Abstract
Wnt signalling regulates several cellular functions including proliferation, differentiation, apoptosis and migration, and is critical for embryonic development. Stem cells are defined by their ability for self-renewal and the ability to be able to give rise to differentiated progeny. Consequently, they are essential for the homeostasis of many organs including the gastrointestinal tract. This review will describe the huge advances in our understanding of how stem cell functions in the gastrointestinal tract are regulated by Wnt signalling, including how deregulated Wnt signalling can hijack these functions to transform cells and lead to cancer.
Keywords: stem cells, Wnt signalling, epithelium, cancer, regeneration, plasticity, intestine, stomach, gastric
1. Introduction
1.1. Adult Stem Cells
Adult stem cells play a vital role in multicellular organisms by maintaining normal tissue homeostasis by ensuring sufficient cells are produced to populate the tissue [1]. They constitute a small population of cells that are defined as having the capacity of both long-term self-renewal and the ability to give rise to at least one type of differentiated cell [2]. Numerous types of precursor cells have been isolated in adult tissues, leading to the concept that organs contain a population, or several populations, of resident stem cells to maintain tissue homeostasis [3].
Prevailing models assume the existence of a single quiescent population of stem cell residing in a niche, however, recent evidence [4] demonstrates the existence of long-lived yet actively cycling stem cell populations. This suggests that clusters of quiescent and active adult stem cells may cohabit the same tissue so that the demands of tissue maintenance and repair can be shared between populations, rather than heaping all cellular responsibilities on a single population [5]. Recent discoveries in the gastrointestinal (GI) tract suggest a model of co-operative stem cell hierarchy whereby proliferative stem cells meet the day-to-day requirements of the tissue and quiescent ‘reserve’ stem cells facilitate tissue regeneration following cytotoxic damage [6].
Several populations of adult stem cells have been identified as the cell of origin for cancer [7]. Stem cells self-renew and have the capacity to promote regeneration, a process similar to tumourigenesis, and therefore it is not surprising that oncogenes, or loss of tumour suppressor genes, are able to hijack these functions to transform these cells [8,9]. Therefore, identification of these cells is also important to help fully understand cancer biology, as well as normal tissue functions.
Although the function of stem cells, and how they are regulated is not fully understood, the past 15 years has revealed many of the complex signalling events controlling stem cells and identified a critical interaction between stem cells and the microenvironment, or niche, in which they reside. A stem cell niche is composed of other cell types including epithelial and stromal cells, signalling factors and extracellular matrix, which in combination with stem cells intrinsic characteristics, defines their properties and potential [10].
The GI tract is a challenging environment for its cellular constituents, most notably the epithelium, which is at the frontline of harsh luminal contents and mechanical stress. Fundamental to prolonged survival, the GI epithelium has evolved the capacity to remove any exhausted cells and replenish the epithelium with spritely participants. Importantly, GI epithelial renewal is powered by resident stem cells, which depend on the coordinated effort of various cellular and biochemical components. This review will focus on the remarkable progress made over the past 15 years in which Wnt signalling has emerged as one of the critical signalling pathways regulating GI tract stem cells. Although the oesophagus is also part of the GI tract, the identification and function of stem cells in this organ has yet to be fully established with the same level of detail as in the stomach and intestine and thus is not covered in this review [11,12].
1.2. Wnt Signalling
Wnt signalling can regulate several cellular functions, including proliferation, differentiation, migration, apoptosis and migration [13]. As such, it is critical during embryonic development, and regulates homeostasis and stem cell function in several adult tissues including the intestine [14,15,16,17], stomach [18], breast [19] and liver [20] (Table 1). The first Wnt gene, Wnt1 (originally named Int-1), was identified in 1982 by Nusse and Varmus who described the cloning of a new murine proto-oncogene [21]. It was then observed that its fruit fly homolog was wingless (Wg), a gene essential in segment polarity during embryonic development [22]. Further investigation since has shown that Wnt1 belongs to a family of highly conserved genes known as the wingless-type mouse mammary tumour virus (MMTV) integration site (Wnt) gene family. There are currently 19 known Wnt genes in the genomes of mice and humans [23], which transmit signals via three different pathways; the canonical Wnt pathway, the non-canonical planar cell polarity pathway, and the non-canonical Wnt/calcium pathway [24]. Although distinct, there is considerable cross-talk between each Wnt pathway, and thus Wnt signalling can be considered more broadly as a signalling network containing distinct arms.
Table 1.
The effects of Wnt ligands on gastrointestinal stem cell populations during homeostasis and cancer.
| Gene | Expression | Homeostasis | Cancer | Reference |
|---|---|---|---|---|
| WNT1 | Gastric epithelium | Gastric stem cell maintenance. Regulates Oct4. | Overexpressed in GC. | [25] |
| WNT2 | Gastric epithelium | Unknown. | Overexpressed in GC and CRC. Promotes cell reprogramming. | [26] |
| WNT2B | Gastric gland base, intestinal stroma | Unknown, Intestinal stem cell maintenance. | Over expressed in CRC and GC. | [27,28,29] |
| WNT3A | Paneth cell/crypt | Intestinal stem cell maintenance. | Over expressed in CRC and GC. | [28,30] |
| WNT4 | Gastric isthmus, intestinal mesenchyme/epithelium | Unknown, intestinal crypt maintenance/regeneration. | Increased expression in GC. | [27,29] |
| WNT5A | Corpus stroma, intestinal villi, colonic epithelium | Gastric stem cell niche, colonic regeneration. | Promotes invasion and prevents anoikis in GC. | [31,32,33] |
| WNT6 | Paneth cell/crypt, gastric gland | Intestinal stem cell maintenance, supports gastric niche. | Over expressed in GC. | [29,33,34] |
| WNT9A | Gastric isthmus and base | Unknown. | Over expressed in CRC and GC. | [29,35] |
| WNT9B | Paneth cell/crypt gastric isthmus | Intestinal stem cell maintenance, unknown. | Unknown. | [29,33] |
| WNT10B | Gastric epithelium | Unknown. | Required for proliferation and migration in GC. | [29,36] |
| WNT11 | Gastric isthmus, surface pit and neck | Unknown. | Increased expression in CRC. | [29,37] |
| WNT16B | Gastric epithelium | Unknown. | Overexpressed in GC. | [38] |
Wnt ligands are secreted glycoproteins that activate signalling pathways through the interaction with a cell surface receptor of the frizzled (FZD) family [39,40]. There are 10 FZD genes in mammals, which can form complexes with other FZD proteins and associate with co-receptors such as low-density lipoprotein-related protein (LRP), most commonly LRP5/6, ROR2 or Ryk to transmit signalling into the cell [41,42]. In the canonical pathways, the absence of Wnt ligands leads to the phosphorylation of β-catenin by the destruction complex, which contains the scaffold protein Axin, APC, GSK3β and CK1-alpha. The phosphorylated β-catenin is targeted for proteasomal degradation. This absence of nuclear β-catenin initiates a complex of TCF/LEF and Groucho to recruit HDACs to repress target genes. Upon binding of Wnt to FZD and LRP co-receptors, the LRP receptors are phosphorylated by CK1-alpha and GSK3β [43] which recruit disheveled (DVL) and axin proteins to the plasma membrane where they become polymerized and activated [44]. The DVL polymers inactivate the destruction complex resulting in the stabilization and accumulation of β-catenin, which translocates to the nucleus [45]. Once in the nucleus, β-catenin forms an active complex with lymphoid enhancer factor (LEF) and T-cell factor (TCF) proteins by disarming TLE/Groucho repression and recruiting histone modifying co-activators, such as Pygo and bcl9 [46] to initiate target gene transcription.
Wnt signalling that is independent of β-catenin is referred to as the non-canonical pathway where a transcriptional response is elicited via an alternative mode of downstream signalling not involving β-catenin–TCF or β-catenin-LEF [24]. There is currently a myriad of non-canonical Wnt pathways which are classified based on the configuration of FZDs and co-receptors involved as well as their downstream effects, however they can be broadly categorized into two pathways; the planar cell polarity (PCP) pathway and the Wnt/Calcium (Wnt/Ca2+) pathway.
The PCP pathway was originally identified in Drosphila when phenotypes to hairs, bristles and ommatidia were observed in mutants including Fzd, Dsh, Celsr, Vangl and Prickle. These genes code for proteins that transmit PCP signalling to regulate cell polarity within the plane of the epithelial sheet making the PCP pathway vital for many developmental processes [47,48]. This pathway is activated when Wnt5a, Wnt7a, Wnt8b or Wnt11 bind to FZD receptors and their respective receptor tyrosine kinases; Ror2 or Ryk [49]. This variety of Wnt ligand, FZD receptors and co-receptors allows for significant variation in the transmission of the signal and depending on which complex is activated determines which cytoplasmic PCP pathway signal transduction components are activated such as c-JUN N-terminal Kinase, DVL, Cdc42, RhoA and Rac1 [50]. PCP signalling is also activated when Fzd7 associates with Syndican4 (Sdc4) and R-Spondin (R-Spo) which transmits Wnt5a signals via the internalization of the whole receptor/ligand complex [51,52,53]. Recent data has indicated that PCP signalling may also promote proliferation in an assortment of different cancers [54,55,56,57,58].
The Wnt/Ca2+ pathway was first identified in zebrafish embryos where over-expression of Wnt5a was observed to increase the frequency of Ca2+ signalling [59] and activate protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase in Xenopus [60]. Wnt/Ca2+ signalling is initiated by the interaction of the Wnt/Fzd receptor complex along with the participating co-receptor Ror1/2 which leads to the production of inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (DAG) as well as calcium via the action of membrane-bound enzyme phospholipase C (PLC). The PLC modifies IP3 and DAG which allows IP3 to diffuse through the cytosol and interact with the calcium channels on the endoplasmic reticulum which results in the efflux of calcium ions; DAG and the intracellular calcium activate PKC. In conjunction with calmodulin, calcium also activates calmodulin dependent protein kinase II (CaMKII). PKC and CaMKII activate numerous regulatory proteins, such as CREB and NFκB, which regulate target gene expression [61].
The formation of Wnt/Fzd/Lrp6 signalling complexes has traditionally been viewed to transduce a linear signal transduction cascade, which centres around stabilization of β-catenin, colloquially known as canonical Wnt signalling. However, recent elegant work from Christof Niehrs and colleagues has shown this pathway has the capacity to bifurcate at the level of Wnt/Lrp6 to initiate other cellular processes independent of β-catenin [62]. For example, Wnt/Lrp6 signalling can sequester up to 70% of the cellular pool of GSK3 in multivesicular bodies, which prevents the many targets of GSK3-mediated phosphorylation being ubiquitylated and degraded [63]. As such, Wnt-dependent stabilization of proteins (Wnt/STOP) peaks during mitosis to slow protein degradation as cells prepare to divide, which influences not only cell-cycle progression but cytoskeletal dynamics, endolysosomal biogenesis and DNA remodelling [62].
The decision of which pathway will be engaged is dependent on the availability of co-receptors and Wnt ligands, thus providing huge complexity and redundancy in the system. As such, there is considerable overlap between the Wnt pathways, for example, Wnt5a/Ca2+ signalling can negatively regulate the canonical Wnt signalling pathway [64,65] however, in other contexts Wnt5a is able to activate β-catenin-dependent transcription in the presence of Fzd4 [66]. In addition, Wnt5a can work in co-operation with the Ror2 receptor to promote β-catenin degradation independently of GSK3 and therefore inhibit Wnt3a-medicated canonical Wnt signalling [67,68].
Despite there being extensive knowledge surrounding canonical Wnt signalling in the GI tract, both in normal homeostasis and cancer, the function of non-canonical Wnt signalling has been less well characterised; this is in part due to the lack of suitable reagents and robust assays. Having said that, Wnt5a, a classical non-canonical Wnt, is critical for mucosal healing and regeneration in the colon following injury. Wnt5a plays an instructive role via potentiating TGFβ signalling to limit the proliferation of intestinal stem cells (ISCs) at the site of injury [31], which highlights the multiplexed control of ISCs by Wnt signalling. Non-canonical Wnt signalling is also involved with migration mediated via Fzd7 [69] as well as being critical for the survival, invasion and metastatic capabilities of colon cancer cells [70]. However, the exact relation of Fzd7 with non-canonical signals in CRC is unknown. Although it is well documented that Wnt5a is overexpressed in both CRC [71] and GC [32] the precise mechanisms of the non-canonical pathways involvement are less well understood. It has been shown that compared to normal tissue, ROR2 is frequently downregulated in gastric carcinoma tissues, which suggests that ROR2 has a tumour suppressive role in gastric carcinoma [72]. The exact underlying mechanisms by which ROR2 acts and how the canonical and non-canonical pathways interact requires further investigation.
