Abstract
Similar to the pattern observed in people with substance abuse disorders, laboratory animals will exhibit escalation of cocaine intake when the drug is available over prolonged periods of time. Here, we investigated the contribution of behavioral contingency of cocaine administration on escalation of cocaine intake and gene expression in the dorsal medial prefrontal cortex (dmPFC) in adult male rats. Rats were allowed to self-administer intravenous cocaine (0.25 mg/infusion) under either limited cocaine- (1h/day), prolonged cocaine- (6h/day), or limited cocaine- (1h/day) plus yoked cocaine-access (5h/day); a control group received access to saline (1h/day). One day after the final self-administration session, the rats were euthanized and the dmPFC was removed for quantification of mRNA expression of critical glutamatergic signaling genes, Homer2, Grin1, and Dlg4, in the dmPFC, as these genes and brain region have been previously implicated in addiction, learning, and memory. All groups with cocaine-access showed escalated cocaine intake during the first 10 minutes of each daily session, and within the first 1h of cocaine administration. Additionally, the limited-access + yoked group exhibited more non-reinforced lever responses during self-administration sessions than the other groups tested. Lastly, Homer2, Grin1, and Dlg4 mRNA were impacted by both duration and mode of cocaine exposure. Only prolonged-access rats exhibited increases in mRNA expression for Homer2, Grin1, and Dlg4 mRNA. Taken together, these findings indicate that both contingent and non-contingent “excessive” cocaine exposure supports escalation behavior, but the behavioral contingency of cocaine-access has distinct effects on the patterning of operant responsiveness and changes in mRNA expression.
Keywords: Cocaine, self-administration, escalation, contingent access, non-contingent access
Introduction
Cocaine addiction is a chronic disorder that persists in spite of negative interpersonal, professional, and physical consequences, with the development of tolerance and increased cocaine intake serving as central diagnostic criteria for stimulant use disorders (APA 2013). It has been argued that one major reason for the escalation of cocaine consumption relates to gradual changes in motivation and learning processes associated with responses to the positive and negative reinforcing properties of cocaine (Koob 2004). In order to model the differences in cocaine use observed in humans, differential access to cocaine self-administration has been employed as an avenue to study the behavioral and neurobiological aspects of drug-taking. Ahmed and Koob (1998) demonstrated that “limited” daily-access (1 h/day) and “prolonged” daily-access conditions (6 h/day) are distinct in their ability to model aspects of drug abuse/addiction in two major ways: 1) rats with prolonged cocaine-access escalate their cocaine consumption across daily sessions, whereas the limited-access rats exhibit stable consumption for several weeks and 2) rats with daily “prolonged” cocaine-access dramatically increased consumption of cocaine for the 1st 10 minutes of self-administration while “limited” access rats did not escalate cocaine consumption within the 1st 10 minutes of self-administration (Ahmed and Koob 1998). This differential pattern of responding has since been replicated with a number of variations to determine the underlying changes in drug-taking behavior (Ahmed and Koob 1999), neurocircuitry (Ahmed et al. 2002; Ben-Shahar et al. 2012; Robinson and Kolb 2004) and cellular/molecular function in rats (Ben-Shahar et al. 2009; Ben-Shahar et al. 2013), as well as in other species (Kirkland Henry et al. 2009; Nakamura et al. 2011).
Despite substantial study, it remains to be determined whether escalated cocaine intake (and the neurobiological changes associated there with) is dependent upon the amount of cocaine exposure or the act of self-administering the cocaine. Indeed, escalated responding and intake of non-drug reinforcers can be achieved by prolonged-access to liquid food, suggesting that the escalation phenomenon may be mediated by behavioral processes underlying the self-administration of appetitive stimuli (Goeders et al. 2009). One way to dissociate the relative contribution of total drug exposure from the behavioral contingency of that exposure is to employ yoked-access procedures (i.e. the administration of cocaine under the control of a separate self-administering rat). For instance, Hemby et al. (1997) investigated the effects of cocaine under response-dependent and –independent conditions in a yoked-triad. Both self-administering and yoked-access rats exhibited elevated levels of dopamine in the nucleus accumbens during the first hour of self-administration, but the self-administering animals exhibited greater dopamine levels than their yoked- counterparts (Hemby et al. 1997). Additionally, relative to rats self-administering cocaine, yoked rats exhibit differential corticosterone levels both in systemic plasma (Galici et al. 2000) and brain (Palamarchouk et al. 2009), have a higher morbidity rate (Dworkin et al. 1995) and exhibit higher indices of distress (measured by ultrasonic vocalization) (Mutschler and Miczek 1998). These studies demonstrate that, despite equivalent cocaine dosing, animals given contingent-access to cocaine exhibit distinct behavioral and neurobiological effects compared to animals with non-contingent access.
To expand upon the role of behavioral contingency in the behavioral sequelae of cocaine exposure, Kippin et al. (2006) employed a novel mixed self-administration/yoked cocaine exposure procedure in which rats received 1-h access to cocaine self-administration before receiving non-contingent cocaine infusions via yoking procedures during its last 5 hours of cocaine self-administration. Although escalated cocaine intake was not observed in this earlier study, both the contingent and non-contingent excessive cocaine exposure groups exhibited greater cue-induced reinstatement than rats with a history of limited cocaine-access only. However, only the prolonged contingent-access rats exhibited greater cocaine-primed reinstatement of responding. In the present study, we employ the prolonged-access and limited-access + yoked procedures to determine the impact of contingent and non-contingent “excessive” cocaine exposure on the escalation of cocaine intake and operant responding for cocaine to determine how behavioral contingency of cocaine delivery influences these aspects of cocaine addiction-related behavior.
