mL) by child sex and child race/ethnicitya
| All Subjects (n=460) |
Black, Non- Hispanic (n=201) |
White, Non- Hispanic (n=90) |
Hispanic (n=137) |
Other Race/Ethnicity (n=29) |
p valueb |
Male (n=251) |
Female (n=209) |
p valueb |
|
|---|---|---|---|---|---|---|---|---|---|
| Methyl paraben | 62.8 (21.7, 303.6) | 83.8 (33.0, 395.6) | 30.7 (12.1, 223.8) | 56.5 (19.7, 200.8) | 92.0 (24.2, 731.9) | <0.01 | 54.3 (19.5, 281.8) | 76.7 (27.5, 328.1) | 0.09 |
| SG corrected | 77.5 (25.7, 323.1) | 113.4 (40.8, 386.6) | 43.6 (13.9, 213.4) | 65.9 (22.9, 235.4) | 142.3 (50.8, 678.4) | <0.01 | 59.4 (21.4, 344.8) | 97.3 (33.5, 312.5) | 0.04* |
| Propyl paraben | 6.8 (2.1, 23.6) | 9.5 (4.0, 32.4) | 2.8 (1.0, 16.7) | 4.8 (1.6, 18.7) | 6.0 (2.9, 33.6) | <0.01 | 5.2 (1.8, 20.3) | 8.9 (2.8, 29.0) | 0.02* |
| SG corrected | 7.9 (2.4, 29.8) | 11.3 (4.3, 39.4) | 3.5 (1.0, 15.0) | 6.0 (1.8, 19.1) | 8.8 (2.7, 42.7) | <0.01 | 5.4 (1.9, 24.7) | 10.4 (3.5, 34.7) | 0.009* |
| Triclosan | 5.5 (2.1, 17.1) | 4.7 (1.9, 14.4) | 6.5 (2.7, 18.1) | 4.2 (2.2, 20.6) | 6.7 (1.7, 19.2) | 0.61 | 4.5 (2.1, 14.3) | 6.6 (2.2, 25.5) | 0.07 |
| SG corrected | 6.0 (2.6, 18.3) | 5.1 (2.6, 15.8) | 7.1 (3.0, 18.8) | 6.6 (2.5, 23.4) | 7.9 (2.9, 23.7) | 0.48 | 5.2 (2.5, 6.7) | 6.7 (3.0, 25.5) | 0.02* |
SG: specific gravity
Medians (25th, 75th percentiles) are presented.
p values are given for the Kruskal-Wallis test statistic for associations between biomarker concentrations and child race/ethnicity, and for associations between biomarker concentrations and child sex.
Note: Child race/ethnicity data were unavailable for 3 subjects.
Association between Maternal Plasma Concentrations and Baseline Characteristics
Maternal plasma concentrations of triclosan, methyl paraben and propyl paraben differed significantly between maternal race/ethnicity groups and between study centers (St. Louis, San Diego and Boston). Maternal plasma triclosan concentration also differed significantly by maternal education. No maternal plasma biomarker concentrations differed by child sex or vitamin D supplement treatment assignment (Table 3 and Online Repository Tables E1A–D).
Association between Child Urinary Concentrations and Baseline Characteristics
Child specific gravity-corrected urinary concentrations differed between child race/ethnicity groups for all biomarkers except triclosan, and differed between male and female children for all biomarkers. Child urinary concentrations of methyl paraben differed by study center, and both child urinary methyl and propyl parabens differed by maternal education. There were no differences in child urinary biomarker concentrations by vitamin D supplement treatment assignment (Table 4 and Online Repository Tables E2A–C)
Frequency of Personal Care Product Use among Children
Personal care product questionnaires were completed within 100 days of the date of urine sample collection for triclosan and parabens measurement for 406 children and questionnaire results are provided in Table 5. Personal care product use varied in frequency; for example, at least weekly use was reported for toothpaste in 97%, for nail polish in 9%, and for hair spray in 7% of children. Personal care product use frequency differed significantly by sex for nail polish, leave-in conditioner, hair oil and hair lotion (p values for Chi square tests = <0.01 for all), with higher frequency of use observed among females than males for all of these products. Personal care product use frequency differed significantly by race/ethnicity for all personal care products except for leave-in conditioner (p value for Chi square test = 0.50).
Table 5.
