Abstract
The outer membrane (OM) of Treponema pallidum, the uncultivatable agent of venereal syphilis, has long been the subject of misconceptions and controversy. Decades ago, researchers postulated that T. pallidum’s poor surface antigenicity is the basis for its ability to cause persistent infection, but they mistakenly attributed this enigmatic property to the presence of a protective outer coat of serum proteins and mucopolysaccharides. Subsequent studies revealed that the OM is the barrier to antibody binding, that it contains a paucity of integral membrane proteins, and that the preponderance of the spirochete’s immunogenic lipoproteins is periplasmic. Since the advent of recombinant DNA technology, the fragility of the OM, its low protein content, and the lack of sequence relatedness between T. pallidum and Gram-negative outer membrane proteins (OMPs) have complicated efforts to characterize molecules residing at the host–pathogen interface. We have overcome these hurdles using the genomic sequence in concert with computational tools to identify proteins predicted to form β-barrels, the hallmark conformation of OMPs in double-membrane organisms and evolutionarily related eukaryotic organelles. We also have employed diverse methodologies to confirm that some candidate OMPs do, in fact, form amphiphilic β-barrels and are surface-exposed in T. pallidum. These studies have led to a structural homology model for BamA and established the bipartite topology of the T. pallidum repeat (Tpr) family of proteins. Recent bioinformatics has identified several structural orthologs for well-characterized Gram-negative OMPs, suggesting that the T. pallidum OMP repertoire is more Gram-negative-like than previously supposed. Lipoprotein adhesins and proteases on the spirochete surface also may contribute to disease pathogenesis and protective immunity.
1 Molecular Architecture of the T. pallidum Cell Envelope
1.1 Experimental Obstacles
Venereal syphilis is a sexually transmitted infection renowned for its protean clinical manifestations and protracted natural history (Radolf et al. 2014), both of which reflect the extraordinary invasiveness and immunoevasiveness of its etiologic agent, Treponema pallidum subsp. pallidum (hereafter referred to as T. pallidum) (Lafond and Lukehart 2006; Radolf et al. 2016). It is also a disease that well into the genomics era presents extraordinary challenges to investigators attempting to unravel its many enigmas (Ho and Lukehart 2011; Radolf et al. 2016). T. pallidum is one of the few major bacterial pathogens of humans that cannot be propagated continuously in artificial medium (Ho and Lukehart 2011; Norris et al. 2001; Radolf et al. 2016). As they have for decades, investigators must employ intratesticular inoculation of rabbits to isolate and propagate the spirochete (Lukehart and Marra 2007). Because T. pallidum cannot be genetically manipulated, experimentalists are restricted primarily to protein-based methodologies to confirm findings and evaluate hypotheses originating from genetic and genomic data. Since the advent of recombinant DNA technology, the fragility of the T. pallidum outer membrane (OM) and its low protein content have served as twin confounders of efforts to characterize molecules residing at the host–pathogen interface (Cameron 2006; Radolf 1995; Radolf et al. 2016).
1.2 Historical Misconceptions
Researchers have long appreciated the importance of the T. pallidum surface in determining the waxing and waning course of syphilis (Radolf et al. 2006). They also have had to overcome a number of misconceptions to clarify its role in disease pathogenesis. Decades ago, investigators recognized that live (i.e., motile) spirochetes react poorly with the antibodies in patient sera (Nelson and Mayer 1949), and they assumed that this property relates to the pathogen’s capacity for immune evasion and persistence (Hardy and Nell 1957; Turner and Hollander 1957). To explain the spirochete’s poor surface antigenicity, the notion evolved that the bacterium acquires a protective coat of serum proteins and host-derived mucopolysaccharides (Alderete and Baseman 1979; Christiansen 1963; Fitzgerald and Johnson 1979). In 1973, the existence of the OM was established unequivocally by transmission electron microscopy (TEM) of ultra-thin sectioned, plastic-embedded organisms (Johnson et al. 1973). The ability to express T. pallidum antigens in Escherichia coli (Norgard and Miller 1983; Stamm et al. 1982; Walfield et al. 1982), the major breakthrough of the early 1980s, attracted to the field many talented molecular biologists intent upon using this powerful new technology to develop a syphilis vaccine. However, these investigators naively assumed that the physical properties and protein content of the syphilis spirochete’s OM are similar to those of E. coli (Radolf et al. 2006). They also incorrectly assumed that treponemal proteins strongly recognized by the human or rabbit syphilitic sera used to screen recombinant libraries were likely to be surface-exposed in T. pallidum (Radolf et al. 2006). The result was the discovery of many notable treponemal antigens, mostly lipoproteins of unknown function at the time, but no OMPs (Cameron 2006; Radolf et al. 2006, 2016).
1.3 The Outer Membrane Hypothesis
As our work with recombinant T. pallidum proteins progressed during the 1980s and early 1990s, several observations led us to question prevailing views about the existence of the outer coat and the nature of the spirochete’s OM. First, while clumps of testicular debris often were observed in proximity to organisms in negatively stained preparations viewed by TEM, a continuous outer coat or layer was not discernible (Hovind-Hougen 1983; Radolf et al. 1986). Subsequently, we confirmed these findings by radioimmunoassay of freshly harvested treponemes collected onto low-protein-binding polycarbonate filters; only negligible amounts of surface-adsorbed immunoglobulins or serum proteins were detected (Cox et al. 1992). Second, it was noted by routine negative staining that the OM was easily disrupted by routine experimental manipulations, such as centrifugation and suspension, or exposure to low concentrations of non-ionic detergents (Cox et al. 1992; Radolf et al. 1988), conditions that do not perturb the OMs of Gram-negative bacteria. Third, removal of OMs using low concentrations of the non-ionic detergent Triton X-114 (TX114) did not result in an appreciable loss of major membrane immunogens detected by immunoblot analysis with syphilitic serum (Fig. 1a) (Radolf et al. 1988). Finally, organisms lacking OMs showed markedly greater reactivity with syphilitic sera than intact treponemes (Cox et al. 1992, 1995; Radolf et al. 1988).
Fig. 1.

The T. pallidum cell envelope. a T. pallidum’s major immunogens are associated with the protoplasmic cylinder, not the outer membrane. Reactivity with human syphilitic serum of proteins extracted with Triton X-114 from whole T. pallidum cells (lane 1), protoplasmic cylinders (lane 2), and solubilized outer membranes (lane 3); reproduced from reference (Radolf et al. 1988). b Freeze-fracture EM reveals scarce intramembranous particles (IMPs) within the T. pallidum OM. Convex and concave leaflets of the OM are indicated. Bar, 0.5 μM. Reproduced from reference (Radolf et al. 1994). c Deep etching reveals that OM intramembranous particles are surface-exposed. Arrowheads indicate the boundaries separating the bacterial surface from the convex fracture face. Particles on the convex fracture face and the treponemal surface are indicated by thin and medium-thickness arrows, respectively. Bar, 0.5 μM. Reproduced from reference (Bourell et al. 1994). d TX-114 phase partitioning reveals that the syphilis spirochete’s major immunogens (based on reactivity with human syphilitic serum) possess hydrophobic character. Lanes: 1. Percoll-purified T. pallidum. 2. TX-114-insoluble material. 3. TX114 detergent-enriched phase proteins. 4. aqueous phase proteins. Reproduced from Reference (Radolf et al. 1988). e Scanning probe microscopy reveals rare particles on the T. pallidum surface; reproduced with permission from reference (Liu et al. 2010). f Cryoelectron microscopy (longitudinal slice) showing, from the inside out, cytoplasmic filaments (red line), cytoplasmic membrane (green line), lipoprotein layer (purple circles), peptidoglycan layer (tan line), flagellar filament (thick blue line), and outer membrane (green line). Bar, 50 nM. Reproduced with permission from reference (Liu et al. 2010). g [3H]palmitate-labeled lipids were extracted from isolated T. pallidum outer membranes and separated by two-dimensional thin layer chromatography. GL glycolipids; CL cardiolipin; PC phosphatidylcholine; PS phosphatidylserine; PG phosphatidylglycerol; O origin. Reproduced from reference (Radolf et al. 1995b)
1.4 Rare Outer Membrane Proteins
Collectively, the above findings led us to hypothesize that the spirochete’s fragile OM, not an outer coat, serves as the barrier to antibody binding. Of course, to do so, it would need to have a much lower protein content than conventional Gram-negative bacterial OMs. The question, then, was how to prove this unorthodox idea given how little was known at the time about the molecular architecture and composition of the T. pallidum cell envelope. Freeze-fracture EM provided part of the solution. This ‘OMP-agnostic’ technique revealed that the density of integral membrane proteins (visualized as intramembranous particles, IMPs) in the T. pallidum OM is ~100-fold less than that of E. coli OMs (Fig. 1b) (Radolf et al. 1989b; Walker et al. 1989). A variant of the freeze-fracture technique, deep etching, showed that these low-abundance particles protrude from the spirochete’s surface (Fig. 1c) (Bourell et al. 1994; Radolf et al. 1989b) and, therefore, can interact directly with host cells, tissue components, and circulating molecules, including antibodies. Subsequent efforts to molecularly characterize these morphological entities became known as “the quest for T. pallidum outer membrane proteins” (Radolf 1995).
1.5 Lipoprotein Immunogens
TX114 phase partitioning led to the other major piece of the surface antigenicity riddle, which continues today—the identification and localization of the syphilis spirochete’s lipoprotein immunogens (Chamberlain et al. 1989a). This technique, developed by Bordier in the 1980s for isolating membrane-associated proteins (Bordier 1981), exploits the relatively low cloud point (~20 °C) of TX114 (Brusca and Radolf 1994). Above the cloud point, TX114 micelles become too large to remain in suspension and can be collected by centrifugation. Membrane proteins incorporated into TX114 micelles at the low temperatures used for solubilization will pellet with the heavier detergent-enriched phase after warming, leaving water-soluble proteins behind in the lighter, aqueous phase. This simple but extremely powerful method revealed that the syphilis spirochete’s major immunogens, as determined by reactivity with syphilitic sera, possess hydrophobic character (i.e., they were recovered in the detergent-enriched phase) (Fig. 1d) (Chamberlain et al. 1989a; Radolf et al. 1988). Within the next several years, DNA sequencing determined that these highly immunogenic membrane proteins are synthesized with signal peptides terminated by lipid modification motifs (Akins et al. 1993; Becker et al. 1994; Purcell et al. 1990; Swancutt et al. 1990; Weigel et al. 1992), now referred to as “lipoboxes” (Setubal et al. 2006). These genetic findings were corroborated at the protein level (i) by radiolabeling of polypeptides in T. pallidum and/or in E. coli with [14C] or [3H]palmitate (Akins et al. 1993; Chamberlain et al. 1989a, b; Purcell et al. 1990; Swancutt et al. 1990); (ii) in some cases, by recovery of radiolabeled fatty acids in the expected 2:1 (ester-to-amide) ratio following sequential alkaline and acid hydrolysis (Chamberlain et al. 1989a; Swancutt et al. 1990); and/or (iii) by showing that processing of native lipoproteins in T. pallidum or lipoproteins expressed in E. coli was prevented by globomycin (Purcell et al. 1990; Swancutt et al. 1990), a specific inhibitor of signal peptidase II, the enzyme that cleaves the signal peptides of lipoproteins at the lipid-modified cysteine residue (Tokunaga et al. 1984). Importantly, in contrast to the lipid-modified proteins, recombinant lipoproteins without their N-terminal acylation signals partitioned into the TX114 aqueous phase (Akins et al. 1993; Chamberlain et al. 1989b; Purcell et al. 1990; Swancutt et al. 1990), demonstrating that the hydrophobic character and membrane association of native lipoproteins were due to their lipid moieties. Parallel freeze-fracture EM experiments showed that, in contrast to proteins with transmembrane domains (e.g., bacteriorhodopsin and bovine rhodopsin), lipoproteins incorporated into liposomes do not form IMPs (Jones et al. 1995). Together, these results indicated that the protein moieties of lipoproteins are extrinsic to the lipid bilayer and, by extrapolation, that the particles observed in freeze-fractured OMs could not be lipoproteins. Immunoelectron microscopy (IEM) and immunofluorescence analysis (IFA) using antisera generated against numerous recombinant lipoproteins (Tpp47 [TP0574], Tpp15 [TP0171], Tpp17 [TP0435], Tpp34 [TP0971], GlpQ [TP0257]) revealed that none of these immunogens could be detected on the spirochete’s surface and, instead, were localized to the periplasmic compartment (Cox et al. 1992, 1995; Deka et al. 2007; Shevchenko et al. 1999). Crucial for these localization experiments was our development of the gel microdroplet method (see below) as a means of maintaining the integrity of the fragile T. pallidum OM throughout the labeling process (Cox et al. 1995; Luthra et al. 2015b).