The dorsomedial prefrontal cortex (dmPFC) is dysregulated in human cocaine addicts (Verdejo-Garcia et al. 2015) and this dysregulation is linked to aberrant learning and plasticity that is attributed to anomalies in glutamate transmission (Kalivas et al. 2005; Pascoli et al. 2014a; Ruan and Yao 2017b). To explore the potential neurobiology underpinning the effects of cocaine on dmPFC as a function of contingency, we measured the levels of mRNA for Homer2, Grin1, and Dlg4 within the dorsomedial prefrontal cortex (dmPFC). Homer2 is a glutamate receptor scaffolding protein that is up-regulated within PFC by both non-contingent and contingent cocaine administration (Ary and Szumlinski, 2007; Ary et al., 2013; Ben-Shahar et al., 2009; Gould et al., 2013) and while it remains to be determined whether or not cocaine-induced increases in PFC Homer2 protein expression reflects increased gene transcription, Homer2 expression bi-directionally regulates both basal and cocaine-induced changes in extracellular glutamate levels within PFC (Ary et al., 2013) to influence cocaine-conditioned approach behavior in place-conditioning models (Ary et al., 2013) and cocaine-primed reinstatement of lever-pressing behavior in operant-conditioning models (Gould et al., 2013). Grin1 mRNA encodes the obligatory N-methyl-D-aspartate (NMDA) receptor sub-unit NR1 within the mammalian brain (Bai and Hoffman 2009); this receptor is widely implicated and critical for many forms of plasticity (Bear 1996; Hopf 2017; Sweatt 2016; Thiels et al. 1996), and is up-regulated in the PFC following cocaine exposure (Ary and Szumlinski 2007; Blanco et al. 2014; Hemby et al. 2005). Dlg4 encodes the sequence for postsynaptic density-95 (PSD-95), a scaffold receptor protein that regulates plasticity and learning through its actions on NMDA receptor density and function (Wang and Peng 2016). PSD-95 expression in the PFC is increased after prolonged withdrawal from cocaine (Ghasemzadeh et al. 2009; McIntosh et al. 2013) and following extinction testing during prolonged exposure to cocaine self-administration (Ghasemzadeh et al. 2011).
Methods
Subjects
Male Sprague-Dawley rats were pair-housed in a 12-h reverse light-dark cycle room and had ad libitum access to food and water (except as noted below). The housing and care of the rats followed the guidelines set forth by the “Guide for the Care and Use of Laboratory Rats, 8th Edition” (NIH 2011).
Surgery
Male Sprague-Dawley rats weighing 300–350g were deeply anesthetized using ketamine (60mg/kg) and xylazine (10mg/kg). Chronic indwelling catheters were constructed using a bent steel cannula with a screw-type connector (Plastics One, Roanoke, VA), SILASTIC tubing (11 cm, i.d. 0.64 mm, o.d. 1.19 mm, Dow Corning, Midland, MI), Prolite polypropylene monofilament mesh (Atrium Medical Corporation, Hudson, NH), a silicon ball 2.5 cm from the end, and methyl methacrylate dental cement. The catheters were implanted and maintained as we have reported previously (Ben-Shahar et al. 2013; Kerstetter et al. 2008). Naïve rats were left in the vivarium and handled daily, but had no access to behavioral training or surgery. They were euthanized at the same age as the rats undergoing behavioral training.
Behavioral Training
Food-training and cocaine self-administration utilized standard operant chambers (Med Associates Inc., St. Albans, VT, USA) and were conducted during a fixed time in the dark phase of the rat’s circadian cycle each day. Before surgical implantation of the jugular catheters, the rats were restricted to 20 g of food for 1 week, and trained on a fixed ratio 1 (FR1) schedule of food reinforcement for two 16 h training sessions where each right lever-press was associated with a 45 mg food pellet (Ben-Shahar et al. 2012). After recovery from the surgery, the rats were placed on a fixed ratio 1 (FR1) schedule of reinforcement for intravenous (IV) cocaine (0.1mL at 0.25 mg/infusion in 0.9% saline) or saline for 1h/day for 5 days. Each active lever-press was associated with a 4 sec infusion of cocaine or saline and a 20 sec timeout was signaled with a 20 sec light cue above the active lever (Ben-Shahar et al. 2012). On the 6th day, the cocaine rats were divided into limited (1 h/day) cocaine-access, prolonged (6 h/day) cocaine-access, and limited-access + yoked-access (1 h access followed by 5 h of “yoked” exposure) treatment groups and continued the FR1 schedule of reinforcement for an additional 15 days. During yoked exposure, rats remained in their chambers for an additional 5 h with the levers retracted to eliminate the opportunity to perform the operant response. During this time, yoked rats received a 4 sec cocaine infusion every time a paired prolonged-access rat self-administered an infusion, but without cue light presentation.