Frequency of personal care product use among children by sex and race/ethnicity
| Product | All children (n=406) | Male (n=223) | Female (n=183) | p value for Chi square test | Black, non-Hispanic (n=180) | White, non-Hispanic (n=77) | Hispanic (n=122) | p value for Chi square test |
|---|---|---|---|---|---|---|---|---|
| Toothpaste | 395 (97) | 218 (98) | 177 (97) | 0.74 | 180 (100) | 76 (99) | 112 (92) | <0.01 |
| Mouth wash | 49 (12) | 24 (11) | 25 (14) | 0.46 | 21 (12) | 4 (5) | 22 (18) | 0.03 |
| Liquid soap | 334 (82) | 186 (83) | 148 (81) | 0.59 | 145 (81) | 75 (97) | 88 (72) | <0.01 |
| Bar soap | 117 (29) | 101 (45) | 76 (42) | 0.51 | 111 (62) | 22 (29) | 38 (31) | <0.01 |
| Hand sanitizer | 216 (53) | 115 (52) | 101 (55) | 0.53 | 110 (61) | 31 (40) | 63 (52) | <0.01 |
| Nail polish | 30 (7) | 2 (1) | 28 (15) | <0.01 | 23 (13) | 2 (3) | 4 (3) | <0.01 |
| Cologne | 33 (8) | 16 (7) | 17 (9) | 0.55 | 10 (6) | 2 (3) | 21 (17) | <0.01 |
| Shampoo or wash-out conditioner | 294 (72) | 155 (70) | 139 (76) | 0.18 | 86 (48) | 73 (95) | 111 (91) | <0.01 |
| Leave-in conditioner | 51 (13) | 11 (5) | 40 (22) | <0.01 | 19 (11) | 11 (14) | 18 (15) | 0.50 |
| Hair spray | 35 (9) | 16 (7) | 19 (10) | 0.33 | 9 (5) | 7 (9) | 18 (15) | 0.01 |
| Hair oil | 89 (22) | 36 (16) | 53 (29) | <0.01 | 66 (37) | 0 (0) | 20 (16) | <0.01 |
| Hair lotion | 63 (16) | 16 (7) | 47 (26) | <0.01 | 50 (28) | 0 (0) | 12 (10) | <0.01 |
| Hand or body lotion | 318 (78) | 169 (76) | 149 (81) | 0.21 | 167 (93) | 36 (47) | 98 (80) | <0.01 |
| Suntan or sunblock lotion | 116 (29) | 63 (28) | 53 (29) | 0.96 | 14 (8) | 40 (52) | 46 (38) | <0.01 |
Number (%) of children reporting use at least once per week is presented for each product.
Note: Results are shown for the 406 children for whom personal care product questionnaires were completed within 100 days of the date of urine sample collection for triclosan and parabens measurement. Results for 24 subjects of other race/ethnicity were excluded from analyses.
Association of Personal Care Product Use and Child Urinary Triclosan and Parabens Concentrations
A comparison of child urinary triclosan and parabens concentrations by frequency of personal care product use is displayed in Table 6. Child urinary tricsosan concentration was not associated with at least weekly use of any personal care product, though there was a trend toward higher triclosan concentrations among children with at least weekly use of hand sanitizer (median 6.8 ng/mL, interquartile range 2.5–20.8) compared to less than weekly use of hand sanitizer (median 4.5 ng/mL, interquartile range 1.7–13.1).
Table 6.
Triclosan and parabens concentrations by personal care product use
| Triclosan (ng/mL) | Methyl Paraben (ng/mL) | Propyl Paraben (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Product | At least weekly use | Less than weekly use | p value | At least weekly use | Less than weekly use | p value | At least weekly use | Less than weekly use | p value |
| Toothpaste | 5.3 (22, 16.5) | 9.6 (2.6, 30.7) | 0.42 | 65.1 (21.9, 333.6) | 28.4 (15.9, 35.3) | 0.01 | 7.0 (2.2, 23.3) | 1.6 (1.4, 3.3) | <0.01 |
| Mouth wash | 7.5 (2.0, 17.5) | 5.1 (2.2, 16.9) | 0.32 | 51.7 (15.1, 163.1) | 65.6 (24.2, 339.2) | 0.09 | 3.5 (1.6, 21.2) | 7.2 (2.2, 23.0) | 0.18 |
