Abstract
Background
We investigated in vitro whether HLA-highly-sensitized patients with end-stage renal disease (ESRD) will be disadvantaged immunologically after a genetically-engineered pig kidney transplant.
Methods
Blood was drawn from patients with a cPRA 99–100% (Gp1, n=10) or cPRA 0% (Gp2, n=12), and from healthy volunteers (Gp3, n=10). Serum IgM and IgG binding was measured (i) to Gal and Neu5Gc glycans by ELISA, and (ii) to pRBC, pAEC, and pPBMC from GTKO/CD46 and GTKO/CD46/CMAHKO pigs by flow cytometry. (iii) T and B cell phenotypes were determined by flow cytometry, and (iv) proliferation of T and B cells CFSE-MLR.
Results
(i) By ELISA, there was no difference in IgM or IgG binding to Gal or Neu5Gc between Gps1 and 2, but binding was significantly reduced in both groups compared to Gp3. (ii) IgM and IgG binding in Gps1 and 2 was also significantly lower to GTKO/CD46 pig cells than in healthy controls, but there were no differences between the 3 groups in binding to GTKO/CD46/CMAHKO cells. (iii and iv) Gp1 patients had more memory T cells than Gp2, but there was no difference in T or B cell proliferation when stimulated by any pig cells. The proliferative responses in all 3 groups were weakest when stimulated by GTKO/CD46/CMAHKO pPBMC.
Conclusions
(i) ESRD was associated with low anti-pig antibody levels. (ii) Xenoreactivity decreased with increased genetic engineering of pig cells. (iii) High cPRA status had no significant effect on antibody binding or T and B cell response.
Introduction
Kidney transplantation is the preferred treatment for most patients with ESRD1–3. Patients highly-sensitized to human leukocyte antigens (HLA), with a high level of calculated panel-reactive antibodies (cPRA), are unlikely to receive a human organ in a timely manner4–7. Those with a cPRA of 99–100% may never receive an allograft8, 9.
Pigs could provide an unlimited source of kidneys. With the development of genetic-engineering, the 3 well-characterized glycan xenoantigens on pig cells (galactose-α1–3 galactose [Gal], N-glycolylneuraminic acid [Neu5Gc], and Sda, a product of beta-1,4-N-acety1-galactosaminyltransferase 2 (β4GalNT2), to be deleted by knockout (KO) technology10, 11. Pigs can also be manipulated to express 1 or more human complement- or coagulation-regulatory proteins, providing additional protection against antibody-mediated rejection12–14. Some previous in vitro studies have indicated that HLA-sensitized patients will not be at greater risk of humoral rejection of a pig organ than HLA-nonsensitized patients15–18. However, other studies suggest some cross-reactivity between anti-HLA and anti-SLA (swine leukocyte antigen) antibodies19–24. Patients with both anti-HLA class I and II antibodies may exhibit increased T cell responses to pig cells25, though others found that HLA sensitization was not indicative of a heightened T cell response to SLA26.
Our present study investigated the impact of (i) cPRA, and (ii) T and B cell reactivity to pig cells in HLA-highly-sensitized (cPRA 99–100%) and nonsensitized (cPRA 0%) prospective kidney transplant recipients.
We compared serum IgM and IgG binding from patients with high cPRA with those with a negative cPRA against red blood cells (RBCs), aortic endothelial cells (AECs), and peripheral blood mononuclear cells (PBMCs) from (i) α1,3-galactosyltransferase gene-knockout (GTKO) pigs that express the human complement-regulatory protein, CD46, or (ii) GTKO/CD46 pigs in which expression of Neu5Gc had been deleted by knockout of the gene for cytidine-monophosphate-N-acetylneuraminic acid hydroxylase (GTKO/CD46/CMAHKO pigs). (RBCs express only glycan antigens, but not SLA class I or class II, whereas AECs and PBMCs express both glycan antigens and SLA.) We also compared the phenotype frequencies and proliferative responses of T or B cells to wild-type (WT, ie, genetically-unmodified), GTKO/CD46, and GTKO/CD46/CMAHKO pig cells.
Our study indicated that a patient with a high cPRA should accept a kidney from a genetically-engineered pig with no increased immune risk when compared to a nonsensitized patient (or any healthy human). These data differ from some other studies, and the possible reasons are discussed.
Methods
Human serum and cell samples
All studies using human blood were approved by the Research Ethics Committee of the University of Pittsburgh (IRB# REN16040230). Blood (40mL) was drawn on a single occasion from 22 subjects awaiting kidney transplantation, and from 10 human volunteers.
Group 1 (n=10) consisted of patients awaiting kidney allotransplantation who had a high cPRA (99–100%); all had undergone previous kidney transplantation. Group 2 (n=12) were patients with a negative cPRA (0%); none had undergone a previous kidney transplant. Group 3 (n=10) were healthy human volunteers (controls). The cPRA was not assessed in the Group 3 subjects, but none had a history suggesting previous HLA or SLA exposure.
All Group 1/2 subjects had been on hemodialysis and had received no exogenous immunosuppressive therapy for >5 years, and were not selected by age, gender, ethnicity, or cause of ESRD.
Human (h) PBMCs from Groups 1–3 subjects were isolated from fresh blood samples27.