Tissue Collection and mRNA Quantification
Twenty-four hours after the last self-administration session, the animals were sacrificed via rapid decapitation, and their brains were frozen over ice and dissected into 0.5 mm sections with a metal brain mold (Braintree Scientific, Braintree, MA). The dmPFC was dissected out at 3.20 to 2.20 mm anterior to Bregma and stored at -80 Celsius. The frozen dmPFC was added to 600 μL of buffer RLT (Qiagen DNA/RNA/Protein extraction kit) and homogenized with the Qiagen TissueRuptor for 30 sec. mRNA was then extracted through use of the AllPrep DNA/RNA/protein extraction kit provided by Qiagen in accordance to the protocol provided by the manufacturer. RNA was eluted from the spin column with 50μL of nuclease-free water. RNA (500 ng/sample) was incubated with 2μL of gDNA Wipeout Buffer (Qiagen) at 42°C for 2 minutes then cooled over ice. 1μL of Reverse Transcription Master Mix (Qiagen), 1μL of RT primer mix (Qiagen), and 4μL of Quantiscript RT Buffer (Qiagen) were added to the reaction mixture and incubated in an Eppendorf MasterCycler at 42°C for 18 minutes to amplify the product, then incubated at 95°C for 3 minutes to inactivate the reverse transcriptase. A reverse transcriptase-negative reaction was carried out in parallel with the samples from 500ng of pooled sample RNA.
Levels of mRNA were assessed in triplicate using quantitative real time pcr (qRT-PCR) (Biorad) on the BioRad CFX96 Touch Real-Time system. Negative controls consisted of a DNA-negative sample and a reverse-transcriptase free sample. Standard curves were run on each pcr plate with 3x serial dilutions ranging from 50.0ng/uL to 1.85ng/uL. The data were normalized using three control genes (Gapdh, βActin, and Tubb5) and dmPFC tissue from naïve age-matched rats according to the equations outlined by Hellemans et al. in 2007.
Statistical Analyses
The self-administration data for individual sessions during differential access to cocaine were compared to baseline responding (average of days 6, 7, & 8 of differential access) at 10 min (i.e. “loading phase”; e.g. Ahmed & Koob, 1998) and 1 h intervals to compare across all four conditions, as well as across the entire 6 h sessions for the prolonged-access and limited-access + yoked groups Separate two-way, between-within (group X day), repeated measures ANOVAs, were conducted for the numbers of cocaine infusions followed by Dunnett post-hoc comparisons to deconstruct significant interactions/main effects using the Prism 6 statistical software (Graphpad). Non-reinforced responding (i.e. during the time-out period) was analyzed separately from total responding via a two-way, between-within (group X day), repeated measures ANOVAs followed by Tukey’s post-hoc comparison for the first 10 min and 1 h of self-administration to assess the efficiency of behavioral responding before (days 4 & 5) and after differential access (days 6 to 20). Inactive lever presses were also analyzed but no significant effects or interactions were detected and in all cases, mean inactive lever presses were < 5 (data not shown). Additionally, self-administration data was separated into separate 10 min blocks on day 6 and day 20 to assess loading during initial daily access. A two-way ANOVA (time block X condition) was conducted to assess the differences in cocaine consumption during 10-minute time blocks for the first hour of self-administration in all four experimental groups during the 6th day and 20th day of self-administration; Tukey’s post-hoc comparison was used to deconstruct significant interactions using the Prism 6 software. Lastly, analyses of normalized quantitative PCR data were performed by one-way MANOVA for Homer2, Grin1, and Dlg4 mRNA and decomposed via post-hoc LSD tests using SPSS Statistics 24 (IBM). All graphics were plotted by using the Prism 6 statistical software (Graphpad).
Results
Cocaine Intake
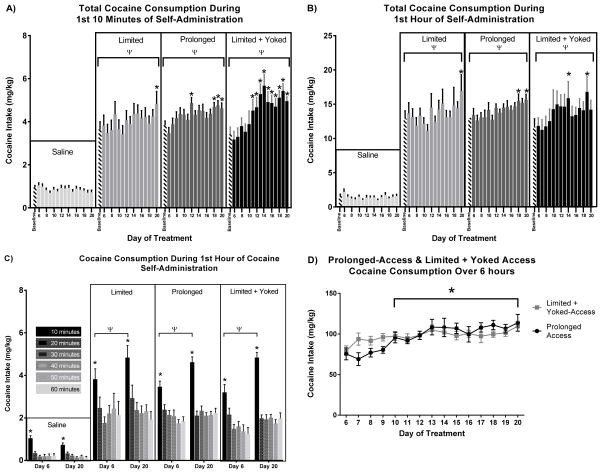
A two-way repeated measures ANOVA of cocaine consumption (mg/kg) during the loading phase of self-administration sessions (first 10 minutes) revealed an interaction between time and treatment (F45, 2055 = 2.88, p < 0.0001 Figure 1A). Post-hoc comparisons revealed increases in cocaine consumption for day 20 vs baseline in the limited-access group, days 12, 18, 19, and 20 vs baseline in prolonged-access group (p<0.05), and days 11 through 20 vs baseline in limited-access + yoked rats (p<0.05). These results indicate that there is an escalation of cocaine consumption in all cocaine groups for the first 10 minutes of self-administration, where the limited-access + yoked rats show escalation earlier and the limited-access rats show escalation later than the prolonged-access rats.
Figure 1. Cocaine consumption during self-administration.