1.6 A Model for the T. pallidum Cell Envelope
By the mid-1990s, it was possible to integrate the above information into a model for the T. pallidum cell envelope that explains the spirochete’s poor surface antigenicity in vitro and its stealth pathogenicity in vivo (Cox et al. 1992; Radolf 1995). The model has two basic components: (i) the OM contains a paucity of integral membrane proteins and surface-exposed lipoproteins and (ii) the preponderance of the spirochete’s major membrane immunogens are lipoproteins, with most tethered by their N-terminal lipids to the periplasmic leaflet of the CM. Over the past two decades, data obtained using electron microscopy, biochemistry, and structural biology have supported the model’s validity. Consistent with the freeze-etch results, scanning probe microscopy of T. pallidum directly visualized sporadic particles on an otherwise smooth bacterial surface (Fig. 1e) (Liu et al. 2010). Cryoelectron microscopy (CryoEM) visualized the native T. pallidum OM as a simple lipid bilayer (Fig. 1f) (Izard et al. 2009; Liu et al. 2010), quite unlike that of Borrelia burgdorferi, whose external surface possesses an easily discernible proteinaceous layer (Liu et al. 2009). In accord with the notion of a dense array of lipoproteins tethered to the CM’s periplasmic leaflet, cryoEM revealed protein “studs” aligned above the CM and below the PG layer (Liu et al. 2010) (Fig. 1f). Tp47, the first T. pallidum protein shown to be lipid-modified (Chamberlain et al. 1989b), was found to be a penicillin-binding protein with DD-carboxypeptidase activity involved in PG remodeling (Deka et al. 2002; Weigel et al. 1994), whereas other lipoprotein immunogens are proven substrate-binding proteins (SBPs) for ABC transporters that shuttle a variety of nutrients across the CM (Becker et al. 1994; Brautigam et al. 2016; Deka et al. 2004a, b, 2006a, b, 2013; Machius et al. 2007; Porcella et al. 1996).
2 The Quest for T. pallidum Outer Membrane Proteins
2.1 Isolation of Outer Membranes
Prior to the availability of the genomic sequence, isolation of T. pallidum OMs seemed the most straightforward approach to identifying rare OMPs (Blanco et al. 1994; Radolf et al. 1995b). The underlying assumption was that rare OMPs enriched in the OM fraction could be identified by SDS-PAGE in combination with peptide sequencing or mass spectrometry and subsequently cloned. Though rational in concept, the results were profoundly disappointing. The most abundant protein in the OM preparations, originally designated T. pallidum rare outer membrane protein 1 (Tromp1) (Blanco et al. 1995), was shown by metal analysis and X-ray crystallography to be the SBP for a transition metal ABC transporter (Deka et al. 1999; Lee et al. 1999, 2002) and, therefore, could not be an OMP. Other OM-enriched proteins were obvious periplasmic contaminants (Shevchenko et al. 1997). Isolation of OMs did, however, yield one valuable dividend—determination of the membrane’s lipid composition. The T. pallidum OM consists principally of phosphatidylcholine, phosphatidylglycerol, phosphatidylserine, and an uncharacterized, poorly immunogenic glycolipid (Fig. 1g) (Radolf et al. 1995b). This lipid profile differs greatly from those of the E. coli (Silhavy et al. 2010) and B. burgdorferi OMs (Radolf et al. 1995a). Notably absent was lipopolysaccharide (LPS), the highly proinflammatory glycolipid responsible for creating the OM permeability barrier in Gram-negatives (Nikaido 2003), subsequently confirmed by the genomic sequence (Fraser et al. 1998). The lack of LPS likely explains the relative permeability of the T. pallidum OM to long-chain fatty acids (LCFAs) compared to that of E. coli (Cox and Radolf 2001). It also helps to explain why Toll-like receptor (TLR)-based innate immune surveillance mechanisms (Kawai and Akira 2010) fail to detect hematogenously disseminating bacteria early during the disease (Radolf et al. 2006) as well as the absence of sepsis-like symptomatology in demonstrably spirochetemic secondary syphilis patients (Cruz et al. 2010; Radolf et al. 2014).
2.2 β-Barrel Predictions
As the 1990s ended, the need for a new line of attack became obvious. The genomic sequence (Fraser et al. 1998) provided the means for this renewed assault, but not without some twists. One surprise was that the spirochete’s genome did not encode orthologs for any well-studied OMPs. Equally unexpected was the finding that it encodes a 12-member paralogous family, designated the T. pallidum repeat (Tpr) family, whose members have sequence homology to the major outer sheath protein (MOSP) of Treponema denticola (Centurion-Lara et al. 1999; Fraser et al. 1998), a known pore-forming protein and adhesin (Anand et al. 2013; Egli et al. 1993; Ellen 2006). The question for investigators, then, was how to take advantage of this powerful new tool to solve the OMP problem. Structural biology eventually provided a solution in the form of the β-barrel, the hallmark conformation of OMPs in all organisms with OMs as well as eukaryotic organelles derived from them (e.g., mitochondria and chloroplasts) (Wimley 2003). By the mid-2000s, algorithms for identifying proteins predicted to form β-barrels with acceptable false-discovery rates were available. As an alternative to unproductive genome mining for sequence orthologs, we devised a consensus computational framework that used a battery of subcellular localization and β-barrel structural prediction tools to identify and rank candidate OMPs (Cox et al. 2010). Recently, using additional β-barrel prediction algorithms (Markov Chain Model for Beta Barrels [MCMBB]) (Bagos et al. 2004b) and Transmembrane β-barrel proteome database [TMBB-DB] (Freeman and Wimley 2012)), along with structural modeling (Swiss-Model and I-TASSER) (Biasini et al. 2014; Yang and Zhang 2015), and domain identification tools (Conserved Domain Database [CDD], pfam, and InterProScan) (Jones et al. 2014), we have modified and expanded the predicted OMPeome of T. pallidum using the Nichols strain as our reference genome (see Table 1 for our current list). Of note, this list should apply to all other syphilis spirochete strains, given their remarkably low degree of sequence divergence (Arora et al. 2016; Smajs et al. 2012). The candidates fall into two classes: T. pallidum repeat proteins (Tprs) and a group of unrelated proteins, most of which are annotated as hypotheticals. Despite their lack of sequence homology, the ‘hypotheticals’ appear to be structural and, presumably, functional orthologs of well-characterized Gram-negative bacterial OMPs. Collectively, these findings suggest that T. pallidum is more Gram-negative-like than previously supposed and that the appearance of Gram-negative-like OMs predated the evolution of proteobacteria.
Table 1.
The predicted Treponema pallidum OMPeome
| TP_ID | Protein annotation | Conserved domains | Structural similarity (PDB ID) | Proposed function | References |
| TP0011 | TPR protein B (TtprB) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013) |
| TP0117 | TPR protein C (TprC) | MOSPC and MOSPN | None | Porin | Anand et al. (2012, 2015), Centurion-Lara et al. (1999, 2013), Gray et al. (2006), Sun et al. (2004) |
| TP0131 | TPR protein D (TprD) | MOSPC and MOSPN | None | Porin | Anand et al. (2012, 2015), Centurion-Lara et al. (1999, 2013), Gray et al. (2006), Sun et al. (2004) |
| TP0313 | TPR protein E (TprE) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013), Gray et al. (2006), Stamm et al. (1998) |
| TP0317 | TPR protein G (TprG) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013), Gray et al. (2006), Stamm et al. (1998) |
| TP0610 | TPR protein H (TprH) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013) |
| TP0620 | TPR protein I (TprI) | MOSPC and MOSPN | None | Porin | Anand et al. (2015), Centurion-Lara et al. (1999, 2013), Gray et al. (2006), Sun et al. (2004) |
| TP0621 | TPR protein J (TprJ) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013), Gray et al. (2006); Stamm et al. (1998) |
| TP0897 | TPR protein K (TprK) | MOSPC and MOSPN | None | Unknown | Centurion-Lara et al. (1999), Cox et al. (2010), Giacani et al. (2012), Hazlett et al. (2001), Pinto et al. (2016) |
| TP1031 | TPR protein L (TprL) | MOSPC and MOSPN | None | Probable porin | Centurion-Lara et al. (1999, 2013) |
| TP0126 | hypothetical protein | None found | 2×27 | OmpW-like ion-channel involved in transport of small hydrophobic molecules | Giacani et al. (2015), Hong et al. (2006) |
| TP0326 | outer membrane protein | Surface antigen (Beta-barrel), Polypeptide Transport domains (POTRA) | 4K3B | BamA, Outer membrane biogenesis | Cameron et al. (2000), Desrosiers et al. (2011), Luthra et al. (2015a) |
| TP0515 | hypothetical protein | LPS-assembly outer membrane protein LptD, Organic solvent tolerance protein OstA | 4Q35 | LPS-assembly protein LptD, substrate unknown | This chapter, Botos et al. (2016), Gu et al. (2015) |
| TP0548 | hypothetical protein | Uncharacterized protein family (UPF0164) | 3BRY | TbuX/FadL-like, long-chain fatty acid transport protein | Cox et al. (2010), van den Berg et al. (2004) |
| TP0733 | hypothetical protein | None found | 2MHL | OprG/OmpW-like ion-channel involved in transport of small hydrophobic molecules | This chapter, Hong et al. (2006) |
| TP0856 | hypothetical protein | Uncharacterized protein family (UPF0164) | 3BS0 | TodX/FadL-like long-chain fatty acid transporter | This chapter, van den Berg et al. (2004, 2005) |
| TP0858 | hypothetical protein | Uncharacterized protein family (UPF0164) | 3DWO | TbuX/FadL-like long-chain fatty acid transporter | Cox et al. (2010), van den Berg et al. (2004), van den Berg (2005) |
| TP0859 | hypothetical protein | Uncharacterized protein family (UPF0164) | 3BRZ | FadL-like long-chain fatty acid transport protein | This chapter, van den Berg et al. (2004), van den Berg (2005) |
| TP0865 | hypothetical protein | Uncharacterized protein family (UPF0164) | 3BRY | TbuX/FadL-like long-chain fatty acid transport protein | This chapter, van den Berg et al. (2004), van den Berg (2005) |
| TP0966 | hypothetical protein | None found | 5AZS | OprJ-like outer membrane efflux protein | This chapter, Yonehara et al. (2016) |
| TP0967 | hypothetical protein | None found | 5AZO | OprN-like outer membrane efflux protein | This chapter, Yonehara et al. (2016) |
| TP0969 | hypothetical protein | OEP (outer membrane efflux protein) family | 2VDE | TolC-like outer membrane efflux protein | Bavro et al. (2008), Cox et al. (2010) |
NA = Not available
Signal peptide and transmembrane helixes were predicted by TOPCONS (Tsirigos et al. 2015), LipoP (Juncker et al. 2003), Phobius (Kall et al. 2007), TMHMM (Krogh et al. 2001), and SignalP (Petersen et al. 2011)
Subcellular localizations were predicted by Cello (Yu et al. 2006) and PsortB (Yu et al. 2010)
Conserved domain identification was done by Conserved Domain Database (CDD) (Marchler-Bauer et al. 2015), pfam (Finn et al. 2016), and InterProScan (Jones et al. 2014)
β-barrel outer membrane protein predictions were performed using TMBETA-RBF (Ou et al. 2008), Markov Chain Model for β-barrels (MCMBB)(Bagos et al. 2004a), and transmembrane β-barrel proteome database (TMBB-DB) (Freeman and Wimley 2012)
2.3 Establishing Authenticity–Biophysical Properties
Bioinformatics is only the starting point for proving that a candidate is a bona fide β-barrel-forming OMP. Establishing authenticity requires demonstrating that a protein has the biophysical properties expected of an OMP and is surface-exposed in T. pallidum (Anand et al. 2012, 2013, 2015; Desrosiers et al. 2011; Luthra et al. 2011). The three essential biophysical properties of an OM-spanning β-barrel are (i) amphiphilicity (i.e., ability to insert into a lipid bilayer), (ii) extensive β-sheet secondary structure, and (iii) adoption of a closed conformation (Wimley 2003). To examine amphiphilicity, we use TX114 phase partitioning of native (i.e., immunoblotting from phase-partitioned cell lysates) and folded recombinant proteins, and we assess the ability of the folded recombinant protein to insert into liposomes. For the latter, we typically use liposomes with a phospholipid composition simulating that of the T. pallidum OM (Radolf et al. 1995b). Proteins that do insert into liposomes can be examined for porin activity; channel formation is a strong evidence for β-barrel formation as well as functional activity (Zeth and Thein 2010). β-sheet content can be assessed quantitatively by far UV circular dichroism (CD) spectroscopy (Shao et al. 1996). Heat modifiability is a technically simple, but powerful, indicator of β-barrel formation; β-barrels are very stable structures that typically migrate faster by SDS-PAGE without than with boiling in final sample buffer (Conlan and Bayley 2003). TEM is an additional means of demonstrating that the folded recombinant protein forms a closed circular structure (Dorset et al. 1983). Incorporation of the folded protein into nanodiscs (a protein scaffold that encloses a lipid bilayer) (Nath et al. 2007) enables one to simultaneously confirm amphiphilicity and ability to circularize (Anand et al. 2015).
2.4 Establishing Authenticity–Surface Exposure in T. pallidum
Because of the many pitfalls inherent in surface labeling spirochetes, particularly an organism with as fragile an OM as T. pallidum, complementary methods always should be used before drawing conclusions about surface-exposure. We typically employ (i) IFA in our gel microdroplet system (see reference (Luthra et al. 2015b) for a detailed description), (ii) proteinase K (PK) accessibility, and (iii) opsonophagocytosis assay using rabbit peritoneal macrophages (Anand et al. 2012, 2013, 2015; Desrosiers et al. 2011; Hazlett et al. 2001, 2005; Lukehart and Miller 1978; Luthra et al. 2011). Each method has strengths and weaknesses and presents its own set of technical challenges. The importance of including control antisera for proteins or protein domains whose locations on the surface or in the periplasm are universally accepted cannot be over-emphasized. Antibodies against the flagellar sheath protein FlaA are often used for this purpose. Opsonophagocytosis is sensitive and, because it uses live organisms, surface-specific. However, background levels of internalization can be high and, as with any complex bioassay, reproducibility can be a problem. With PK accessibility experiments, use of motile organisms is extremely important; we use videomicroscopy to document motility throughout the PK incubation period (Desrosiers et al. 2011). Importantly, opsonophagocytosis assay and PK accessibility only determine whether an antigen is surface-exposed. In addition to being able to detect proteins expressed on the T. pallidum surface in low copy numbers, the gel microdroplet assay allows localization of periplasmic proteins or the periplasmic domains of bipartite OMPs following controlled removal of OMs. Moreover, when performed in a double-labeling format with an antibody directed against FlaA or another periplasmic marker, the method enables one to assess the intactness of individual organisms thought to be surface-labeled (Cox et al. 2010; Hazlett et al. 2005). This is important because even under optimal circumstances, a small percentage of organisms (usually ~5%) have disrupted OMs.
