Abstract
Treatment-related changes can be difficult to differentiate from progressive glioma using MRI with contrast (CE). The purpose of this study is to compare the sensitivity and specificity of 18F-DOPA-PET and MRI in patients with recurrent glioma. Thirteen patients with MRI findings suspicious for recurrent glioma were prospectively enrolled and underwent 18F-DOPA-PET and MRI for neurosurgical planning. Stereotactic biopsies were obtained from regions of concordant and discordant PET and MRI CE, all within regions of T2/FLAIR signal hyperintensity. The sensitivity and specificity of 18F-DOPA-PET and CE were calculated based on histopathologic analysis. Receiver operating characteristic curve analysis revealed optimal tumor to normal (T/N) and SUVmax thresholds. In the 37 specimens obtained, 51% exhibited MRI contrast enhancement (M+) and 78% demonstrated 18F-DOPA-PET avidity (P+). Imaging characteristics included M-P− in 16%, M-P+ in 32%, M+P+ in 46% and M+P− in 5%. Histopathologic review of biopsies revealed grade II components in 16%, grade III in 43%, grade IV in 30% and no tumor in 11%. MRI CE sensitivity for recurrent tumor was 52% and specificity was 50%. PET sensitivity for tumor was 82% and specificity was 50%. A T/N threshold > 2.0 altered sensitivity to 76% and specificity to 100% and SUVmax > 1.36 improved sensitivity and specificity to 94 and 75%, respectively. 18F-DOPA-PET can provide increased sensitivity and specificity compared with MRI CE for visualizing the spatial distribution of recurrent gliomas. Future studies will incorporate 18F-DOPA-PET into re-irradiation target volume delineation for RT planning.
Keywords: Glioma, Recurrent, 18F-DOPA PET, MRI, Stereotactic
Introduction
In 2016, an estimated 23,770 new central nervous system tumors will be diagnosed and 16,060 will succumb to their disease [1]. Gliomas are the most common primary brain tumor diagnosed in children and adults. Despite modern therapy, most patients will eventually experience tumor progression. For patients with high-grade gliomas, progression-free survival remains short at 7 months [2, 3]. While low-grade tumors tend to have a better prognosis, at least half of low-grade glioma patients will experience tumor progression [4, 5].
Although the rate of tumor progression is high for glioma patients, the radiographic determination of tumor progression can be difficult. Following completion of surgery, chemotherapy and radiotherapy for primary disease, the volume of peritumoral edema can be substantial and differentiating treatment-related changes from true tumor progression can be impossible with conventional MRI, as both can result in increased contrast enhancement and T2 signal abnormality. Because of these shortcomings, neurosurgical intervention is often needed to confirm the diagnosis.
The limitations of conventional MRI and invasive nature of neurosurgical intervention intensify the need for an alternative imaging modality with greater insight into tumor kinetics [6–8]. A more accurate imaging modality could not only better identify patients with recurrent disease, but could also enhance localization of recurrent tumor within the typically large areas of post-treatment T2 signal change. 18F-fluoro-deoxyglucose (18F-FDG) positron emission tomography (PET) is commonly used to visualize tumors outside the brain. However, the high rate of brain glucose metabolism limits its utility in the setting of brain tumors. In contrast, brain tumors exhibit high uptake of amino acid tracers relative to the normal surrounding brain [9, 10]. Unlike gadolinium, 3,4-dihydroxy-6-[18F]-fluoro-L-pheny-lalanine (18F-DOPA) is transported across the intact blood brain barrier [11]. A previous report of primarily newly-diagnosed gliomas demonstrated that 18F-DOPA-PET SUV-max more accurately identified regions of high-grade disease than MRI in patients with astrocytomas [12]. Limited data exist to compare 18F-DOPA-PET with conventional MRI in patients with suspected recurrent gliomas [13]. We hypothesize that 18F-DOPA-PET offers enhanced sensitivity and specificity for spatially delineating recurrent glioma than with conventional MRI.
Materials and methods
Clinical trial enrollment
Informed consent was obtained and patients were enrolled in one of two institutional clinical trials evaluating the impact of 18F-DOPA-PET on neurosurgical planning for newly-diagnosed or recurrent gliomas. In order to be included for analysis in the present study, patients needed to be at least 7 years old with MRI findings compatible with recurrent glioma using RANO criteria and a planned craniotomy with resection or biopsy [8]. Any contraindication to MRI or PET would exclude patients from the trials.