| Liquid soap | 5.7 (2.2, 16.6) | 4.3 (2.2, 2.1) | 0.91 | 62.9 (24.3, 300.0) | 64.7 (19.6, 383.0) | 0.81 | 7.0 (2.2, 23.2) | 4.7 (1.9, 15.8) | 0.51 |
| Bar soap | 4.8 (2.2, 17.0) | 5.6 (2.2, 16.6) | 0.97 | 72.4 (27.5, 374.8) | 56.3 (19.6, 240.9) | 0.09 | 6.9 (2.2, 24.7) | 6.7 (1.9, 22.9) | 0.37 |
| Hand sanitizer | 6.8 (2.5, 20.8) | 4.5 (1.7, 13.1) | 0.05 | 71.6 (21.6, 362.3) | 58.8 (22.6, 239.7) | 0.18 | 8.1 (2.4, 29.0) | 6.3 (1.6, 19.4) | 0.04 |
| Nail polish | 6.2 (2.7, 15.8) | 5.1 (2.2, 17.1) | 0.70 | 140.0 (54.5, 490.8) | 61.4 (20.8, 286.8) | 0.02 | 19.0 (6.5, 48.0) | 6.6 (1.9, 21.3) | <0.01 |
| Cologne | 7.6 (2.8, 30.0) | 5.1 (2.1, 16.9) | 0.15 | 76.7 (27.4, 356.0) | 62.9 (21.6, 303.5) | 0.63 | 4.9 (2.2, 34.9) | 6.9 (2.1, 22.3) | 0.93 |
| Shampoo or wash-out conditioner | 5.7 (2.2, 17.4) | 4.6 (2.3, 14.6) | 0.66 | 56.8 (18.5, 244.3) | 98.3 (29.3, 477.2) | <0.01 | 5.8 (1.6, 20.1) | 9.5 (3.7, 28.3) | <0.01 |
| Leave-in conditioner | 6.6 (2.1, 24.4) | 5.1 (2.2, 16.8) | 0.45 | 63.3 (17.6, 186.0) | 62.9 (22.8, 333.6) | 0.63 | 5.6 (1.6, 19.3) | 6.9 (2.2, 23.1) | 0.57 |
| Hair spray | 5.0 (2.7, 24.5) | 5.3 (2.2, 17.1) | 0.80 | 103.8 (32.9, 388.2) | 62.7 (21.6, 302.2) | 0.41 | 10.8 (2.6, 50.1) | 6.8 (2.1, 21.6) | 0.21 |
| Hair oil | 7.2 (2.5, 14.5) | 5.0 (2.2, 17.5) | 0.60 | 72.4 (28.8, 314.1) | 61.6 (21.5, 303.5) | 0.49 | 9.3 (2.8, 24.7) | 6.3 (1.7, 22.3) | 0.06 |
| Hair lotion | 5.3 (2.9, 13.4) | 5.2 (2.2, 17.1) | 0.61 | 110.7 (51.7, 459.0) | 57.7 (19.0, 287.6) | 0.01 | 15.2 (4.8, 46.1) | 6.3 (1.8, 20.0) | <0.01 |
| Hand or body lotion | 5.8 (2.4, 18.0) | 3.9 (1.7, 15.3) | 0.26 | 72.2 (28.2, 373.4) | 36.5 (12.9, 168.4) | <0.01 | 7.4 (2.4, 27.5) | 3.6 (1.2, 13.1) | <0.01 |
| Suntan or sunblock lotion | 5.5 (2.4, 16.7) | 5.2 (2.2, 17.0) | 0.96 | 55.6 (17.9, 286.8) | 66.7 (24.4, 320.2) | 0.25 | 4.6 (1.3, 21.5) | 7.4 (2.4, 24.3) | 0.07 |
Medians (25th, 75th percentiles) are displayed.
p values are for Wilcoxin rank sum test.
Note: Results are shown for the 406 children for whom personal care product questionnaires were completed within 100 days of the date of urine sample collection for triclosan and parabens measurement.
Child urinary concentrations of both methyl and propyl parabens were significantly higher in children with at least weekly use of toothpaste, nail polish, hair lotion and hand or body lotion compared to children who used these products less than weekly. Propyl paraben concentrations were higher in children with at least weekly use of hand sanitizer compared to children with less than weekly use of hand sanitizer. Interestingly, both child urinary methyl and propyl parabens concentrations were lower in children with at least weekly use of shampoo or wash-out conditioner compared to those with less than weekly use of shampoo or wash-out conditioner.
Association between Maternal Plasma and Child Urinary Triclosan and Parabens Concentrations
Results of Spearman correlation analyses of the association between maternal plasma biomarker concentrations during pregnancy and child urinary biomarker concentrations at age 3–4 years are shown in Table 7. There were statistically significant positive correlations between plasma and specific gravity-corrected urinary concentrations of methyl paraben (Spearman rho 0.13, p = 0.005) and propyl paraben (Spearman rho 0.12, p = 0.008).
Table 7.