3 The Expanding Repertoire of Rare Outer Membrane Proteins
3.1 T. pallidum Repeat Proteins (Tprs)
3.1.1 TprC/D, TprI, and TprF
Among the Tprs, our original consensus computational matrix (Cox et al. 2010) identified TprC/D (TprC [TP0117] and TprD [TP0131] are identical in the Nichols strain) and TprI (TP0620) as the strongest candidate OMPs. We verified these predictions for the native and recombinant proteins using the methods described above (Anand et al. 2012, 2015). In T. pallidum, native TprC/D and TprI are low abundance (~200 copies each per cell), trimeric, amphiphilic, and surface-exposed, while the folded recombinants form β-sheet-rich, heat-modifiable trimers that partition into the TX114 detergent-enriched phase and insert readily into artificial membranes. As with T. denticola MOSP (Anand et al. 2013; Egli et al. 1993), integration of TprC/D and TprI into liposomes results in increases in permeability comparable to those produced by the archetypal porin, E. coli OmpF (Anand et al. 2012, 2015; Nikaido 2003). With classical porins, the entire polypeptide forms the β-barrel (Nikaido 2003). However, to our surprise, the NCBI conserved domain database (CDD) revealed that Tpr C/D and TprI contain N- and C-terminal regions related to the corresponding domains of T. denticola MOSP (Fig. 2a) (Anand et al. 2012, 2015). When examined separately as recombinant proteins, the MOSPC domains of TprC and TprI formed amphiphilic β-barrels with porin activity in vitro (Fig. 2b) and were surface-exposed in T. pallidum (Fig. 2c). The MOSPN domains, in contrast, were a mixture of α-helix and β-sheet, lacked amphiphilic character, and were periplasmic in T. pallidum (Fig. 2c). Consistent with these results, TprF, a truncated protein which contains only a MOSPN domain (Fig. 2a), lacked amphiphilicity (Fig. 2b), failed to increase liposome permeability (Fig. 2b) and was found by small-angle X-ray scattering (SAXS) analysis to have an elongated structure (Anand et al. 2015). Because TprF is identical to the MOSPN domains of TprC and TprI along most of its length, this elongated structure almost certainly applies to the N-terminal halves of TprC and TprI as well (Anand et al. 2015).
Fig. 2.

Bipartite topology of Tpr C/D and I (Nichols strain). a Domain architectures of T. denticola major outer surface protein (MOSP) and TprC/D/I/F subfamily members. The signal sequences of all three proteins are shown in blue. The portions of TprC and TprI colored in black and yellow, respectively, denote the TprC- and TprI-specific regions of each protein (TprCSp and TprISp). The green regions in TprI and TprF denote regions present in TprI and TprF but not TprC (TprI/FSp). Reproduced with permission from reference (Anand et al. 2015)). b The MOSPC domains of TrpC and TprI are solely responsible for membrane insertion and pore formation by the full-length proteins. Liposomes were reconstituted with folded, full-length recombinant proteins (TprCFl and TprIFl), TprF, or the MOSPC (TprCC and TprIC) or MOSPN (TprCN and TprIN) domains of TprC and TprI followed by sucrose density gradient ultracentrifugation and immunoblot analysis. The top fractions (TF) contain liposome-incorporated material, whereas the middle and bottom fractions (MF and BF, respectively) contain unincorporated material. The bar graphs show pore formation by the same proteins, along with E. coli OmpF (positive) and OmpA (negative) controls, measured by efflux of encapsulated into liposomes (100% efflux = the degree of quenching obtained by detergent lysis). Statistical significance compared with E. coli OmpF was assigned according to the following scheme: * P < 0.05; ** P < 0.0001. Reproduced from Reference (Anand et al. 2015). c Bipartite topology of native TprC and TprI in live treponemes. Motile T. pallidum were encapsulated in gel microdroplets and probed with 1:100 dilutions of rat antisera against TprCN, TprCC, or FlaA without (intact I) or with the removal of OMs (Permeabilization P) by pre-incubation with 0.10% Triton X-100. Antibody binding was detected with goat anti-rat Alexa Fluor 488 (green) conjugate. Given that TprCC antibodies are highly cross-reactive with TprIC, both TprC and TprI are being labeled. Reproduced from reference (Anand et al. 2015)
By conventional TX114 phase partitioning of T. pallidum, both native TprC/D and TprI fractionate with the detergent-insoluble material (Fig. 3a), which contains the peptidoglycan sacculus (Fig. 3b) (Radolf et al. 1989a). However, if TprC and TprI were first dissociated from the sacculus by extraction with the detergent n-dodecyl-β-D-maltoside (DDM), they then partitioned into the TX114 detergent-enriched phase (Fig. 3a). These results, in combination with those described above for the recombinant proteins, indicate that, with both Tprs, the C-terminal β-barrels insert into the OM, while the N-terminal portions extend downward, anchoring the barrels to the PG sacculus within the periplasm (Fig. 3c); structural modeling predicts that the β-barrels contain 10 transmembrane strands (Fig. 3d). Interestingly, pre-extraction with DDM did not release TprF (Fig. 3a), indicating that it is even more tightly bound to the sacculus than TprC and TprI. Heterologous expression studies performed with E. coli surrogates provided additional support for the bipartite model. When TprC/D, TprI, and TprF were placed downstream of PelB leader sequences, TprC/D and TprI were OM-associated with only their C-terminal β-barrels surface-exposed, while TprF was exclusively periplasmic (Fig. 4). Finally, since MOSP is considered the parental Tpr ortholog, we also examined its domain architecture (Anand et al. 2013). Our finding that only MOSPC forms a β-barrel with porin activity and is surface-exposed in T. denticola strongly suggests that the bipartite architectural model applies to the entire Tpr family.
Fig. 3.

Native TprC and TprI are amphiphilic but tethered to the peptidoglycan sacculus, whereas TprF is tightly bound to the peptidoglycan sacculus. a Triton X-114 phase partitioning of T. pallidum lysates without (−) or with (+) pre-solubilization with 2% DDM. Whole cells (WC), Triton X-114-insoluble material (Ins), and aqueous and Triton X-114-enriched phases (Aq and Det, respectively) were separated by SDS-PAGE followed by immunoblotting using antisera specific for TprC (top), TprI (middle), or TprI and F (bottom). Arrowheads in bottom panel indicate TprF; TprI is the larger protein. Reproduced from reference (Anand et al. 2015). b Extensively washed Triton X-114-insoluble material visualized in negatively stained whole mounts by transmission electron microscopy. Previous studies have shown that this material contains the peptidoglycan sacculus (Radolf et al. 1989a). Reproduced from reference (Anand et al. 2015). c Bipartite model for Tpr C/D and TprI. d Structural model of TprC (Nichols) generated using TMBpro (Randall et al. 2008) predicts a 10-stranded β-barrel
Fig. 4.

TprC and TprI, but not TprF, expressed in E. coli with PelB signal sequences display bipartite topology. IFA of intact (I) or permeabilized (P) E. coli C41 (DE3) expressing TprC, TprI, or TprF with a PelB signal sequence were probed with rat antisera against TprCN, TprCC, and Skp (periplasmic control). Antibody binding was detected with goat anti-rat Alexa Fluor 488 conjugate. Reproduced from reference (Anand et al. 2015)
3.1.2 TprK
When the T. pallidum Nichols genomic sequence became available and the existence of the Tpr family came to light (Fraser et al. 1998), TprK drew immediate attention because of its sequence relatedness to T. denticola MOSP and its relatively high level of expression, determined by semi-quantitative qRT-PCR, among tpr genes (Centurion-Lara et al. 1999). In their landmark study, Centurion-Lara et al. (1999) reported that antibodies against TprK promote opsonophagocytosis of treponemes by rabbit peritoneal macrophages and that immunization with a large N-terminal fragment of TprK induces partial protection against T. pallidum challenge. The subsequent discovery that TprK undergoes intra-strain variation, generating subpopulations of organisms with variant TprK sequences as infection proceeds (Centurion-Lara et al. 2000; LaFond et al. 2003, 2006a; Stamm and Bergen 2000), suggested that the protein plays a fundamental role in immune evasion by T. pallidum (Deitsch et al. 2009; Ho and Lukehart 2011; Lafond and Lukehart 2006). Importantly, accumulation of TprK sequence variants occurs in syphilis patients as well as infected rabbits (LaFond et al. 2003, 2006a; Myint et al. 2004; Pinto et al. 2016). Indeed, an extraordinary recent study from Portugal in which DNAs for genomic sequencing were ‘captured’ from genital ulcer swabs described “rampant” sequence variation in tprK genes (Pinto et al. 2016). Variability in tprK/TprK sequences is not randomly distributed. It occurs in seven discrete variable (V) regions separated by stretches of conserved sequences (Fig. 5), with some V-regions displaying greater sequence diversity than others (Deitsch et al. 2009; LaFond et al. 2003; Pinto et al. 2016). DNA sequence cassettes that correspond to V-region sequences were discovered in an area of the T. pallidum chromosome located away from the tprK gene (Centurion-Lara et al. 2004). The authors proposed that these cassettes serve as unidirectional donor sites for the generation of variable regions by nonreciprocal gene conversion (Fig. 5) (Deitsch et al. 2009; Ho and Lukehart 2011; Lafond and Lukehart 2006). Generation of TprK variants differs widely among T. pallidum strains and, surprisingly, appears to occur at particularly low frequency in the Nichols reference strain (Giacani et al. 2012; LaFond et al. 2006a), perhaps because of extensive passage in rabbits. Sequence variability in the tprk donor and expression sites may also explain these strain differences (Giacani et al. 2012). It is important to note that the Lisbon genomic sequences described above were obtained in a relatively confined locale (i.e., Lisbon) and, thus, may be derived from the same T. pallidum strain circulating in a circumscribed social network.
Fig. 5.

Variation in TprK is attributed to gene conversion wherein variant DNA segments adjacent to tprD recombine with variable regions (V1–V7) of tprK to generate new TprK mosaics. Reproduced with permission from reference (Ho and Lukehart 2011)
The TprK protein elicits both cellular and humoral immunity in infected animals (LaFond et al. 2006b; Morgan et al. 2002a, 2003). TprK antibodies are specifically targeted to the V-regions (Morgan et al. 2002b, 2003), which are thought to be located on extracellular loops (Centurion-Lara et al. 2013); slight changes in the amino acid sequence of a V-region can abrogate antibody binding (LaFond et al. 2006b). Consistent with the notion that immune pressure selects for variants, immunization of rabbits with peptides to V6 resulted in enhanced sequence variability (Giacani et al. 2010). The finding in a rabbit model of secondary syphilis that TprK variability is significantly greater at sites of dissemination compared to the inoculum is further evidence that immune pressure selects for variants and that sequence variation facilitates immune evasion (Reid et al. 2014).
Nevertheless, TprK presents something of a conundrum. While the genetic data collectively constitute a powerful argument that TprK is an authentic OMP, the information available about the protein does not agree with this assertion. In contrast to TprC/D and TprI, both recombinant and native TprK (Nichols) are hydrophilic by TX114 phase partitioning (Cox et al. 2010; Hazlett et al. 2001). In accord with these results, localization data obtained by proteinase K susceptibility and IFA in the Nichols strain place the native protein in the periplasm (Cox et al. 2010; Hazlett et al. 2001). Like other full-length Tprs, TprK is predicted to have a bipartite domain architecture (Anand et al. 2013). Three of the V domains are predicted to be upstream of the MOSPC domain, a location that would be of no value for immune evasion. Also worth noting is that the portions of the protein reported to confer partial protection (Centurion-Lara et al. 1999; Morgan et al. 2002a) are from the hydrophilic N-terminal half one would expect to be periplasmic based on our bipartite model for the Tprs; indeed, in other hands (Hazlett et al. 2001), this portion of the protein is not protective. However, why a periplasmic protein would undergo such extensive sequence variation is, without question, perplexing. Definitive structural and topologic analyses of intra- and interstrain TprK variants, including localization of epitopes subject to sequence variation, are needed to resolve these discordances.
3.1.3 Why a Family?
Finally, why so many Tprs? One obvious answer is that sequence diversity in the extracellular loops of the β-barrel domains of the full-length Tprs, in conjunction with differential expression, enhances the spirochete’s capacity for immune evasion (see below). A second, non-mutually exclusive possibility is physiological. Sequence diversity in the strands that form the walls of the barrel lumen would be expected to affect the conductance properties and substrate specificities of the channel (Nikaido 2003; van den Berg 2012). Indeed, in addition to a role in immune evasion, we propose that the Tprs function collectively as a family of OM transporters whose varied substrate specificities enable the spirochete, an extreme auxotroph (Fraser et al. 1998; Norris et al. 2001), to import the wide range of water-soluble small molecules needed for survival in diverse microenvironments.