Stereotactic neurosurgical planning and tissue acquisition
Patients underwent 18F-DOPA-PET as previously described [12]. CT images were obtained for attenuation correction of PET data. 10 min after injection of the radioisotope, a 10-min 3D PET acquisition was acquired. PET sinograms were reconstructed with a fully 3D-OSEM algorithm into a 300 mm field of view with a pixel size of 1.17 and slice thickness of 1.96 mm. Before the operation, conventional MRI scans were acquired using a 3D T1-weighted spoiled gradient series (field of view 24 × 24 cm, matrix size 256 × 256) after administration of gadolinium–diethylen-etriamine pentaacetic acid contrast (Magnevist). Following this, both a 2D T2-weighted fast spin echo series and a T2-weighted fluid-attenuated inversion recovery (FLAIR) series with contiguous 3-mm-thick slices were obtained.
Tumor SUVmax and normal SUVmean were used to generate the tumor-to-normal (T/N) ratios, which were used guide thresholding and contouring of the tumor on PET imaging by an expert nuclear medicine radiologist. The normal reference brain volume was defined by contouring the contralateral normal brain tissue at the level of the centrum semiovale up through the most superior portion of the brain. An experienced nuclear medicine radiologist contoured the 18F-DOPA PET volumes based on a series of predetermined PET thresholds (ranging from 1.1 to 1.8) using the mean SUV from normal contralateral brain. A T/N > 2.0 was used to identify a sub-region of the 18F-DOPA PET contour that corresponded to the area of highest grade disease components. The 18F-DOPA-PET scan was rigidly registered to the T1 MRI scan using MIM Maestro (MIM Software, Inc, Cleveland, OH) software and the images were transferred to the Stealth Station Neuro-navigation System (Medtronic Sofamor Danek). An experienced Radiation Oncologist contoured the T1 CE and T2 FLAIR abnormalities.
Tissue targets were planned from regions containing the following: T1 contrast enhancement (CE) with PET avidity (M+P+), T1 nonenhancing region with PET avidity (M-P+), a region with T1 CE but not PET uptake (M+P−) and a region with neither contrast enhancement nor PET uptake (M-P−). All biopsies were taken from regions of T2 signal hyperintensity. A representative fusion of PET and MRI volumes is shown in Fig. 1. Patients underwent craniotomy with either a stereotactic biopsy or attempted resection. Locations were only biopsied if the neurosurgeon deemed the location to be low risk for morbidity. For patients who underwent resection, 1–3 simulated stereotactic biopsy sample locations ~ 1 cm3 in size were planned and acquired during the resection, whereas patients undergoing biopsy had at least three locations sampled. Each sample obtained was processed for histopathology and graded independently by an expert neuropathologist blinded to radiographic imaging using hematoxylin and eosin (H&E) staining.
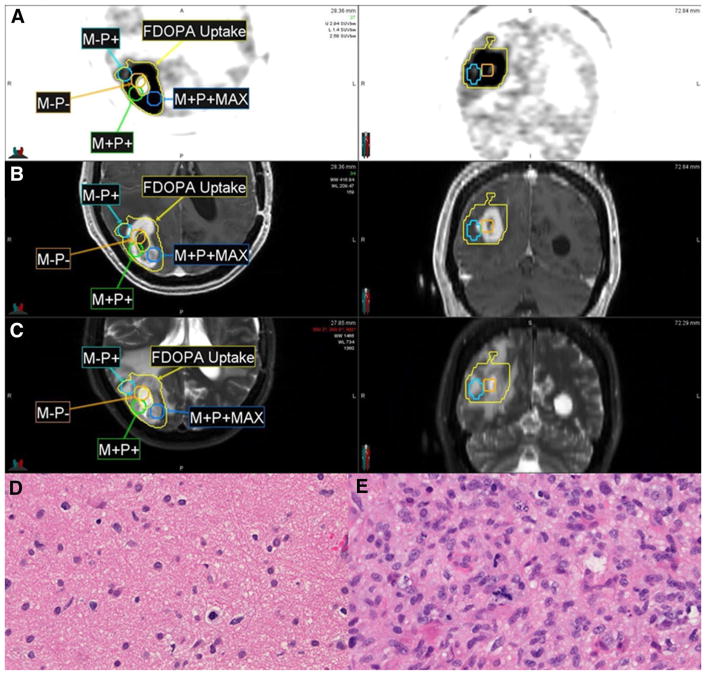
Fig. 1.