Association between maternal plasma and child urinary biomarker concentrations
| Child urinary concentrations not adjusted for specific gravity | Child urinary concentrations adjusted for specific gravity | |||
|---|---|---|---|---|
| Spearman rho | p value | Spearman rho | p value | |
| Methyl paraben | 0.13 | 0.007 | 0.13 | 0.005 |
| Propyl paraben | 0.12 | 0.012 | 0.12 | 0.008 |
| Triclosan | 0.005 | 0.91 | −0.03 | 0.54 |
Association between Triclosan and Parabens Concentrations and Allergic Outcomes
Total IgE
There was no association between child urinary or maternal plasma methyl paraben, propyl paraben or triclosan concentrations and total IgE at age 3 years (Online Repository Tables E3–E4).
Environmental Sensitization
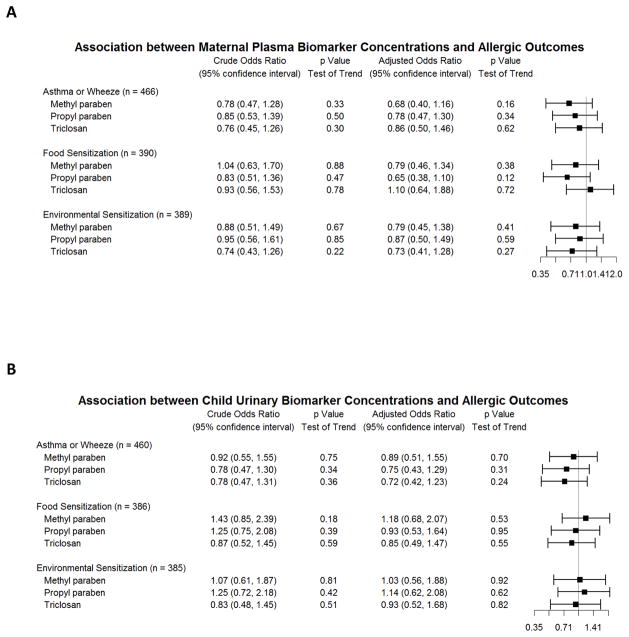
Methyl paraben, propyl paraben and triclosan demonstrated a trend towards an inverse association between maternal plasma concentrations during pregnancy and environmental sensitization in children, though this effect was not statistically significant in any analysis (Figure 1A, Online Repository Table E3). We did not identify an association between child urinary concentrations of triclosan or any paraben with environmental sensitization (Figure 1B, Online Repository Table E4).
Figure 1.
Associations between biomarker concentrations and allergic outcomes at age 3 years: asthma and recurrent wheeze, food sensitization and environmental sensitization. Odds ratios comparing the 3rd to 1st tertile of each biomarker concentration in logistic regression models are displayed. (A) Association of maternal plasma biomarker concentrations and allergic outcomes. Adjusted model includes maternal race/ethnicity, maternal education, maternal age, treatment assignment, and study center as covariates. (B) Association of child urinary biomarker concentrations and allergic outcomes. Crude model adjusted for urine specific gravity only. Adjusted model includes urine specific gravity, child sex, child race/ethnicity, child age at urine collection, maternal education, treatment assignment, and study center as covariates.
Food sensitization
Methyl paraben and propyl paraben demonstrated a trend towards an inverse association between maternal plasma concentrations during pregnancy and food sensitization in children, though these associations were not statistically significant. We did not identify an association between maternal plasma triclosan, or child urinary triclosan or parabens with food sensitization. Detailed results are provided in Figures 1A–B and Online Repository Tables E3–4.
Asthma or recurrent wheeze
We did not identify an association of either maternal plasma or child urinary concentrations of triclosan or parabens with the composite asthma and/or recurrent wheeze outcome at age 3 (Figures 1A–B and Online Repository Tables E3–4). There remained no association when physician-diagnosed asthma and recurrent wheeze were analyzed separately (Online Repository Tables E3–4).