2. Intestinal Stem Cells and Wnt Signalling
2.1. Biology of the Intestinal Epithelium
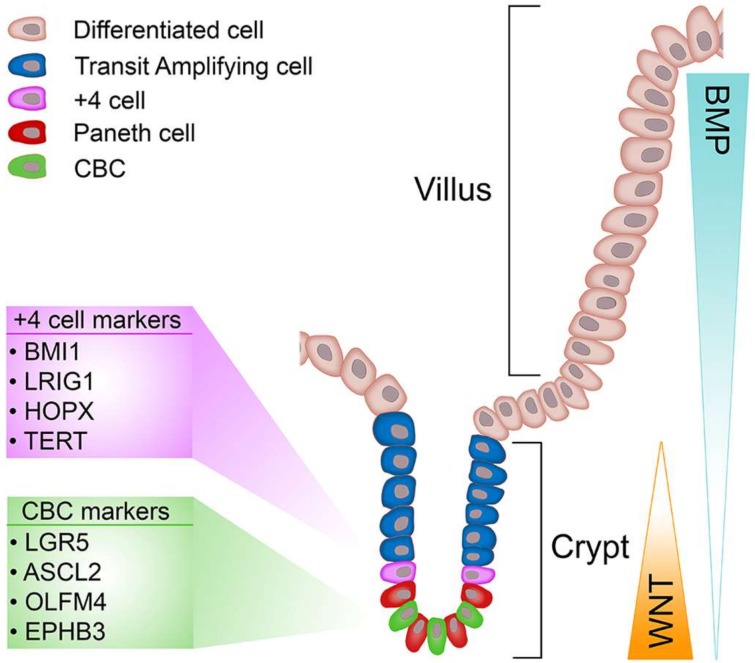
The epithelial lining of the intestine allows efficient exchange and absorption of nutrients whilst simultaneously excluding passage of harmful molecules and organisms, and undergoing constant renewal [73]. For these reasons the intestinal epithelium represents an excellent model to study the processes that regulate cell renewal, differentiation and homeostasis. The bulk of the simple columnar epithelium of the small intestine is composed of finger-like projections known as villi that extend into the intestinal lumen and house the various cell types needed for nutrient exchange and absorption [74]. At the bases of villi are mucosal invaginations, the crypts of Lieberkühn, (referred from herein as crypts) which are home to progenitor cells, differentiated Paneth cells and ISCs (Figure 1). The colon lacks villi, but still retains the crypt-like structures, which house the stem cells, located in the base, and the differentiated progeny [75]. The colon absorbs water, including water with ions, vitamins and nutrients dissolved in it from host gut bacteria in all the differentiated cells. Variable chemical, biological and mechanical stresses encountered by the intestinal epithelium stimulate a perineal renewal along a vertical (crypt-villus) intestinal axis every four to five days, which serves as a protective mechanism to rid the epithelium of any cells that have undergone genotoxic insult. The driving force behind epithelial renewal of the intestine are ISCs that proliferate daily [4,76] to generate a population of unspecified transit-amplifying (TA) cells that rapidly divide while migrating vertically along an epithelial conveyer belt to produce new secretory, enteroendocrine and absorptive lineages that replenish exhausted cells at the villus tips (or top of the crypt in the colon), which undergo apoptosis and are shed into the lumen. However, secretory Paneth cells in the small intestine, or cKit+/Reg4+ cells in the colon [77,78], do not follow the rapid renewal and migration pattern displayed by other intestinal cell types; Paneth cells are renewed every 3-6 weeks by committed secretory progenitor cells located at the base of the TA compartment, which mature into fully differentiated Paneth cells as they migrate toward the crypt base. Paneth cells play important roles in controlling the ISC microenvironment through secretion of antimicrobial peptides (defensins and lysozyme) and various growth factors that confer ‘stemness’ (Wnt, EGF and Notch) [79,80]. Of these factors, extensive research demonstrates Wnt signalling as a critical regulator of ISC maintenance. However, when Wnt signalling is deregulated it can provide favourable conditions to transform cells [13].
Figure 1.
Stem cell populations and signalling gradients of the gastrointestinal epithelium. Schematic of the small intestinal epithelium depicting the various cell types, stem cell populations and signalling gradients.
2.2. A Brief Perspective of Wnt Signalling in the Intestine
To understand why Wnt signalling plays such an instrumental role in ISC biology, we need to understand how and why Wnt appeared on the radar of gastrointestinal researchers. Near the turn of the 20th century, several groups mapped and functionally linked APC (previously discussed), located on chromosome 5q21, to sporadic colorectal cancer (CRC) and familial adenomatous polyposis (FAP), the latter being an autosomal dominant condition that drives the formation of hundreds to thousands of small benign tumours in the large intestine, which can progress to cancer [81,82]. Following these discoveries, immunoprecipitation experiments reveal complexes of APC bound to β-catenin [83], suggesting a role for APC in Wnt signal regulation. Importantly, seminal work published back-to-back in Science show APC mutant colorectal cancers fail to regulate β-catenin, which permit constitutive transcriptional activation of a β-catenin/TCF-4 complex, thus driving Wnt signalling in colorectal cancer tissue [84,85]. However, until this point, the requirement of Wnt signalling in the normal intestinal epithelium had not been tested. In the normal intestine, the role of Tcf-4 (encoded by Tcf7l2) was elucidated by Korinek et al., which reveal neonatal Tcf7l2−/− mice exhibit a complete absence of the prospective intestinal crypt and stem cell compartments leading to death shortly after birth, highlighting for the first time the necessity of Wnt signalling for intestinal homeostasis and stem cell maintenance [16]. These critical findings were soon reinforced by several key studies that embraced the Cre-LoxP system [86] to permit the investigation of Wnt signalling in the adult intestinal epithelium, which collectively demonstrate that manipulation of key regulators of the Wnt pathway impose an aberrant Wnt-signal gradient, which significantly disrupts intestinal crypt-villus architecture and homeostasis. In addition, FAP patients, in which one allele of APC is mutated and Wnt signalling activated when the second is lost via loss-of-heterozygosity, present with polyps in the stomach as well as the intestine, illustrating a role for Wnt in the regulation of gastric stem cells as well [87]. Together these highlight the importance of Wnt signalling for regulating stem cell function in the GI tract, which is the focus of this review [88,89,90,91,92].
2.3. Identification and Dynamics of Intestinal Stem Cells
Two prevailing models of ISC identity have competed for legitimacy over the past four decades. The identification of slender, undifferentiated, mitotically active crypt base columnar cells (CBCs) provides the foundation for the “stem cell zone model”. Cheng and Leblond observed the gradual presence of radiolabelled phagosomes, initially detected only in CBCs, in multiple cell lineages. However, the various labelled cell types were observed in separate crypts, negating multipotency of CBCs [93]. Additional evidence in support of CBC stemness came decades later through chemical mutagenesis of the Dlb-1 locus, which generates long-lived clones comprised of all four intestinal cell lineages. This was interpreted as evidence for CBC cells being self-renewing multipotent stem cells [94]. In contrast, studies from Christopher Potten’s group propose cells positioned directly above the uppermost Paneth cell, between +2 and +7, average of +4, relative to the crypt base embody ISCs [76]. Potten’s +4 cells actively divide (daily), but can retain DNA incorporated labels, a trait normally synonymous with cellular quiescence [95]. Moreover, the ability of +4 cells to retain DNA labels while actively proliferating is thought to occur through selective segregation of old (labelled) and new (unlabelled) DNA strands into stem cells and their daughter progeny, respectively, which was proposed as a mechanism to reduce accumulation of DNA damage in long-lived stem cells [96,97]. Furthermore, the radiosensitivity of +4 cells is seen to be a desirable quality for stem cells as it would prevent the accumulation of DNA replication errors that could be inherited by daughter cells. Despite the recent discovery of specific markers for both candidate stem cell populations, demonstrating which of the two models is correct has been immensely complex [73]. However, only recently has a unifying theory that incorporates aspects of both models started to emerge and how each stem cell pool contributes to intestinal homeostasis and regeneration (discussed below).
2.4. Markers of CBCs
While it was known that the cellular source of intestinal renewal resided within the crypts, the precise identity of ISCs remained elusive until the beginning of the 21st century. In a landmark study, Barker et al. confirmed a previously identified Wnt target gene, Lgr5 [92,98], to mark a population of CBCs, which were decades earlier postulated to represent ISCs [93]. Each intestinal crypt harbours approximately 14 Lgr5+ cells, some 10% of which occupy the +4 position, which intercalate between Paneth cells. The generation of a Lgr5-EGFP-IRES-CreERT2 mouse was used to demonstrate via lineage tracing that mitotically active Lgr5+ cells are self-renewing, long-lived and multipotent [4]. Detailed investigations of the endogenous population dynamics of Lgr5+ cells using the Confetti reporter mouse and mathematical modelling reveal Lgr5+ cells drift towards clonality and divide symmetrically, implying functional equivalence within the Lgr5+ ISC pool [99]. Intra-vital imaging further refines this model, showing ISCs compete for access to essential niche factors (Wnt, EGF, Notch) at the crypt base, resulting in central ISCs having a survival advantage over spatially displaced counterparts (border cells), which will maintain stemness and adopt a progenitor fate respectively [100]. Furthermore, as Lgr5+ cells from the Lgr5-EGFP-IRES-CreERT2 knock-in mouse express GFP, FACS isolated Lgr5GFP+ cells can generate three-dimensional self-renewing ex vivo organoid cultures, complete with a full repertoire of intestinal lineages in the correct ratios and anatomical position, thus providing additional evidence of Lgr5 as a marker of ISCs [80,101]. Subsequent investigations have shown Lgr5 marks stem cell populations in many other tissues such as the hair follicle [102], stomach [103], ovary [104], kidney [105], liver [106], mammary [107], pancreas [108], inner ear [109] and taste buds [110].
The utility of Lgr5 as a marker of ISCs has allowed multiple aspects of ISC biology to be better understood, such as the molecular regulators of stem cell identity. Gene and proteomic signatures of Lgr5+ ISCs [111] identify the transcription factor achaete scute-like 2 (Ascl2) as a master regulator of ISCs, which co-operate with Tcf-4 and β-catenin to establish a Wnt-driven stem cell program at the crypt base [111,112]. Like Lgr5, Ascl2 specifically marks CBCs and transgenic overexpression or conditional deletion of Ascl2 result in crypt hypertrophy and rapid stem cell death respectively [113]. However, in the latter scenario, no gross intestinal phenotype is observed due to ‘escaper’ crypts that can replenish the ISC pool [113]. Intriguingly, although Ascl2 can increase the number of ISCs and its expression is positively correlated with CRC progression [114], forced expression of Ascl2 in a mouse model of colon cancer doesn’t accelerate tumourigenesis, which suggests ectopic expression in cells that do not normally express Ascl2, rather than overexpression, explain the aetiology of Ascl2 in CRC [115].
The ephrin family of signalling molecules regulate cell-position cues in a wide variety of tissues, including the intestinal crypt-villus axis. Interactions between Eph receptors and ephrin ligands usually facilitate cell repulsion [116] and within the crypt, Wnt signalling inversely regulates the expression of EphB receptors and ephrinB ligands, creating correct compartmentalisation of undifferentiated and differentiated cells [92]. Indeed, EphB3 is enriched in Lgr5+ cells and EphB2 is expressed uniformly throughout the crypt, whereas the ephrinB1 and B2 ligands are expressed in the villi. Deletion of EphB2 and EphB3 triggers a mislocalisation of Paneth cells combined with an abnormal intermingling of proliferative and differentiated cells [117]. Furthermore, paralleling the results in normal crypts, the graded expression of EphB2 in CRCs identifies tumour cell populations displaying ISC-like or differentiated-like phenotypes [118].
2.5. Markers of +4 Cells
Shortly after the discovery of Lgr5, several other markers (Bmi-1, Lrig1, Hopx, m-Tert) were reported to preferentially mark a population of quiescent cells located above the uppermost Paneth cell, the +4 position, which do not contribute substantially to epithelial homeostasis, but can support epithelial reconstitution following injury [119,120,121,122].
The polycomb complex protein Bmi-1, which regulates hematopoietic and neural stem cell self-renewal, was observed to mark a radio-resistant +4 cell population [120]. In vivo lineage tracing with Bmi-IRES-CreERT2 mice demonstrate Bmi-1+ cells are multipotent, but do not contribute to epithelial homeostasis under basal conditions [120,123]. While Bmi-1+ cells do participate in epithelial regeneration in response to injury (discussed later), the validity of Bmi-1 as a specific marker of +4 cells was brought into question. Independent analysis show Bmi-1 expression overlaps substantially with Lgr5+ cells [111,119,122,124] and lineage tracing can initiate anywhere in the crypt, including Lgr5+ cells, and over time, most of these clonal populations are shed from the epithelium demonstrating they originated in short-lived progenitors/stem cells [111]. However, hierarchical clustering of ISC populations by bulk and single-cell mRNA-seq show enteroendocrine (EE) gene signature enrichment in Bmi-1+ cells, which display clonogenic potential ex vivo, suggesting that stem cell activity resides in a subset of Bmi-1+ cells [125,126]. In addition, Bmi-1+ cells are not sensitive to Wnt signalling, as demonstrated by the failure of crypt expansion and atrophy following R-Spo and Dickkopf-1 (Dkk1) treatment respectively, which indicate a functional distinction between Lgr5+ and Bmi-1+ cells [123].
Increased telomerase expression is a desirable trait for stem cells, which buffers against replication-induced senescence. Indeed, loss of telomerase induces crypt-villus atrophy, suggesting a functional role for telomerase maintenance in ISCs [127,128]. Prompted by this hypothesis, Montgomery et al. report a population of rare quiescent cells located in the lower crypt, around the +4 position, labelled by mouse-telomerase reverse transcriptase (m-Tert) [122]. m-Tert+ cells are radio-resistant and distinct from Lgr5+ cells. In vivo lineage tracing demonstrates a small percentage of m-Tert+ cells are actively cycling stem cells that contribute to intestinal homeostasis [122]. Second-generation Tert knock-in reporter animals (TertTCE/+) confirm previous reports of label-retaining qualities displayed by Tert+ cells, but also reveal Tert expression overlaps with ChgA+ cells, which is consistent with multi-capable EE lineages [27,125]. Furthermore, injury-induced activation of Tert+ cells requires Wnt2b signalling, highlighting Wnt as a critical component of successful epithelial recovery following injury [27]. Collectively, these results support m-Tert as being a marker of an independent, quiescent damage-inducible population of ISCs [73].
A prominent mitogen that regulates ISCs is Epidermal Growth Factor (EGF) signalling [101]. As such, EGF must be diligently regulated to avoid excessive signalling, which is linked to many epithelial malignancies [129,130]. The leucine-rich repeats and immunoglobulin-like domain 1 (Lrig1) gene encodes a single-pass transmembrane receptor that functions as an inhibitor of ErbB signalling in several tissues and marks interfollicular epidermal stem cells [131]. Lineage tracing with Lrig1-IRES-CreERT2 mice, initiated at +4 position, produce long-lived multipotent clonal units throughout the small intestine [119]. Indeed, models of intestinal injury mobilise Lrig1+ cells to proliferate and regenerate the colonic epithelium [119]. Furthermore, comparative expression analysis of Lrig1+ and Lgr5+ cells reveal different transcriptome profiles, supporting their independence as makers of intestinal (small & large) stem cells [119]. However, an independent study reports contrasting data, bringing into question the specificity of Lrig1 as a marker of ISCs [132]. In-situ and immunohistochemical analyses confirmed the expression of Lrig1 in the crypts, however, transcriptome analysis reveal an enrichment of Lrig1 expression in Lgr5+ cells [132], which is consistent with fluorescent in situ hybridisation (FISH) showing Lrig1 expression throughout the intestinal crypt [111]. Together these studies highlight an important role for Lrig1 in regulating ErbB signalling in the intestinal crypt, however the broad expression of Lrig1 and enrichment in Lgr5+ cells suggest it is not a specific marker of +4 ISCs.