Pig (p) cell sources and preparation of pRBCs, pAECs, and pPBMCs
RBCs, AECs, and PBMCs were collected from (i) wild-type (WT), (ii) GTKO/CD46, and (iii) GTKO/CD46/CMAHKO pigs (Revivicor, Blacksburg, VA).
Quantitation of total IgA, IgE, IgG, and IgM in serum
The total IgA, IgG, IgM in serum samples was quantitated by rate nephelometry (Beckman IMMAGE 800 Immunochemistry System, UPMC Clinical Laboratory). Serum IgE was quantitated by a solid-phase chemiluminescent immunometric assay (Siemens Immuline 2000XPI; UPMC Clinical Laboratory).
Determination of anti-Gal and anti-Neu5Gc antibody levels in human serum
Anti-Gal and anti-Neu5Gc antibodies were measured by anti-Gal and anti-Neu5Gc ELISA for IgM and IgG28.
Binding of human anti-pig IgM and IgG antibodies to pRBCs, pAECs, and pPBMCs
Binding of antibodies to GTKO/CD46 and GTKO/CD46/CMAHKO pig cells was measured by flow cytometry using relative median fluorescence intensity (RMFI)29.
Measurement of T and B cell frequency and phenotypes in hPBMC
Surface staining of PBMCs from the human subjects was performed by flow cytometry (see Supplementary Methods).
Measurement of human T and B cell proliferative responses (CFSE-MLR) to pPBMC by flow cytometry
For CFSE-MLR, human PBMCs were used as responder cells, with stimulator cells (pPBMC) from WT, GTKO/CD46, or GTKO/CD46/CMAHKO pigs30.
Statistical analysis
Normally distributed data are presented as mean and standard deviation (SD). Study groups were compared using Student t-test (for normally distributed continuous variables) or nonparametric Mann-Whitney test (for not normally distributed variables) to determine statistical significance. For correlation, a Pearson correlation test was used. A P value of <0.05 was considered significant.
Results
Antibody Studies
Quantitation of total immunoglobulins in human sera
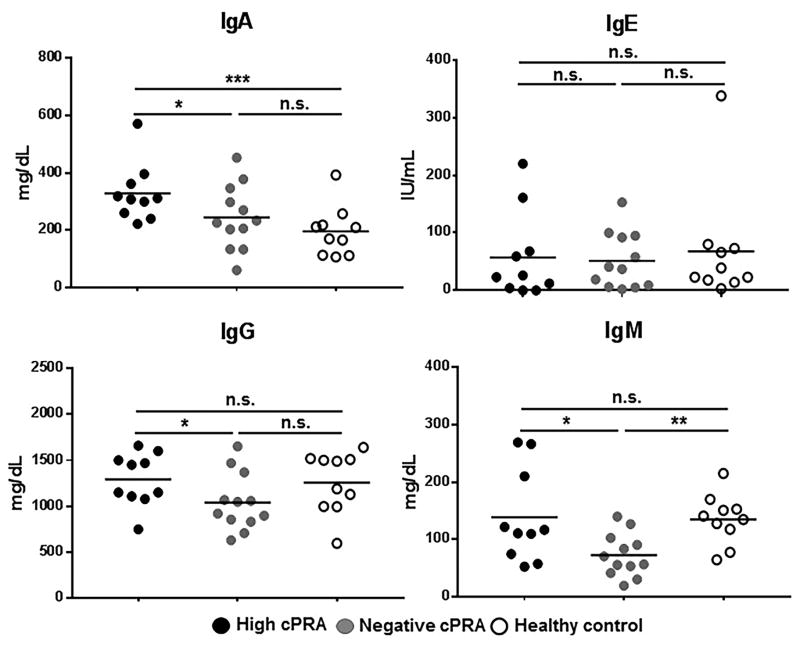
Total IgA, IgE, IgG, and IgM levels in serum from all subjects were compared (Figure 1, Table S1). Total IgA in Group 1 was higher than in both Groups 2 and 3 (P<0.05 and P<0.005, respectively). There was no difference in total IgE levels between the 3 groups. Group 1 had significantly higher levels of IgG and IgM than Group 2 (P<0.05), but no difference from Group 3. There were no significant differences in IgA, IgE, and IgG between Groups 2 and 3, but the level of IgM in Group 2 was significantly lower than in Group 3 (P<0.01).
Figure 1.
The amount of total IgA, IgE, IgG, IgM antibodies in all subjects was measured in high cPRA subjects (Group 1, n=10), in negative cPRA subjects (Group 2, n=12), and healthy volunteers (controls, Group 3, n=10) (ns=not significant; *P<0.05). Only 1 patient (in Group 2) had an IgA nephropathy. (A high anti-HLA IgA has been associated with less rejection by binding IgG antibodies61–64, and a correlation of increased IgA levels with chronic kidney disease has been noted in the past65).
These data suggest that hemodialysis (in Groups 1/2) does not reduce the total immunoglobulin levels compared to healthy control subjects (Group 3). However, there was evidence that ESRD patients with a high cPRA had higher levels of total IgA, IgG, and IgM than patients with a negative cPRA, but only IgA was higher than in the healthy controls.