Stratification by Sex
Environmental sensitization
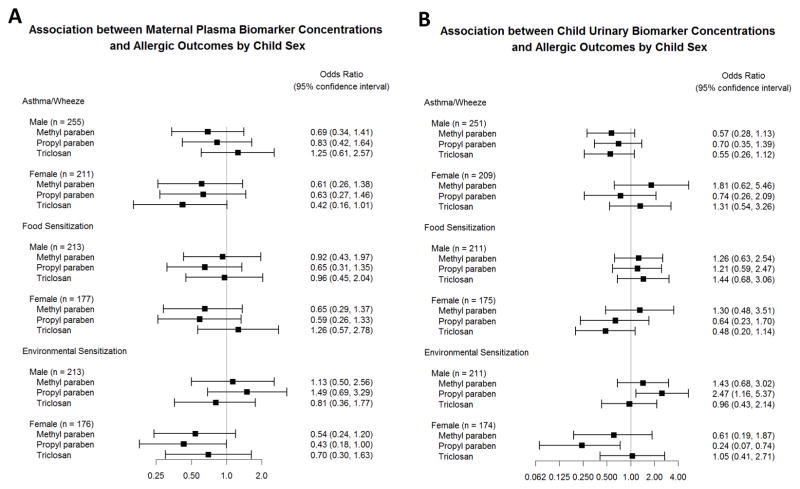
For both child urinary and maternal plasma parabens, there was a trend towards higher odds of environmental sensitization associated with increasing biomarker concentrations in males compared to females (Figures 2A–B, Online Repository Tables E5A–C and E6A–C). For example, the adjusted odds of environmental sensitization comparing the third tertile of urinary propyl paraben concentration to the first was 2.47 in males (95% confidence interval 1.16–5.37) and 0.24 in females (95% confidence interval 0.07–0.74). The interaction between sex and chemical concentration reached statistical significance only for the effects of child urinary propyl paraben and maternal plasma propyl paraben on environmental sensitization (p = 0.001 and p = 0.02, respectively, for interaction).
Figure 2.
Associations between biomarker concentrations (odds ratios comparing 3rd to 1st biomarker tertiles) and allergic outcomes at age 3 years stratified by child sex. (A) Association of maternal plasma biomarker concentrations and allergic outcomes in logistic regression models adjusted for maternal race/ethnicity, maternal education, maternal age, treatment assignment, and study center. (B) Association of child urinary biomarker concentrations and allergic outcomes in logistic regression models adjusted for urine specific gravity, child sex, child race/ethnicity, child age at urine collection, maternal education, treatment assignment, and study center.
Food sensitization
There was a trend towards higher odds of food sensitization associated with increased propyl paraben and triclosan in males compared to females (Figures 2A–B, Online Repository Tables E5A–C and E6A–C), though these associations were not statistically significant.
Asthma or recurrent wheeze
Results of analyses stratified by sex are presented in Figure 2A–B and Online Repository Tables E5A–C and E6A–C. Increasing concentrations of child urinary methyl paraben were associated with increased odds of asthma or wheeze in females, but decreased odds of asthma or wheeze in males (p value for interaction: 0.02). Maternal plasma triclosan trended towards the opposite effect, with increasing concentrations associated with increased odds of asthma or wheeze in males, but decreased odds of asthma or wheeze in females (p value for interaction: 0.05).
DISCUSSION
In this longitudinal prospective study of a large and diverse study population, we hypothesized that higher plasma and urine concentrations of triclosan and parabens are associated with increased risk of allergic disease. Contrary to our expectation, we did not identify an association between either prenatal maternal plasma or child urinary triclosan or parabens concentrations and asthma, recurrent wheeze, environmental or food sensitization in children at age 3 years in the overall study population.
We observed a trend toward a negative association between triclosan and parabens concentrations and odds of allergic sensitization. Prior cross-sectional studies that measured urinary triclosan and parabens concentrations have largely provided evidence of a positive association between exposure and risk of allergic disease14–17. It is possible that cross-sectional studies suggested a positive association between triclosan and parabens exposure and allergic disease because abnormal skin barrier function in eczema predisposes to increased biomarker absorption27, without these biomarkers playing a pathologic role in development of atopy. One study utilizing NHANES data found a negative association between parabens and triclosan urinary concentrations and prevalence of nonatopic asthma and wheeze in children age 6 to 1814. A weak protective effect of exposure to these antimicrobial chemicals may be mediated by a reduction in infections or other beneficial modification of microbial composition14, though these explanations are speculative.
Covariates in adjusted analyses were chosen a priori, and differences between crude and adjusted associations between biomarker concentrations and allergic outcomes suggest that significant confounding was present in crude analyses. Bivariable analyses suggest that race/ethnicity, maternal education, and study site were the biggest confounders among included covariates (results not shown).
We found evidence of effect measure modification by sex on the association of chemical concentrations with risk of allergic disease. There was a pattern of higher odds of environmental sensitization associated with increasing parabens concentrations in both maternal plasma and child urine among males, but not females, though this interaction reached statistical significance only for propyl paraben. This is in accordance with sex-specific differences observed in a prior study utilizing NHANES data14. Sexual dimorphism is well-documented in childhood asthma and recurrent wheeze28 and there is evidence of sexual dimorphism with regard to environmental and food sensitization, with males generally at higher risk than females29–32. Other endocrine-disrupting compounds including phthalates and bisphenol A have different effects on males and females with regard to diverse outcomes33. It is possible that there is interplay between endocrine and antimicrobial effects of triclosan and parabens, in which differences in hormone function, microbiome composition and consequences of microbial interactions account for sex differences in risk of allergic disease, though this is speculative34.