Representative 18F-DOPA-PET (a), T1 post-contrast MRI (b), and T2 MRI (c) demonstrating regions of concordant and discordant MRI and PET findings. The regions shown include a 0.5-cm radial expansion upon the stereotactic biopsy coordinate. The M-P+ biopsy specimen (SUVmax 2.73; d) showed lower cellularity compared with the M+P+MAX specimen (SUVmax 4.66; e). M-P−: non-enhancing, PET-negative; M-P+: non-enhancing, PET-positive; M+P+: enhancing, PET-positive
Data analysis
For each biopsied sample, a 5 mm radius contour was created around the stereotactic coordinate point from the center of the location with MIM Maestro to account for the biopsy sample size and uncertainties in the physical biopsy volume. Statistical analysis was performed using JMP 10.0. Sensitivity was calculated by taking true positive results divided by the sum of true positive and false negatives. Specificity was calculated by taking true negative results and dividing by the sum of true negatives and false positives. A receiver operating characteristic (ROC) curve was constructed for evaluation of optimal SUVmax and T/N PET thresholds. Fisher’s exact test was used to compare categorical variables and ANOVA was used for comparison of continuous and categorical variables.
Results
Patient characteristics
Of the 47 patients enrolled in the two clinical trials, 13 had a prior diagnosis of glioma and presented with imaging findings worrisome for recurrent disease. Characteristics of the patients and tumors treated are shown in Table 1. The median age at initial diagnosis was 40 (range 14–64). Primary surgical resection commonly achieved GTR (54%) and pathology revealed astrocytoma histology in most patients. The most common adjuvant treatment course consisted of radiotherapy (69%) with concurrent and adjuvant temozolomide (54%). Characteristics at the time of recurrence are shown in Table 1. The median time to first recurrence was 25 months (range 6–143 months). The median time from initial diagnosis to trial enrollment was 30 months (range 10–247 months). Patients were most commonly enrolled on the trial at the time of first recurrence (69%). One of the two patients with initial low-grade disease had high-grade transformation at the time of recurrence. The highest grade of recurrent disease was II in 8%, III in 38% and IV in 54%. All recurrent tumors had an astrocytic component.
Table 1.
Patient and tumor characteristics
| N = 13 | % | |
|---|---|---|
| Age (years) | ||
| Median (range) | 40 (14–64) | |
| Gender | ||
| Male | 9 | 69 |
| Female | 4 | 31 |
| Initial surgery | ||
| Biopsy | 2 | 15 |
| STR | 4 | 31 |
| GTR | 7 | 54 |
| Initial histology | ||
| Glioblastoma, NOS | 3 | 23 |
| Glioblastoma, IDH wild type | 3 | 23 |
| Glioblastoma, IDH mutant | 1 | 8 |
| Diffuse astrotycoma, IDH mutant | 1 | 8 |
| Anaplastic astrocytoma, IDH wild type | 1 | 8 |
| Anaplastic astrocytoma, IDH mutant | 2 | 15 |
| Anaplastic oligodendroglioma, NOS | 1 | 8 |
| Pleomorphic xanthoastrocytoma | 1 | 8 |
| Initial grade | ||
| II | 2 | 15 |
| III | 4 | 31 |
| IV | 7 | 54 |
| IDH status | ||
| Mutant | 4 | 31 |
| Wild type | 3 | 23 |
| Unknown | 6 | 46 |
| 1p/19q status | ||
| Codeleted | 1 | 8 |
| Single deletion | 1 | 8 |
| Intact | 4 | 31 |
| Unknown | 7 | 54 |
| MGMT status | ||
| Methylated | 1 | 8 |
| Unmethylated | 2 | 15 |
| Unknown | 10 | 77 |
| Initial chemotherapy | ||
| TMZ | 7 | 54 |
| TMZ + dasatinib | 1 | 8 |
| None | 5 | 38 |
| RT delivered | ||