The Hopx gene codes for an atypical homeobox protein, which is expressed mainly in the +4 cells of the intestine [121]. Hopx+ cells are quiescent, radio-resistant and can restore the epithelium following radiation-induced damage. Unlike Bmi-1+ cells, in vivo lineage tracing from Hopx-IRES-CreERT2 mice produce multipotent, long-lived clonal tracings throughout the intestinal epithelium [121]. Furthermore, expression profiling of Hopx+ cells and immediate progeny revealed Hopx-derived cells express high levels of Lgr5 [121]. Conversely, ex vivo organoid cultures derived from isolated Lgr5+ intestinal stem cells can give rise to Hopx+ cells. Taken together, these observations support a model in which actively proliferating Lgr5+ cells and quiescent Hopx+ cells occupy separate positions within the intestinal crypt and can interconvert to maintain epithelial homeostasis and regeneration.
Overall, the discrepancies between Potten’s original observations of +4 cells and more recent reports highlight differences in radiosensitivity and mitotic activity, which make it difficult to distinguish whether markers of +4 cells truly represent a distinct class of stem cell or whether they are simply progeny of Lgr5+ CBCs that inherit and express different molecular features as they migrate away from the crypt base. However, the gastric epithelium also has distinct populations of stem cells, with several genes identified as markers of reserve stem cells, suggesting that redundancy in stem cells could be an evolutionary conserved function in the GI tract to help it cope with the harsh conditions encountered in this tissue.
2.6. Intestinal Plasticity
Following the identification of various ISC markers, questions about ISC hierarchy have been intensely studied. For instance, are ISCs functionally equivalent and are they required during homeostasis and/or regeneration? Given the remarkable capacity of the intestine to regenerate [133,134], models of intestinal regeneration have been utilised to tease-apart the functional requirement of various ISC populations. Calvin Kuo’s group took advantage of differences in sensitivity to ionising radiation to show Bmi-1+ cells are mobilised through epigenetic remodelling to restock the depleted Lgr5+ population and restore the epithelium [123,125]. Similarly, Hopx+ cells can restore the intestinal epithelium following damage, supporting the notion of a dedicated reserve stem cell pool [106]. However, using a diphtheria-toxin (DT) inducible transgenic mouse (Lgr5-EGFP-DTR) to selectively ablate Lgr5+ cells highlights the requirement of Lgr5+ cells during intestinal regeneration [135]. Under these conditions (DT + 10γ radiation), the regenerative capability of the intestinal epithelium is significantly impaired, demonstrating that Bmi-1+ and/or Hopx+ cells are unable to mediate efficient intestinal regeneration [135]. Of note, Lgr5 expression is detected in a modest proportion of +4 cells [124], which would also be targeted in experiments using the Lgr5-EGFP-DTR mouse. Interestingly, using the same mouse model to selectively ablate the Lgr5+ population under basal conditions reveal no gross alterations to epithelial homeostasis, which is compensated by the activation of Bmi-1+ cells [136]. Importantly, the rapid replacement of Lgr5+ ISCs following ablation has been reconciled by the inherent plasticity of lineage-specific precursor populations. For instance, label-retaining secretory precursor cells, which express a range of ISC markers (Lrig1, Bmi-1, Lgr5), under basal conditions give rise to short-lived clones comprised of cells of the secretory lineage [137,138]. Much like the +4 ‘reserve’ population, following injury, secretory precursor cells can generate all epithelial lineages, demonstrating they can de-differentiate into functional multipotent stem cells that subsequently reconstitute the intestinal epithelium [137,138]. Similarly, cells expressing the enterocyte differentiation marker alkaline-phosphatase intestinal (Alpi) can be induced to de-differentiate and replenish the Lgr5+ ISC pool following injury [139]. Collectively, these studies suggest a model where actively cycling Lgr5+ CBCs are responsible for the daily turn-over of the intestinal epithelium, whereas a quiescent subset of CBC progeny can restock the active stem cell pool and intestinal epithelium following cytotoxic insult [6]. More importantly, these studies highlight that ISC identity is not an intrinsic quality, but rather defined by a cell’s location within the crypt and exposure to extrinsic ISC promoting factors, including Wnt signals. This current model reconciles the once irreconcilable stem cell zone and +4 models.
2.7. Wnt Signalling Is Critical for ISCs
Wnt signalling plays a critical role in the mammalian intestinal epithelium where it regulates stem cell behaviour, proliferation, cellular differentiation, apoptosis and migration (Table 1). Indeed, much of our understanding concerning the role of Wnt signalling in homeostasis, stem cell biology and disease have originated from studies in the intestinal epithelium as it is a highly dynamic and well characterised system amenable to vast experimentation.
As mentioned earlier, the prominent phenotype observed in Tcf-4 mutant animals highlight a critical role for Wnt signalling in the intestine. Indeed, Tcf-4 knockdown downregulates key Wnt target genes involved in proliferation and cell-cycle progression such as Myc, which normally represses the action of cell-cycle inhibitors such as p21, permitting cell-cycle arrest [92]. Conditional deletion of Myc from the intestinal epithelium causes reduced biosynthetic activity and crypt atrophy, which is countered by rapidly clearing Myc-deficient cells from the epithelium, highlighting a critical role for Wnt signalling in maintaining ISC proliferation [140]. Similarly, over-expression of Dkk1 suppresses Wnt/β-catenin target gene expression resulting in severe lack of proliferative crypts, reminiscent of the phenotype observed in Tcf-4 mutant mice [16,89,90]. Conversely, hyperactivation of Wnt signalling is sufficient to impose a crypt-progenitor phenotype characterised by mis-localisation of differentiated cell types, impaired differentiation and crypt hyperplasia. Such phenotypes are typically achieved by establishing a situation where β-catenin is unable to be regulated by the destruction complex or by administering the potent Wnt agonist R-Spo [17,141]. However, the latter context is sufficient to bolster the resilience of ISCs following chemoradiotherapy, thereby improving recovery while targeting tumour tissue [142]. Furthermore, conditional deletion of CTNNB1 (encodes for β-catenin) from the intestinal epithelium disrupts secretory cell specification and triggers rapid repopulation of the epithelium with β-catenin-proficient cells [88]. Studies investigating the mechanisms of how Wnt/β-catenin signalling activates key target genes in the intestine have identified other components of the Tcf/β-catenin transcription complex that play key roles in intestinal homeostasis. Both Brahma-related gene-1 (Brg1) and leukaemia-associated Mllt10/Af10-Dot1l are essential Tcf/β-catenin co-activators required for target gene transcription [143,144]. Targeted deletion of Brig1 or Mllt10/Af10-Dot1l from ISCs results in rapid epithelial repopulation and proliferative defects observed in intestinal crypts respectively [143,144].
Competition for niche space is a defining feature in determining ISC identity. Wnt is a central component of the ISC niche, which is provided by neighbouring Paneth cells [80], yet, ablation of Paneth cells reveals no obvious changes to ISCs or intestinal homeostasis [145]. This may have been due to incomplete deletion of all Paneth cells, but nonetheless, conditional deletion of Wnt3, the Wnt released by Paneth cells that is required for stem cell function [80], or inhibition of Wnt secretion (conditional deletion of Porcupine or Wntless) from the epithelium also reveal no change to ISC homeostasis. These data suggest that the surrounding stroma can provide an alternate source of Wnt ligands, and it was recently demonstrated that Wnt2b is secreted by sub-epithelial mesenchymal cells to activate ISCs in situations of Paneth cell shortage [15,28,146]. As mentioned, Wnt is paramount to ISC maintenance, which is reflected by the necessity of Wnt and R-Spo in establishing Lgr5+-derived organoid cultures [101]. Indeed, mesenchymal cells can rescue Wnt-depleted organoid cultures through supplying ISCs with critical Wnts and R-Spo ligands [15]. From the perspective of the ISC, Wnt ligands expressed by Paneth cells (Wnt3a, 6 and 9b) are directly sequestered via binding to Fzd receptors expressed on the surface of ISCs, rather than via intercellular diffusion [33,80,91,147]. We have shown Fzd7 to be the major Fzd expressed by Lgr5+ ISC and deletion of Fzd7 is sufficient to induce transient depletion of the ISC pool [14]. In addition to its function as a marker of ISCs, Lgr5 encodes for a 7-transmembrane protein that participates in the Wnt receptor complex where it binds R-Spo [148,149,150] to prohibit the ubiquitylation of Fzd receptors by E3 ligases Znrf3/Rnf43, thereby stabilising Fzd expression, and thus potentiating Wnt signal strength [151,152]. Hence, the R-Spo/Lgr5/Znrf3/Rnf43 signalling axis fine-tunes the level of Wnt at the crypt base, ensuring highest levels are achieved in cells destined for an ISC fate. Indeed, perturbations to any of these Wnt signal regulators induces dramatic changes to the dynamics of the ISC pool and ultimately intestinal homeostasis [141,148,151,152,153]. Recent work has elucidated the roles of Wnt and R-Spo to suggest that R-Spo ligands adopt the primary instructive role in ISC maintenance and proliferation, whilst Wnt ligands confer basal proliferative competence to ISCs through maintaining suitable levels of the R-Spo receptor Lgr5. Thus, in this model, intestinal homeostasis is dictated by the abundance of R-Spo ligand, with Wnt serving to prime the cells which will respond to this signal by upregulating Lgr5 [154].
2.8. Aberrant Wnt Signalling Triggers Intestinal Tumourigenesis
As mentioned previously, deregulated Wnt signalling is linked to the progression of cancer, most notably CRC [155] (Table 1). Of the three broad phases that encompass CRC tumour evolution, the initiating or ‘breakthrough’ phase is often triggered by truncating mutations to APC, providing cells with a growth advantage that progress to small adenomas in the colon [156]. Subsequent mutation to key driver-genes (Kras, Smad4, p53) permit cells to invade and grow in otherwise hostile environments, which over many years progress to adenocarcinoma [157]. Hyperactivated Wnt signalling in CRC can also arise from mutations to AXIN2 [158] or CTNNB1 (β-catenin) [85], but account for a small percentage of cases in comparison to APC. While the kinetics by which APC is mutated differs between FAP and sporadic CRC patients, tumour cells with truncating APC mutations that maintain a ‘just-right’ level of Wnt signalling are positively selected for optimal tumour growth [159]. Mouse models of both FAP and sporadic CRC show substantial variation in disease onset and severity based on the nature of Apc mutation [160,161], which supports the idea of a ‘just-right’ or ‘Goldilocks’ amount of Wnt signalling as a driver of tumour formation, rather than an “on-off” switch of excessive Wnt activation. Conditional truncation of Apc in the intestinal epithelium instantly transforms the epithelium, causing an expansion of ISCs and disruption to epithelial homeostasis [17]. The growth of Apc-mutant cells requires the Wnt target Myc, which upon co-deletion rescues the intestinal phenotypes induced by Apc mutation [162]. Furthermore, restoration of wild-type Apc alone is sufficient to block intestinal tumour growth, despite concurrent mutations in oncogenes Kras, Smad4 and the tumour suppressor p53, which highlight the clinical importance of controlling Wnt in CRC [163].
Although these early studies provide invaluable insight into the consequences following Apc mutation in the intestine, cancer genome sequencing has revealed new kinds of CRC mutations that deregulate Wnt signalling [164,165]. Whole-exome sequencing of patient tumour-matched samples reveal loss-of-function (LOF) mutations to RNF43, which negatively regulate Fzd receptors, in 18.9% of CRCs, showing a higher prevalence in microsatellite instable (MSI) tumours [164]. Moreover, RNF43 mutations are mutually exclusive to APC mutations [164]. Conditional deletion of both Znrf3 and Rnf43 from the intestinal epithelium induces rapid expansion of Lgr5+ ISCs and subsequent hyperactivation of Wnt signalling, quickly leading to adenoma formation [151]. Similarly, recurrent gene fusions between R-SPO2 and R-SPO3 are observed in 10% of CRCs [165], which like RNF43, are mutually exclusive to APC mutations, indicating a role for R-SPO in Wnt activation in CRC formation. As such, human CRC xenografts treated with a R-SPO3 blocking antibody promotes cell differentiation and decreased Lgr5+ ISC function. Interestingly, a modest reduction in the expression of several Wnt target genes, AXIN2, MYC and CCND1, is observed following anti-R-SPO3 treatment, suggesting therapies targeting R-SPO in the intestine will be well tolerated [166].
Considering the remarkable plasticity of the intestine and long-standing cancer-stem-cell (CSC) theories [7], a question that persists in the field is whether all epithelial cells are equivalent in their tumour forming capability, or are stem cells the only cell type endowed with the potential to give rise to a tumour? Support for the latter hypothesis was shown following targeted activation of Wnt signalling in Lgr5+ or Bmi-1+ ISCs, which lead to rapid adenoma formation [8,120]. In contrast, activation of Wnt signalling in enterocytes failed to yield established adenomas [8]. More recently, two independent approaches have shown LGR5+ cell ablation in human and mouse CRC organoid xenografts is sufficient to induce tumour remission, further supporting a ‘bottom-up’ CSC model for CRC [167]. However, following the cessation of LGR5+ cell ablation, mobilisation of LGR5- cells quickly reinstated tumour growth [168,169], which may undermine the clinical translation of CSC-targeted therapies. Indeed, plasticity within CRC has been previously reported, owing to extrinsic cues from the tumour microenvironment that induce transformation of differentiated epithelial cells through Wnt activation [170]. Similarly, terminally differentiated enterocytes or DCLK1+ tuft-cells re-express ISC markers (Lgr5, Ascl2, Rnf43) and acquire tumour-forming capacity following simultaneous stimulation of Wnt and inflammation pathways, which support a ‘top-down’ model of CRC initiation [171,172]. Likewise, TGFβ signalling from the stroma limits the capacity of terminally differentiated enterocytes to de-differentiate following Wnt and MAPK activation, stressing the importance of targeting both cell intrinsic and extrinsic cues in CRC cells [173]. Moreover, elegant work from Simon Leedham’s group show genetic disruption of intestinal morphogen gradients via GREM1 overexpression enables the persistence of Lgr5- cells to form ectopic crypts on the flanks of villi. This demonstrates selective pressures that disturb homeostatic balance, such as morphogen dysregulation, can cause committed progenitor cells and even some differentiated cells to regain stem cell properties [174]. Collectively, these studies depict a challenging obstacle to successfully treat CRC given the plasticity of differentiated cells to acquire stem cell properties. Thus, it may be an attractive therapeutic approach to target the CSCs and also the niche that provides these signalling cues, including the Wnt pathway.