We found significant differences by sex and by race/ethnicity in the frequency of personal care product use among children, and significant differences in child urinary concentrations of parabens by frequency of personal care product use. Though we were not able to ascertain the triclosan or parabens contents of specific products, many of the included personal care products frequently contain these chemicals1,2. Of note, at least weekly vs less than weekly use of nail polish, hair lotion, hand and body lotion and hand sanitizer were associated with higher child urinary parabens concentrations. Use of these products differed significantly by race/ethnicity and could help explain differences in parabens concentration that were seen between race/ethnicity groups. Similarly, use of nail polish and hair lotion was more frequent in females than males, and was associated with higher concentrations of child urinary parabens. Exposure to nail polish and hair lotion could contribute to the higher concentrations of urinary parabens that were seen in females compared to males. Interestingly, we found that at least weekly vs less than weekly use of shampoo or wash-out conditioner was associated with lower concentrations of child urinary parabens. This could be because use of such products leads to removal of products such as hair lotion that may contain parabens. Frequency of shampoo or wash-out conditioner use differed by race/ethnicity and could help explain differences in child urinary parabens concentrations that were seen between race/ethnicity groups. Differences in urinary triclosan and parabens concentrations by sex and by race/ethnicity could be due to several other factors, including dietary and metabolic differences. In accordance with prior studies, we demonstrate here the ubiquity of exposure to triclosan and parabens, with triclosan, methyl paraben and propyl paraben detectable in the majority of subjects. Interestingly, for methyl and propyl parabens, plasma concentrations during pregnancy of participating mothers correlated with urinary concentrations in their children sampled more than three years later. This is consistent with findings from a cross-sectional study of Swedish mothers and their 6 year-old children, which also found significant correlations between mother and child urinary concentrations of methyl paraben and propyl paraben35, and suggests that exposure to these chemicals may be fairly consistent over several years, and may be household-wide.
This study has limitations. We cannot establish or rule out causality in this observational study, as it is possible that people who differ in their risk of developing allergic outcomes also differ in the extent of their exposure to triclosan or parabens. We studied the outcome of sensitization based on serum IgE concentrations, and our results may not be generalizable to clinical outcomes of food allergy or environmental allergy. We studied asthma and recurrent wheeze at age 3 years; however, many children who wheeze at this age do not go on to develop asthma later in childhood. This could produce misclassification bias, as some children who will not go on to have persistent asthma are included in the asthma or recurrent wheeze outcome group, and this misclassification would be expected to result in underestimation of the association between biomarker concentrations and asthma development.
We assessed chemical exposure through concentrations of the biomarkers in urine in children and plasma in pregnant mothers, as urine samples from pregnant mothers were not available. Urinary biomarkers are accepted as markers of exposure to triclosan and parabens1,36, though measurement in plasma is not as well-established. The fact that in a subset of plasma samples analyzed for free concentrations of the biomarkers, the major species of triclosan and parabens were conjugates suggests that plasma concentrations of these biomarkers reflect true exposures and are not the result of contamination23–25. Plasma concentrations of these non-persistent chemicals are normally lower than urinary concentrations37–39, which may result in misclassification of exposure status and a larger number of non-detectable concentrations. Replacement of non-detectable concentrations for both maternal plasma and child urinary measurements may have resulted in additional misclassification. Intraclass correlations are moderate-to-high for measurement of urinary triclosan and parabens over pregnancy40– 43, and moderate-to-high in non-pregnant populations44,45, including children age 6–10 years of age46. Nonetheless, obtaining a single spot urine collection in children and two plasma samples in pregnant mothers may have resulted in exposure misclassification due to variability over time within individuals. Similarly, personal care product questionnaire responses obtained within 100 days of urinary biomarker measurement were analyzed. As triclosan and parabens are excreted within hours of exposure1,47, this approach assumes that personal care product use was consistent between the time of urine collection and questionnaire completion. Additionally, maternal plasma concentrations may not accurately reflect in utero exposure, though significant correlations between maternal plasma and amniotic fluid methyl paraben, propyl paraben and triclosan have been demonstrated48. These sources of misclassification would be expected to be non-differential, thus biasing results towards the null. Finally, we measured triclosan and parabens prenatally and at age 3–4 years. To our knowledge, the stability of triclosan and parabens concentrations from birth to early childhood is unknown, and our measurements may not accurately reflect exposure during infancy, a potential period of high vulnerability to chemical exposures; though prior studies demonstrated a cross-sectional association between allergic outcomes and chemical concentrations even at older ages14–17.