Human IgM and IgG binding to synthetic glycans by ELISA
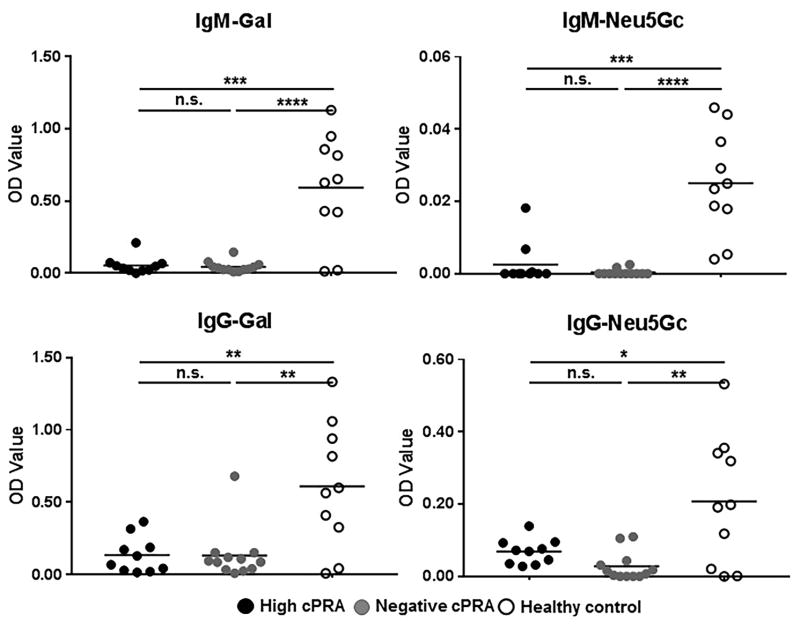
The high (Group 1) and the negative (Group 2) cPRA patients exhibited no significant differences in IgM or IgG binding to either Gal or Neu5Gc (Figure 2). The control Group 3, however, had significantly higher IgM and IgG binding to both glycans than either study group (P<0.005 for IgM, P<0.05 for IgG). Thus, the binding of human IgM or IgG to these glycans did not correlate with the level of human antibodies directed to HLA.
Figure 2.
Anti-Gal and anti-Neu5Gc IgM/IgG were measured by ELISA in high cPRA subjects (Group 1, n=10), in negative cPRA subjects (Group 2, n=12), and in healthy controls (Group 3, n= 10) (ns=not significant; *P<0.05, **P<0.01, ***P<0.005, ****P<0.001).
In contrast to total immunoglobulin levels, these data strongly suggest that chronic hemodialysis or the presence of ESRD results in reduced anti-glycan immunoglobulin levels in patients awaiting kidney transplantation (discussed below).
Human IgM and IgG binding to GTKO/CD46 and GTKO/CD46/CMAHKO pig cells by flow cytometry
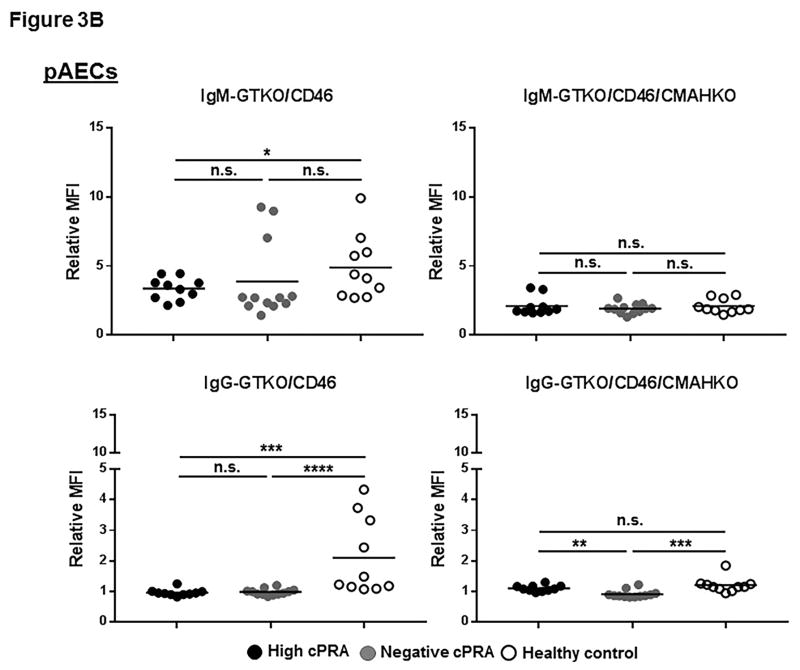
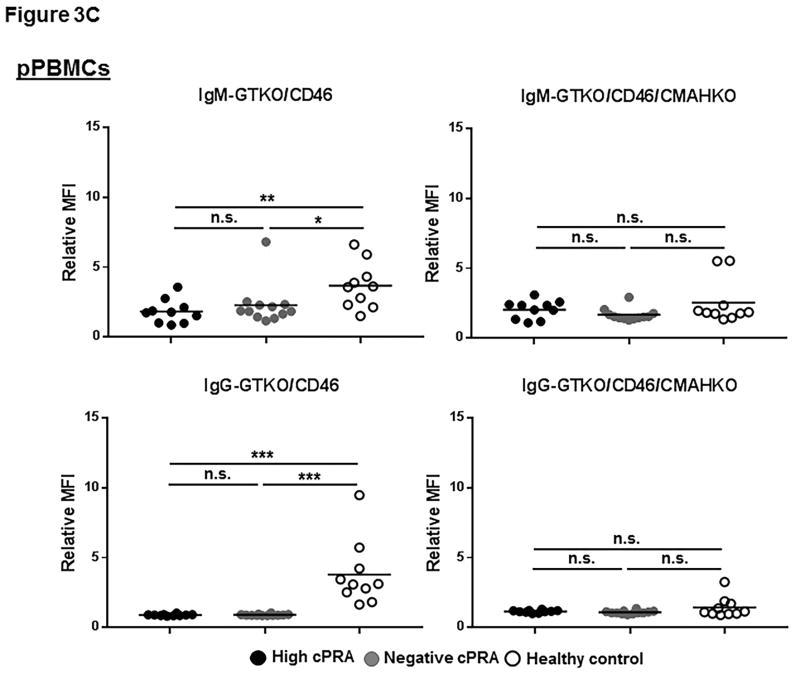
Binding to GTKO/CD46 pig cells
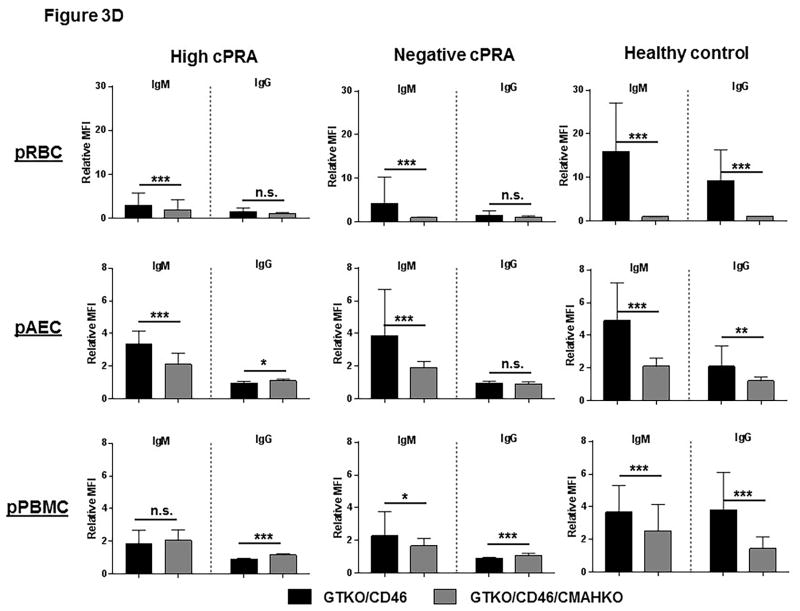
In patients with high or negative cPRA, there was a very low level of IgM binding to all of the 3 types of cells from GTKO/CD46 pigs, and almost no binding of IgG (Figures 3A–C, Table S2), with no significant difference in binding of either IgM or IgG between Groups 1 and 2. Binding of IgM and IgG in Group 3 was significantly higher. These data correlate with the results of ELISA.
Figure 3.