3. Gastric Stem Cells and Wnt Signalling
3.1. Gastric Biology
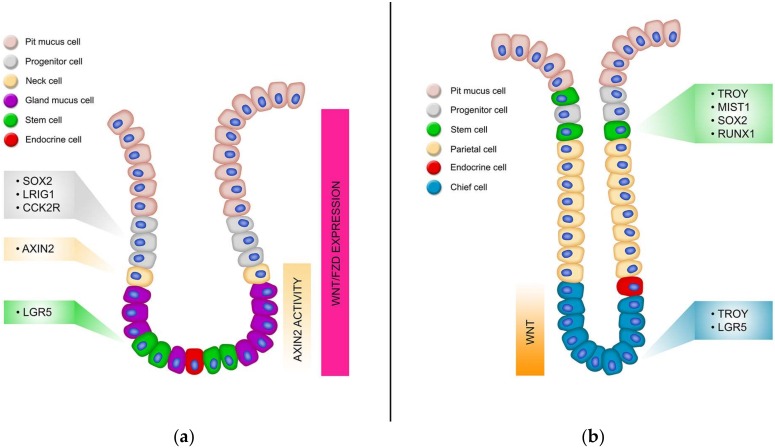
Due to the dynamic function of the stomach, the gastric epithelium is replenished continually, fuelled by a small population of resident adult stem cells [175]. Like the intestine, the gastric epithelium is organized into numerous glandular mucosal invaginations referred to as gastric units, which house stem cells and various differentiated cells responsible for the regular function of the stomach. Gastric units comprise of the flask-shaped gland, which can be subdivided into the isthmus, neck, base and the pit which is continuous with the surface epithelium (Figure 1B). The four terminally differentiated cell types include parietal cells which secrete hydrochloric acid and a number of other factors including HB-EGF, TGF-α and Shh [176], chief cells with pepsinogen granules secreting active pepsin, two types of gastric mucus cells (surface mucous cells and mucous neck cells) secreting Muc5AC and Trefoil Factor-1 (Tff1) or Muc6 and Tff2 respectively [177] as well as several types of hormone-secreting enteroendocrine cells [103]. These lineages differentiate from progenitor cell populations located in the upper neck region of the gastric glands [178].
The glandular stomach is comprised of the proximal corpus and the distal antrum with the precise cellular composition, architecture and turnover rate varying significantly between the two (Figure 2). The interface between the corpus and the antrum is not abrupt but marked by transitional mucosae that shares features of both [179]. The gastric units of the antrum comprise several short glands that feed into a single extended pit. They have a simple cellular composition characterized by abundant gastric alkaline mucous-secreting cells, endocrine cells (G-cells), and low numbers of acid-secreting parietal cells [180,181]. The hydrochloric–secreting parietal cells aide in digestion of food and maintain the acidic pH of the stomach. Large quantities of mucous produced by the mucous-secreting cells protect the duodenum from hydrochloric acid following the emptying of the stomach while the G-cells secrete a variety of hormones that regulate digestion and absorption, such as Ghrelin and Somatostatin [182].
Figure 2.
Stem cell populations and signalling gradients of the gastric epithelium. Schematic of the gastric epithelium depicting the various cell types, stem cell populations and signalling gradients. The antral (a) and corpal (b) epithelium are shown on the left and right respectively.
In contrast, the corpus gastric units are comprised of several longer tubular glands that feed into short pits. The corpus epithelium is more heterogeneous than that of the antrum, containing many more parietal cells in addition to chief cells, base and neck located mucus cells, and endererndrone cells [175]. Mucous neck cells function in a secretory fashion as well as an intermediate progenitor for chief cells. The chief cells work in conjunction with the HCl-secreting parietal cells and release chymosin and pepsinogen, which is converted to pepsin in acidic conditions thus reflecting its role as the major digestive unit of the stomach [160].
The mechanisms and factors that regulate the homeostasis of the gastric epithelium have only been partially characterized. However, aberrations in these mechanisms, most frequently due to chronic inflammation, can lead to oxyntic atrophy (loss parietal cells) and give rise to Spasmolytic Polypeptide-expressing Metaplasia (SPEM); an event linked to the initiation of both dysplasia and neoplasia [183]. SPEM was first studied in models of acute parietal cell loss using compounds (DMP-777 and L635) to induce parietal cell death via targeted inhibition of protoniophores [184], with similar parietal cell toxicity effects observed following high-dose tamoxifen administration [185]. Although the current understanding is that gastric metaplasia is triggered by the loss of the parietal cells, it has been noted that loss of chief cells also occurs during oxyntic atrophy and recent data postulates that the two processes may be linked. Lineage tracing from chief cells, using the Mist1-CreERT2:LSL-LacZ mice identified that SPEM lineages were mainly derived from chief cells which undergo zymogen granule atrophy and changes in gene expression, including upregulation of Tff2 [186]. Thus, SPEM is a feature that develops in response to gastric mucosal injury, suggesting SPEM aids in regeneration, and in common to the intestine, this is a process that can be hi-jacked by oncogenes or loss of tumour suppressors to initiate tumourigenesis, and consequently SPEM is linked with an increased risk of gastric neoplasia [184,187].
3.2. Gastric Stem Cells
Gastric epithelial cells are exposed to considerable chemical stress, including low pH and exogenous toxins, and physical stresses from peristaltic contractions of the stomach. To function in this situation, the epithelium has a rapid turnover of cells fuelled by resident populations of stem cells. The corpus and antrum gland are functionally and morphologically distinct and therefore have very different stem cell populations, and turnover rates, with the corpus taking 10–30 days and the antrum much faster at around 7–10 days [175]. Early studies used radioactive nucleotides to label differentiated progenitor populations to investigate gastric epithelial proliferation dynamics [188]. However, the subsequent generation of transgenic mice to mark the progeny of specific cell types via lineage tracing, which is considered the gold-standard to assay stem cell function in vivo, has permitted the identification of several gastric stem cell markers, which will be the focus of the following sections.
The search for gastric stem cell markers revealed that there were several populations of active stem cells and reserve stem cells per gland, often with common genes marking populations in both the antrum and corpus. Homeostasis of the corpal epithelium is maintained by stem cells located in the highly proliferative isthmus region marked by Mist1, Runx1, Sox2 and Troy, although lineage tracing has been observed occasionally from Troy+ cells located in the base [32]. However, there is a population of reserve stem cells located in the base of the corpus gland which can give rise to whole corpus glands in response to injury which is marked by several genes including Mist1, Lgr5, Runx1, Sox2 and Troy [189,190,191,192]. Four stem cell markers have been identified in the antrum; Lgr5, Sox2, Lrig1 and CCKBR [103,119,189,193], which all contribute to homeostasis of this tissue. Below is a description of each of the gastric stem cell markers identified to date.
3.3. Lgr5
In 2010 Barker et al identified a small population of rapidly cycling cells that expressed the Wnt target gene Lgr5 at the base of antral glands [103]. To determine if this population of cells were stem cells they generated Lgr5-EGFP-IRES-CreERT2/Rosa26lacZ mice and performed lineage tracing in vivo. Lgr5+ cells were indeed able to give rise to all the differentiated cells of the antral epithelium, demonstrating they are long-lived multipotent stem cells of the gastric epithelium [88]. Lgr5+ gastric stem cells share similar proliferation kinetics with their intestinal counterparts, demonstrating a drift towards clonality and regular mitotic activity [194]. Isolated Lgr5+ stem cells form long-lived gastric organoids that closely resembled the antral epithelium, demonstrating this population of cells has potent stem cell qualities [88]. These gastric organoid cultures require Wnt3a and R-Spo in the culture medium highlighting that Wnt is needed to maintain the gastric epithelium [103,195].
Similar expression patterns were seen in adult human antrum via in-situ hybridization, suggesting LGR5 also marks gastric stem cells in humans [103]. However, transgenic silencing of the Lgr5-EGFP-IRES-CreERT2 locus resulted in variegated expression of this locus and thus limited the investigation into the full extent of Lgr5 as a stem cell marker. To overcome this, Leushacke et al. recently developed a non-variegated mouse model, Lgr5-2A-CreERT2, to allow further investigation of Lgr5. They were now able to detect that Lgr5 was also expressed in a sub-population of chief cells at the base of corpal glands [191]. Lineage tracing showed that although this population of cells do not give rise to fully traced glands during homeostasis, they were activated in response to damage, after which they could give rise to whole corpus glands [191]. This highlights Lgr5-expressing cells as reserve stem cells in the corpus but as more active stem cells in the antrum where they contribute to daily tissue homeostasis. Ablation of Lgr5+ cells in the corpus resulted in compromised tissue architecture and a substantial reduction in the capacity to form corpal stomach organoids [191]. Although these data indicate the importance of Lgr5 in regulating homeostasis in the corpus they also suggest that reserve stem cells are functioning to maintain corpus function when Lgr5 is deleted. This is consistent with the present model of several populations of stem cells co-operating during homeostasis and damage in the gastric epithelium.
3.4. RunX1
The transcription factor Runx1 is required for the generation of hematopoietic stem cells and endothelial cells [196], and is expressed in several epithelial tissues including breast [197] and skin [198]. Investigations into the expression of Runx1 in the gastric epithelium via in-situ hybridization (ISH) and immunofluorescence analyses on tissues from C57BL/6 mice revealed that it marks a population of highly proliferative cells located in the isthmus [199]. An element of the Runx1 promoter called eR1, was identified as an enhancer of Runx1 in hematopoietic stem cells [200,201]. Lineage tracing experiments on the gastric tissue from eR1-CreERT2;Rosa-LSL-tdTomato and eR1-CreERT2;Rosa-LSL-EYFP mice revealed that eR1 marked a population of stem cells in the isthmus of the corpus and the antrum [185]. These eR1+ stem cells continuously gave rise to differentiated cells to maintain the gastric unit as well as generated gastric organoid cultures in vitro when co-cultured with Wnt3a, EGF, Noggin and R-Spo. Rarer Runx1+ chief cells at the base of the corpus can also function as reserve stem cells that function, along with other populations of gastric stem cells, during repair [185].
3.5. Sox2
The transcription factor Sox2 is expressed in a variety of adult self-renewing epithelia including oesophageal, lung, intestinal and stomach [189]. Sox2-CreERT2;Rosa26-LSL-EYFP mice identified that Sox2 is expressed in the corpus and antrum of the gastric epithelium, with some clusters of EYFP expression demonstrating that some rare Sox2+ cells had expanded over the 4-day pulse period [175]. 15–22 months after the treatment fully traced glands in both the corpus and antrum were observed, suggesting that Sox2+ cells have the ability for self-renewal and can give rise to the mature cell types of the glandular stomach. The fully traced corpus glands were seen less frequently than the antrum glands which is consistent with the reported slower turnover rate of corpus cells (3–194 days) compared with antral cells (1–60 days).
Co-labelling experiments with antibodies that recognized known differentiated cell types of the stomach showed that Sox2+ cells were negative for all differentiation markers in the antrum and corpus and therefore are uncommitted stem cells. However, EYFP+ glands originating from lineage-traced Sox2+ cells 6 months after the treatment showed co-staining of EYFP+ cells with markers of enteroendocrine cells, mucus cells, parietal cells and chief cells. It can therefore be concluded that Sox2+ cells are multipotent stem cells in both the antrum and the corpus and the observation that labelled glands were still detectable up to 460 days after the treatment highlight Sox2+ cells self-renewing ability.
Since Barker et al demonstrated that Lgr5 cells are multipotent stem cells in the antrum, it was important to assess if there was any overlap in the expression patterns of Sox2 and Lgr5. IHC for GFP and Sox2 on sections of antrum tissue from Lgr5-GFP-IRES-CreERT2 mice showed no overlap in the expression of Lgr5 and Sox2, suggesting these genes mark district types of stem cells in the antrum. In the corpus, Troy expression was increased, whilst expression of Sox2 remained unchanged in Lgr5 depleted corpus, suggesting these two populations may contribute to Lgr5-independent renewal [191].
3.6. Lrig1
Leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1), an ErbB negative regulator, marks a population of multipotent cells in the corpus and antrum. Upon injury, these cells can proliferate to replace damaged gastric glands. Lineage tracing from Lrig1-CreERT2;R26RLacZ mice identified that Lrig1 marks a quiescent, long-lived intestinal stem cell population unique from Lgr5 [119]. Also, Lrig1 marks short- (24 h) and long-term (2 months) cells in the gastric epithelium. Further studies utilized an Lrig1-CreERT2;R26R-YFP mouse to demonstrate that Lrig1+ cells were located in the isthmus of the corpus, and towards the bottom, but not the base, of the antrum [202]. Lrig1 is co-expressed with a subset of proliferating cells in both the corpus and the antrum, and a subset of parietal cells in the corpus [188]. Lrig1+ cells could give rise to long term (1-year post recombination) gastric lineage epithelial cells in both the corpus and the antrum [188], illustrating it is a marker of stem cells during homeostasis and injury repair in the gastric epithelium.
Lrig1+ cells can also contribute to the recovery of gastric glands in the corpus after acute oxyntic injury induced by DMP-777, which is a parietal cell-specific protonophore. DMP-777 treatment of Lrig1-CreERT2;R26R-YFP mice identified that Lrig1+ cells gave rise to parietal cells (H/K-ATPase), neck cells (GS-II lectin), proliferating cells, and metaplastic cells (CD44v9) following tissue repair [188]. However, Lrig1 knockout mice treated for three days with DMP-777, were able to recover from acute gastric mucosal injury, suggesting that Lrig1 protein is not required for regeneration of gastric mucosa after oxyntic atrophy and lineage differentiation [188].
3.7. Troy
Tumour necrosis factor receptor superfamily, member 19 (TROY), is a member of the TNF-receptor superfamily. In the stomach, Troy was originally reported to label cells at the base of corpus glands and pulse labelling from Troy-eGFP-IRES-CreERT2 mice demonstrated that Troy was expressed in a small population of fully differentiated chief cells [192]. Although Troy+ chief cells do not proliferate, or if so very rarely, they can form organoids, suggesting they have stem cell properties in specific situations [178]. Indeed, lineage tracing in Troy-eGFP-IRES-CreERT2 mice showed that fully traced glands did originate from Troy+ chief cells but at a very slow rate, often taking up to 3 months or onward after recombination [178]. These data suggest Troy+ chief cells act as reserve stem cells in the corpus. In support of this, treatment of Troy-eGFP-IRES-CreERT2 mice with 5-fluorouracil (5-FU) to selectively kill proliferative cells in the isthmus, greatly enhanced the rate and number of lineage tracing events originating from Troy+ chief cells in the corpus base [178]. Whole-labelled glands have also been reported to originate from a rare population of Troy+ cells in the isthmus of the corpus [32], suggesting there are two populations of Troy+ cells in the corpus; one in the isthmus which is able to lineage trace during normal homeostasis [174], and another in the base of the glands which functions predominantly as a population of reserve stem cells during healing [203].