3.2 BamA
In 2000, Cameron et al. discovered TP92 (TP0326) using a differential screening strategy to identify E. coli clones expressing T. pallidum opsonic targets (Cameron et al. 2000). In this seminal report, they noted that TP0326 had sequence similarity to Gram-negative OMPs whose function(s), at that time, were poorly defined. A decade later, TP0326 emerged from our consensus computational framework as one of the most probable β-barrel formers encoded in the T. pallidum genome (Cox et al. 2010). By that time, it was apparent that TP0326 belongs to the Omp85 superfamily whose members play a central role in OM biogenesis in double-membrane organisms and eukaryotic organelles (i.e., mitochondria and chloroplasts) derived from them (Heinz and Lithgow 2014). Subsequently, we demonstrated that, like other members of the superfamily, TP0326/BamA has a dual-domain architecture (Fig. 6a) consisting of an amphiphilic, surface-exposed C-terminal β-barrel and a periplasmic region containing five polypeptide transport-associated (POTRA) repeats presumed to associate with other BAM complex subunits and interact with the chaperones that shuttle nascent OMPs across the periplasm (Desrosiers et al. 2011). A homology model based upon the solved structure of Neisseria gonorrhoeae BamA (Noinaj et al. 2013) predicted that TP0326 contains a 16-stranded β-barrel with characteristic BamA features (Fig. 6b) (Luthra et al. 2015a). One is the shortened and incompletely hydrogen-bonded β1 and β16 strands that theoretically can separate to allow insertion of nascent OMPs into the OM bilayer. Another is the putative dome formed by three of the barrel’s eight extracellular loops (L4, L6, and L7) that occlude the extracellular opening, ‘forcing’ OMP precursors within the channel to move laterally through the separated barrel strands.
Fig. 6.

TP0326/BamA, the central component of the T. pallidum β-barrel assembly machine (BAM) complex. a Schematic of BamA bipartite topology showing five N-terminal periplasmic POTRA domains and a C-terminal β-barrel. b A homology model based upon the solved structure of Neisseria gonorrhoeae BamA (Noinaj et al. 2013) predicts that TP0326 contains a 16-stranded β-barrel with characteristic BamA features, including three extracellular loops (L4, L6, and L7) that occlude the barrel’s extracellular opening. Reproduced from reference (Luthra et al. 2015a). c L4 is an immunodominant surface feature of BamA. Multiple-sequence alignment of the L4 regions of BamAs from geographically diverse T. pallidum strains, sequences amplified from skin biopsy specimens from two secondary syphilis patients (Cali-77 and Cali-84) enrolled at our Cali, Colombia, study site, and the Gauthier strain of the T. pallidum subsp. pertenue. All strains of T. pallidum subsp. pertenue in the database have same the L4 sequence. Immunoblot relativities of pooled IRS and pooled sera from U.S. (HSSU) and Colombian (HSSC) HIV-negative patients with secondary syphilis against L4, L6, and L7 loop peptides and a control peptide L3β6 derived from a portion of the barrel not surface-exposed. NRS and NHS, normal rabbit and normal human serum, respectively. Reproduced from reference (Luthra et al. 2015a). d Identification of the BAM complex in T. pallidum. Lysates solubilized with graded concentrations of the detergent DDM were separated by Blue-Native PAGE followed by immunoblot analysis with antisera directed against the POTRA arm of TP0326/BamA. Reproduced from reference (Desrosiers et al. 2011)
L4 contains a surface-exposed, immunodominant epitope and proven opsonic target (Luthra et al. 2015a). Antibodies directed against this epitope could be responsible for the partial protection against intradermal challenge with T. pallidum observed in the initial TP92 report (Cameron et al. 2000). The L4 epitope also provides potential insights into the selective pressures acting on spirochetes within at-risk populations. Some T. pallidum strains have BamA β-barrels in which a single amino acid substitution in the immunodominant L4 epitope markedly reduces the reactivity of sera from patients infected with strains containing Nichols-like BamA β-barrels (Fig. 6c) (Luthra et al. 2015a). Petrosova et al. (2012) noted that the β-barrel in the TP0326 of Mexico A is identical to that in subsp. pertenue strains. They proposed that the subsp. pertenue TP0326 β-barrel was introduced into the subsp. pallidum genome as a result of recombination in an individual co-infected with yaws and venereal syphilis. Because of its diminished susceptibility to pre-existing Nichols anti-L4 antibodies, the resulting subsp. pallidum strain would theoretically be capable of making inroads into populations in which Nichols-like strains predominate.
In genetically manipulable microorganisms, including Borrelia burgdorferi (Dunn et al. 2015), BamA was shown to be the central component of a molecular machine whose subunits work cooperatively to catalyze the insertion of newly exported OMPs into the OM (Rollauer et al. 2015). The large size of the BAM complex in T. pallidum (~400 kDa vs. ~200 kDa E. coli) (Fig. 6d), along with the absence of orthologs for known BAM subunits, strongly suggests that the mechanisms in T. pallidum for chaperoning nascent OMPs into the OM differ substantially from those in Gram-negative prototypes (Desrosiers et al. 2011; Luthra et al. 2015a). In addition to the BAM complex, E. coli also contains a translocation and assembly module (TAM) required for export of autotransporters to the cell surface (Webb et al. 2012a). The TAM complex consists of TamA, also a member of the OMP85 superfamily, and a cytoplasmic membrane protein, TamB, containing a periplasmic DUF490 domain that interacts with the POTRA arm of TamA (Selkrig et al. 2015). In T. pallidum, tp0325 encodes a large (108 kDa) cytoplasmic membrane-anchored protein with a DUF490 domain. Webb et al. (2012b) proposed that T. pallidum evolved a BAM–TAM chimeric OM biogenesis molecular machine. Strongly supporting this conjecture, Akins and coworkers (Iqbal et al. 2016) recently demonstrated that the TamB ortholog of B. burgdorferi is part of the Lyme disease spirochete’s BAM complex.
3.3 TP0515/LptD
As noted earlier, the genomic sequence confirmed that T. pallidum lacks the LPS biosynthetic pathway (consisting of up to 100 genes) (Fraser et al. 1998; Whitfield and Trent 2014). TP0515 is a structural ortholog of LptD, the OM component of the Gram-negative apparatus that conveys LPS from its site of synthesis at the inner membrane to its ultimate destination in the outer leaflet of the OM (Okuda et al. 2016). Like other LptDs (Gu et al. 2015), TP0515 is predicted to consist of a periplasmic N-terminal domain adopting a β-jellyroll fold and a crenelated 26-stranded β-barrel in which the first and last strands are incompletely hydrogen-bonded, enabling lateral opening of the barrel, as with BamA (Fig. 7a). In Gram-negative bacteria, LptD forms a heterodimeric translocon with LptE, an OM-tethered lipoprotein that resides within the lumen of the LptD barrel (Okuda et al. 2016). Perplexingly, the T. pallidum genome lacks a recognizable LptE but encodes orthologs for the other five components of the LPS transport pathway (Fig. 7b). TP0784 (LptC), TP0785 (LptA), and the soluble (i.e., periplasmic) N-terminal portion of LptD are predicted to form a contiguous structure that bridges the inner and outer membranes (Fig. 7b). By analogy with the ‘PEZ’ model developed for Gram-negative bacteria (Okuda et al. 2016), once the as yet unidentified cargo reaches the outer leaflet of the CM, TP0883 (LptF), TP0884 (LptG), and TP0786 (LptB) form an ATP-dependent machine that pushes it toward LptC and then across the periplasmic bridge. What could be the cargo for this system in an organism lacking LPS? One possibility is the previously identified OM glycolipids (Radolf et al. 1995b). Conceivably, the biochemical differences between these glycolipids and conventional LPS either obviate the need for LptE or, by analogy with the BAM complex, necessitate a markedly different functional ortholog. Interestingly, Leptospira species, which do contain true LPS, encode LptE (Haake and Zuckert 2015).
Fig. 7.
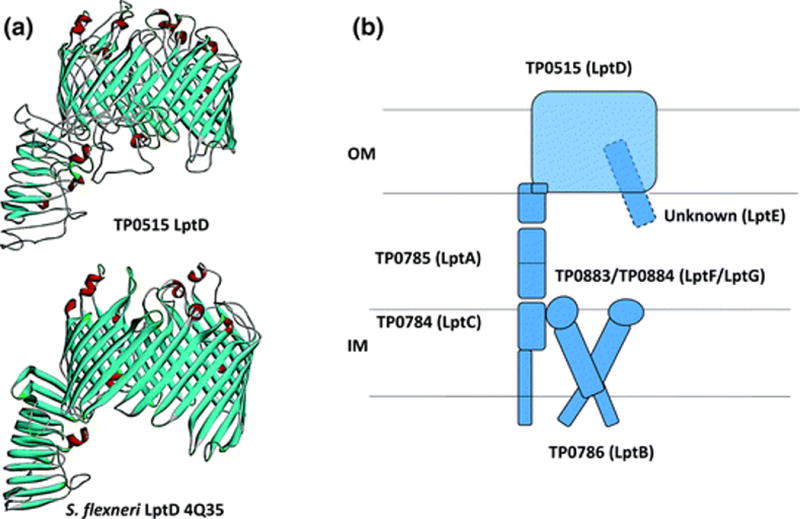
T. pallidum’s LptD ortholog. a Homology model for TP0515 based upon the solved structure of Shigella flexneri LptD (PDB 4Q35). The model and figure were generated using I-TASSER (Yang and Zhang 2015) and Discovery Studio, respectively. b T. pallidum contains orthologs for all of the components of the E. coli LptD complex except LptE
3.4 FadLs
Although biomembranes typically are highly permeable to long-chain fatty acids (LCFAs), Gram-negative OMs are a notable exception because of the permeability barrier created by an outer leaflet composed of LPS (Nikaido 2003). Consequently, transport of LCFAs across the OM of Gram-negative bacteria requires a dedicated transporter, FadL, a crenelated 14-stranded β-barrel that opens laterally to allow diffusion of hydrophobic molecules into the interior of the OM lipid bilayer (Fig. 8) (van den Berg et al. 2004; van den Berg 2005). How T. pallidum obtains LCFAs, which it cannot synthesize (Fraser et al. 1998), has been a longstanding question. As noted earlier, the syphilis spirochete’s OM is more permeable to LCFAs than that of E. coli, suggesting that T. pallidum could obtain LCFAs by diffusion (Cox and Radolf 2001). However, the discovery that T. pallidum appears to harbor five FadL structural orthologs (TP0548, TP0856, TP0858, TP0859, and TP0865) argues that diffusion of LCFAs alone is likely not sufficient to meet the bacterium’s needs. Structural models (Fig. 8) predict that all five proteins possess an N-terminal hatch domain or plug within the barrel lumen, a characteristic FadL feature (van den Berg et al. 2004; van den Berg 2005). At least four are predicted to possess the characteristic FadL kink in the third transmembrane strand for lateral exit of substrate (van den Berg et al. 2004; van den Berg 2005) (Fig. 8). The question, then, is why five orthologs? Members of the FadL family are substrate-specific transporters (Hearn et al. 2008). If all five T. pallidum proteins are FadLs, the implication is that they are required to import distinct classes of essential hydrophobic nutrients. It is also possible that one or more FadLs interact with a “tetratricopeptide repeat” (TPR) protein-associated transporter (TP0956/TatT and TP0957/TatP) postulated to convey hydrophobic substrates across the periplasm (Brautigam et al. 2012; Deka et al. 2012).
Fig. 8.
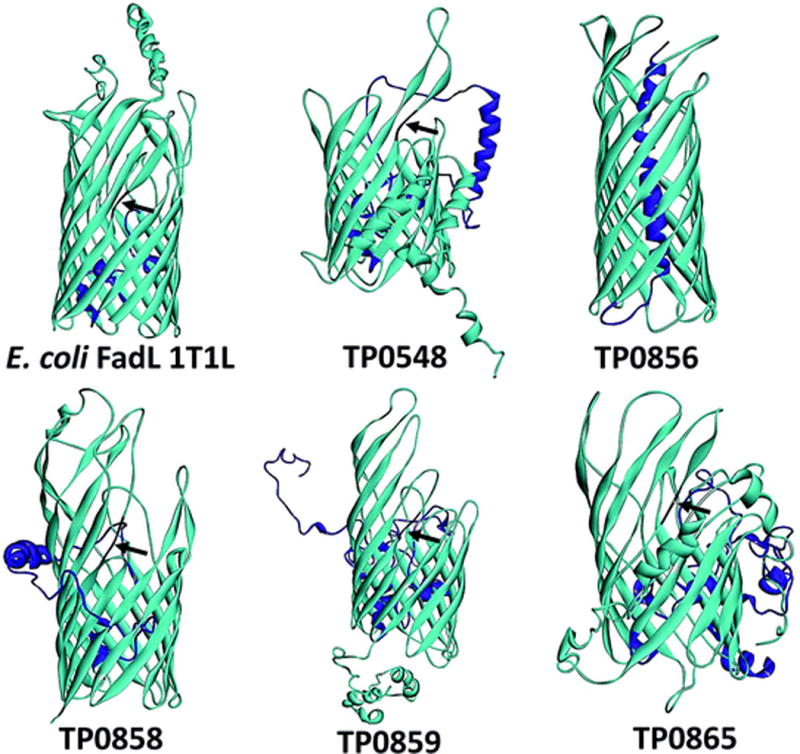
T. pallidum contains five FadL orthologs. Structural models and figures for TP0548, TP0856, TP0858, TP0859, and TP0865 were generated using I-TASSER (Yang and Zhang 2015) and Discovery Studio (BIOVIA 2015), respectively. The N-terminal hatch domains are shown in magenta, while the kinks in the third transmembrane strands of TP0548, TP0858, TP0859, and TP0865 are shown in black and indicated by arrowheads. E. coli FadL (PDB 1T1L) is shown for comparison
3.5 Efllux Pumps
Bacteria live in complex communities in which they must protect themselves against toxic compounds, including antibiotics, elaborated by their microbial and fungal competitors (Sassone-Corsi and Raffatellu 2015). Among the resistance mechanisms they employ to defend themselves against cytotoxic molecules are tripartite efflux pumps, cell-envelope-spanning machines consisting of an OMP, a cytoplasmic membrane protein, and a “fusion protein” or adaptor that connects the two (Nikaido and Takatsuka 2009). The prototype RND-type efflux OMP, TolC, is a trimer in which each protomer contributes four transmembrane strands of a 12-stranded β-barrel (Nikaido and Takatsuka 2009; Phan et al. 2015). Our analysis (Fig. 9a) reveals that T. pallidum contains orthologs for OprJ (TP0966) and OrpN (TP0967) from Pseudomonas aeruginosa (Yonehara et al. 2016) as well as E. coli TolC (TP0969) (Koronakis et al. 2000). We have also identified orthologs for the AcrA adaptor (TP0965) and AcrB cytoplasmic membrane protein (TP0790) predicted to complex with TP0969 to form a complete TolC-like RND pump (Fig. 9b). Because of its rapid invasiveness, T. pallidum is usually conceived of as a ‘loner’ pathogen that lives in otherwise sterile microenvironments. However, during sexual activity, syphilis spirochetes are inoculated into areas that teem with commensal microorganisms (Radolf et al. 2014). Efflux pumps, hitherto unknown to exist in T. pallidum, appear to be part of its strategy for resisting the efforts by commensals to repel the interloper.