| Yes, initial course | 9 | 69 |
| Yes, recurrence | 2 | 15 |
| No | 2 | 15 |
| Initial RT dose (Gy) | ||
| Median (range) | 59.4 (54–60) | |
| Time to first recurrence (months) | ||
| Median (range) | 25 (6–143) | |
| Time from diagnosis to study enrollment (months) | ||
| Median (range) | 30 (10–247) | |
| Number of recurrences | ||
| 1 | 9 | 69 |
| 2 | 1 | 8 |
| 5 | 2 | 15 |
| 6 | 1 | 8 |
| Prior salvage chemotherapy | ||
| Yes | 3 | 23 |
| No | 10 | 77 |
| Number of salvage chemotherapy courses | ||
| 3 | 2 | 67 |
| 4 | 1 | 33 |
| Salvage chemotherapy agentsa | ||
| TMZ | 5 | 50 |
| CCNU | 2 | 20 |
| PCV | 1 | 10 |
| Bevacizumab | 1 | 10 |
| Prior salvage surgery | ||
| None | 9 | 69 |
| 1 | 1 | 8 |
| 2 | 0 | 0 |
| 3 | 3 | 23 |
| Type of salvage surgeryb | ||
| Biopsy | 1 | 10 |
| STR | 5 | 50 |
| GTR | 4 | 40 |
| Type of study surgery | ||
| Biopsy | 2 | 15 |
| STR | 3 | 23 |
| GTR | 8 | 62 |
| Recurrence gradec | ||
| II | 1 | 8 |
| III | 5 | 38 |
| IV | 7 | 54 |
| Recurrence histologyd | ||
| Astrocytoma | 10 | 77 |
| Oligoastrocytoma | 3 | 23 |
| Oligodendroglioma | 0 | 0 |
STR subtotal resection, GTR gross total resection, IDH isocitrate dehydrogenase, MGMT O-6-methylguanine-DNA-methyltransferase, TMZ temozolomide, CCNU lomustine, PCV procarbacine, cyclophos-phamide, vincristine, RT radiation therapy
Denominator is total number of courses of chemotherapy
Denominator is total number of surgeries performed
The highest grade tissue obtained at the time of study surgery or during any prior procedure
Classified using 2007 WHO criteria. Histologic classification using 2016 WHO criteria was not possible due to a lack of mutation status testing performed on recurrent specimens
Comparison of contoured PET avidity and MRI contrast enhancement
A total of 37 specimens were obtained for analysis. Of these, 19 (51%) were taken from regions of MRI contrast enhancement and 29 (78%) from regions of 18F-DOPA PET avidity as determined by an expert nuclear medicine radiologist. Discordant imaging characteristics included M-P+ in 12 (32%) and M+P− in 2 (5%). Concordance between MRI and PET were seen as M+P+ in 17 (46%) and M-P− in 6 (16%). Sensitivity and specificity analyses are shown in Table 2. MRI sensitivity was 52% and specificity was 50%. PET sensitivity was 82% and specificity was 50%.
Table 2.
Radiographic and histologic correlations
| MRI+ | MRI− | ||||
| PET+ | 17 | 12 | 29 | ||
| PET− | 2 | 6 | 8 | ||
| 19 | 18 | p = 0.13 | |||
| Tumor+ | Tumor− | ||||
| PET+ | 27 | 2 | 29 | Sensitivity | 82% |
| PET− | 6 | 2 | 8 | Specificity | 50% |
| 33 | 4 | p = 0.20 | |||
| MRI+ | 17 | 2 | 19 | Sensitivity | 52% |
| MRI− | 16 | 2 | 18 | Specificity | 50% |
| 33 | 4 | p = 0.95 |
PET threshold analysis
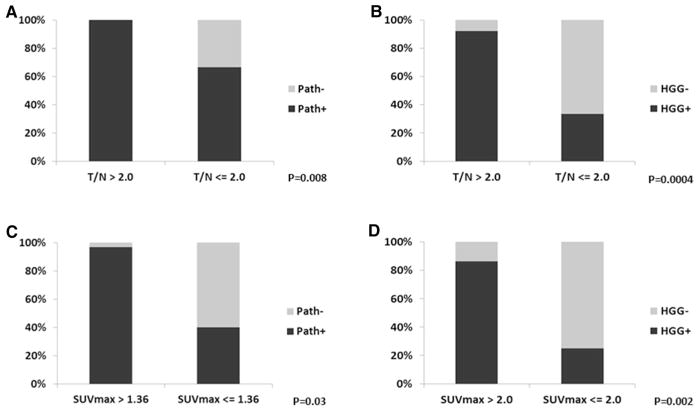
An exploratory analysis of PET tumor-to-normal (T/N) and SUVmax thresholds was performed using a ROC curve analysis. Increasing T/N ratios correlated with the presence of recurrent tumor on biopsy with the ANOVA test (p = 0.002). A T/N ratio of > 1.0 significantly correlated with the presence of recurrent tumor (p = 0.009), with sensitivity of 100% and specificity of 50%. ROC analysis revealed the optimal T/N threshold to be > 2.0, resulting in a sensitivity of 76% and specificity of 100% (p = 0.008; Fig. 2a). An optimal PET SUVmax threshold using ROC analysis was 1.36, which strongly correlated with the presence of recurrent tumor on biopsy (p = 0.03; Fig. 2c) with a sensitivity of 94% and specificity of 75%.