3.8. Mist1
Mist1 is a basic helix-loop-helix transcription factor. Using Mist1-CreERT2-R26-mTmG mice it was observed that Mist1 is expressed predominantly in chief cells of the lower two thirds of the corpus, with a rare population also observed in the isthmus [174]. Lineage tracing revealed bi-directional expansion from a single Mist1+ cells in the isthmus which gave rise to long term, fully traced glands containing all the differentiated cell types of the corpus [174], illustrating Mist1 marks stem cells located in the isthmus. Mist1-CreERT2;R26-Confetti mice revealed that Mist1+ isthmus cells gave rise to blocks of colour expansion, whilst recombination in chief cells did not, thus demonstrating that Mist1+ cells located in the isthmus are the predominant stem cell in the corpus rather than chief cells [174]. In support of this, isthmal Mist1+ cells were able to form gastric organoids while Mist1+ chief cells remained as single cells and did not form organoids. To determine if Mist1+ cells were giving rise to Lgr5+ cells when cultured for organoid growth, Lgr5 cells were first ablated in Mist1-CreERT2;Lgr5-DTR;R26-TdTomato mice, however this did not prevent robust organoid culture from isthmus Mist1+ cells highlighting Mist1+ cells are capable of self-renewal in the absence of Lgr5+ cells.
3.9. CCK2R
Cholecystokinin B receptor (CCK2R) is the receptor for gastrin and pro-gastrin which are expressed by G-cells in the antral stomach. CCK2R-CreERT-BAC mice revealed that CCK2R expressing cells were located at +3 to +7 position above the base of the antral gastric units [193]. Lineage tracing from CCK2R-CreERT2-BAC;Rosa26TdTomato mice revealed that CCK2R+ cells were able to give rise to full antral gastric units containing all differentiated cells, and were thus markers of antral stem cells [179]. In support of this, FACS sorted CCK2R+ cells could grow gastric organoids, whilst CCK2R negative cells failed to do so. CCK2R+ cells were negative (or very low) for Lgr5 expression, suggesting they are a distinct population, and are also located slightly above the Lgr5+ population and were more proliferative [179]. However, treatment of Lgr5neg or low/CCK2R+ gastric cells with progastrin resulted in rapid interconversion of these cells into Lgr5high cells, thus expanding the pool of active stem cells in the antrum.
3.10. How Wnt Signalling Regulates Gastric Stem Cells
Wnt signalling is well characterised as a critical signalling pathway regulating homeostasis and stem cell function in the intestine (Table 1). Among the first reports to demonstrate this were loss of stem cells and proliferative cells when the Wnt antagonist Dickkopf1 (Dkk1) was overexpressed in the intestine [89,90], and the failure of crypts to form in Tcf4−/− mice [16]. However, there is growing evidence that Wnt is also important in the gastric epithelium. Gastric organoid cultures require Wnt3a and R-Spo in the culture media [103,195] demonstrating that gastric stem cells require Wnt. Indeed, the first stem cell marker identified in the intestine, Lgr5, is also a marker of gastric stem cells, and has been identified as a Wnt target gene, and a co-receptor involved in Wnt signalling whereby it promotes Wnt signalling by associating with R-Spo to titrate RNF43/ZNRF3 and thus reduce the turnover of Fzd receptors on the cell surface [103,148]. These data highlight that Wnt signalling is active in the gastric epithelium, with further investigation identifying that Wnt signalling is higher in the antrum than the corpus [18], although Troy+ cells in the base of the corpus also express Wnt target genes and stem cell signature genes [192].
There is also evidence of co-operation between Wnt1 and the gastric stem cell marker, and Wnt target gene CD44. Overexpression of Wnt1 dramatically improves the spheroid formation of gastric cancer cell lines as well as enriches the side population cells with stem-like properties. Of interest, CD44 and β-catenin are upregulated in cells overexpressing Wnt1 [204]. Therefore, a subpopulation of gastric stem cells may be present whereby Wnt1 overexpression increases the relative prevalence of these cells. CD44 was also upregulated during reprogramming of chief cells, induced by deletion of Mist1 as they underwent SPEM, suggesting Wnt may be regulating this process [205]. However, CD44 is also regulated by other pathways in the gastric epithelium, including p-ErK–CD44–pStat3 signalling that has been demonstrated to regulate SPEM [191].
Detailed analysis of Wnt signalling in the epithelium of the antrum using in situ hybridization (ISH) showed that several Wnt ligands were expressed (Wnt2b, 3a, 4, 5a, 9a, 9b and 11), and all 10 Fzd receptors [29]. The expression patterns of some Fzd receptors was focused in specific areas of the gastric units, for example Fzd10 was expressed predominantly in the pit region, Fzd6 predominantly in the base, Fzd5 expression was markedly decreased in the gland base, whilst Fzd6 and Fzd7 expression was mainly confined to the bottom two thirds of the gastric unit [192]. These specific expression patterns suggest a distinct role for Wnt signalling in each area of the gastric unit, which has yet to be fully elucidated and will no doubt become clearer in future studies.
Using an Axin2-CreERT2 mouse and ISH, the location of canonical Wnt responsive cells in the antrum has recently been revealed to be constrained to the gland neck and base [192]. Axin2+ cells were undifferentiated, and lineage tracing revealed they could give rise to full glands containing differentiated cells, and thus marked a population of stem cells. Interestingly, only a proportion of Axin2+ cells co-express Lgr5, and Axin2+ cells were still able to lineage trace in Lgr5DTR mice in which Lgr5+ cells had been depleted, indicating two distinct populations of Wnt sensitive stem cells located in the antral gland base [192]. Axin2+/Lgr5− cell activity was regulated by R-Spo3 secreted by the underlying myofibroblasts in vivo, however, this was not observed for less proliferative Lgr5+ cells that were also insensitive to R-Spo1 or R-Spo3 treatment in vivo [192].
Despite evidence that Wnt signalling is active in the gastric epithelium, it has only recently been discovered which Fzd receptor transmits these signals. In addition to its expression in the intestinal epithelium, [14], we have recently shown that Fzd7 is also expressed in the antrum of the gastric epithelium and deletion of this receptor in vivo is deleterious and triggers rapid repopulation of the epithelium [18]. This was the first observation that the gastric epithelium has the capacity to repopulate following genetic insult, in this case deletion of Fzd7, and also the first demonstration that Wnt signalling is critical in the regulation of the antral epithelium [18]. Fzd7 can transmit signalling via the canonical and non-canonical pathways. However, in the gastric epithelium Fzd7 is most likely to be transmitting signals via the canonical pathways since gastric organoid death upon Fzd7 deletion could be rescued by activating the canonical Wnt pathway at the level of GSK3β [18]. The expression of several canonical Wnt target genes are reduced following Fzd7 deletion, however, this does not preclude that non-canonical signalling may still be involved in regulating gastric stem cells, and homeostasis. Indeed, Wnt5a regulates gastric tumour progression [206], whilst Wnt5a-Ror2 regulates mesenchymal stem cells to promote the growth of gastric cancer cells [207], suggesting this pathway may also regulate gastric stem cells. In the intestine, Fzd7 is required for the function of Lgr5+ cells [14]. However, although Fzd7 clearly regulates gastric homeostasis, the exact population of stem cells that require Fzd7 has yet to be identified.
3.11. The Oncogenic Role of Wnt Signalling in Gastric Cancer
Gastric cancer is a common malignancy with approximately 1,000,000 new cases diagnosed annually and is the third most common form of cancer-related death world-wide [208]. The largest proportion of cases are reported from East Asia, South America and Eastern Europe [209]. Gastric cancer is divided histopathologically into two classes: diffuse-type and intestinal-type [210]. However, these broad classifications are dwarfed by the complex pathogenic, environmental and genomic milieu that ultimately shape the characteristics of any given gastric lesion. In terms of medical interventions, surgical resection is the first option, but is only effective if gastric tumours are detected early, which unfortunately is seldom. Encouragingly, various therapies targeting essential signalling pathways have provided some therapeutic benefit to patients, but are often fraught with drug-acquired resistance and tumour relapse, highlighting the need to identify other druggable rate-limiting pathways utilized by gastric cancer cells. Wnt signalling is deregulated in gastric cancer at several points along the pathway including the ligand/receptor interface, regulatory proteins and transcriptional machinery. Moreover, the link between aberrant Wnt signalling and cancer is well established in many solid tumours including breast, liver and the colon. However, its functional relevance in gastric cancer is less well characterized. In the following section, we review the extent of Wnt signal deregulation in the stomach and the cellular and molecular consequences.
Chronic infection with the gram-negative bacteria Helicobacter pylori, which has evolved the capacity to survive in arguably the most hostile environment of the human body, the stomach, is a major risk factor for the development of gastric cancer [211]. Following the successful colonization of the stomach lumen, H. pylori migrates proximally to the stomach epithelium where it delivers bacterial virulence factors that modulate epithelial biology and inflammatory responses for its own benefit. Of the virulence factors produced by H. pylori, cytotoxin-associated gene product (CagA) and its associated type IV secretion system (T4SS) have been shown to activate Wnt signalling and promote gastric tumourigenesis and progression [211,212]. Thomas Meyer’s group demonstrate that virulent strains of H. pylori directly inject CagA into gastric epithelial cells, including Lgr5+ gastric stem cells [213]. Indeed, the number Lgr5+ gastric cells is significantly increased following infection with H. pylori, which was associated with an increase in Wnt signalling activity and enhanced clonogenic potential of ex vivo cultures [213]. Further refinement of previous host-pathogen interaction models reveal a novel population of Wnt responsive Axin2+ gastric stem cells significantly expand following H. pylori infection, which is associated with an increase in R-Spo3 signalling from subepithelial myofibroblasts [29]. Indeed, both R-SPO and LGR5 (the receptor for R-SPO) expression are positively correlated with poor patient survival and outcome [214,215]. Gastric epithelial cells infected with H. pylori induce the expression and subsequent activation of other Wnt receptor components such as Fzd [216] and Lrp [217], which propagate Wnt signalling and cell transformation. Collectively, these data provide a rational therapeutic foundation to target Wnt signalling at the level of the receptor complex in H. pylori infected gastric cancer cells.
Independent of H. pylori-mediated activation of Wnt signalling, mutation and epigenetic modification at multiple tiers of the Wnt pathway feature in subsets of gastric cancer patients [218]. For instance, LOF mutations to the E3 ubiquitin-protein ligase RNF43 are reported in 54% and 8% of MSI and microsattelite stable (MSS) gastric tumours respectively [219]. The functional consequence of RNF43 mutation allows for FZD receptor stabilization, hyper-sensitivity to available Wnt ligands and subsequent Wnt activation [151]. Diagnostically, RNF43 mutations occur in early-stage gastric lesions [220], which could be used to stratify patients who may benefit from compounds that block the secretion of Wnt ligands, which have substantial efficacy in other ‘Wnt-addicted’ RNF43 mutant cancers [221,222]. Another common mechanism to de-regulate Wnt signalling is via forced stabilization of β-catenin, which facilitates robust transcription of Wnt target genes [223], many of which regulate multiple hallmarks of cancer [224]. Stabilisation and accumulation of β-catenin can occur via truncating APC [219,225], which is a core component of the β-catenin destruction complex. Of note, allelic imbalance of APC can be detected in the blood and saliva of patients with gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) [226], which place APC as an excellent prognostic marker. Gastric tumours expressing nuclear β-catenin often harbor gain-of-function mutations in CTNNB1 (encoding β-catenin) [227,228], which permit β-catenin to escape proteosomal degradation and initiate transcription of target genes involved in cell proliferation and growth [229,230]. As such, targeted inhibition of β-catenin in gastric cancer cells is sufficient to inhibit Wnt signalling, cell proliferation and induce apoptosis via suppression of Survivin [231].
Other regulators of the Wnt pathway such as Dickkopf (DKK) and secreted Frizzled-related protein (sFRP) are epigenetically silenced via promoter hypermethylation in gastric cancer patients, which permit excessive Wnt activation. Importantly, treatment with de-methylating agents or exogenous expression of sFRP or Dkk is sufficient to arrest gastric cancer growth in vitro and in tumour xenografts [232,233,234]. Interestingly, potent anti-tumour effects of functional Dkk and/or sFRP are observed in gastric cancer cells with and without truncating mutations to APC, which provide compelling evidence that Wnt signalling can be further modulated in APC mutant gastric cancer cells.
Despite the low incidence of genetic lesions to Wnts and Fzd receptors reported in gastric cancer, aberrant expression of Wnts and Fzd receptors is often observed in gastric tumours [25,218]. The prototypical ‘non-canonical’ Wnt, Wnt5a, which is synonymous with Wnt/β-catenin inhibition and promotion of cell motility and migration [235], is overexpressed in gastric cancer and correlates with poor outcome [236]. Hayakawa and colleagues elegantly demonstrate Wnt5a, which is secreted from a perivascular niche, permit Apc/Kras mutant Mist1+ cells in the corpal stomach to circumvent anoikis, providing a permissive space for anchorage-independent growth. In support, Wnt5a-deficient bone marrow chimeras or treatment with polyclonal anti-Wnt5a antibody is sufficient to reduce gastric tumourigenesis [32,178]. Like its binding counterpart, Fzd receptors show de-regulated expression in gastric cancer patients [237], which is likely attributed to previously discussed Wnt-activating mutations. Of the ten mammalian FZDs, FZD7 is overexpressed in gastric cancer at various stages of disease progression [237,238,239], which reflects its promiscuous capability to transduce Wnt signalling via all major signalling arms [14,52]. Together, these data demonstrate gastric cancer cells commonly display aberrant levels of Wnt signalling, which is rate-limiting for efficient tumour growth and thus, warrants further investigation into safely targeting Wnt signalling in gastric cancer.