Fig. 9.

T. pallidum TolC/OprJ/OprN orthologs and putative TolC complex. a Structural models for T. pallidum’s OprJ (TP0966), OprN (TP0967), and TolC (TP0969) orthologs. The models for TP0966 and TP0967 are based upon Pseudomonas aeruginosa OprJ (PDB 5AZS) and OprN (PDB 5AZO), while that for TP0969 is based upon E. coli TolC (PDB 2VDE). Structural models and figures were generated using SWISS-MODEL (Biasini et al. 2014) and Discovery Studio (BIOVIA 2015), respectively. b Schematic for T. pallidum’s putative AcrAB–TolC complex
3.6 OmpWs
OmpW is a small monomeric OMP commonly found in Gram-negative bacteria (Nandi et al. 2005). The crystal structure of E. coli OmpW revealed an 8-stranded β-barrel with a long, narrow hydrophobic channel (Fig. 10); these results, along with the demonstration of ion-channel properties in planar lipid bilayers, suggested that OmpW functions to transport small hydrophobic molecules across the OM (Hong et al. 2006). In subsequent studies, OmpWs have been shown to be functionally versatile proteins. They can protect bacteria against environmental stress (Asakura et al. 2008) and enhance virulence by conferring resistance to phagocytosis (Wu et al. 2013) and killing by the alternative complement pathway (Li et al. 2016). Interestingly, the T. pallidum genome encodes two OmpW orthologs, TP0126 and TP0733 (Fig. 10). In a recent report, Giacani and coworkers (Giacani et al. 2015) demonstrated that folded recombinant TP0126 has extensive β-sheet secondary structure and that changes in length of a polyG tract within the promoter may promote phase variation of the protein.
Fig. 10.
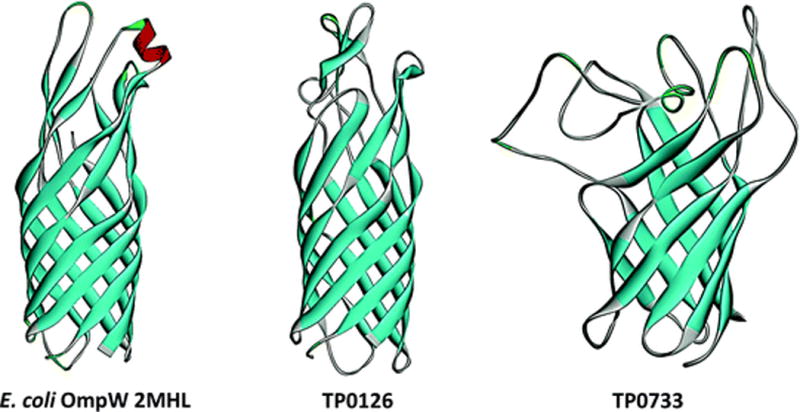
OmpW orthologs in T. pallidum. Structural models for TP0126 and TP0733 are based upon Pseudomonas aeruginosa OprG (2×27) and E. coli OmpW (2MHL). Structural models and figures were generated using SWISS-MODEL (Biasini et al. 2014) and Discovery Studio (BIOVIA 2015), respectively
4 Outer Membrane Lipoproteins
In Gram-negative bacteria, mature lipoproteins typically either remain anchored to the periplasmic leaflet of the CM or are transported to the inner leaflet of the OM by the Lol (localization of lipoproteins) system (Okuda and Tokuda 2011; Silhavy et al. 2010). The Lol system in E. coli (Okuda and Tokuda 2011) consists of an ABC transporter (LolCDE) that uses the energy from ATP hydrolysis to extract the proteins from the CM and transfer them to a soluble periplasmic carrier, LolA. A hydrophobic pocket in LolA binds the lipoprotein via its hydrophobic N-terminus and delivers it to the OM, where it is passed “mouth-to-mouth” to the OM lipoprotein receptor LolB. Through an unknown mechanism, LolB then catalyzes insertion of the lipoprotein via its N-terminal lipids into the periplasmic leaflet of the OM. Lipoproteins that contain a “Lol avoidance” (or CM “retention”) signal, an aspartate at the +2 position in E. coli, are not taken up by the Lol transporter and remain CM-associated. Most E. coli lipoproteins lack this signal and, consequently, are routed to the OM. Not all Gram-negative bacteria adhere to the +2 rule. In Pseudomonas aeruginosa, Lys and Ser at positions 3 and 4, respectively, are the typical CM retention signals (Narita and Tokuda 2007).
T. pallidum contains orthologs for LolCDE and LolA, but no discernible ortholog for LolB (Luthra et al. 2011). Nevertheless, the presence of a Lol system suggests that some T. pallidum lipoproteins make their way to the OM. We serendipitously identified one such lipoprotein, TP0453 (p30.5), when we used a hydrophobic, photoactivatable probe, 3-(trifluoromethyl)-3-(m-[125I] iodophenyl-diazarene, in a pre-genomic attempt to identify OM-associated proteins (Hazlett et al. 2005). Although this approach was intended to identify OM-spanning proteins, the only radiolabeled protein was the lipoprotein TP0453. PK accessibility demonstrated that TP0453 is not surface-exposed. Double-label (i.e., FlaA and anti-TP0453 antibodies) localization experiments in the gel microdroplet assay confirmed this, but also revealed that subcritical micellar concentrations of Triton X-100 (i.e., 0.02%), which would be expected to preferentially perturb the outer leaflet of the OM, markedly increased the percentage of treponemes labeled by anti-TP0453 antibodies without a corresponding increase in labeling of FlaA. We concluded that TP0453 is anchored to the inner leaflet of the OM. Subsequent investigations revealed that the TP0453 polypeptide possesses unique physical properties (Hazlett et al. 2005; Luthra et al. 2011). As noted earlier, polypeptide moieties of spirochetal lipoproteins typically are hydrophilic. The polypeptide portion of TP0453, however, is amphiphilic by TX114 phase partitioning and liposome incorporation. The X-ray crystal structure of TP0453 revealed that it exists in closed and open conformations (Luthra et al. 2011). Membrane insertion occurs as a result of lateral movement of amphipathic helices on the surface of the closed conformer; these movements also expose a large hydrophobic pocket. We postulated that TP0453 functions as a carrier of lipids, glycolipids, and/or derivatives during OM biogenesis. We also proposed that the ability of the TP0453 polypeptide to spontaneously insert into membranes obviated the need for LolB.
In Gram-negative bacteria, lipoproteins translocate the OM via an extension of the Sec-dependent general export pathway, which involves 12 to 16 different proteins comprising the type II secretion machine or “secreton” that bridges the CM and OM (Douzi et al. 2012). Importantly, since lipoproteins must engage the secreton from the periplasmic side of the CM, the Lol and type II pathways are considered to be mutually exclusive (Douzi et al. 2012). The T. pallidum genome does not encode the Type II pathway (Fraser et al. 1998), and there are no other known mechanisms whereby lipoproteins can reach the surface of a double-membrane organism. However, this, does not, in itself, rule out the possibility that T. pallidum can express lipoproteins on its surface. B. burgdorferi also lacks a secreton but clearly expresses numerous outer surface lipoproteins (Radolf et al. 2012), and it has been proposed that an OM-situated “flippase” translocates folded lipoproteins delivered by the Lol system (Zuckert 2014). The need for LolA–lipoprotein complexes to engage the flippase from the underside of the OM perhaps explains why neither spirochete has a LolB (Zuckert 2014).
In recent years, three putative T. pallidum outer surface lipoproteins have been identified. One is the multifunctional TP0751. First identified as a laminin-binding adhesion (Cameron et al. 2005), TP0751 is also a zinc-dependent metalloprotease (hence, its designation as “pallilysin”) that forms a complex with TP0750 (which contains a Von Willebrand factor type A domain) capable of degrading clots and extracellular matrix, activities that could facilitate spirochetal dissemination as well as attachment (Houston et al. 2012, 2014). Unfortunately, expression levels of pallilysin in T. pallidum (unpublished observations) or following heterologous expression in B. burgdorferi (Parker et al. 2016) are so low that it cannot be detected by IFA or chemiluminescence-based immunoblot analysis. Also noteworthy, the recently solved X-ray structure revealed that pallilysin is a member of the lipocalin superfamily (Parker et al. 2016). In E. coli, the lipocalin Blc is a lipoprotein tethered to the inner leaflet of the OM where it binds lysophospholipids (Campanacci et al. 2006). On the other hand, three lines of evidence support its surface location: (i) pallilysin antibodies yielded positive results in opsonophagocytosis assays (Houston et al. 2012); (ii) FLAG-tagged pallilysin expressed in B. burgdorferi could be detected by flow cytometry on the surface of intact Lyme disease spirochetes and promoted their attachment to HUVEC monolayers in “gain of function” experiments (Parker et al. 2016); and (iii) most convincing is the recent demonstration that immunization of rabbits with pallilysin protected against dissemination of T. pallidum following intradermal challenge (Lithgow et al. 2017).
TP0136, a second putative T. pallidum outer surface lipoprotein, has been characterized as a fibronectin-binding lipoprotein adhesin with sequence heterogeneity among T. pallidum strains (Arora et al. 2016; Brinkman et al. 2008; Ke et al. 2015). In contrast to IEM data published by Palzkill and coworkers (Brinkman et al. 2008), we found no evidence for surface-exposure of TP0136 in the Nichols strain using our gel microdroplet assay (Cox et al. 2010). TP0435/Tpp17, the third, has been known for years as a major treponemal antigen with great utility for serodiagnosis of syphilis (Sena et al. 2015). Evidence that it functions as a cytadhesin was obtained using B. burgdorferi as a surrogate genetic host (Chan et al. 2016). The case for surface-exposure of at least small amounts of native TP0435 in T. pallidum was bolstered by IEM and opsonophagocytosis assays with T. pallidum (Chan et al. 2016). In considering these findings, one must bear in mind that Tpp17 is a structural ortholog for NlpE (Brautigam et al. 2015), a lipoprotein tethered to the inner leaflet of the OM in Gram-negative bacteria (Hirano et al. 2007) and that, in our localization studies, we did not observe evidence for surface-exposure (Cox et al. 1995).
5 Concluding Remarks
5.1 Immune Evasion
While at one time it was thought that the OM of T. pallidum is antigenically inert (Cox et al. 1992; Penn et al. 1985), we now know that this simplistic notion is incorrect and that syphilitic infection does, in fact, induce antibodies capable of promoting bacterial clearance (so-called “functional” antibodies) (Radolf and Lukehart 2006). After many false starts, the field has progressed to the point where we can topologically map humoral responses to bona fide OMPs and identify protective B cell epitopes. Besides their obvious relevance to vaccine development, such studies are essential to addressing a central issue of syphilis pathogenesis—why, unlike ‘classic’ extracellular pathogens, spirochetes not only fail to be rapidly cleared, but actually continue to disseminate in the presence of surface-reactive antibodies (Lafond and Lukehart 2006; Radolf et al. 2006; Salazar et al. 2002).
Immunolabeling and opsonophagocytosis assays have shown that T. pallidum populations consist of antibody-binding and non-binding subpopulations and that the minority of organisms that bind antibodies do so in minute amounts and with delayed kinetics (Lukehart et al. 1992; Cox et al. 2010). One can envision a scenario at sites of infection whereby the nonbinders evade host antibodies, replenishing the ranks of spirochetes that do bind sufficient amounts of antibodies to be cleared (Radolf et al. 2016). If correct, then understanding the basis for the heterogeneity of T. pallidum’s surface antigenicity becomes critical to unraveling the immune evasion enigma. Now that we have a much clearer understanding of the spirochete’s OMP repertoire, a “one size fits all” explanation seems unlikely. The picture emerging appears to be multifactorial, involving (i) poor target availability due to low copy numbers (Anand et al. 2012, 2015; Desrosiers et al. 2011; Luthra et al. 2015a), (ii) limited production of antibodies against surface-exposed epitopes along with skewed production of antibodies against periplasmic domains (Anand et al. 2012, 2015; Desrosiers et al. 2011; Luthra et al. 2015a), (iii) organism-to-organism variation in the levels of expression of OMPs and outer surface lipoproteins (Chan et al. 2016; Giacani et al. 2007, 2015; Pinto et al. 2016), and (iv) in the case of TprK, antigenic variation as a result of intra-genomic recombination (Deitsch et al. 2009; Ho and Lukehart 2011; Pinto et al. 2016). Conceivably, when the antibody repertoire covers a sufficiently broad range of surface epitopes on a population-wide basis, humoral responses eventually drive down spirochete burdens and keep them suppressed (Radolf et al. 2016).