IgM/IgG binding to (A) pRBC, (B) pAEC, and (C) pPBMC isolated from GTKO/CD46 and GTKO/CD46/CMAHKO pigs was measured by flow cytometry (presented as median fluorescence intensity [RMFI]) in high cPRA subjects (Group 1, n=10), in negative cPRA subjects (Group 2, n=12), and healthy controls (Group 3, n=10). (D) The impact of pig genetics on IgM/IgG binding to pRBC, pAEC, and pPBMC (ns=not significant, *P<0.05, **P<0.01, ***P<0.005, ****P<0.001).
Binding to GTKO/CD46/CMAHKO pig cells
There was no significant difference in IgM/IgG binding to all 3 types of cells from GTKO/CD46/CMAHKO pigs among the 3 groups (Figures 3A–C). The only exception was that IgG in the high cPRA group showed more binding to pAEC than the negative cPRA group (P<0.01) (Figure 3B). IgG binding in Group 3 sera was higher than in the Group 2 sera (Figure 3B), though the binding in all 3 groups was very low.
These data indicate that the level of human IgM/IgG binding to GTKO/CD46/CMAHKO pig cells did not correlate with cPRA status.
Healthy controls had higher IgM (P<0.05) and IgG (P<0.005) antibody binding to pRBC and pPBMC from GTKO/CD46 pigs (Figures 3A,C). This contrasted to the near absence of antibody binding (IgM and IgG) in healthy controls to all cell targets from GTKO/CD46/CMAHKO pigs. Absence of Neu5Gc expression, therefore, significantly reduced binding of healthy human sera to all pig cells tested (Figure 3D), but not binding of sera of patients with ESRD undergoing hemodialysis.
Whereas binding of both IgM and IgG from sera in Group 3 to GTKO/CD46/CMAHKO pig cells was always reduced compared to binding to GTKO/CD46 pig cells, this was not always the case in sera from Groups 1/2 (Figure 3D). Although IgM binding in sera from Groups 1/2 was always reduced (or unchanged) to GTKO/CD46/CMAHKO cells (compared to GTKO/CD46 cells), IgG binding was sometimes minimally increased (<15%) (P<0.005 for both high and negative cPRA IgG binding to pPBMCs, P<0.05 for high cPRA IgG binding to pAECs), although the level of binding was always low.