Functional proof that deregulated Wnt signalling can trigger gastric tumourigenesis was first demonstrated following genetic deletion of Gsk3-β in the mouse gastric epithelium, which developed intestinal type adenomas [103,240]. The Apc floxed allele has also been used to illustrate that gastric tumourigenesis can be triggered upon deregulation of Wnt signalling, however, Mist1Cre;Apcfl/fl mice only develop tumours in the antrum [241], despite robust recombination and deregulation of Wnt signalling in both the corpus and antrum [32], highlighting that the antrum is more sensitive to Wnt signalling. Sox2Cre;Apcfl/fl mice develop gastric tumours in the corpus and antrum, suggesting that each stem cell population in the gastric epithelium has a distinct response to this level of deregulated Wnt signalling, as gastric tumourigenesis was not observed in the corpus of Mist1Cre;Apcfl/fl mice [25]. Gastric tumours did develop in the corpus of Mist1Cre;Apcfl/fl;KrasG12D mice and TroyCre;Apcfl/fl;KrasG12D illustrating a co-operation between Wnt and Kras in gastric tumourigenesis, and that stem cells are the cell of origin in the gastric epithelium [32]. Indeed, Lrig1Cre;Apcfl/+ mice also developed gastric tumours in the antrum, as well as the intestine [242].
4. Summary
There is currently a vast body of data highlighting the critical role of Wnt signalling in regulating many functions of stem cells in the gastric and intestinal epithelium, including survival, proliferation and clonality. These are also cell functions which are hijacked via aberrant Wnt signalling during transformation by oncogenes or loss of tumour suppressors, which has also been well documented in gastric and intestinal cancer, highlighting the importance for strict regulation of the Wnt pathway to prevent pathologies. It also highlights the great potential to harness these regulatory properties to enable scientists and clinicians to manipulate cell function to their own specific needs. For example, a transient increase in proliferation could greatly aid regenerative medicine [243], whilst well defined Wnt activity is critical for the culture of organoids and tumour organoids from several tissues including the stomach and intestine [244]. To fully understand how Wnt signalling regulates stem cells, and thus triggers pathologies, current and future projects are trying to elucidate the complex interactions between the different stem cell populations and how signalling pathways interact with each other to regulate these functions [245]. A good barometer of the journey left to travel on this research path is the relatively few specific roles that can be assigned to a single Wnt ligand or receptor which will eventually illuminate the full extent of how this pathway network regulates this import population of cells.
Acknowledgments
The authors wish to thank Gavan Mitchell for helping generate the figures in this manuscript. Funding is gratefully acknowledged from the following; National Health and Medical Research Council of Australia (NHMRC) project grants (566679 and APP1099302 awarded to E.V.); Melbourne Health project grants (605030 and PG-002 awarded to E.V./T.J.P.), and an Early Career Researcher grant (GIA-033) awarded to D.J.F.; Cancer Council of Victoria project grants (CCV, APP1020716) awarded to E.V. and T.J.P., CCV Fellowship awarded to D.J.F. and Cardiff University Research Fellowship awarded to T.J.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blanpain C., Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/S0092-8674(00)81692-X. [DOI] [PubMed] [Google Scholar]
- 3.Choumerianou D.M., Dimitriou H., Kalmanti M. Stem cells: Promises versus limitations. Tissue Eng. Part B Rev. 2008;14:53–60. doi: 10.1089/teb.2007.0216. [DOI] [PubMed] [Google Scholar]
- 4.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 5.Li L., Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tetteh P.W., Farin H.F., Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015;25:100–108. doi: 10.1016/j.tcb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Visvader J.E. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 8.Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 9.Phesse T.J., Buchert M., Stuart E., Flanagan D.J., Faux M., Afshar-Sterle S., Walker F., Zhang H.H., Nowell C.J., Jorissen R., et al. Partial inhibition of gp130-Jak-Stat3 signaling prevents Wnt-β-catenin-mediated intestinal tumor growth and regeneration. Sci. Signal. 2014;7:ra92. doi: 10.1126/scisignal.2005411. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/S0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 11.Doupe D.P., Alcolea M.P., Roshan A., Zhang G., Klein A.M., Simons B.D., Jones P.H. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa Y., Sethi N., Sepulveda A.R., Bass A.J., Wang T.C. Oesophageal adenocarcinoma and gastric cancer: Should we mind the gap? Nat. Rev. 2016;16:305–318. doi: 10.1038/nrc.2016.24. [DOI] [PubMed] [Google Scholar]
- 13.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan D.J., Phesse T.J., Barker N., Schwab R.H.M., Amin N., Malaterre J., Stange D.E., Nowell C.J., Currie S.A., Saw J.T.S., et al. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells. Stem Cell Rep. 2015;4:759–767. doi: 10.1016/j.stemcr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Aliyev J., Wu Y., Bunte R., Rossant J., et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 16.Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P.J., Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 17.Sansom O.J., Reed K.R., Hayes A.J., Ireland H., Brinkmann H., Newton I.P., Batlle E., Simon-Assmann P., Clevers H., Nathke I.S., et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan D.J., Barker N., Nowell C., Clevers H., Ernst M., Phesse T.J., Vincan E. Loss of the Wnt receptor Frizzled7 in the gastric epithelium is deleterious and triggers rapid repopulation in vivo. Dis. Model. Mech. 2017 doi: 10.1242/dmm.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Amerongen R., Bowman A.N., Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Burke Z.D., Reed K.R., Phesse T.J., Sansom O.J., Clarke A.R., Tosh D. Liver zonation occurs through a β-catenin-dependent, c-myc-independent mechanism. Gastroenterology. 2009;136:2316–2324. doi: 10.1053/j.gastro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 21.Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 22.Nusslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 23.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 25.Mao J., Fan S., Ma W., Fan P., Wang B., Zhang J., Wang H., Tang B., Zhang Q., Yu X., et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int. J. Oncol. 2001;19:1003–1007. doi: 10.3892/ijo.19.5.1003. [DOI] [PubMed] [Google Scholar]
- 27.Suh H.N., Kim M.J., Jung Y.S., Lien E.M., Jun S., Park J.I. Quiescence exit of Tert(+) stem cells by Wnt/β-Catenin is indispensable for intestinal regeneration. Cell Rep. 2017;21:2571–2584. doi: 10.1016/j.celrep.2017.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Sigal M., Logan C.Y., Kapalczynska M., Mollenkopf H.J., Berger H., Wiedenmann B., Nusse R., Amieva M.R., Meyer T.F. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451–455. doi: 10.1038/nature23642. [DOI] [PubMed] [Google Scholar]
- 30.Qi L., Sun B., Liu Z., Cheng R., Li Y., Zhao X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J. Exp. Clin. Cancer Res. 2014;33:107. doi: 10.1186/s13046-014-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi H., Ajima R., Luo C.T., Yamaguchi T.P., Stappenbeck T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa Y., Ariyama H., Stancikova J., Sakitani K., Asfaha S., Renz B.W., Dubeykovskaya Z.A., Shibata W., Wang H., Westphalen C.B., et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregorieff A., Pinto D., Begthel H., Destree O., Kielman M., Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Yuan G., Regel I., Lian F., Friedrich T., Hitkova I., Hofheinz R.D., Strobel P., Langer R., Keller G., Rocken C., et al. WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene. 2013;32:375–387. doi: 10.1038/onc.2012.40. [DOI] [PubMed] [Google Scholar]
- 35.An C.H., Kim S.S., Kang M.R., Kim Y.R., Kim H.S., Yoo N.J., Lee S.H. Frameshift mutations of ATBF1, WNT9A, CYLD and PARK2 in gastric and colorectal carcinomas with high microsatellite instability. Pathology. 2010;42:583–585. doi: 10.3109/00313025.2010.508735. [DOI] [PubMed] [Google Scholar]
- 36.Wu X.D., Bie Q.L., Zhang B., Yan Z.H., Han Z.J. Wnt10B is critical for the progression of gastric cancer. Oncol. Lett. 2017;13:4231–4237. doi: 10.3892/ol.2017.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishioka M., Ueno K., Hazama S., Okada T., Sakai K., Suehiro Y., Okayama N., Hirata H., Oka M., Imai K., et al. Possible involvement of Wnt11 in colorectal cancer progression. Mol. Carcinog. 2013;52:207–217. doi: 10.1002/mc.21845. [DOI] [PubMed] [Google Scholar]
- 38.Norollahi S.E., Alipour M., Rashidy-Pour A., Samadani A.A., Larijani L.V. Regulatory Fluctuation of WNT16 Gene Expression Is Associated with Human Gastric Adenocarcinoma. J. Gastrointest. Cancer. 2017 doi: 10.1007/s12029-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 39.Adler P.N., Vinson C., Park W.J., Conover S., Klein L. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics. 1990;126:401–416. doi: 10.1093/genetics/126.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinson C.R., Adler P.N. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 41.Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cell. 2004;13:149–156. doi: 10.1016/S1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 42.Wehrli M., Dougan S.T., Caldwell K., O’Keefe L., Schwartz S., Vaizel-Ohayon D., Schejter E., Tomlinson A., DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35071131. [DOI] [PubMed] [Google Scholar]
- 43.Janda C.Y., Waghray D., Levin A.M., Thomas C., Garcia K.C. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li V.S., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J., Maurice M.M., Mahmoudi T., et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Potten C.S., Wilson J.W., Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 46.Potten C.S., Booth D., Haley J.D. Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br. J. Cancer. 1997;75:1454–1459. doi: 10.1038/bjc.1997.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walck-Shannon E., Hardin J. Cell intercalation from top to bottom. Nat. Rev. Mol. Cell Biol. 2014;15:34–48. doi: 10.1038/nrm3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vladar E.K., Antic D., Axelrod J.D. Planar cell polarity signaling: The developing cell’s compass. Cold Spring Harb. Perspect. Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebbagh M., Borg J.P. Insight into planar cell polarity. Exp. Cell Res. 2014;328:284–295. doi: 10.1016/j.yexcr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Anastas J.N., Moon R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 51.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B., Koshida I., Suzuki K., Yamada G., Schwabe G.C., et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 52.Phesse T., Flanagan D., Vincan E. Frizzled7: A promising Achilles’ heel for targeting the Wnt receptor complex to treat cancer. Cancers. 2016;8 doi: 10.3390/cancers8050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohkawara B., Glinka A., Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell. 2011;20:303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Puvirajesinghe T.M., Bertucci F., Jain A., Scerbo P., Belotti E., Audebert S., Sebbagh M., Lopez M., Brech A., Finetti P., et al. Identification of p62/SQSTM1 as a component of non-canonical Wnt VANGL2-JNK signalling in breast cancer. Nat. Commun. 2016;7:10318. doi: 10.1038/ncomms10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi T., Yanagisawa K., Sugiyama R., Hosono Y., Shimada Y., Arima C., Kato S., Tomida S., Suzuki M., Osada H., et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S., Chen L., Cui B., Chuang H.Y., Yu J., Wang-Rodriguez J., Tang L., Chen G., Basak G.W., Kipps T.J. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gentile A., Lazzari L., Benvenuti S., Trusolino L., Comoglio P.M. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71:3132–3141. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 58.Rasmussen N.R., Debebe Z., Wright T.M., Brooks S.A., Sendor A.B., Brannon A.R., Hakimi A.A., Hsieh J.J., Choueiri T.K., Tamboli P., et al. Expression of Ror2 mediates invasive phenotypes in renal cell carcinoma. PLoS ONE. 2014;9:e116101. doi: 10.1371/journal.pone.0116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slusarski D.C., Yang-Snyder J., Busa W.B., Moon R.T. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 60.Sheldahl L.C., Park M., Malbon C.C., Moon R.T. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 1999;9:695–698. doi: 10.1016/S0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 61.Kreusser M.M., Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acebron S.P., Niehrs C. β-Catenin-independent roles of Wnt/LRP6 signalling. Trends Cell Biol. 2016;26:956–967. doi: 10.1016/j.tcb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Acebron S.P., Karaulanov E., Berger B.S., Huang Y.L., Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell. 2014;54:663–674. doi: 10.1016/j.molcel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Ishitani T., Ninomiya-Tsuji J., Nagai S., Nishita M., Meneghini M., Barker N., Waterman M., Bowerman B., Clevers H., Shibuya H., et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 65.Ishitani T., Kishida S., Hyodo-Miura J., Ueno N., Yasuda J., Waterman M., Shibuya H., Moon R.T., Ninomiya-Tsuji J., Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/β-catenin signaling. Mol. Cell. Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mikels A.J., Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grumolato L., Liu G., Mong P., Mudbhary R., Biswas R., Arroyave R., Vijayakumar S., Economides A.N., Aaronson S.A. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P.J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincan E., Swain R.K., Brabletz T., Steinbeisser H. Frizzled7 dictates embryonic morphogenesis: Implications for colorectal cancer progression. Front. Biosci. J. Virtual Lib. 2007;12:4558–4567. doi: 10.2741/2410. [DOI] [PubMed] [Google Scholar]
- 70.Ueno K., Hazama S., Mitomori S., Nishioka M., Suehiro Y., Hirata H., Oka M., Imai K., Dahiya R., Hinoda Y. Down-regulation of Frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br. J. Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holcombe R.F., Marsh J.L., Waterman M.L., Lin F., Milovanovic T., Truong T. Expression of Wnt ligands and Frizzled receptors in colonic mucosa and in colon carcinoma. Mol. Pathol. 2002;55:220–226. doi: 10.1136/mp.55.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan L., Du Q., Yao J., Liu R. ROR2 inhibits the proliferation of gastric carcinoma cells via activation of non-canonical Wnt signaling. Exp. Ther. Med. 2016;12:4128–4134. doi: 10.3892/etm.2016.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barker N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 74.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Pinto D., Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Potten C.S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 77.Rothenberg M.E., Nusse Y., Kalisky T., Lee J.J., Dalerba P., Scheeren F., Lobo N., Kulkarni S., Sim S., Qian D., et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142:1195–1205. doi: 10.1053/j.gastro.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki N., Sachs N., Wiebrands K., Ellenbroek S.I., Fumagalli A., Lyubimova A., Begthel H., van den Born M., van Es J.H., Karthaus W.R., et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. USA. 2016;113:E5399–E5407. doi: 10.1073/pnas.1607327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ireland H., Houghton C., Howard L., Winton D.J. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn. 2005;233:1332–1336. doi: 10.1002/dvdy.20446. [DOI] [PubMed] [Google Scholar]