5.2 Functional and Regulatory Considerations
Early on, we recognized that our ultrastructural model raises physiologic- and virulence-related issues that could not be ignored: how does the spirochete meet its nutritional requirements and carry out its complex parasitic program with a minimalist OM (Radolf 1995)? A partial answer may lie with the bacterium’s extremely slow (~30 h) rate of replication (Magnuson et al. 1948), presumably an evolutionary ‘compromise’ between the density of surface molecules needed for viability and the demands of stealth. Multifunctionality of surface molecules, a well-accepted facet of Gram-negative OMP biology (Galdiero et al. 2012; Smith et al. 2007), is likely another piece of the puzzle. In addition to being nutrient transporters, the OMPs described herein could mediate a multitude of interactions and functions at the host–pathogen interface. Of course, the answers ultimately lie in further detailed characterization of T. pallidum’s OMP repertoire. One of the take-home lessons from our recent bioinformatics exercises is that the syphilis spirochete’s OMP repertoire seemingly encompasses a much greater degree, as well as greater redundancy, of transport capabilities than previously recognized. One might postulate that this diversity serves the ends of stealth pathogenicity in two ways: by enhancing nutrient acquisition in the myriad niches T. pallidum is known to inhabit within its obligate human host (Radolf et al. 2014) and promoting persistence in these niches through differential expression driven by host environmental pressures (Giacani et al. 2009, 2015; Radolf et al. 2016). Given the limitations of animal models for experimental syphilis and ethical issues involved in translational human research, devising strategies to elucidate in vivo expression profiles and underlying regulatory mechanisms is going to be a formidable challenge. Mining of the spirochete genome, the obvious starting point, has revealed that the spirochete’s regulatory apparatus is far more intricate than previously suspected. As is true of all bacteria, T. pallidum appears to sense its environment to a large extent through the lens of intermediary metabolism (Radolf et al. 2016). OMPs, the bacterium’s eyes for sensing nutrient availability, undoubtedly will be part of these regulatory networks.
Acknowledgments
Funded in part by R01 AI-26756 from the National Institutes of Health (NIAID) and the Department of Research, Connecticut Children’s Medical Center.
Contributor Information
Justin D. Radolf, Departments of Medicine, Pediatrics, Molecular Biology and Biophysics, Genetics and Genomic Sciences, and Immunology, UConn Health, Farmington CT 06030-3715, USA
Sanjiv Kumar, Department of Medicine, UConn Health, Farmington CT 06030-3715, USA.
References
- Akins DR, Purcell BK, Mitra MM, Norgard MV, Radolf JD. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993;61:1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Baseman JB. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979;26:1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Luthra A, Dunham-Ems S, Caimano MJ, Karanian C, LeDoyt M, Cruz AR, Salazar JC, Radolf JD. TprC/D (Tp0117/131), a trimeric, pore-forming rare outer membrane protein of Treponema pallidum, has a bipartite domain structure. J Bacteriol. 2012;194:2321–2333. doi: 10.1128/JB.00101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Luthra A, Edmond ME, Ledoyt M, Caimano MJ, Radolf JD. The major outer sheath protein (Msp) of Treponema denticola has a bipartite domain architecture and exists as periplasmic and outer membrane-spanning conformers. J Bacteriol. 2013;195:2060–2071. doi: 10.1128/JB.00078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, LeDoyt M, Karanian C, Luthra A, Koszelak-Rosenblum M, Malkowski MG, Puthenveetil R, Vinogradova O, Radolf JD. Bipartite topology of Treponema pallidum repeat proteins C/D and I: outer membrane insertion, trimerization, and porin function require a C-terminal β-barrel domain. J Biol Chem. 2015;290:12313–12331. doi: 10.1074/jbc.M114.629188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora N, Schuenemann VJ, Jager G, Peltzer A, Seitz A, Herbig A, Strouhal M, Grillova L, Sanchez-Buso L, Kuhnert D, Bos KI, Davis LR, Mikalova L, Bruisten S, Komericki P, French P, Grant PR, Pando MA, Vaulet LG, Fermepin MR, Martinez A, Centurion Lara A, Giacani L, Norris SJ, Smajs D, Bosshard PP, Gonzalez-Candelas F, Nieselt K, Krause J, Bagheri HC. Origin of modern syphilis and emergence of a pandemic Treponema pallidum cluster. Nat Microbiol. 2016;2:16245. doi: 10.1038/nmicrobiol.2016.245. [DOI] [PubMed] [Google Scholar]
- Asakura H, Kawamoto K, Haishima Y, Igimi S, Yamamoto S, Makino S. Differential expression of the outer membrane protein W (OmpW) stress response in enterohemorrhagic Escherichia coli O157:H7 corresponds to the viable but non-culturable state. Res Microbiol. 2008;159:709–717. doi: 10.1016/j.resmic.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Bagos PG, Liakopoulos TD, Hamodrakas SJ. Finding B-barrel outer membrane proteins with a markov chain model. WSEAS Trans Biol Biomed. 2004a;2:186–189. [Google Scholar]
- Bagos PG, Liakopoulos TD, Spyropoulos IC, Hamodrakas SJ. A hidden Markov model method, capable of predicting and discriminating beta-barrel outer membrane proteins. BMC Bioinform. 2004b;5:29. doi: 10.1186/1471-2105-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavro VN, Pietras Z, Furnham N, Perez-Cano L, Fernandez-Recio J, Pei XY, Misra R, Luisi B. Assembly and channel opening in a bacterial drug efflux machine. Mol Cell. 2008;30:114–121. doi: 10.1016/j.molcel.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PS, Akins DR, Radolf JD, Norgard MV. Similarity between the 38-kilodalton lipoprotein of Treponema pallidum and the glucose/galactose-binding (MglB) protein of Escherichia coli. Infect Immun. 1994;62:1381–1391. doi: 10.1128/iai.62.4.1381-1391.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biovia DS. Discovery studio modeling environment. San Diego, CA, USA: Dassault Systèmes; 2015. [Google Scholar]
- Blanco DR, Reimann K, Skare J, Champion CI, Foley D, Exner MM, Hancock RE, Miller JN, Lovett MA. Isolation of the outer membranes from Treponema pallidum and Treponema vincentii. J Bacteriol. 1994;176:6088–6099. doi: 10.1128/jb.176.19.6088-6099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco DR, Champion CI, Exner MM, Erdjument-Bromage H, Hancock RE, Tempst P, Miller JN, Lovett MA. Porin activity and sequence analysis of a 31-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1) J Bacteriol. 1995;177:3556–3562. doi: 10.1128/jb.177.12.3556-3562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, Buchanan SK. Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens. Structure. 2016;24:965–976. doi: 10.1016/j.str.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourell KW, Schulz W, Norgard MV, Radolf JD. Treponema pallidum rare outer membrane proteins: analysis of mobility by freeze-fracture electron microscopy. J Bacteriol. 1994;176:1598–1608. doi: 10.1128/jb.176.6.1598-1608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam CA, Deka RK, Schuck P, Tomchick DR, Norgard MV. Structural and thermodynamic characterization of the interaction between two periplasmic Treponema pallidum lipoproteins that are components of a TPR-protein-associated TRAP transporter (TPAT) J Mol Biol. 2012;420:70–86. doi: 10.1016/j.jmb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam CA, Deka RK, Liu WZ, Norgard MV. Insights into the potential function and membrane organization of the TP0435 (Tp17) lipoprotein from Treponema pallidum derived from structural and biophysical analyses. Protein Sci. 2015;24:11–19. doi: 10.1002/pro.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam CA, Deka RK, Liu WZ, Norgard MV. The Tp0684 (MglB-2) lipoprotein of Treponema pallidum: a glucose-binding protein with divergent topology. PLoS ONE. 2016;11:e0161022. doi: 10.1371/journal.pone.0161022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman MB, McGill MA, Pettersson J, Rogers A, Matejkova P, Smajs D, Weinstock GM, Norris SJ, Palzkill T. A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect Immun. 2008;76:1848–1857. doi: 10.1128/IAI.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca JS, Radolf JD. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181:1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- Cameron CE, Brouwer NL, Tisch LM, Kurowa JM. Defining the interation of the Treponema pallidum adhesin TP0751 with laminin. Infect Immun. 2005;73:7485–7494. doi: 10.1128/IAI.73.11.7485-7494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CE. The T pallidum outer membrane and outer membrane proteins. In: Radolf JD, Lukehart SA, editors. Pathogenic treponema: molecular and cellular biology, Caister. Academic Press; Norwich, UK: 2006. pp. 237–266. [Google Scholar]
- Campanacci V, Bishop RE, Blangy S, Tegoni M, Cambillau C. The membrane bound bacterial lipocalin Blc is a functional dimer with binding preference for lysophospholipids. FEBS Lett. 2006;580:4877–4883. doi: 10.1016/j.febslet.2006.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis WC, Lukehart SA. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J Exp Med. 1999;189:647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion-Lara A, Godornes C, Castro C, Van Voorhis WC, Lukehart SA. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect Immun. 2000;68:824–831. doi: 10.1128/iai.68.2.824-831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, Van Voorhis WC, Lukehart SA. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol Microbiol. 2004;52:1579–1596. doi: 10.1111/j.1365-2958.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- Centurion-Lara A, Giacani L, Godornes C, Molini BJ, Brinck Reid T, Lukehart SA. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl Trop Dis. 2013;7:e2222. doi: 10.1371/journal.pntd.0002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain NR, Brandt ME, Erwin AL, Radolf JD, Norgard MV. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989a;57:2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain NR, DeOgny L, Slaughter C, Radolf JD, Norgard MV. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989b;57:2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Nasereddin T, Alter L, Centurion-Lara A, Giacani L, Parveen N. Treponema pallidum Lipoprotein TP0435 Expressed in Borrelia burgdorferi produces multiple surface/periplasmic isoforms and mediates adherence. Sci Rep. 2016;6:25593. doi: 10.1038/srep25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S. Protective layer covering pathogenic treponemata. Lancet. 1963;1:423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- Conlan S, Bayley H. Folding of a monomeric porin, OmpG, in detergent solution. Biochemistry. 2003;42:9453–9465. doi: 10.1021/bi0344228. [DOI] [PubMed] [Google Scholar]
- Cox DL, Chang P, McDowall AW, Radolf JD. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992;60:1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Akins DR, Porcella SF, Norgard MV, Radolf JD. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- Cox DL, Radolf JD. Insertion of fluorescent fatty acid probes into the outer membranes of the pathogenic spirochaetes Treponema pallidum and Borrelia burgdorferi. Microbiology. 2001;147:1161–1169. doi: 10.1099/00221287-147-5-1161. [DOI] [PubMed] [Google Scholar]
- Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, Caimano MJ, Radolf JD. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect Immun. 2010;78:5178–5194. doi: 10.1128/IAI.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AR, Pillay A, Zuluaga AV, Ramirez LG, Duque JE, Aristizabal GE, Fiel-Gan MD, Jaramillo R, Trujillo R, Valencia C, Jagodzinski L, Cox DL, Radolf JD, Salazar JC. Secondary syphilis in Cali, Colombia: new concepts in disease pathogenesis. PLoS Negl Trop Dis. 2010;4:e690. doi: 10.1371/journal.pntd.0000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitsch KW, Lukehart SA, Stringer JR. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol. 2009;7:493–503. doi: 10.1038/nrmicro2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka RK, Lee YH, Hagman KE, Shevchenko D, Lingwood CA, Hasemann CA, Norgard MV, Radolf JD. Physicochemical evidence that Treponema pallidum TroA is a zinc-containing metalloprotein that lacks porin-like structure. J Bacteriol. 1999;181:4420–4423. doi: 10.1128/jb.181.14.4420-4423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka RK, Machius M, Norgard MV, Tomchick DR. Crystal structure of the 47-kDa lipoprotein of Treponema pallidum reveals a novel penicillin-binding protein. J Biol Chem. 2002;277:41857–41864. doi: 10.1074/jbc.M207402200. [DOI] [PubMed] [Google Scholar]
- Deka RK, Goldberg MS, Hagman KE, Norgard MV. The Tp38 (TpMglB-2) lipoprotein binds glucose in a manner consistent with receptor function in Treponema pallidum. J Bacteriol. 2004a;186:2303–2308. doi: 10.1128/JB.186.8.2303-2308.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka RK, Neil L, Hagman KE, Machius M, Tomchick DR, Brautigam CA, Norgard MV. Structural evidence that the 32-kilodalton lipoprotein (Tp32) of Treponema pallidum is an L-methionine-binding protein. J Biol Chem. 2004b;279:55644–55650. doi: 10.1074/jbc.M409263200. [DOI] [PubMed] [Google Scholar]
- Deka RK, Brautigam CA, Yang XF, Blevins JS, Machius M, Tomchick DR, Norgard MV. The PnrA (Tp0319; TmpC) lipoprotein represents a new family of bacterial purine nucleoside receptor encoded within an ATP-binding cassette (ABC)-like operon in Treponema pallidum. J Biol Chem. 2006;281:8072–8081. doi: 10.1074/jbc.M511405200. [DOI] [PubMed] [Google Scholar]
- Deka RK, Brautigam CA, Tomson FL, Lumpkins SB, Tomchick DR, Machius M, Norgard MV. Crystal structure of the Tp34 (TP0971) lipoprotein of Treponema pallidum: implications of its metal-bound state and affinity for human lactoferrin. J Biol Chem. 2007;282:5944–5958. doi: 10.1074/jbc.M610215200. [DOI] [PubMed] [Google Scholar]