A) 1st 10 min of self-administration. Limited-access rats escalated cocaine consumption on day 20 of self-administration. Prolonged-access rats exhibited escalated cocaine consumption on days 12, 14, 15, 18, 19, and 20 of self-administration. Limited + yoked rats escalated cocaine consumption in blocks between days 11 and 20 of self-administration. (* p<0.05) B) 1st 1 hour of self-administration. Prolonged-access rats exhibited escalated cocaine consumption in time block 5. Limited + yoked rats exhibited escalated cocaine consumption in time blocks 2, 3, 4, and 5. Saline and limited access rats did not escalate cocaine intake in the first hour of cocaine self-administration. (* p<0.05) C) Cocaine consumption recorded every 10 minutes during 1st hour of self-administration. Limited-access, prolonged-access, and limited + yoked-access rats all displayed increased cocaine consumption during the first 10 minutes of self-administration sessions on both day 6 and day 20 of self-administration. Additionally, prolonged-access and limited + yoked-access rats exhibited increased cocaine consumption within the 1st 10 minutes of self-administration between day 6 and day 20. (* p<0.05, ψ p <0.05) D) Total daily cocaine infusions for prolonged and limited + yoked access rats. Total cocaine consumption did not differ between prolonged and limited + yoked access rats. Cocaine consumption escalated in both groups beginning on the 10th day of extended access to cocaine. (* p<0.05)
Two-way repeated measures ANOVA of cocaine consumption (mg/kg) for the first hour of cocaine self-administration revealed a significant interaction between time and treatment (F45, 1875 = 1.385, p<0.05; Figure 1B). Dunnett’s multiple comparisons post-hoc analysis showed increased cocaine consumption in the limited-access rats for day 20 vs baseline (p<0.05), prolonged-access rats for days 18 & 20 vs baseline (p<0.05) and in limited-access + yoked rats for days 14 & 19 vs baseline (p<0.05). These data also indicate that there is an escalation of cocaine consumption in prolonged-access and limited-access + yoked rats that differ in the time of onset.
A two-way ANOVA was run to assess differences in cocaine consumption (mg/kg) during 10 min time blocks for the 1st 1 h of self-administration on day 6 and day 20 of the experiment. The results revealed a significant interaction between time block and condition (F35, 1644 = 1.968, p < 0.001). Tukey’s post-hoc comparison revealed that rats in the limited-access, prolonged-access, and limited-access + yoked conditions all consumed more cocaine in the first 10 minutes of self-administration than during any other 10-minute block (p < 0.01). Additionally, rats in the prolonged-access and limited-access + yoked conditions consumed more cocaine in the first 10 minutes of day 20 than during the first 10 minutes of day 6 of the self-administration procedure (p < 0.0001). These results indicate that rats in all cocaine-access conditions consumed more cocaine in the first 10 minutes of self-administration than in the rest of the first hour, and also that rats in the prolonged-access and limited-access + yoked conditions escalated cocaine consumption between day 6 and day 20 of self-administration.
A two-way repeated measures ANOVA was run to assess any differences in total cocaine consumption (mg/kg) over 6 h for the prolonged-access rats versus the limited-access + yoked rats. The results revealed a significant interaction between time and treatment (F14, 392 = 1.779, p < 0.05), an effect of time (F14, 392 = 6.874, p < 0.0001), but no effect for treatment (F1, 28 = 0.0318, p = 0.8597). Dunnett’s post-hoc comparison revealed that the prolonged-access and limited-access + yoked conditions exhibited escalated cocaine intake from day 12 to day 20. And that there was no difference between prolonged-access and limited-access + yoked conditions. These data indicate that both groups had increased cocaine exposure from baseline and, expectedly as the majority of daily intake in both groups was controlled by prolonged-access rats, there is no observable difference in cocaine exposure between these two conditions (Figure 1D).
Non-reinforced Lever Responding
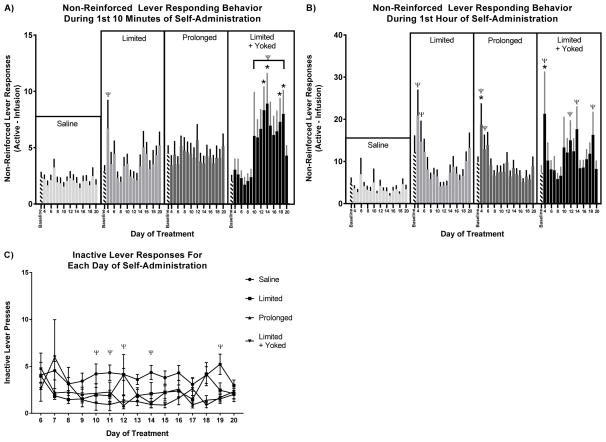
A two-way repeated measures ANOVA of the numbers of non-reinforced active lever-responses (i.e. responses during the time-out period) during the first 10 min of self-administration revealed an interaction between time and treatment (F45, 2085 = 2.061, p<0.0001) (Figure 2A). Dunnett Post-hoc comparisons indicated significant differences in the non-reinforced lever-responding for only the limited-access + yoked-access rats on days 12, 13, 14, 15, 18, and 19 versus baseline (days 6, 7, & 8) (p<0.05) (Figure 2A). These data indicate that for the first 10 min of self-administration, the limited-access + yoked animals exhibited significantly more non-reinforced lever responding than limited-access and prolonged-access conditions well after differential-access to cocaine was initiated.
Figure 2. Non-reinforced lever responding behavior.