- 80.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su L.K., Kinzler K.W., Vogelstein B., Preisinger A.C., Moser A.R., Luongo C., Gould K.A., Dove W.F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 82.Kinzler K.W., Nilbert M.C., Vogelstein B., Bryan T.M., Levy D.B., Smith K.J., Preisinger A.C., Hamilton S.R., Hedge P., Markham A., et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 83.Rubinfeld B., Souza B., Albert I., Muller O., Chamberlain S.H., Masiarz F.R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 84.Korinek V., Barker N., Morin P.J., van Wichen D., de Weger R., Kinzler K.W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 85.Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K.W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 86.Sauer B., Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe H., Enjoji M., Yao T., Ohsato K. Gastric lesions in familial adenomatosis coli: Their incidence and histologic analysis. Hum. Pathol. 1978;9:269–283. doi: 10.1016/S0046-8177(78)80085-9. [DOI] [PubMed] [Google Scholar]
- 88.Ireland H., Kemp R., Houghton C., Howard L., Clarke A.R., Sansom O.J., Winton D.J. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: Effect of loss of β-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 89.Kuhnert F., Davis C.R., Wang H.T., Chu P., Lee M., Yuan J., Nusse R., Kuo C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Es J.H., Jay P., Gregorieff A., van Gijn M.E., Jonkheer S., Hatzis P., Thiele A., van den Born M., Begthel H., Brabletz T., et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 92.Van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., van der Horn K., Batlle E., Coudreuse D., Haramis A.P., et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 93.Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 94.Bjerknes M., Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/S0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 95.Potten C.S. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat. Res. 2004;161:123–136. doi: 10.1667/RR3104. [DOI] [PubMed] [Google Scholar]
- 96.Potten C.S., Gandara R., Mahida Y.R., Loeffler M., Wright N.A. The stem cells of small intestinal crypts: Where are they? Cell Prolif. 2009;42:731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 98.Van der Flier L.G., Sabates-Bellver J., Oving I., Haegebarth A., De Palo M., Anti M., Van Gijn M.E., Suijkerbuijk S., Van de Wetering M., Marra G., et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 99.Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 100.Ritsma L., Ellenbroek S.I.J., Zomer A., Snippert H.J., de Sauvage F.J., Simons B.D., Clevers H., van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 102.Jaks V., Barker N., Kasper M., van Es J.H., Snippert H.J., Clevers H., Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 103.Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Ng A., Tan S., Singh G., Rizk P., Swathi Y., Tan T.Z., Huang R.Y., Leushacke M., Barker N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014;16:745–757. doi: 10.1038/ncb3000. [DOI] [PubMed] [Google Scholar]
- 105.Barker N., Rookmaaker M.B., Kujala P., Ng A., Leushacke M., Snippert H., van de Wetering M., Tan S., Van Es J.H., Huch M., et al. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012;2:540–552. doi: 10.1016/j.celrep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 106.Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Visser K.E., Ciampricotti M., Michalak E.M., Tan D.W., Speksnijder E.N., Hau C.S., Clevers H., Barker N., Jonkers J. Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J. Pathol. 2012;228:300–309. doi: 10.1002/path.4096. [DOI] [PubMed] [Google Scholar]
- 108.Huch M., Bonfanti P., Boj S.F., Sato T., Loomans C.J., van de Wetering M., Sojoodi M., Li V.S., Schuijers J., Gracanin A., et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chai R., Kuo B., Wang T., Liaw E.J., Xia A., Jan T.A., Liu Z., Taketo M.M., Oghalai J.S., Nusse R., et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yee K.K., Li Y., Redding K.M., Iwatsuki K., Margolskee R.F., Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 2013;31:992–1000. doi: 10.1002/stem.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munoz J., Stange D.E., Schepers A.G., van de Wetering M., Koo B.K., Itzkovitz S., Volckmann R., Kung K.S., Koster J., Radulescu S., et al. The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuijers J., Junker J.P., Mokry M., Hatzis P., Koo B.K., Sasselli V., van der Flier L.G., Cuppen E., van Oudenaarden A., Clevers H. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 2015;16:158–170. doi: 10.1016/j.stem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 113.van der Flier L.G., van Gijn M.E., Hatzis P., Kujala P., Haegebarth A., Stange D.E., Begthel H., van den Born M., Guryev V., Oving I., et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 114.Jubb A.M., Chalasani S., Frantz G.D., Smits R., Grabsch H.I., Kavi V., Maughan N.J., Hillan K.J., Quirke P., Koeppen H. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene. 2006;25:3445–3457. doi: 10.1038/sj.onc.1209382. [DOI] [PubMed] [Google Scholar]
- 115.Reed K.R., Tunster S.J., Young M., Carrico A., John R.M., Clarke A.R. Entopic overexpression of Ascl2 does not accelerate tumourigenesis in ApcMin mice. Gut. 2012;61:1435–1438. doi: 10.1136/gutjnl-2011-300842. [DOI] [PubMed] [Google Scholar]
- 116.Flanagan J.G., Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 117.Batlle E., Henderson J.T., Beghtel H., van den Born M.M., Sancho E., Huls G., Meeldijk J., Robertson J., van de Wetering M., Pawson T., et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/S0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 118.Merlos-Suarez A., Barriga F.M., Jung P., Iglesias M., Cespedes M.V., Rossell D., Sevillano M., Hernando-Momblona X., da Silva-Diz V., Munoz P., et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 119.Powell A.E., Wang Y., Li Y., Poulin E.J., Means A.L., Washington M.K., Higginbotham J.N., Juchheim A., Prasad N., Levy S.E., et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montgomery R.K., Carlone D.L., Richmond C.A., Farilla L., Kranendonk M.E., Henderson D.E., Baffour-Awuah N.Y., Ambruzs D.M., Fogli L.K., Algra S., et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan K.S., Chia L.A., Li X., Ootani A., Su J., Lee J.Y., Su N., Luo Y., Heilshorn S.C., Amieva M.R., et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Itzkovitz S., Lyubimova A., Blat I.C., Maynard M., van Es J., Lees J., Jacks T., Clevers H., van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 2012;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jadhav U., Saxena M., O’Neill N.K., Saadatpour A., Yuan G.C., Herbert Z., Murata K., Shivdasani R.A. Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells. Cell Stem Cell. 2017;21:65–77. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan K.S., Gevaert O., Zheng G.X.Y., Anchang B., Probert C.S., Larkin K.A., Davies P.S., Cheng Z.F., Kaddis J.S., Han A., et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21:78–90. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Breault D.T., Min I.M., Carlone D.L., Farilla L.G., Ambruzs D.M., Henderson D.E., Algra S., Montgomery R.K., Wagers A.J., Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc. Natl. Acad. Sci. USA. 2008;105:10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rudolph K.L., Chang S., Lee H.W., Blasco M., Gottlieb G.J., Greider C., DePinho R.A. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/S0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 129.Ljuslinder I., Golovleva I., Palmqvist R., Oberg A., Stenling R., Jonsson Y., Hedman H., Henriksson R., Malmer B. LRIG1 expression in colorectal cancer. Acta Oncol. 2007;46:1118–1122. doi: 10.1080/02841860701426823. [DOI] [PubMed] [Google Scholar]
- 130.Miller J.K., Shattuck D.L., Ingalla E.Q., Yen L., Borowsky A.D., Young L.J., Cardiff R.D., Carraway K.L., 3rd., Sweeney C. Suppression of the negative regulator LRIG1 contributes to ErbB2 overexpression in breast cancer. Cancer Res. 2008;68:8286–8294. doi: 10.1158/0008-5472.CAN-07-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jensen K.B., Collins C.A., Nascimento E., Tan D.W., Frye M., Itami S., Watt F.M. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wong V.W., Stange D.E., Page M.E., Buczacki S., Wabik A., Itami S., van de Wetering M., Poulsom R., Wright N.A., Trotter M.W., et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat. Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bernal N.P., Stehr W., Zhang Y., Profitt S., Erwin C.R., Warner B.W. Evidence for active Wnt signaling during postresection intestinal adaptation. J. Pediatr. Surg. 2005;40:1025–1029. doi: 10.1016/j.jpedsurg.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 134.Withers H.R. Regeneration of intestinal mucosa after irradiation. Cancer. 1971;28:75–81. doi: 10.1002/1097-0142(197107)28:1<75::AID-CNCR2820280115>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 135.Metcalfe C., Kljavin N.M., Ybarra R., de Sauvage F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Es J.H., Sato T., van de Wetering M., Lyubimova A., Nee A.N., Gregorieff A., Sasaki N., Zeinstra L., van den Born M., Korving J., et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buczacki S.J., Zecchini H.I., Nicholson A.M., Russell R., Vermeulen L., Kemp R., Winton D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 139.Tetteh P.W., Basak O., Farin H.F., Wiebrands K., Kretzschmar K., Begthel H., van den Born M., Korving J., de Sauvage F., van Es J.H., et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 140.Muncan V., Sansom O.J., Tertoolen L., Phesse T.J., Begthel H., Sancho E., Cole A.M., Gregorieff A., de Alboran I.M., Clevers H., et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol. 2006;26:8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim K.A., Kakitani M., Zhao J., Oshima T., Tang T., Binnerts M., Liu Y., Boyle B., Park E., Emtage P., et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 142.Zhou W.J., Geng Z.H., Spence J.R., Geng J.G. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature. 2013;501:107–111. doi: 10.1038/nature12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holik A.Z., Krzystyniak J., Young M., Richardson K., Jarde T., Chambon P., Shorning B.Y., Clarke A.R. Brg1 is required for stem cell maintenance in the murine intestinal epithelium in a tissue-specific manner. Stem Cells. 2013;31:2457–2466. doi: 10.1002/stem.1498. [DOI] [PubMed] [Google Scholar]
- 144.Mahmoudi T., Li V.S., Ng S.S., Taouatas N., Vries R.G., Mohammed S., Heck A.J., Clevers H. The kinase TNIK is an essential activator of Wnt target genes. EMBO J. 2009;28:3329–3340. doi: 10.1038/emboj.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Durand A., Donahue B., Peignon G., Letourneur F., Cagnard N., Slomianny C., Perret C., Shroyer N.F., Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc. Natl. Acad. Sci. USA. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Valenta T., Degirmenci B., Moor A.E., Herr P., Zimmerli D., Moor M.B., Hausmann G., Cantu C., Aguet M., Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15:911–918. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 147.Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V., de Punder K., Angers S., Peters P.J., Maurice M.M., et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 148.De Lau W., Barker N., Low T.Y., Koo B.K., Li V.S., Teunissen H., Kujala P., Haegebarth A., Peters P.J., van de Wetering M., et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 149.Carmon K.S., Gong X., Lin Q., Thomas A., Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Glinka A., Dolde C., Kirsch N., Huang Y.L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C.M., Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koo B.K., Spit M., Jordens I., Low T.Y., Stange D.E., van de Wetering M., van Es J.H., Mohammed S., Heck A.J., Maurice M.M., et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 152.Hao H.X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 153.Barry E.R., Morikawa T., Butler B.L., Shrestha K., de la Rosa R., Yan K.S., Fuchs C.S., Magness S.T., Smits R., Ogino S., et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan K.S., Janda C.Y., Chang J., Zheng G.X.Y., Larkin K.A., Luca V.C., Chia L.A., Mah A.T., Han A., Terry J.M., et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Polakis P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vogelstein B., Kinzler K.W. The path to cancer—Three strikes and you’re out. N. Engl. J. Med. 2015;373:1895–1898. doi: 10.1056/NEJMp1508811. [DOI] [PubMed] [Google Scholar]
- 157.Kinzler K.W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 158.Liu W., Dong X., Mai M., Seelan R.S., Taniguchi K., Krishnadath K.K., Halling K.C., Cunningham J.M., Boardman L.A., Qian C., et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat. Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 159.Albuquerque C., Breukel C., van der Luijt R., Fidalgo P., Lage P., Slors F.J., Leitao C.N., Fodde R., Smits R. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum. Mol. Genet. 2002;11:1549–1560. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 160.Leedham S.J., Rodenas-Cuadrado P., Howarth K., Lewis A., Mallappa S., Segditsas S., Davis H., Jeffery R., Rodriguez-Justo M., Keshav S., et al. A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut. 2013;62:83–93. doi: 10.1136/gutjnl-2011-301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lewis A., Segditsas S., Deheragoda M., Pollard P., Jeffery R., Nye E., Lockstone H., Davis H., Clark S., Stamp G., et al. Severe polyposis in Apc(1322T) mice is associated with submaximal Wnt signalling and increased expression of the stem cell marker Lgr5. Gut. 2010;59:1680–1686. doi: 10.1136/gut.2009.193680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sansom O.J., Meniel V.S., Muncan V., Phesse T.J., Wilkins J.A., Reed K.R., Vass J.K., Athineos D., Clevers H., Clarke A.R. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 163.Dow L.E., O’Rourke K.P., Simon J., Tschaharganeh D.F., van Es J.H., Clevers H., Lowe S.W. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161:1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giannakis M., Hodis E., Jasmine Mu X., Yamauchi M., Rosenbluh J., Cibulskis K., Saksena G., Lawrence M.S., Qian Z.R., Nishihara R., et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014;46:1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seshagiri S., Stawiski E.W., Durinck S., Modrusan Z., Storm E.E., Conboy C.B., Chaudhuri S., Guan Y., Janakiraman V., Jaiswal B.S., et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Storm E.E., Durinck S., de Sousa e Melo F., Tremayne J., Kljavin N., Tan C., Ye X., Chiu C., Pham T., Hongo J.A., et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- 167.Preston S.L., Wong W.M., Chan A.O., Poulsom R., Jeffery R., Goodlad R.A., Mandir N., Elia G., Novelli M., Bodmer W.F., et al. Bottom-up histogenesis of colorectal adenomas: Origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 2003;63:3819–3825. [PubMed] [Google Scholar]