Fig. 2.
Radiographic and pathologic correlations for MRI and 18F-DOPA-PET. A T/N ratio above 2.0 was associated with the presence of recurrent tumor denoted as “Path+” (a), particularly with a high-grade component (b). An SUVmax above 1.36 was similarly associated with the presence of recurrent tumor (c), and an SUVmax above 2.0 correlated with HGG (d)
Radiographic and pathologic correlations
The presence of MRI contrast enhancement was associated with increasing recurrent tumor grade (p = 0.004). Regions of MRI contrast enhancement correlated with the presence of high-grade recurrent tumor (p = 0.03) with a sensitivity of 63% and specificity of 80%. The contoured region of PET avidity did not correlate with recurrent tumor grade (p = 0.35, Table 3). However, a T/N greater than 2.0 (p = 0.001) and SUVmax greater than 2.0 (p = 0.003) correlated with increasing recurrent tumor grade (Table 3). A T/N greater than 2.0 correlated with the presence of high-grade recurrent tumor (p = 0.0004) with a sensitivity of 85% and specificity of 80% (Fig. 2b). A PET SUVmax above 2.0 was associated with 93% sensitivity and 60% specificity (p = 0.002) for the presence of high-grade recurrent tumor (Fig. 2d). Although neither PET avidity (p = 0.12) nor the presence of MRI contrast enhancement (p = 0.51) correlated with tumor histology, an SUVmax greater than 2.0 (p = 0.01) and T/N greater than 2.0 (p = 0.003) correlated with an increasing likelihood of a pure astrocytoma (Table 3).
Table 3.
Correlation between imaging findings and tumor histology
| No tumor | II | III | IV | No tumor | Oligoastrocytoma | Astrocytoma | HGG | No HGG | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI+ | 2 | 0 | 8 | 9 | 2 | 1 | 16 | 17 | 2 | Sensitivity | 63% | |
| MRI− | 2 | 6 | 8 | 2 | 2 | 3 | 13 | 10 | 8 | Specificity | 80% | |
| p = 0.004 | p = 0.51 | p = 0.03 | ||||||||||
| PET+ | 2 | 4 | 13 | 10 | 2 | 2 | 25 | 23 | 6 | Sensitivity | 85% | |
| PET− | 2 | 2 | 3 | 1 | 2 | 2 | 4 | 4 | 4 | Specificity | 40% | |
| p = 0.35 | p = 0.12 | p = 0.17 | ||||||||||
| SUVmax > 2.0 | 1 | 3 | 14 | 11 | 1 | 2 | 26 | 25 | 4 | Sensitivity | 93% | |
| SUVmax ≤ 2.0 | 3 | 3 | 2 | 0 | 3 | 2 | 3 | 2 | 6 | Specificity | 60% | |
| p = 0.003 | p = 0.01 | p = 0.002 | ||||||||||
| T/N > 2.0 | 0 | 2 | 12 | 11 | 0 | 2 | 23 | 23 | 2 | Sensitivity | 85% | |
| T/N ≤ 2.0 | 4 | 4 | 4 | 0 | 4 | 2 | 6 | 4 | 8 | Specificity | 80% | |
| p = 0.001 | p = 0.003 | p = 0.0004 |
A total of eight specimens were obtained in the two patients initially diagnosed with a low-grade glioma. Malignant transformation to high-grade disease was seen in three specimens, which did not correlate with MRI contrast enhancement (p = 1.0), PET avidity (p = 0.14), SUVmax greater than 2.0 (p = 0.14) or T/N greater than 2.0 (p = 0.14) in this small subset of patients.