CONCLUSIONS
In summary, this longitudinal prospective study did not identify an association in the overall study population between prenatal or early life triclosan or parabens concentrations and asthma, recurrent wheeze, environmental or food sensitization in children at age 3 years. There was some evidence of differential effects of triclosan or parabens exposure on allergic outcomes by sex, which warrants further exploration.
Supplementary Material
Clinical Implications.
This longitudinal prospective study did not find evidence that prenatal or early life triclosan or parabens exposures are associated with development of early childhood allergic disease.
Acknowledgments
Funding: VDAART was funded by U01HL091528 from the National Heart, Lung, and Blood Institute. Additional funding came from AAAAI/FARE, Hood Foundation, NIH grant K23AI110522 and NIH grant 5T32AI007306-30.
We thank Prabha Dwidendi, Xiaoliu Zhou, and Tao Jia for technical assistance in the quantification of triclosan and parabens biomarkers.
Abbreviations used
- CDC
Centers for Disease Control and Prevention
- LOD
Limit of detection
- NHANES
National Health and Nutrition Examination Survey
- SG
Specific gravity
- VDAART
Vitamin D Antenatal Asthma Reduction Trial
Footnotes
Disclaimer:
The findings expressed in this article are the opinions of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Competing Financial Interests: Robert S. Zeiger reports research support to Kaiser Permanente Research and Evaluation: Aerocrine, AstraZeneca, Genentech, GSK, MedImmune, Merck. Consultant activities: AstraZeneca, Genentech, Novartis, GSK, Theravance, Regeneron, Teva.
Leonard B. Bacharier reports grants from NIH; personal fees from Aerocrine, personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees from Merck, personal fees from Cephalon, personal fees from DBV Technologies, personal fees from Teva, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from WebMD/Medscape, personal fees from Sanofi, personal fees from Vectura, personal fees from Circassia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosmetic Ingredient Review. Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 3.Russell AD. Whither triclosan? J Antimicrob Chemother. 2004;53:693–5. doi: 10.1093/jac/dkh171. [DOI] [PubMed] [Google Scholar]
- 4.Bredin J, Davin-Régli A, Pagès JM. Propyl paraben induces potassium efflux in Escherichia coli. J Antimicrob Chemother. 2005;55:1013–5. doi: 10.1093/jac/dki110. [DOI] [PubMed] [Google Scholar]
- 5.Gee RH, Charles A, Taylor N, Darbre PD. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 2008;28:78–91. doi: 10.1002/jat.1316. [DOI] [PubMed] [Google Scholar]
- 6.Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, et al. The effects of triclosan on puberty and thyroid hormones in male wistar rats. Toxicol Sci. 2009;107:56–64. doi: 10.1093/toxsci/kfn225. [DOI] [PubMed] [Google Scholar]
- 7.Crofton KM, Paul KB, DeVito MJ, Hedge JM. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 2007;24:194–7. doi: 10.1016/j.etap.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Beland FA, Fang JL. Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol Vitr. 2016;32:310–9. doi: 10.1016/j.tiv.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12–9. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- 10.Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: A critical review of the literature. Crit Rev Toxicol. 2010;40:1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- 11.Dann AB, Hontela A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J Appl Toxicology. 2011;31(4):285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 12.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. Population: NHANES 2005–2006. Environ Health Perspect. 2010;118:679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Perspect. 2011;119:390–6. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130(2):453–60. e7. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spanier AJ, Fausnight T, Camacho TF, Braun JM. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014;35:475–81. doi: 10.2500/aap.2014.35.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage J, Johns C, Hauser R, Litonjua A. Urinary triclosan levels and recent asthma exacerbations. Ann Allergy Asthma Immunol. 2014;112:179–181. e2. doi: 10.1016/j.anai.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Bertelsen RJ, Longnecker MP, Løvik M, Calafat AM, Carlsen KH, London SJ, et al. Triclosan exposure and allergic sensitization in Norwegian children. Allergy Eur J Allergy Clin Immunol. 2013;68:84–91. doi: 10.1111/all.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litonjua A, Lange N, Carey V, Al E. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin trials. 2014;38:37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2006;844:53–9. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 21.Kuklenyik Z, Needham LL, Ye X, Calafat AM. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Anal Chem. 2005;77:5407–13. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Tao LJ, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta. 2008;76:865–71. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Calafat AM. Contemporary issues in exposure assessment using biomonitoring. Curr Epidemiol Rep. 2016;3:145–53. doi: 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidry V, Longnecker M, Aase H, Al E. Measurement of Total and Free Urinary Phenol and Paraben Concentrations over the Course of Pregnancy: Assessing Reliability and Contamination of Specimens in the Norwegian Mother and Child Cohort Study. Env Heal Perspect. 2015;123:705–11. doi: 10.1289/ehp.1408325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, et al. Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect. 2015;123:A166–8. doi: 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 27.Overgaard L, Main K, Frederiksen H, Stender S, Szecsi P, Williams H, et al. Children with atopic dermatitis and frequent emollient use have increased urinary levels of low-molecular-weight phthalate metabolites and parabens. Allergy. 2017 doi: 10.1111/all.13157. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Tse SM, Rifas-Shiman SL, Coull BA, Litonjua AA, Oken E, Gold DR. Sex-specific risk factors for childhood wheeze and longitudinal phenotypes of wheeze. Journal of Allergy and Clinical Immunology. 2016;138(6):1561–8. e6. doi: 10.1016/j.jaci.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy. 1993;23:941–8. doi: 10.1111/j.1365-2222.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldhahn K, Bockelbrink A, Nocon M, Almqvist C, DunnGalvin A, Willich SN, et al. Sex-specific differences in allergic sensitization to house dust mites: a meta-analysis. Ann Allergy Asthma Immunol. 2009;102:487–94. doi: 10.1016/S1081-1206(10)60122-6. [DOI] [PubMed] [Google Scholar]
- 31.Alduraywish SA, Lodge CJ, Vicendese D, Lowe AJ, Erbas B, Matheson MC, et al. Sensitization to milk, egg and peanut from birth to 18 years: A longitudinal study of a cohort at risk of allergic disease. Pediatr Allergy Immunol. 2016;27:83–91. doi: 10.1111/pai.12480. [DOI] [PubMed] [Google Scholar]
- 32.Schnabel E, Sausenthaler S, Schaaf B, Schäfer T, Lehmann I, Behrendt H, et al. Prospective association between food sensitization and food allergy: Results of the LISA birth cohort study. Clin Exp Allergy. 2010;40:450–7. doi: 10.1111/j.1365-2222.2009.03400.x. [DOI] [PubMed] [Google Scholar]
- 33.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2016:121. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 35.Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int. 2014;73:323–33. doi: 10.1016/j.envint.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114:1843–6. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hines EP, Mendola P, von Ehrenstein OS, Ye X, Calafat AM, Fenton SE. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod Toxicol. 2015;54:120–8. doi: 10.1016/j.reprotox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frederiksen H, Jørgensen N, Andersson AM. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J Expo Sci Environ Epidemiol. 2011;21:262–71. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 39.Ye X, Zhou X, Wong LY, Calafat AM. Concentrations of bisphenol a and seven other phenols in pooled sera from 3–11 year old children: 2001–2002 national health and nutrition examination survey. Environ Sci Technol. 2012;46(22):12664–71. doi: 10.1021/es303109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120:1538–43. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ Sci Technol. 2013;47:3439–47. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertelsen RJ, Engel SM, Jusko TA, Calafat AM, Hoppin JA, London SJ, et al. Reliability of triclosan measures in repeated urine samples from Norwegian pregnant women. J Expo Sci Environ Epidemiol. 2014:1–5. doi: 10.1038/jes.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss L, Arbuckle TE, Fisher M, Ramsay T, Mallick R, Hauser R, et al. Temporal variability and sources of triclosan exposure in pregnancy. Int J Hyg Environ Health. 2015;218:507–13. doi: 10.1016/j.ijheh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, et al. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: Personal care product ingredients. Toxicol Lett. 2014;231:261–9. doi: 10.1016/j.toxlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Dewalque L, Pirard C, Vandepaer S, Charlier C. Temporal variability of urinary concentrations of phthalate metabolites, parabens and benzophenone-3 in a Belgian adult population. Environ Res. 2015;142:414–23. doi: 10.1016/j.envres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Janjua NR, Frederiksen H, Skakkebæk NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31(2):118–30. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 48.Shekhar S, Sood S, Showkat S, Lite C, Chandrasekhar A, Vairamani M, et al. Detection of phenolic endocrine disrupting chemicals from maternal blood plasma and amniotic fluid in Indian population. Gen Comp Endocrinol. 2016;241:100–7. doi: 10.1016/j.ygcen.2016.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.