- 168.de Sousa e Melo F., Kurtova A.V., Harnoss J.M., Kljavin N., Hoeck J.D., Hung J., Anderson J.E., Storm E.E., Modrusan Z., Koeppen H., et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543:676–680. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 169.Shimokawa M., Ohta Y., Nishikori S., Matano M., Takano A., Fujii M., Date S., Sugimoto S., Kanai T., Sato T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545:187–192. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 170.Vermeulen L., De Sousa E.M.F., van der Heijden M., Cameron K., de Jong J.H., Borovski T., Tuynman J.B., Todaro M., Merz C., Rodermond H., et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 171.Schwitalla S., Fingerle A.A., Cammareri P., Nebelsiek T., Goktuna S.I., Ziegler P.K., Canli O., Heijmans J., Huels D.J., Moreaux G., et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 172.Westphalen C.B., Asfaha S., Hayakawa Y., Takemoto Y., Lukin D.J., Nuber A.H., Brandtner A., Setlik W., Remotti H., Muley A., et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cammareri P., Vincent D.F., Hodder M.C., Ridgway R.A., Murgia C., Nobis M., Campbell A.D., Varga J., Huels D.J., Subramani C., et al. TGFβ pathway limits dedifferentiation following WNT and MAPK pathway activation to suppress intestinal tumourigenesis. Cell Death Differ. 2017;24:1681–1693. doi: 10.1038/cdd.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Biswas S., Davis H., Irshad S., Sandberg T., Worthley D., Leedham S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J. Pathol. 2015;237:135–145. doi: 10.1002/path.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim T.H., Shivdasani R.A. Stomach development, stem cells and disease. Development. 2016;143:554–565. doi: 10.1242/dev.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Beauchamp R.D., Barnard J.A., McCutchen C.M., Cherner J.A., Coffey R.J., Jr. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J. Clin. Investig. 1989;84:1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramsey V.G., Doherty J.M., Chen C.C., Stappenbeck T.S., Konieczny S.F., Mills J.C. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 178.Hanaki H., Yamamoto H., Sakane H., Matsumoto S., Ohdan H., Sato A., Kikuchi A. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol. Cancer Ther. 2012;11:298–307. doi: 10.1158/1535-7163.MCT-11-0682. [DOI] [PubMed] [Google Scholar]
- 179.Karam S.M. Cell lineage relationship in the stomach of normal and genetically manipulated mice. Braz. J. Med. Biol. Res. 1998;31:271–279. doi: 10.1590/S0100-879X1998000200010. [DOI] [PubMed] [Google Scholar]
- 180.Lee E.R. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am. J. Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- 181.Lee E.R., Leblond C.P. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am. J. Anat. 1985;172:241–259. doi: 10.1002/aja.1001720306. [DOI] [PubMed] [Google Scholar]
- 182.Mills J.C., Shivdasani R.A. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goldenring J.R., Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G999–G1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 184.Nam K.T., Lee H.J., Sousa J.F., Weis V.G., O’Neal R.L., Finke P.E., Romero-Gallo J., Shi G., Mills J.C., Peek R.M., Jr., et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huh W.J., Khurana S.S., Geahlen J.H., Kohli K., Waller R.A., Mills J.C. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mills J.C., Goldenring J.R. Metaplasia in the Stomach Arises from Gastric Chief Cells. Cell. Mol. Gastroenterol. Hepatol. 2017;4:85–88. doi: 10.1016/j.jcmgh.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lennerz J.K., Kim S.H., Oates E.L., Huh W.J., Doherty J.M., Tian X., Bredemeyer A.J., Goldenring J.R., Lauwers G.Y., Shin Y.K., et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am. J. Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Karam S.M., Leblond C.P. Identifying and counting epithelial cell types in the "corpus" of the mouse stomach. Anat. Rec. 1992;232:231–246. doi: 10.1002/ar.1092320208. [DOI] [PubMed] [Google Scholar]
- 189.Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Laoukili J., Kooistra M.R., Bras A., Kauw J., Kerkhoven R.M., Morrison A., Clevers H., Medema R.H. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 191.Leushacke M., Tan S.H., Wong A., Swathi Y., Hajamohideen A., Tan L.T., Barker N. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 2017;19:774. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 192.Stange D.E., Koo B.K., Huch M., Sibbel G., Basak O., Lyubimova A., Kujala P., Bartfeld S., Koster J., Geahlen J.H., et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hayakawa Y., Jin G., Wang H., Chen X., Westphalen C.B., Asfaha S., Renz B.W., Ariyama H., Dubeykovskaya Z.A., Takemoto Y., et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Leushacke M., Ng A., Galle J., Loeffler M., Barker N. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 2013;5:349–356. doi: 10.1016/j.celrep.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 195.Flanagan D.J., Schwab R.H., Tran B.M., Phesse T.J., Vincan E. Isolation and Culture of Adult Intestinal, Gastric, and Liver Organoids for Cre-recombinase-Mediated Gene Deletion. Methods Mol. Biol. 2016 doi: 10.1007/7651_2016_14. [DOI] [PubMed] [Google Scholar]
- 196.Chen M.J., Yokomizo T., Zeigler B.M., Dzierzak E., Speck N.A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Banerji S., Cibulskis K., Rangel-Escareno C., Brown K.K., Carter S.L., Frederick A.M., Lawrence M.S., Sivachenko A.Y., Sougnez C., Zou L., et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Osorio K.M., Lilja K.C., Tumbar T. Runx1 modulates adult hair follicle stem cell emergence and maintenance from distinct embryonic skin compartments. J. Cell Biol. 2011;193:235–250. doi: 10.1083/jcb.201006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Matsuo J., Kimura S., Yamamura A., Koh C.P., Hossain M.Z., Heng D.L., Kohu K., Voon D.C., Hiai H., Unno M., et al. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 200.Ng C.E., Yokomizo T., Yamashita N., Cirovic B., Jin H., Wen Z., Ito Y., Osato M. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells. 2010;28:1869–1881. doi: 10.1002/stem.507. [DOI] [PubMed] [Google Scholar]
- 201.Nottingham W.T., Jarratt A., Burgess M., Speck C.L., Cheng J.F., Prabhakar S., Rubin E.M., Li P.S., Sloane-Stanley J., Kong A.S.J., et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007;110:4188–4197. doi: 10.1182/blood-2007-07-100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Choi E., Lantz T.L., Vlacich G., Keeley T.M., Samuelson L.C., Coffey R.J., Goldenring J.R., Powell A.E. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut. 2017 doi: 10.1136/gutjnl-2017-313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Phesse T.J., Sansom O.J. Lgr5 joins the club of gastric stem cell markers in the corpus. Nat. Cell Biol. 2017;19:752–754. doi: 10.1038/ncb3567. [DOI] [PubMed] [Google Scholar]
- 204.Mao J., Fan S., Ma W., Fan P., Wang B., Zhang J., Wang H., Tang B., Wang H., Zhang Q., et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2015;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khurana S.S., Riehl T.E., Moore B.D., Fassan M., Rugge M., Romero-Gallo J., Noto J., Peek R.M., Jr., Stenson W.F., Mills J.C. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J. Biol. Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yamamoto H., Kitadai Y., Yamamoto H., Oue N., Ohdan H., Yasui W., Kikuchi A. Laminin γ2 Mediates Wnt5a-Induced Invasion of Gastric Cancer Cells. Gastroenterology. 2009;137:242–252. doi: 10.1053/j.gastro.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 207.Takiguchi G., Nishita M., Kurita K., Kakeji Y., Minami Y. Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci. 2016;107:290–297. doi: 10.1111/cas.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guggenheim D.E., Shah M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 209.Rahman R., Asombang A.W., Ibdah J.A. Characteristics of gastric cancer in Asia. World J. Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 211.Amieva M., Peek R.M., Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Franco A.T., Israel D.A., Washington M.K., Krishna U., Fox J.G., Rogers A.B., Neish A.S., Collier-Hyams L., Perez-Perez G.I., Hatakeyama M., et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sigal M., Rothenberg M.E., Logan C.Y., Lee J.Y., Honaker R.W., Cooper R.L., Passarelli B., Camorlinga M., Bouley D.M., Alvarez G., et al. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148:1392–1404. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 214.Wilhelm F., Simon E., Boger C., Behrens H.M., Kruger S., Rocken C. Novel insights into gastric cancer: Methylation of R-Spondins and regulation of LGR5 by SP1. Mol. Cancer Res. 2017;15:776–785. doi: 10.1158/1541-7786.MCR-16-0472. [DOI] [PubMed] [Google Scholar]
- 215.Xi H.Q., Cui J.X., Shen W.S., Wu X.S., Bian S.B., Li J.Y., Song Z., Wei B., Chen L. Increased expression of Lgr5 is associated with chemotherapy resistance in human gastric cancer. Oncol. Rep. 2014;32:181–188. doi: 10.3892/or.2014.3207. [DOI] [PubMed] [Google Scholar]
- 216.Geng Y., Lu X., Wu X., Xue L., Wang X., Xu J. MicroRNA-27b suppresses Helicobacter pylori-induced gastric tumorigenesis through negatively regulating Frizzled7. Oncol. Rep. 2016;35:2441–2450. doi: 10.3892/or.2016.4572. [DOI] [PubMed] [Google Scholar]
- 217.Gnad T., Feoktistova M., Leverkus M., Lendeckel U., Naumann M. Helicobacter pylori-induced activation of β-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol. Cancer. 2010;9:31. doi: 10.1186/1476-4598-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Flanagan D.J., Vincan E., Phesse T.J. Winding back Wnt signalling: Potential therapeutic targets for treating gastric cancers. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang K., Yuen S.T., Xu J., Lee S.P., Yan H.H., Shi S.T., Siu H.C., Deng S., Chu K.M., Law S., et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 220.Min B.H., Hwang J., Kim N.K., Park G., Kang S.Y., Ahn S., Ahn S., Ha S.Y., Lee Y.K., Kushima R., et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. J. Pathol. 2016;240:304–314. doi: 10.1002/path.4777. [DOI] [PubMed] [Google Scholar]
- 221.Jiang X., Hao H.X., Growney J.D., Woolfenden S., Bottiglio C., Ng N., Lu B., Hsieh M.H., Bagdasarian L., Meyer R., et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. USA. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Steinhart Z., Pavlovic Z., Chandrashekhar M., Hart T., Wang X., Zhang X., Robitaille M., Brown K.R., Jaksani S., Overmeer R., et al. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat. Med. 2017;23:60–68. doi: 10.1038/nm.4219. [DOI] [PubMed] [Google Scholar]
- 223.Cadigan K.M., Waterman M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 225.The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li J., Woods S.L., Healey S., Beesley J., Chen X., Lee J.S., Sivakumaran H., Wayte N., Nones K., Waterfall J.J., et al. Point mutations in exon 1B of APC reveal gastric adenocarcinoma and proximal polyposis of the stomach as a familial adenomatous polyposis variant. Am. J. Hum. Genet. 2016;98:830–842. doi: 10.1016/j.ajhg.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clements W.M., Wang J., Sarnaik A., Kim O.J., MacDonald J., Fenoglio-Preiser C., Groden J., Lowy A.M. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 228.Woo D.K., Kim H.S., Lee H.S., Kang Y.H., Yang H.K., Kim W.H. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int. J. Cancer. 2001;95:108–113. doi: 10.1002/1097-0215(20010320)95:2<108::AID-IJC1019>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 229.Akama Y., Yasui W., Yokozaki H., Kuniyasu H., Kitahara K., Ishikawa T., Tahara E. Frequent amplification of the cyclin E gene in human gastric carcinomas. Jpn. J. Cancer Res. 1995;86:617–621. doi: 10.1111/j.1349-7006.1995.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.He T. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 231.Jiang H., Xia J., Kang J., Ding Y., Wu W. Short hairpin RNA targeting beta-catenin suppresses cell proliferation and induces apoptosis in human gastric carcinoma cells. Scand. J. Gastroenterol. 2009;44:1452–1462. doi: 10.3109/00365520903342166. [DOI] [PubMed] [Google Scholar]
- 232.Cheng Y.Y., Yu J., Wong Y.P., Man E.P., To K.F., Jin V.X., Li J., Tao Q., Sung J.J., Chan F.K., et al. Frequent epigenetic inactivation of secreted frizzled-related protein 2 (SFRP2) by promoter methylation in human gastric cancer. Br. J. Cancer. 2007;97:895–901. doi: 10.1038/sj.bjc.6603968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nojima M., Suzuki H., Toyota M., Watanabe Y., Maruyama R., Sasaki S., Sasaki Y., Mita H., Nishikawa N., Yamaguchi K., et al. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26:4699–4713. doi: 10.1038/sj.onc.1210259. [DOI] [PubMed] [Google Scholar]
- 234.Wang B., Liu J., Ma L.N., Xiao H.L., Wang Y.Z., Li Y., Wang Z., Fan L., Lan C., Yang M., et al. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44(+) gastric cancer cells via attenuating Wnt signaling. J. Gastroenterol. 2012 doi: 10.1007/s00535-012-0711-z. [DOI] [PubMed] [Google Scholar]
- 235.Moon R.T. An introduction to non-canonical Wnt and Frizzled signaling. Semin. Cell Dev. Biol. 2002;13:215. doi: 10.1016/S1084-9521(02)00040-X. [DOI] [Google Scholar]
- 236.Kurayoshi M., Oue N., Yamamoto H., Kishida M., Inoue A., Asahara T., Yasui W., Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 237.Zhao C.M., Hayakawa Y., Kodama Y., Muthupalani S., Westphalen C.B., Andersen G.T., Flatberg A., Johannessen H., Friedman R.A., Renz B.W., et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kirikoshi H., Sekihara H., Katoh M. Expression profiles of 10 members of Frizzled gene family in human gastric cancer. Int. J. Oncol. 2001;19:767–771. doi: 10.3892/ijo.19.4.767. [DOI] [PubMed] [Google Scholar]
- 239.Schmuck R., Warneke V., Behrens H.M., Simon E., Weichert W., Rocken C. Genotypic and phenotypic characterization of side population of gastric cancer cell lines. Am. J. Pathol. 2011;178:1792–1804. doi: 10.1016/j.ajpath.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Radulescu S., Ridgway R.A., Cordero J., Athineos D., Salgueiro P., Poulsom R., Neumann J., Jung A., Patel S., Woodgett J., et al. Acute WNT signalling activation perturbs differentiation within the adult stomach and rapidly leads to tumour formation. Oncogene. 2012 doi: 10.1038/onc.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hayakawa Y., Sakitani K., Konishi M., Asfaha S., Niikura R., Tomita H., Renz B.W., Tailor Y., Macchini M., Middelhoff M., et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Powell A.E., Vlacich G., Zhao Z.Y., McKinley E.T., Washington M.K., Manning H.C., Coffey R.J. Inducible loss of one Apc allele in Lrig1-expressing progenitor cells results in multiple distal colonic tumors with features of familial adenomatous polyposis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G16–G23. doi: 10.1152/ajpgi.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Phesse T.J., Sansom O.J. Responding to R-Spondin: Slit2 potentiates intestinal regeneration. Cell Stem Cell. 2013;13:512–514. doi: 10.1016/j.stem.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 244.Vincan E., Schwab R.H.M., Flanagan D.J., Moselen J.M., Tran B.M., Barker N., Phesse T.J. The central role of Wnt signaling and organoid technology in personalizing anticancer therapy. Prog. Mol. Biol. Transl. Sci. 2018;153:299–319. doi: 10.1016/bs.pmbts.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 245.Phesse T.J., Durban V.M., Sansom O.J. Defining key concepts of intestinal and epithelial cancer biology through the use of mouse models. Carcinogenesis. 2017;38:953–965. doi: 10.1093/carcin/bgx080. [DOI] [PMC free article] [PubMed] [Google Scholar]