These data suggest that many healthy subject sera (Group 3) have antibodies that bind to Neu5Gc, whereas sera from many ESRD patients on hemodialysis (Groups 1/2) do not. The observation that in some ESRD patients IgG binding was sometimes increased to GTKO/CD46/CMAHKO pAECs and pPBMCs (but not to pRBCs) suggests that the binding may have been to nonglycan antigens, eg, SLA.
In the flow cytometry studies (Figures 3A–C), there were occasional patients in Groups 1/2 with aberrantly higher levels of IgM or IgG binding to GTKO/hCD46 pig cells. However, these occasional patients did not necessarily have a greater number of memory T cells or a greater than average T cell proliferative response.
Cellular Studies
Phenotypes and quantitation of T and B cells in hPBMCs by flow cytometry
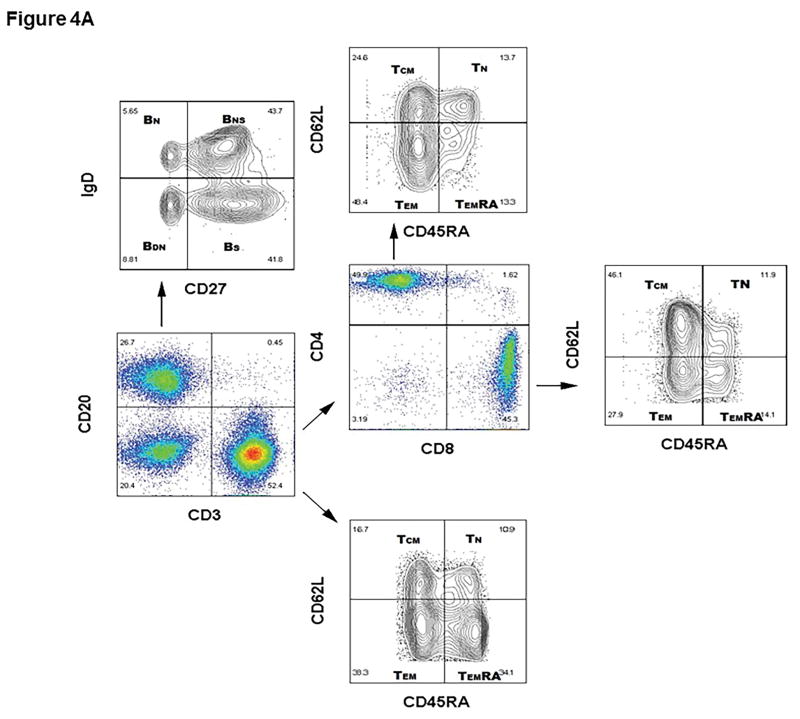
One representative flow gating strategy in a high cPRA subject to analyze the phenotypes and quantitation of T and B cells is shown in Figure 4A.
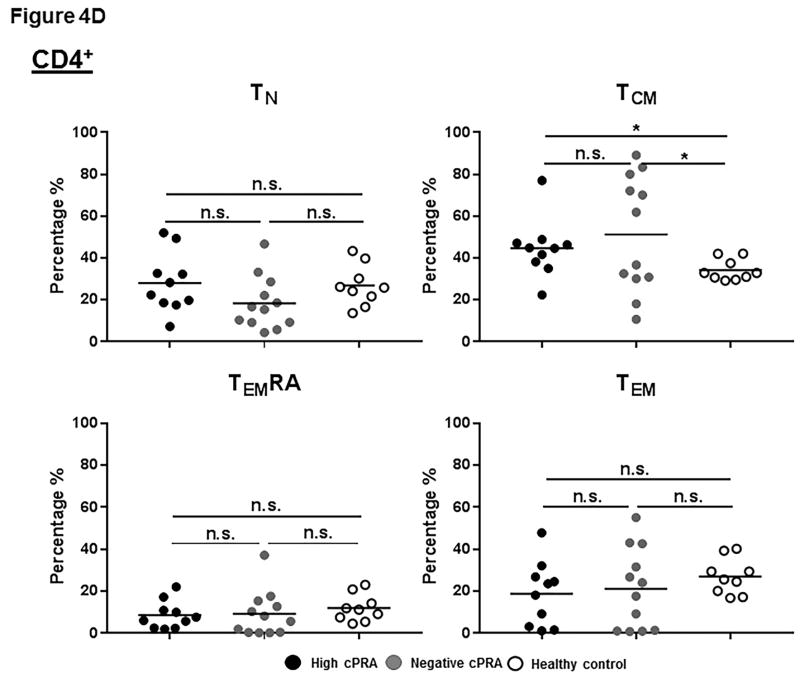
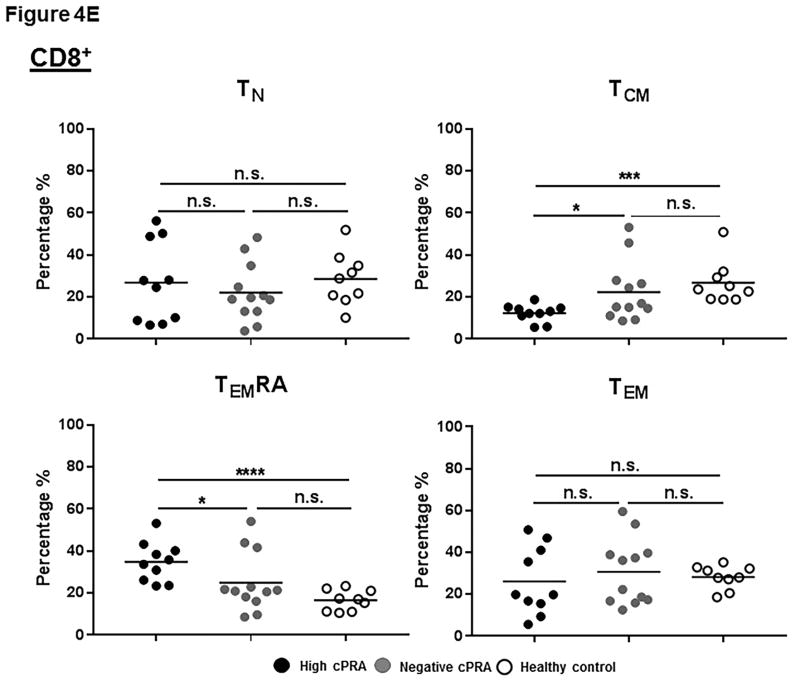
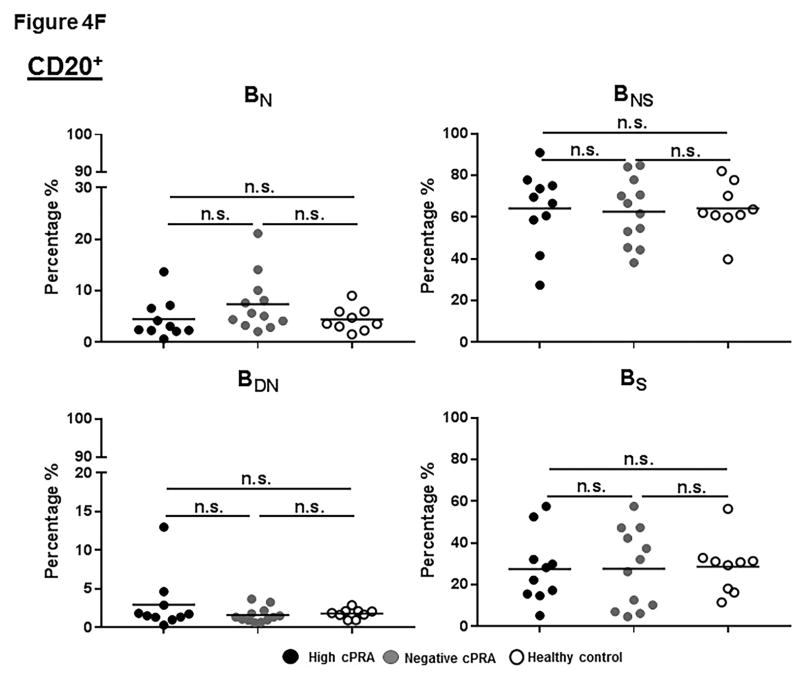
Figure 4.
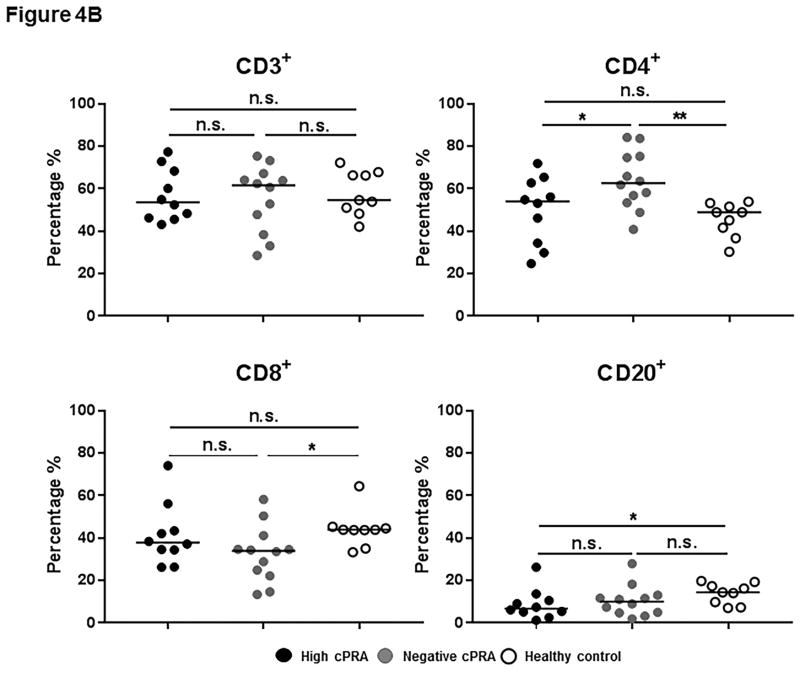
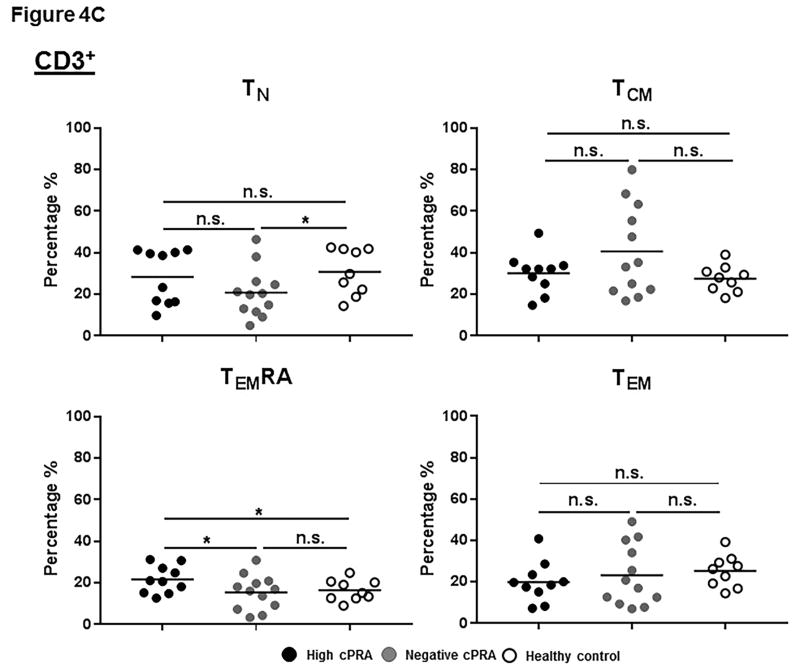