- Deka RK, Brautigam CA, Goldberg M, Schuck P, Tomchick DR, Norgard MV. Structural, bioinformatic, and in vivo analyses of two Treponema pallidum lipoproteins reveal a unique TRAP transporter. J Mol Biol. 2012;416:678–696. doi: 10.1016/j.jmb.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka RK, Brautigam CA, Liu WZ, Tomchick DR, Norgard MV. The TP0796 lipoprotein of Treponema pallidum is a bimetal-dependent FAD pyrophosphatase with a potential role in flavin homeostasis. J Biol Chem. 2013;288:11106–11121. doi: 10.1074/jbc.M113.449975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers DC, Anand A, Luthra A, Dunham-Ems SM, LeDoyt M, Cummings MA, Eshghi A, Cameron CE, Cruz AR, Salazar JC, Caimano MJ, Radolf JD. TP0326, a Treponema pallidum beta-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol Microbiol. 2011;80:1496–1515. doi: 10.1111/j.1365-2958.2011.07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorset DL, Engel A, Haner M, Massalski A, Rosenbusch JP. Two-dimensional crystal packing of matrix porin. A channel forming protein in Escherichia coli outer membranes. J Mol Biol. 1983;165:701–710. doi: 10.1016/s0022-2836(83)80275-7. [DOI] [PubMed] [Google Scholar]
- Douzi B, Filloux A, Voulhoux R. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci. 2012;367:1059–1072. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JP, Kenedy MR, Iqbal H, Akins DR. Characterization of the β-barrel assembly machine accessory lipoproteins from Borrelia burgdorferi. BMC Microbiol. 2015;15:70. doi: 10.1186/s12866-015-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli C, Leung WK, Muller KH, Hancock RE, McBride BC. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect Immun. 1993;61:1694–1699. doi: 10.1128/iai.61.5.1694-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen RP. Virulence determinants of oral treponemes. In: Radolf JD, Lukehart SA, editors. Pathogenic treponema molecular and cellular biology. Norwich, UK: Caister Academic Press; 2006. pp. 357–386. [Google Scholar]
- Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald TJ, Johnson RC. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979;24:244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Jr, Wimley WC. TMBB-DB: a transmembrane β-barrel proteome database. Bioinformatics. 2012;28:2425–2430. doi: 10.1093/bioinformatics/bts478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero S, Falanga A, Cantisani M, Tarallo R, Della Pepa ME, D’Oriano V, Galdiero M. Microbe-host interactions: structure and role of Gram-negative bacterial porins. Curr Protein Pept Sci. 2012;13:843–854. doi: 10.2174/138920312804871120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L, Lukehart S, Centurion-Lara A. Length of guanosine homopolymeric repeats modulates promoter activity of subfamily II tpr genes of Treponema pallidum ssp. pallidum. FEMS Immunol Med Microbiol. 2007;51:289–301. doi: 10.1111/j.1574-695X.2007.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L, Godornes C, Puray-Chavez M, Guerra-Giraldez C, Tompa M, Lukehart SA, Centurion-Lara A. TP0262 is a modulator of promoter activity of tpr Subfamily II genes of Treponema pallidum ssp. pallidum. Mol Microbiol. 2009;72:1087–1099. doi: 10.1111/j.1365-2958.2009.06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L, Molini BJ, Kim EY, Godornes BC, Leader BT, Tantalo LC, Centurion-Lara A, Lukehart SA. Antigenic variation in Treponema pallidum: TprK sequence diversity accumulates in response to immune pressure during experimental syphilis. J Immunol. 2010;184:3822–3829. doi: 10.4049/jimmunol.0902788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L, Brandt SL, Puray-Chavez M, Reid TB, Godornes C, Molini BJ, Benzler M, Hartig JS, Lukehart SA, Centurion-Lara A. Comparative investigation of the genomic regions involved in antigenic variation of the TprK antigen among treponemal species, subspecies, and strains. J Bacteriol. 2012;194:4208–4225. doi: 10.1128/JB.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacani L, Brandt SL, Ke W, Reid TB, Molini BJ, Iverson-Cabral S, Ciccarese G, Drago F, Lukehart SA, Centurion-Lara A. Transcription of TP0126, Treponema pallidum putative OmpW homolog, is regulated by the length of a homopolymeric guanosine repeat. Infect Immun. 2015;83:2275–2289. doi: 10.1128/IAI.00360-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RR, Mulligan CJ, Molini BJ, Sun ES, Giacani L, Godornes C, Kitchen A, Lukehart SA, Centurion-Lara A. Molecular evolution of the tprC, D, I, K, G, and J genes in the pathogenic genus Treponema. Mol Biol Evol. 2006;23:2220–2233. doi: 10.1093/molbev/msl092. [DOI] [PubMed] [Google Scholar]
- Gu Y, Stansfeld PJ, Zeng Y, Dong H, Wang W, Dong C. Lipopolysaccharide is inserted into the outer membrane through an intramembrane hole, a lumen gate, and the lateral opening of LptD. Structure. 2015;23:496–504. doi: 10.1016/j.str.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Zuckert WR. The leptospiral outer membrane. Curr Top Microbiol Immunol. 2015;387:187–221. doi: 10.1007/978-3-662-45059-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy PH, Jr, Nell EE. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957;66:160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hazlett KR, Sellati TJ, Nguyen TT, Cox DL, Clawson ML, Caimano MJ, Radolf JD. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J Exp Med. 2001;193:1015–1026. doi: 10.1084/jem.193.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett KR, Cox DL, Decaffmeyer M, Bennett MP, Desrosiers DC, La Vake CJ, La Vake ME, Bourell KW, Robinson EJ, Brasseur R, Radolf JD. TP0453, a concealed outer membrane protein of Treponema pallidum, enhances membrane permeability. J Bacteriol. 2005;187:6499–6508. doi: 10.1128/JB.187.18.6499-6508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn EM, Patel DR, van den Berg B. Outer-membrane transport of aromatic hydrocarbons as a first step in biodegradation. Proc Natl Acad Sci U S A. 2008;105:8601–8606. doi: 10.1073/pnas.0801264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E, Lithgow T. A comprehensive analysis of the Omp85/TpsB protein superfamily structural diversity, taxonomic occurrence, and evolution. Front Microbiol. 2014;5:370. doi: 10.3389/fmicb.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Hossain MM, Takeda K, Tokuda H, Miki K. Structural studies of the Cpx pathway activator NlpE on the outer membrane of Escherichia coli. Structure. 2007;15:963–976. doi: 10.1016/j.str.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584–4592. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Patel DR, Tamm LK, van den Berg B. The outer membrane protein OmpW forms an eight-stranded b-barrel with a hydrophobic channel. J Biol Chem. 2006;281:7568–7577. doi: 10.1074/jbc.M512365200. [DOI] [PubMed] [Google Scholar]
- Houston S, Hof R, Honeyman L, Hassler J, Cameron CE. Activation and proteolytic activity of the Treponema pallidum metalloprotease, pallilysin. PLoS Pathog. 2012;8:e1002822. doi: 10.1371/journal.ppat.1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston S, Russell S, Hof R, Roberts AK, Cullen P, Irvine K, Smith DS, Borchers CH, Tonkin ML, Boulanger MJ, Cameron CE. The multifunctional role of the pallilysin-associated Treponema pallidum protein, Tp0750, in promoting fibrinolysis and extracellular matrix component degradation. Mol Microbiol. 2014;91:618–634. doi: 10.1111/mmi.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovind-Hougen K. Morphology. In: Schell RF, Musher DM, editors. Pathogenesis and immunology of treponemal infection. Marcel Dekker; New York: 1983. pp. 3–28. [Google Scholar]
- Iqbal H, Kenedy MR, Lybecker M, Akins DR. The TamB ortholog of Borrelia burgdorferi interacts with the beta-barrel assembly machine (BAM) complex protein BamA. Mol Microbiol. 2016 doi: 10.1111/mmi.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard J, Renken C, Hsieh CE, Desrosiers DC, Dunham-Ems S, La Vake C, Gebhardt LL, Limberger RJ, Cox DL, Marko M, Radolf JD. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. J Bacteriol. 2009;191:7566–7580. doi: 10.1128/JB.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Ritzi DM, Livermore BP. Outer envelope of virulent Treponema pallidum. Infect Immun. 1973;8:291–295. doi: 10.1128/iai.8.2.291-295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Bourell KW, Norgard MV, Radolf JD. Membrane topology of Borrelia burgdorferi and Treponema pallidum lipoproteins. Infect Immun. 1995;63:2424–2434. doi: 10.1128/iai.63.7.2424-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003;12:1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Ke W, Molini BJ, Lukehart SA, Giacani L. Treponema pallidum subsp. pallidum TP0136 protein is heterogeneous among isolates and binds cellular and plasma fibronectin via its NH2-terminal end. PLoS Negl Trop Dis. 2015;9:e0003662. doi: 10.1371/journal.pntd.0003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- LaFond RE, Centurion-Lara A, Godornes C, Rompalo AM, Van Voorhis WC, Lukehart SA. Sequence diversity of Treponema pallidum subsp. pallidum tprK in human syphilis lesions and rabbit-propagated isolates. J Bacteriol. 2003;185:6262–6268. doi: 10.1128/JB.185.21.6262-6268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFond RE, Centurion-Lara A, Godornes C, Van Voorhis WC, Lukehart SA. TprK sequence diversity accumulates during infection of rabbits with Treponema pallidum subsp. pallidum Nichols strain. Infect Immun. 2006a;74:1896–1906. doi: 10.1128/IAI.74.3.1896-1906.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFond RE, Molini BJ, Van Voorhis WC, Lukehart SA. Antigenic variation of TprK V regions abrogates specific antibody binding in syphilis. Infect Immun. 2006b;74:6244–6251. doi: 10.1128/IAI.00827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Deka RK, Norgard MV, Radolf JD, Hasemann CA. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nature Structural Biology. 1999;6:628–633. doi: 10.1038/10677. [DOI] [PubMed] [Google Scholar]
- Lee YH, Dorwart MR, Hazlett KR, Deka RK, Norgard MV, Radolf JD, Hasemann CA. The crystal structure of Zn(II)-free Treponema pallidum TroA, a periplasmic metal-binding protein, reveals a closed conformation. J Bacteriol. 2002;184:2300–2304. doi: 10.1128/JB.184.8.2300-2304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wen L, Li C, Chen R, Ye Z, Zhao J, Pan J. Contribution of the outer membrane protein OmpW in Escherichia coli to complement resistance from binding to factor H. Microb Pathog. 2016;98:57–62. doi: 10.1016/j.micpath.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Lithgow KV, Hof R, Wetherell C, Phillips D, Houston S, Cameron CE. A defined syphilis vaccine candidate inhibits dissemination of Treponema pallidum subps. pallidum. Nat Commun. 2017;8:14272. doi: 10.1038/ncomms14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, Norris SJ. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J Bacteriol. 2009;191:5026–5036. doi: 10.1128/JB.00340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, Norris SJ. Cellular architecture of Treponema pallidum: novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J Mol Biol. 2010;403:546–561. doi: 10.1016/j.jmb.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart SA, Miller JN. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978;121:2014–2024. [PubMed] [Google Scholar]
- Lukehart SA, Shaffer JM, Baker-Zander SA. A subpopulation of Treponema pallidum is resistant to phagocytosis: possible mechanism of persistence. J Infect Dis. 1992;166:1449–1453. doi: 10.1093/infdis/166.6.1449. [DOI] [PubMed] [Google Scholar]
- Lukehart SA, Marra CM. Isolation and laboratory maintenance of Treponema pallidum. Curr Protoc Microbiol. 2007 doi: 10.1002/9780471729259.mc12a01s7. Chapter 12: Unit 12A 11. [DOI] [PubMed] [Google Scholar]
- Luthra A, Zhu G, Desrosiers DC, Eggers CH, Mulay V, Anand A, McArthur FA, Romano FB, Caimano MJ, Heuck AP, Malkowski MG, Radolf JD. The transition from closed to open conformation of Treponema pallidum outer membrane-associated lipoprotein TP0453 involves membrane sensing and integration by two amphipathic helices. J Biol Chem. 2011;286:41656–41668. doi: 10.1074/jbc.M111.305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra A, Anand A, Hawley KL, LeDoyt M, La Vake CJ, Caimano MJ, Cruz AR, Salazar JC, Radolf JD. A homology model reveals novel structural features and an immunodominant surface loop/opsonic target in the Treponema pallidum BamA ortholog TP_0326. J Bacteriol. 2015a;197:1906–1920. doi: 10.1128/JB.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra A, Anand A, Radolf JD. Treponema pallidum in gel microdroplets: A method for topological analysis of BamA (TP0326) and localization of rare outer membrane proteins. Methods Mol Biol. 2015b;1329:67–75. doi: 10.1007/978-1-4939-2871-2_6. [DOI] [PubMed] [Google Scholar]
- Machius M, Brautigam CA, Tomchick DR, Ward P, Otwinowski Z, Blevins JS, Deka RK, Norgard MV. Structural and biochemical basis for polyamine binding to the Tp0655 lipoprotein of Treponema pallidum: putative role for Tp0655 (TpPotD) as a polyamine receptor. J Mol Biol. 2007;373:681–694. doi: 10.1016/j.jmb.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson HJ, Eagle H, Fleischman R. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain) and a consideration of its rate of multiplication in vivo. Am J Syph Gonorrhea Vener Dis. 1948;32:1–18. [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Lukehart SA, Van Voorhis WC. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect Immun. 2002a;70:6811–6816. doi: 10.1128/IAI.70.12.6811-6816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J Immunol. 2002b;169:952–957. doi: 10.4049/jimmunol.169.2.952. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Lukehart SA, Van Voorhis WC. Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect Immun. 2003;71:5605–5612. doi: 10.1128/IAI.71.10.5605-5612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint M, Bashiri H, Harrington RD, Marra CM. Relapse of secondary syphilis after benzathine penicillin G: molecular analysis. Sex Transm Dis. 2004;31:196–199. doi: 10.1097/01.olq.0000114941.37942.4c. [DOI] [PubMed] [Google Scholar]