A) 1st 10 min of self-administration. Only limited + yoked rats exhibited high levels of non-reinforced cocaine responding on days 14, 15, 18, and 19 during the first 10 minutes of cocaine self-administration. B) 1st 1 hour of self-administration. All cocaine-access groups showed an initial non-reinforced active-lever responding for the first 1 hour of self-administration during cocaine acquisition on day 4. C) Inactive lever responses over entire day. There were no differences in the inactive lever responses within or between groups across all days.
A two-way repeated measures ANOVA of the non-reinforced lever responding during the first 1 h of self-administration revealed a significant interaction between time and treatment (F45, 2040 = 2.388, p<0.0001; Figure 2B). Dunnett’s multiple comparisons post-hoc analysis indicated a significant decrease in the number of non-reinforced active lever responses compared to day 4 of self-administration for days 8–14 & 16–18 for rats with limited-access, days 7–11 & 13–20 for prolonged-access, and days 8 & 9 for limited-access + yoked rats (p<0.05). These data indicate that before differential access, all cocaine-access animals exhibited inefficient behavioral responding at day 4. However, only limited-access + yoked animals continued the pattern of inefficient behavioral responding throughout the experiment.
Quantitative Real-Time PCR of mRNA levels
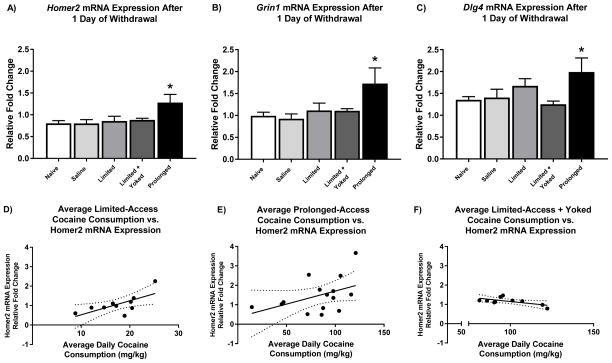
A one-way MANOVA of mRNA expression for Homer2, Grin1, and Dlg4 resulted in a significant main effect of condition (Hotelling’s trace = 0.652, F12, 110) = 1.991, p < 0.05). LSD post-hoc pairwise comparisons revealed greater Homer2 mRNA expression in the prolonged-access group relative to the naïve, saline, and limited-access + yoked groups (figure 3A) LSD post-hoc pair-wise comparisons also revealed increased Grin1 mRNA expression in the prolonged-access group compared to naïve, saline, limited-access, and limited-access + yoked groups (figure 3B). Lastly, LSD post-hoc pair-wise comparisons revealed increased levels of Dlg4 mRNA expression in the prolonged-access group compared to the naïve and limited-access + yoked access groups (figure 3C). These data indicate that prolonged-access rats have a unique molecular phenotype, even though rats in the limited-access + yoked group received equivalent amounts of cocaine and escalated cocaine consumption at about the same rate as the prolonged-access rats.
Figure 3. mRNA expression for glutamatergic genes in the dmPFC.
A) Prolonged-access to cocaine resulted in increased levels of Homer2 mRNA within the dmPFC after 1 day of withdrawal. B) Prolonged-access to cocaine resulted in increased levels of Grin1 mRNA within the dmPFC after 1 day of withdrawal. C) Prolonged-access to cocaine resulted in increased levels of Dlg4 mRNA within the dmPFC after 1 day of withdrawal. D) Homer2 mRNA was positively correlated with total cocaine infusions in the prolonged access group (R2 = 0.1975, p < 0.05). E) Homer2 mRNA was positively correlated with total cocaine infusions in the limited access group (R2 = 0.4763, p < 0.05). F) Homer2 mRNA was negatively correlated with total cocaine infusions in the limited + yoked access group (R2 = 0.4046, p < 0.05).
Additionally, Pearson correlation coefficients were calculated for total cocaine consumption versus mRNA expression. Homer2 expression was correlated positively with cocaine exposure for both prolonged-access groups (R2 = 0.1975, p < 0.05), and limited-access (R2 = 0.4763, p < 0.05) groups, whereas mRNA expression correlated negatively for the limited + yoked access group (R2 = 0.4046, p < 0.05) (Figure 3D, E, F). Pearson correlation coefficients failed to reveal significant correlations between total cocaine consumption versus Grin1 and Dlg4 mRNA (ps >0.05).
Discussion
The major finding of the present study is that rats self-administering cocaine under all three cocaine-access conditions exhibited escalation of cocaine intake, but with distinct temporal and molecular profiles. Rats in all self-administration conditions escalated their cocaine intake during the loading phase of self-administration (aka the first 10 minutes, Figure 1A,C), during the first 1 h of self-administration, and over entire daily sessions (Figures 1B, D). Additionally, both prolonged-access and limited-access + yoked conditions escalated cocaine intake faster than limited-access animals. However, despite equivalent “excessive” cocaine exposure, the associated patterns of active lever responding are distinct between prolonged-access and limited-access + yoked groups, with the limited-access + yoked condition exhibiting more non-reinforced lever responding on the active lever during the first 10 min of self-administration (i.e. during the time-out period which did not result in cocaine infusion; see Figure 2), suggesting that partially distinctive behavioral mechanisms may underlie the escalation of cocaine intake induced by contingent versus non-contingent cocaine exposure. Furthermore, we observed an overall increase in Homer2, Grin1, and Dlg4 mRNA expression only in the prolonged-access rats and total cocaine exposure and Homer2 mRNA expression are positively correlated in both prolonged- and limited-access conditions but negatively correlated in the limited-access + yoked condition indicating distinct neurobiological consequences of both amount and mode of cocaine exposure. Thus, the present study demonstrates that escalation of cocaine-taking induced by differential cocaine-access, both with respect to session duration and behavioral contingency of cocaine delivery, can produce distinct behavioral and neurobiological consequences.