Discussion
In this series of patients with recurrent gliomas, 18F-DOPA-PET visualized biopsy-proven recurrent disease better than MRI contrast enhancement. Regions of high PET avidity with an SUVmax > 1.36 or T/N > 2.0 had better sensitivity and specificity for tumor than MRI contrast enhancement. However, the presence of high-grade tumor correlated significantly with both MRI contrast enhancement and increasing 18F-DOPA-PET avidity, with higher sensitivity than MRI when SUVmax was > 2.0 or T/N was > 2.0. Taken together, these data support the use of 18F-DOPA-PET in the localization of recurrent gliomas for neurosurgical biopsy targeting, resection planning and radiotherapy target volume delineation.
MRI is the most common imaging modality used for diagnosis and surveillance of gliomas. In patients with newly-diagnosed gliomas, tumor is known to extend beyond contrast enhancement into peritumoral edema [14]. Recurrences are contained within 2 cm of contrast enhancement in 78–90% of patients [15, 16]. The remainder of tumor is considered nonenhancing, which typically resides in an edematous region seen as an increase in T2/FLAIR signal on MRI. Although historical criteria used to identify patients with tumor progression did not take into account nonenhancing tumor, its significance has become widely accepted and has been incorporated into updated response assessment criteria [8, 17]. However, after surgery, chemotherapy and radiotherapy, the volume of FLAIR hyperintensity can be substantial and differentiating delayed treatment-related changes from tumor progression can be problematic, as both can cause increased contrast enhancement. An imaging modality that could better visualize the true extent of recurrent disease would therefore be advantageous.
Amino acid PET is a metabolic imaging modality that has been reported to better visualize gliomas than MRI alone. Unlike contrast enhancement, it is preferentially transported into tumor tissue and has low baseline uptake in the surrounding normal brain. In this study of patients with suspected recurrent glioma, 18F-DOPA-PET sensitivity exceeded that of MRI contrast enhancement. When a T/N threshold of 2.0 or SUVmax threshold of 1.36 was used, both sensitivity and specificity were greater than MRI. A prior study of 35 patients with suspected recurrent gliomas underwent MRI and 18F-DOPA-PET [13]. They reported MRI contrast enhancement sensitivity and specificity to be 92 and 44%, respectively, and PET sensitivity and specificity to be 100 and 89%, respectively. In contrast to our study, recurrent disease was evaluated with a single biopsy or serial imaging and no effort was made to evaluate the distribution of tumor in regions of discordant imaging. Thus, the data presented here offer greater insight into the spatial distribution of tumoral components in recurrent gliomas.
In addition to improved tumor localization, the metabolic information gained with amino acid PET has the potential to noninvasively assess tumor grade and histology [12, 18]. While MRI contrast enhancement correlated with tumor grade in this cohort of recurrent glioma patients, it did not correlate with histology. Because nearly all tumors exhibited some increase in PET avidity, PET uptake did not independently correlate with tumor grade or histology. A prior 18F-DOPA-PET study reported similar findings with a visual, non-quantitative assessment of avidity [19]. Unlike MRI contrast, amino acid PET tracers can be transported through the intact blood brain barrier in lower-grade tumors [11]. Despite this, when a quantitative assessment of PET thresholds was explored, an SUVmax > 2.0 or T/N > 2.0 correlated with increasing tumor grade and astrocytic histology. Specific thresholds for recurrent high-grade tumor have not yet been clearly established in the literature. While some groups report no correlation between SUVmax or T/N with initial tumor grade or histology, they have correlated higher T/N ratios and SUVmax values with poor survival [19–21]. Furthermore, our group and several others have shown that increasing SUVmax values and T/N ratios correlate with the presence of higher tumor grade in primarily newly-diagnosed gliomas [12, 22–24]. Ultimately, these data suggest that most recurrent gliomas exhibit some degree of 18F-DOPA uptake with increasing uptake seen in higher-grade, astrocytic (i.e. more aggressive) tumors.
The benefits of more accurate imaging in patients with suspected recurrent glioma are numerous. In patients with questionable radiographic findings, an imaging modality that better correlates with active tumor could more accurately differentiate tumor progression from pseudoprogression or radionecrosis than MRI. Furthermore, more reliable tumor localization can allow for more extensive resections while minimizing unnecessary morbidity. Given that re-resection can produce new neurologic deficits in 8–39% of patients, knowing the true extent of tumor may help to better predict postoperative deficits while maximizing the extent of resection and potentially enhancing survival [25–27]. Moreover, better tumor localization could direct patients toward noninvasive therapy in lieu of surgery when the risk of morbidity exceeds the expected benefit of resection.