- Nandi B, Nandy RK, Sarkar A, Ghose AC. Structural features, properties and regulation of the outer-membrane protein W (OmpW) of Vibrio cholerae. Microbiology. 2005;151:2975–2986. doi: 10.1099/mic.0.27995-0. [DOI] [PubMed] [Google Scholar]
- Narita S, Tokuda H. Amino acids at positions 3 and 4 determine the membrane specificity of Pseudomonas aeruginosa lipoproteins. J Biol Chem. 2007;282:13372–13378. doi: 10.1074/jbc.M611839200. [DOI] [PubMed] [Google Scholar]
- Nath A, Atkins WM, Sligar SG. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry. 2007;46:2059–2069. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Jr, Mayer MM. Immobilization of Treponema pallidum in vitro by antibody produced in syphilitic infection. J Exp Med. 1949;89:369–393. doi: 10.1084/jem.89.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta. 2009;1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, Lithgow T, Buchanan SK. Structural insight into the biogenesis of β-barrel membrane proteins. Nature. 2013;501:385–390. doi: 10.1038/nature12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard MV, Miller JN. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983;42:435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ, Cox DL, Weinstock GM. Biology of Treponema pallidum: correlation of functional activities with genome sequence data. J Mol Microbiol Biotechnol. 2001;3:37–62. [PubMed] [Google Scholar]
- Okuda S, Tokuda H. Lipoprotein sorting in bacteria. Annu Rev Microbiol. 2011;65:239–259. doi: 10.1146/annurev-micro-090110-102859. [DOI] [PubMed] [Google Scholar]
- Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol. 2016;14:337–345. doi: 10.1038/nrmicro.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YY, Gromiha MM, Chen SA, Suwa M. TMBETADISC-RBF: Discrimination of b-barrel membrane proteins using RBF networks and PSSM profiles. Comput Biol Chem. 2008;32:227–231. doi: 10.1016/j.compbiolchem.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Parker ML, Houston S, Petrosova H, Lithgow KV, Hof R, Wetherell C, Kao WC, Lin YP, Moriarty TJ, Ebady R, Cameron CE, Boulanger MJ. The structure of Treponema pallidum Tp0751 (Pallilysin) reveals a non-canonical lipocalin fold that mediates adhesion to extracellular matrix components and interactions with host cells. PLoS Pathog. 2016;12:e1005919. doi: 10.1371/journal.ppat.1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn CW, Cockayne A, Bailey MJ. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985;131:2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Petrosova H, Zobanikova M, Cejkova D, Mikalova L, Pospisilova P, Strouhal M, Chen L, Qin X, Muzny DM, Weinstock GM, Smajs D. Whole genome sequence of Treponema pallidum ssp. pallidum, strain Mexico A, suggests recombination between yaws and syphilis strains. PLoS Negl Trop Dis. 2012;6:e1832. doi: 10.1371/journal.pntd.0001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan G, Picard M, Broutin I. Focus on the Outer Membrane Factor OprM, the Forgotten Player from Efflux Pumps Assemblies. Antibiotics (Basel) 2015;4:544–566. doi: 10.3390/antibiotics4040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M, Borges V, Antelo M, Pinheiro M, Nunes A, Azevedo J, Borrego MJ, Mendonca J, Carpinteiro D, Vieira L, Gomes JP. Genome-scale analysis of the non-cultivable Treponema pallidum reveals extensive within-patient genetic variation. Nat Microbiol. 2016;2:16190. doi: 10.1038/nmicrobiol.2016.190. [DOI] [PubMed] [Google Scholar]
- Porcella SF, Popova TG, Hagman KE, Penn CW, Radolf JD, Norgard MV. A mgl-like operon in Treponema pallidum, the syphilis spirochete. Gene. 1996;177:115–121. doi: 10.1016/0378-1119(96)00286-7. [DOI] [PubMed] [Google Scholar]
- Purcell BK, Swancutt MA, Radolf JD. Lipid modification of the 15 kiloDalton major membrane immunogen of Treponema pallidum. Mol Microbiol. 1990;4:1371–1379. doi: 10.1111/j.1365-2958.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Fehniger TE, Silverblatt FJ, Miller JN, Lovett MA. The surface of virulent Treponema pallidum: resistance to antibody binding in the absence of complement and surface association of recombinant antigen 4D. Infect Immun. 1986;52:579–585. doi: 10.1128/iai.52.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Chamberlain NR, Clausell A, Norgard MV. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988;56:490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Moomaw C, Slaughter CA, Norgard MV. Penicillin-binding proteins and peptidoglycan of Treponema pallidum subsp. pallidum. Infect Immun. 1989a;57:1248–1254. doi: 10.1128/iai.57.4.1248-1254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Norgard MV, Schulz WW. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989b;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Bourell KW, Akins DR, Brusca JS, Norgard MV. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol. 1994;176:21–31. doi: 10.1128/jb.176.1.21-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Goldberg MS, Bourell K, Baker SI, Jones JD, Norgard MV. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995a;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Robinson EJ, Bourell KW, Akins DR, Porcella SF, Weigel LM, Jones JD, Norgard MV. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995b;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Lukehart SA. Immunology of Syphilis. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponemes: Cellular and Molecular Biology. Caister Academic Press; Norfolk, UK: 2006. pp. 285–322. [Google Scholar]
- Radolf JD, Hazlett KRO, Lukehart SA. Pathogenesis of Syphilis. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponemes: Cellular and Molecular Biology. Caister Academic Press; Norfolk, UK: 2006. pp. 197–236. [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Tramont EC, Salazar JC. Syphilis (Treponema pallidum) In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. Churchill Livingtone, Elsevier; Philadelphia: 2014. pp. 2684–2709. [Google Scholar]
- Radolf JD, Deka RK, Anand A, Smajs D, Norgard MV, Yang XF. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol. 2016;14:744–759. doi: 10.1038/nrmicro.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Cheng J, Sweredoski M, Baldi P. TMBpro: secondary structure, beta-contact and tertiary structure prediction of transmembrane beta-barrel proteins. Bioinformatics. 2008;24:513–520. doi: 10.1093/bioinformatics/btm548. [DOI] [PubMed] [Google Scholar]
- Reid TB, Molini BJ, Fernandez MC, Lukehart SA. Antigenic variation of TprK facilitates development of secondary syphilis. Infect Immun. 2014;82:4959–4967. doi: 10.1128/IAI.02236-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollauer SE, Sooreshjani MA, Noinaj N, Buchanan SK. Outer membrane protein biogenesis in Gram-negative bacteria. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2015.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Hazlett KR, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002;4:1133–1140. doi: 10.1016/s1286-4579(02)01638-6. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi M, Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J Immunol. 2015;194:4081–4087. doi: 10.4049/jimmunol.1403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkrig J, Belousoff MJ, Headey SJ, Heinz E, Shiota T, Shen HH, Beckham SA, Bamert RS, Phan MD, Schembri MA, Wilce MC, Scanlon MJ, Strugnell RA, Lithgow T. Conserved features in TamA enable interaction with TamB to drive the activity of the translocation and assembly module. Sci rep. 2015;5:12905. doi: 10.1038/srep12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena AC, Pillay A, Cox DL, Radolf JD. Treponema and Brachyspira, human host-associated spirochetes. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. ASM Press; Washington, D.C: 2015. pp. 1055–1081. [Google Scholar]
- Setubal JC, Reis M, Matsunaga J, Haake DA. Lipoprotein computational prediction in spirochaetal genomes. Microbiology. 2006;152:113–121. doi: 10.1099/mic.0.28317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Kinnally KW, Mannella CA. Circular dichroism studies of the mitochondrial channel, VDAC, from Neurospora crassa. Biophys J. 1996;71:778–786. doi: 10.1016/S0006-3495(96)79277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko DV, Akins DR, Robinson EJ, Li M, Shevchenko OV, Radolf JD. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun. 1997;65:4179–4189. doi: 10.1128/iai.65.10.4179-4189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko DV, Sellati TJ, Cox DL, Shevchenko OV, Robinson EJ, Radolf JD. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect Immun. 1999;67:2266–2276. doi: 10.1128/iai.67.5.2266-2276.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smajs D, Norris SJ, Weinstock GM. Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect Genet Evol. 2012;12:191–202. doi: 10.1016/j.meegid.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett. 2007;273:1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- Stamm LV, Bergen HL. The sequence-variable, single-copy tprK gene of Treponema pallidum Nichols strain UNC and Street strain 14 encodes heterogeneous TprK proteins. Infect Immun. 2000;68:6482–6486. doi: 10.1128/iai.68.11.6482-6486.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm LV, Folds JD, Bassford PJ., Jr Expression of Treponema pallidum antigens in Escherichia coli K-12. Infect Immun. 1982;36:1238–1241. doi: 10.1128/iai.36.3.1238-1241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm LV, Greene SR, Bergen HL, Hardham JM, Barnes NY. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol Lett. 1998;169:155–163. doi: 10.1111/j.1574-6968.1998.tb13312.x. [DOI] [PubMed] [Google Scholar]
- Sun ES, Molini BJ, Barrett LK, Centurion-Lara A, Lukehart SA, Van Voorhis WC. Subfamily I Treponema pallidum repeat protein family: sequence variation and immunity. Microbes Infect. 2004;6:725–737. doi: 10.1016/j.micinf.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Swancutt MA, Radolf JD, Norgard MV. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990;58:384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Loranger JM, Wu HC. A distinct signal peptidase for prolipoprotein in Escherichia coli. J Cell Biochem. 1984;24:113–120. doi: 10.1002/jcb.240240203. [DOI] [PubMed] [Google Scholar]
- Tsirigos KD, Peters C, Shu N, Kall L, Elofsson A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015;43:W401–W407. doi: 10.1093/nar/gkv485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TB, Hollander DH. Biology of the treponematoses. World Health Organization; Geneva: 1957. [PubMed] [Google Scholar]
- van den Berg B. The FadL family: unusual transporters for unusual substrates. Curr Opin Struct Biol. 2005;15:401–407. doi: 10.1016/j.sbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- van den Berg B. Structural basis for outer membrane sugar uptake in pseudomonads. J Biol Chem. 2012;287:41044–41052. doi: 10.1074/jbc.M112.408518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B, Black PN, Clemons WM, Jr, Rapoport TA. Crystal structure of the long-chain fatty acid transporter FadL. Science. 2004;304:1506–1509. doi: 10.1126/science.1097524. [DOI] [PubMed] [Google Scholar]
- Walfield AM, Hanff PA, Lovett MA. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982;216:522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- Walker EM, Zampighi GA, Blanco DR, Miller JN, Lovett MA. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989;171:5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CT, Heinz E, Lithgow T. Evolution of the beta-barrel assembly machinery. Trends Microbiol. 2012;20:612–620. doi: 10.1016/j.tim.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Weigel LM, Brandt ME, Norgard MV. Analysis of the N-terminal region of the 47-kilodalton integral membrane lipoprotein of Treponema pallidum. Infect Immun. 1992;60:1568–1576. doi: 10.1128/iai.60.4.1568-1576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel LM, Radolf JD, Norgard MV. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc Natl Acad Sci U S A. 1994;91:11611–11615. doi: 10.1073/pnas.91.24.11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. struct Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- Wimley WC. The versatile β-barrel membrane protein. Curr Opin Struct Biol. 2003;13:404–411. doi: 10.1016/s0959-440x(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Wu XB, Tian LH, Zou HJ, Wang CY, Yu ZQ, Tang CH, Zhao FK, Pan JY. Outer membrane protein OmpW of Escherichia coli is required for resistance to phagocytosis. Res Microbiol. 2013;164:848–855. doi: 10.1016/j.resmic.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Y. Protein structure and function prediction using I-TASSER. Curr Protoc Bioinform. 2015;52:5 8 1–15. doi: 10.1002/0471250953.bi0508s52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara R, Yamashita E, Nakagawa A. Crystal structures of OprN and OprJ, outer membrane factors of multidrug tripartite efflux pumps of Pseudomonas aeruginosa. Proteins. 2016;84:759–769. doi: 10.1002/prot.25022. [DOI] [PubMed] [Google Scholar]
- Yu CS, Chen YC, Lu CH, Hwang JK. Prediction of protein subcellular localization. Proteins. 2006;64:643–651. doi: 10.1002/prot.21018. [DOI] [PubMed] [Google Scholar]
- Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeth K, Thein M. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem J. 2010;431:13–22. doi: 10.1042/BJ20100371. [DOI] [PubMed] [Google Scholar]
- Zuckert WR. Secretion of bacterial lipoproteins: through the cytoplasmic membrane, the periplasm and beyond. Biochim Biophys Acta. 2014;1843:1509–1516. doi: 10.1016/j.bbamcr.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