The finding that all cocaine-taking groups exhibited an escalation of drug intake, but at different rates and to different degrees, is generally consistent with prior findings. Although the capacity of prolonged drug-access to escalate drug-taking, over that observed under limited-access conditions, is a highly replicable finding (e.g. Ahmed and Koob 1998; Ben-Shahar et al. 2004; Ben-Shahar et al. 2013), an escalation of cocaine intake is also reported in rats self-administering cocaine during slightly longer (2 h) daily sessions, albeit to a lesser extent than counterparts with daily 6-h-access (Mandt et al. 2015). Further, some rat strains exhibit escalation under daily 1 h sessions of cocaine-access (Perry et al., 2006), particularly when allowed to self-administer cocaine over a protracted test period (e.g. 75 days) (Belin et al. 2009). The present report extends the literature on escalation by demonstrating that cocaine intake during the initial 10 minutes of the self-administration paradigm (i.e., the loading phase; see Ahmed & Koob, 1998) also escalates with drug experience in all conditions but at somewhat different rates (Figure 1). Thus, it appears that the loading phase (i.e. first 10 min) is more sensitive to escalation of intake than the overall duration of daily access.
The escalation of cocaine consumption observed in the limited-access + yoked condition was additionally associated with a pronounced, but transient, increase in non-reinforced lever-responding during cocaine access (Figures 2A, B). Marked differences in non-reinforced responding have been reported in the absence of differences in cocaine intake; e.g. in female relative to male rats (Fuchs et al., 2005; Kippin et al., 2006; Kosten & Zhang, 2008); thus, the two measures appear generally dissociable. In the case of the limited-access + yoked rats in the present study, the rats had acquired responding for cocaine, accompanied by low levels of non-reinforced responding which was markedly elevated, particularly during the loading phase (Figure 2A), following exposure to the yoking procedure. This disruption of “efficient” (i.e. lever presses are not tied to the drug reinforcer) operant behavior elicited by the addition of non-contingent cocaine exposure is likely due to discontinuous reinforcement schedules. Further, contextual cues can modulate escalation behavior; when rats are allowed alternating days of 1 h and 6 h access to cocaine with differential cues, they only escalate during the 6 h sessions (Beckmann et al., 2012). An equally-viable explanation for increased non-reinforced lever responding induced by non-contingent drug exposure may pertain to differences in the aversive, stress-inducing, and glucocorticoid-releasing properties of cocaine delivered under yoked procedures (Twining et al. 2009) which have been implicated in the processes underlying escalation (Mantsch et al., 2007, 2008). Thus, the combination of yoked cocaine with subsequent re-introduction to the operant chamber with differential cues (i.e. lever extension and operant light) appears to serve as a potent elicitor of lever-responding.
Given the central role of drug-induced neuroadaptations, particularly alterations in glutamate function, in theories of addiction (see e.g. Kalivas & Volkow, 2005), it is critical to discern the role of behavioral contingency in neurobiological changes associated with escalating drug intake. Here, we identified differences between the prolonged-access and limited-access + yoked conditions; we examined the dmPFC which has been implicated in the escalation of cocaine intake (Smith et al. 2008) for changes in the expression levels of molecular markers implicated in addiction (i.e. Homer2, Grin1, and Dlg4 mRNA). Consistent with prior findings (Ben-Shahar et al., 2009), we observed increased levels of Homer2 mRNA only in the prolonged-access condition, (Figure 3A). Furthermore, the level of intake in the limited- and prolonged-access conditions correlated positively with Homer2 mRNA expression, whereas cocaine exposure in the limited-access + yoked group correlated negatively with Homer2 mRNA expression (Figure 3D–F). In addition, we also observed increases in Grin1 and Dlg4 mRNA within the dmPFC of only in the prolonged-access rats (Figures 3B–C). Overall, the differences between prolonged-access and limited-access + yoked conditions are consistent with other studies employing yoked procedures (Krawczyk et al. 2013; Ma et al. 2013; McFarland et al. 2003; Radley et al. 2015) but the present finding furthers this literature by demonstrating that contingent and non-contingent cocaine exposure induces distinct neurobiological changes even when both are associated with escalation of cocaine intake, with only the prolonged access condition producing elevation of several genes that are suggestive of enhanced glutamatergic signaling.
The increases in expression of glutamate-related genes observed specifically in following prolonged contingent access to cocaine to current enhanced glutamate neurotransmission and cocaine-specific neuroplasticity in the prefrontal cortex. Repeated contingent access to cocaine results in cocaine-specific synaptic plasticity including: increased dendritic spine density (Frankfurt et al. 2011), increased long-term potentiation (LTP) in the PFC (Huang et al. 2006), and lowered induction threshold for inducing cocaine-specific LTP (Ruan and Yao 2017a). Lastly, optogenetic reversal of cocaine-induced plasticity within the PFC eliminates cocaine seeking behaviors (Pascoli et al. 2014b), indicating that cocaine-specific plasticity of glutamatergic receptors develops with repeated contingent-access to cocaine, and is necessary for cocaine-seeking behavior. We have previously demonstrated that prolonged-access to cocaine enhances baseline glutamate neurotransmission (Shin et al. 2016) after 30 days of withdrawal, and increased expression of the NMDA GluN2b receptor subunit at 3 and 30 days of withdrawal (Szumlinski et al. 2016). Other groups have also shown increases in NMDA as well as AMPA and Kainate receptor subunits after withdrawal from contingent cocaine self-administration (Crespo et al. 2002; Ghasemzadeh et al. 2009; Tang et al. 2004).