(A–E) Determination of T and B cell subsets in hPBMCs. (A) Representative results to illustrate gating strategy for measurement of T and B cell phenotypes in human PBMC (hPBMC) by flow cytometry; (B)The frequencies of CD3+, CD4+, and CD8+T cells, and B cells in high cPRA subjects (n=10), negative cPRA subjects (n=12), and healthy controls (n=9) were determined by flow cytometry. Naïve and memory (C) CD3+T cell, (D) CD4+T cell, and (E) CD8+T cell populations present in hPBMC from subjects in the 3 study groups were identified and quantified (presented as percentage of total CD3+T cells, CD4+T cells, and CD8+T cells, respectively) by flow cytometry. (F) The ratios of B cell phenotypes in each hPBMC sample of all study groups were identified and quantified (presented as percentage of total CD20+B cells) by flow cytometry. (n=10 for high cPRA subjects, n=12 for negative cPRA subjects, and n=9 for healthy controls, ns=not significant; *P<0.05, **P<0.01, ***P<0.005, ****P<0.001).
CD3+T cells
There was no significant difference in the frequency of CD3+T cells among the 3 groups (Figure 4B, Table S3). However, there was a higher frequency of CD4+T cells in Group 2 compared with Group 1 (P<0.05) and Group 3 (P<0.01), while there was no significant difference between Groups 1 and 3. Even though Group 3 had a higher frequency of CD8+T cells than Group 2 (P<0.05), there was no significant difference between Groups 1 and 3 or between Groups 1 and 2.
CD3+T cell subsets