Many patients are treated with reirradiation after other salvage therapies have been rendered ineffective or hazardous. This is typically delivered using stereotactic, hypofractionated techniques targeting solely contrast enhancing tumor [28, 29]. Because nonenhancing tumor is left untreated, approximately 30–40% of recurrences are located outside the reirradiation target volume [30, 31]. As the risk of radionecrosis rises substantially with increasing reirradiation volume, incorporating all of the T2/FLAIR abnormality would likely increase toxicity to unacceptable levels [29]. In contrast, nonenhancing 18F-DOPA-PET avid regions are often significantly smaller than T2/FLAIR volumes [12]. Thus, incorporating 18F-DOPA-PET into the delineation of recurrent glioma treatment volumes could potentially improve tumor control without substantially increasing toxicity.
Although this prospective study has numerous strengths, several limitations exist. This study was primarily designed to compare the spatial distribution of MRI contrast enhancement with 18F-DOPA-PET avidity. Thus, the volume of T2/ FLAIR abnormality was not explicitly compared with the distribution of 18F-DOPA uptake. However, all biopsies were taken from regions of T2 hyperintensity. Furthermore, our group and several others have shown that the T2/FLAIR abnormality is significantly larger than amino acid PET-avid regions [12, 32, 33]. Moreover, our prior study showed no 18F-DOPA-PET avidity outside the T2/FLAIR abnormality and very limited extension of tumor beyond 18F-DOPA-PET avidity into the T2/FLAIR volume at all. The lack of comparison with perfusion and diffusion MRI sequences represents a limitation of this study. Because sensitivity and specificity analyses were based on individual biopsy specimens, patients with more biopsy specimens obtained were more heavily weighted than those with fewer specimens obtained. Similarly, the low frequency of negative biopsies, and overall low number of biopsies performed per patient could limit our statistical robustness. Though this has the potential to influence the results, no clear evidence of bias was seen upon detailed review of the database. The inclusion of low- and high-grade gliomas in this series may influence the interpretation of results, as the radiographic appearance of recurrent disease may be influenced by histology. Because mutation testing was not performed for the recurrent glioma samples obtained during the study, and classification was performed by an expert neuropathologist using the then-current 2007 WHO classification, reclassification of recurrent tumors using the updated 2016 WHO criteria was not performed. Lastly, all patients enrolled on this trial had MRI findings concerning for recurrent disease and all ultimately had biopsy confirmation of recurrence. Thus, these results do not comment on the utility of 18F-DOPA-PET to differentiate pseudoprogression from tumor progression.
In conclusion, 18F-DOPA-PET avidity has better sensitivity than MRI contrast enhancement for the detection of recurrent glioma. Furthermore, a T/N ratio > 2.0 and SUV-max > 1.36 had better sensitivity and specificity than MRI in predicting the presence of tumor. Enhanced sensitivity was also seen for the detection of high-grade disease when the T/N or SUVmax were > 2.0. Future studies will use 18F-DOPA-PET avidity and thresholds to guide neurosurgical resections and radiotherapy target volume delineation for reirradiation in patients with recurrent gliomas.
Supplementary Material
Acknowledgments
This work has been accepted for presentation at the 2017 ASTRO Annual Meeting (September 24–27, 2017) in San Diego, California.