Briefly, Homer2 is a gene encoding for a scaffolding protein that links metabotropic glutamate receptors (mGluRs) and NMDA ionotropic glutamate receptors and has been implicated in addictive behaviors (c.f., Szumlinski et al., 2008). Homer2a/b protein within the mPFC is increased following prolonged-access to cocaine (Ben Shahar et al., 2009) and repeated cocaine injections (Ary and Szumlinski 2007). Further, viral-mediated Homer2b overexpression in the mPFC increases basal glutamate levels and cocaine-preference in mice, whereas Homer2b knockdown reduces basal glutamate in this area (Ary et al. 2013). Additionally, Grin1 encodes for the obligatory NR1 subunit of NMDA receptors, while Dlg4 encodes for PSD-95, a scaffolding protein that regulates NMDA receptor function (Bai and Hoffman 2009). Increases in NR1 protein within the PFC have previously been observed in response to repeated cocaine injections (Kovacs et al. 2010) and cocaine self-administration (Hemby et al. 2005). Furthermore, NR1 is essential for cocaine-mediated learning; mice expressing a mutant version of the NR1 subunit (which reduces calcium flow through the NMDA receptor) fail to form conditioned place preference and locomotor sensitization in response to repeated cocaine exposure (Heusner and Palmiter 2005). PSD-95 is critical for synaptic plasticity and regulation of NMDA receptor location and function (Wang and Peng 2016). PSD-95 is also implicated in behavioral plasticity associated with chronic cocaine administration (Yao et al. 2004), extinction of cocaine self-administration (Knackstedt et al. 2010; Ghasemzadeh et al. 2011), as well as prolonged withdrawal from cocaine (Ghasemzadeh et al. 2009; McIntosh et al. 2013). Thus, our RNA data is generally consistent with findings examining protein levels of glutamatergic signaling molecules with the increases in RNA observed here coinciding or preceding latent increases in protein.
Escalation of drug consumption is an important diagnostic criterion of addiction in humans and an integral component in various theories of addiction. Therefore, understanding the behavioral and neurobiological underpinnings of escalation is likely to facilitate addiction management programs in humans. In addition to facilitating cocaine intake, prolonged daily access to cocaine is associated with several behavioral changes, such as reduced brain reward function (Ahmed et al. 2002; Ahmed and Koob 2005), increased breakpoints for cocaine reinforcement under progressive ratio schedules (Paterson and Markou 2004; Wee et al. 2009), diminished aversive properties of cocaine (Ben-Shahar et al. 2008), increased extinction responding during protracted withdrawal (Ferrario et al. 2005), as well as increased responding during cocaine-primed and cue-induced reinstatement of cocaine-seeking (Ahmed and Cador 2006; Kippin et al. 2006; Knackstedt and Kalivas 2007; Mantsch et al. 2004). Similarly, other approaches to modeling “excessive” intake also produce increases in measures of cocaine-taking and -seeking behaviors (Deroche-Gamonet et al. 2004; Roberts et al. 2007). Further study is required to determine the relations between nature of cocaine exposure and induction of addiction-like behavior, as well as between behavioral and molecular outcomes. To this end, the present study demonstrates behavioral contingency plays an important role in the nature of the behavioral and molecular changes induced by cocaine exposure.
Acknowledgments
This research was supported by NIH grants DA-027115 and DA-027525 (TEK) and DA024038 (KKS), as well as funding from and the W.M. Keck Foundation (TEK). We would also like to thank Xiang Li, Danay Andresen-Baker, Dana Hutchison, Amanda Navarro, and Joseph Clark for their assistance with the behavioral experiments.