TN cells were less frequent in Group 2 than in Group 3 (P<0.05), while there was no significant difference between Groups 1 and 2 (Figure 4C). There was no significant difference in frequency of TCM cells among the 3 groups. Group 1 had the highest frequency of TEMRA cells (P<0.05). No differences in TEM numbers were observed.
CD4+T cells
There were no significant differences in the frequencies of TN, TEM and TEMRA of CD4+T-cells among the 3 groups (Figure 4D, Table S3). However, Groups 1 and 2 had significantly higher TCM cell numbers than Group 3 (P<0.05), but there was no significant difference between Groups 1 and 2.
CD8+T cells
There were no differences in TN and TEM between the groups (Figure 4E, Table S3). Group 1 had the highest frequency of TEMRA cells within the CD8+T cell population, but had a lower frequency of TCM cells.
As with IgM binding to GTKO/CD46 cells, there were occasional ‘outliers’ (with a higher percentage of a specific cell type) in the Group 1/2 populations, suggesting that these subjects might have a stronger T cell proliferative response to pig antigens. However, patients with a higher percentage of a specific T memory cell did not necessarily have a stronger T cell proliferative response.
CD20+B cells
The frequency of CD20+B cells in Group 1 was significantly lower than in Group 3 (P<0.05), but not different from Group 2 (Figure 4B). Moreover, there was no significant difference in B cell subsets among the 3 groups (Figure 4F, Table S3).