Funding This work was supported by a generous Grant from Brains Together for a Cure, the National Cancer Institute (CA178200) and by a Mayo Brain Tumor NIH SPORE Grant (CA108961).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-018-2750-7) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest No actual or potential conflicts of interest exist pertaining to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. J Am Med Assoc. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 4.Youland RS, Schomas DA, Brown PD, Nwachukwu C, Buckner JC, Giannini C, Parney IF, Laack NN. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15:1102–1110. doi: 10.1093/neuonc/not080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta MP. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30:3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 7.Watling CJ, Lee DH, Macdonald DR, Cairncross JG. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol. 1994;12:1886–1889. doi: 10.1200/JCO.1994.12.9.1886. [DOI] [PubMed] [Google Scholar]
- 8.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–911. [PubMed] [Google Scholar]
- 10.Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 2016;18:426–434. doi: 10.1093/neuonc/nov148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, Giannini C, Laack NN, Parney IF, Lowe VJ, Brinkmann DH, Sarkaria JN. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111:11–18. doi: 10.1007/s11060-012-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C, Kemp BJ, Grams MP, Morris JM, Hoover JM, Hu LS, Sarkaria JN, Brinkmann DH. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15:1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karunanithi S, Sharma P, Kumar A, Khangembam BC, Bandopadhyaya GP, Kumar R, Goenka A, Gupta DK, Malhotra A, Bal C. Comparative diagnostic accuracy of contrast-enhanced MRI and (18)F-FDOPA PET-CT in recurrent glioma. Eur Radiol. 2013;23:2628–2635. doi: 10.1007/s00330-013-2838-6. [DOI] [PubMed] [Google Scholar]
- 14.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 16.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 18.Bell C, Dowson N, Puttick S, Gal Y, Thomas P, Fay M, Smith J, Rose S. Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl Med Biol. 2015;42:788–795. doi: 10.1016/j.nucmedbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Beuthien-Baumann B, Bredow J, Burchert W, Fuchtner F, Bergmann R, Alheit HD, Reiss G, Hliscs R, Steinmeier R, Franke WG, Johannsen B, Kotzerke J. 3-O-methyl-6-[18F]fluoro-L-DOPA and its evaluation in brain tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30:1004–1008. doi: 10.1007/s00259-003-1205-2. [DOI] [PubMed] [Google Scholar]
- 20.Becherer A, Karanikas G, Szabo M, Zettinig G, Asenbaum S, Marosi C, Henk C, Wunderbaldinger P, Czech T, Wadsak W, Kletter K. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30:1561–1567. doi: 10.1007/s00259-003-1259-1. [DOI] [PubMed] [Google Scholar]
- 21.Karunanithi S, Sharma P, Kumar A, Gupta DK, Khangembam BC, Ballal S, Kumar R, Bal C. Can (18)F-FDOPA PET/CT predict survival in patients with suspected recurrent glioma? a prospective study. Eur J Radiol. 2014;83:219–225. doi: 10.1016/j.ejrad.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Chiaravalloti A, Fiorentini A, Villani V, Carapella C, Pace A, Di Pietro B, Di Russo C, Palumbo B, Floris R, Schillaci O. Factors affecting (1)(8)F FDOPA standardized uptake value in patients with primary brain tumors after treatment. Nucl Med Biol. 2015;42:355–359. doi: 10.1016/j.nucmedbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Janvier L, Olivier P, Blonski M, Morel O, Vignaud JM, Karcher G, Taillandier L, Verger A. Correlation of SUV-derived indices with tumoral aggressiveness of gliomas in static 18F-FDOPA PET: use in clinical practice. Clin Nucl Med. 2015;40:e429–435. doi: 10.1097/RLU.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 24.Nioche C, Soret M, Gontier E, Lahutte M, Dutertre G, Dulou R, Capelle L, Guillevin R, Foehrenbach H, Buvat I. Evaluation of quantitative criteria for glioma grading with static and dynamic 18F-FDopa PET/CT. Clin Nucl Med. 2013;38:81–87. doi: 10.1097/RLU.0b013e318279fd5a. [DOI] [PubMed] [Google Scholar]
- 25.Chen MW, Morsy AA, Liang S, Ng WH. Re-do craniotomy for recurrent grade IV glioblastomas: impact and outcomes from the National Neuroscience Institute Singapore. World Neurosurg. 2016;87:439–445. doi: 10.1016/j.wneu.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 26.Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, Ashby LS, Brachman D, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120:846–853. doi: 10.3171/2013.12.JNS13184. [DOI] [PubMed] [Google Scholar]
- 27.Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, Meyer B, Simon M. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd SF, Laing RW, Cosgrove VP, Warrington AP, Hines F, Ashley SE, Brada M. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37:393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 30.Cho KH, Hall WA, Gerbi BJ, Higgins PD, McGuire WA, Clark HB. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45:1133–1141. doi: 10.1016/s0360-3016(99)00336-3. [DOI] [PubMed] [Google Scholar]
- 31.Niyazi M, Jansen NL, Rottler M, Ganswindt U, Belka C. Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat Oncol. 2014;9:299. doi: 10.1186/s13014-014-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64–74. doi: 10.1016/j.ijrobp.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Arbizu J, Tejada S, Marti-Climent JM, Diez-Valle R, Prieto E, Quincoces G, Vigil C, Idoate MA, Zubieta JL, Penuelas I, Richter JA. Quantitative volumetric analysis of gliomas with sequential MRI and (1)(1)C-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging. 2012;39:771–781. doi: 10.1007/s00259-011-2049-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.