References
- Ahmed S, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:9. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–6. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–12. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–90. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci. 2013;33:8101–13. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain research. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bai G, Hoffman PW. Frontiers in Neuroscience Transcriptional Regulation of NMDA Receptor Expression. In: Van Dongen AM, editor. Biology of the NMDA Receptor. CRC Press/Taylor & Francis Taylor & Francis Group, LLC; Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13453–9. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–8. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus E, Waldroup S, Ettenberg A. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:7. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento A, Miller B, Webb S, Wroten M, Silva H, Caruana A, Gordon E, Ploense K, Ditzhazy J, Kippin T, Szumlinski K. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33:495–506a. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Szumlinski K, Lominac K, Cohen A, Gordon E, Ploense K, DeMartini J, Bernstein N, Rudy N, Nabhan A, Sacramento A, Pagano K, Carosso G, Woodward N. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addiction biology. 2012;17:746–57. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E, Pavon FJ, Palomino A, Luque-Rojas MJ, Serrano A, Rivera P, Bilbao A, Alen F, Vida M, Suarez J, Rodriguez de Fonseca F. Cocaine-induced behavioral sensitization is associated with changes in the expression of endocannabinoid and glutamatergic signaling systems in the mouse prefrontal cortex. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014:18. doi: 10.1093/ijnp/pyu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Annals of the New York Academy of Sciences. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117:262–6. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and Behavioral Plasticity Associated with the Transition from Controlled to Escalated Cocaine Use. Biological Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Flores C, Wen X, Labelle-Dumais C, Kolb B. Chronic phencyclidine treatment increases dendritic spine density in prefrontal cortex and nucleus accumbens neurons. Synapse. 2007;61:978–984. doi: 10.1002/syn.20452. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Salas-Ramirez K, Friedman E, Luine V. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse. 2011;65:955–61. doi: 10.1002/syn.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain research. 2011;1413:60–71. doi: 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009;92:383–92. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2009;93:67–74. doi: 10.1016/j.pbb.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome biology. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain research. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–7. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes, brain, and behavior. 2017;16:118–138. doi: 10.1111/gbb.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Lin H-J, Hsu K-S. Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cerebral Cortex. 2006;17:1877–1888. doi: 10.1093/cercor/bhl096. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–50. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–7. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–47. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt L, Kalivas P. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:7. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–92. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Allostatic view of motivation: implications for psychopathology. Nebraska Symposium on Motivation Nebraska Symposium on Motivation. 2004;50:1–18. [PubMed] [Google Scholar]
- Kovacs K, Lajtha A, Sershen H. Effect of nicotine and cocaine on neurofilaments and receptors in whole brain tissue and synaptoneurosome preparations. Brain research bulletin. 2010;82:109–17. doi: 10.1016/j.brainresbull.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, Di Prospero C, Chiang C, Martinez A, Jones AA, Doudnikoff E, Caille S, Bezard E, Georges F, Dumont EC. D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. J Neurosci. 2013;33:11960–71. doi: 10.1523/JNEUROSCI.1784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Henley SM, Toll J, Jentsch JD, Evans CJ, Levine MS, Cepeda C. Drug-primed reinstatement of cocaine seeking in mice: increased excitability of medium-sized spiny neurons in the nucleus accumbens. ASN neuro. 2013;5:257–71. doi: 10.1042/AN20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Copenhagen LI, Zahniser NR, Allen RM. Escalation of cocaine consumption in short and long access self-administration procedures. Drug Alcohol Depend. 2015;149:166–72. doi: 10.1016/j.drugalcdep.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J, Yuferov V, Mathieu-Kia A, Ho A, Kreek M. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:11. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh S, Howell L, Hemby SE. Dopaminergic dysregulation in prefrontal cortex of rhesus monkeys following cocaine self-administration. Frontiers in psychiatry. 2013;4:88. doi: 10.3389/fpsyt.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998;136:402–8. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Gao S, Okamura H, Nakahara D. Intrathecal cocaine delivery enables long-access self-administration with binge-like behavior in mice. Psychopharmacology (Berl) 2011;213:119–29. doi: 10.1007/s00213-010-2021-6. [DOI] [PubMed] [Google Scholar]
- NIH. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; 2011. [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014a;509:459–64. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014b;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology. 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT. The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J Neurosci. 2015;35:11897–910. doi: 10.1523/JNEUROSCI.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Morgan D, Liu Y. How to make a rat addicted to cocaine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ruan H, Yao W-D. Cocaine promotes coincidence detection and lowers induction threshold during Hebbian associative synaptic potentiation in prefrontal cortex. Journal of Neuroscience. 2017a;37:986–997. doi: 10.1523/JNEUROSCI.2257-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Yao WD. Cocaine Promotes Coincidence Detection and Lowers Induction Threshold during Hebbian Associative Synaptic Potentiation in Prefrontal Cortex. J Neurosci. 2017b;37:986–997. doi: 10.1523/JNEUROSCI.2257-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology. 2016;102:103–10. doi: 10.1016/j.neuropharm.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ward SJ, Roberts DC. Lesions of the dorsomedial frontal cortex block sensitization to the positive-reinforcing effects of cocaine. Pharmacol Biochem Behav. 2008;88:238–46. doi: 10.1016/j.pbb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Neural plasticity and behavior - sixty years of conceptual advances. J Neurochem. 2016;139(Suppl 2):179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochemical pharmacology. 2008;75:112–33. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Wroten MG, Miller BW, Sacramento AD, Cohen M, Ben-Shahar O, Kippin TE. Cocaine Self-Administration Elevates GluN2B within dmPFC Mediating Heightened Cue-Elicited Operant Responding. Journal of drug abuse. 2016:2. doi: 10.21767/2471-853x.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–33. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Xie X, Yeckel MF, Barrionuevo G, Berger TW. NMDA receptor-dependent LTD in different subfields of hippocampus in vivo and in vitro. Hippocampus. 1996;6:43–51. doi: 10.1002/(SICI)1098-1063(1996)6:1<43::AID-HIPO8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 2009;123:913–25. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Verdejo-Roman J, Albein-Urios N, Martinez-Gonzalez JM, Gutierrez B, Soriano-Mas C. Neural substrates of cognitive flexibility in cocaine and gambling addictions. The British journal of psychiatry: the journal of mental science. 2015;207:158–64. doi: 10.1192/bjp.bp.114.152223. [DOI] [PubMed] [Google Scholar]
- Wang H, Peng RY. Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Military Medical Research. 2016;3:26. doi: 10.1186/s40779-016-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. The Journal of pharmacology and experimental therapeutics. 2007;320:1134–43. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–38. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]