Human T and B cell proliferation in response to pPBMCs (by CFSE-MLR)
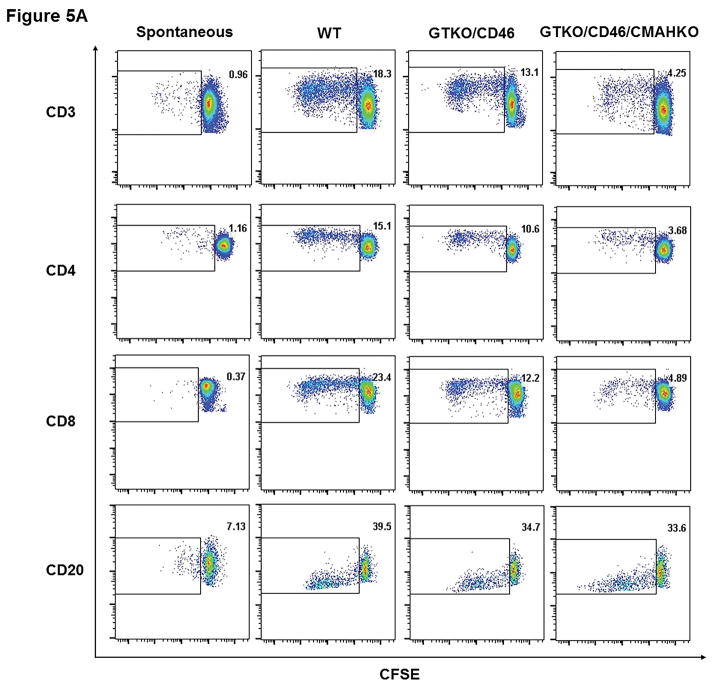
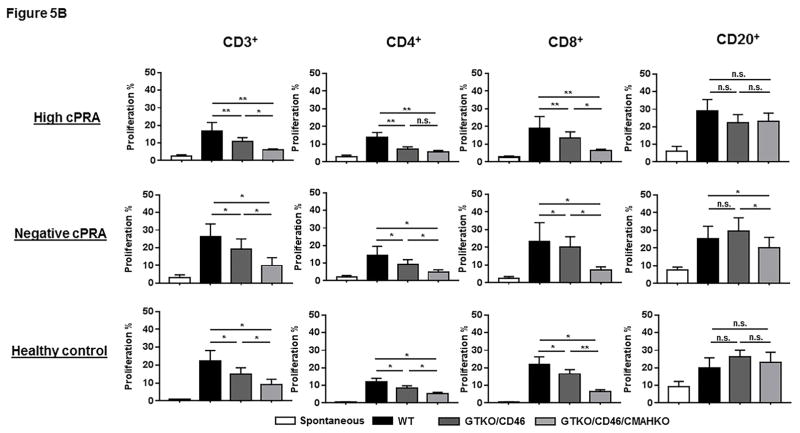
Dot-plot representative of the proliferation of CD3+, CD4+, CD8+ T cells, and CD20+ cells in hPBMC of one high cPRA subject responding to pPBMC from WT, GTKO/CD46, and GTKOCD46/CMAHKO is shown in Figure 5A. The proliferation of T cells (CD3+, including subsets CD4+ and CD8+) in Groups 1–3 was weakest when stimulated with GTKO/CD46/CMAHKO pPBMCs (Figure 5B, Table S4). However, the relative proliferation of human T cells (CD3+, including the proliferation of subsets CD4+ and CD8+T cells) to the various pig cells (WT, etc.) was similar in the 3 groups (data not shown). The CD3+T cell proliferative response in Group 1 patients was rather weaker (but not significantly) than that of Group 2.
Figure 5.
The response of hPBMC to pPBMC by CFSE-MLR. (A) Representative dot-plot of the proliferation of CD3+, CD4+, CD8+ T cells, and CD20+ cells in hPBMC of a hPRA subject responding to pPBMC from WT, GTKO/CD46, and GTKOCD46/CMAHKO; (B) Percentage proliferation of CD3+, CD4+, CD8+ T cells and CD20+ cells in hPBMC in all study groups responding to pPBMC from WT, GTKO/CD46, and GTKOCD46/CMAHKO pigs was identified and quantified. (n=10 high cPRA subjects responding to WT [n=7] and to GTKO/CD46 and GTKO/CD46/CMAHKO [n=10], n=7 for negative cPRA subjects responding to WT [n=5] and to GTKO/CD46 and GTKO/CD46/CMAHKO [n=7], n=9 for healthy controls responding to WT [n=6] and to GTKO/CD46 and GTKO/CD46/CMAHKO [n=9].) (ns=not significant; *P<0.05, **P<0.01).
These data suggest that the human T cell proliferative response to pPBMCs is not affected by the cPRA, but is influenced by the type of pig stimulator cells. High cPRA patients did not have a T cell proliferative response that was stronger than patients with a negative cPRA (not shown).
The B cell (CD20+) proliferative response showed very few differences between the 3 human groups, nor was there a great difference in response to the 3 pig cell types (Figure 5A,B).
Correlation data
Correlation of IgM/IgG binding with T/B cell proliferation, and the ratio of T/B cell phenotypes with proliferation are reported in Supplementary Methods and Results.
Discussion
In the USA, despite the new allocation rules, HLA-highly-sensitized patients remain disadvantaged in obtaining a renal allograft. We aimed to determine whether patients with a cPRA 99–100% would be disadvantaged if a pig xenograft was transplanted compared to those with a cPRA 0%. We also investigated whether high cPRA patients demonstrated differences in the T and B cell responses that might affect the outcome of a xenograft.
In concordance with previous studies29, 31, in healthy humans (Group 3) we found significantly diminished antibody binding to GTKO/CD46/CMAHKO pig cells compared to GTKO/CD46 pig cells (Figure 3D). In the Group 1/2 patients, IgM binding was also reduced to GTKO/CD46/CMAHKO pig cells, but IgG binding was sometimes unchanged or even slightly increased. Importantly, there was no greater binding of IgM or IgG xenoreactive antibodies in the sera of high cPRA patients than negative cPRA patients.
Double-KO pig cells (GTKO and CMAHKO) do not stimulate human responder cells more than in an allograft model, and the T cell responses are similarly effectively reduced by conventional immunosuppressive agents11. In our current study, the anti-pig T cell response, as measured by CFSE-MLR, was also significantly reduced compared to that to WT pig cells (Figure 5). HLA-highly-sensitized patients did not have a stronger T cell response to pig cells.
A previous study indicated that healthy adult blood contains comparable percentages of CD45RA− (TCM, TEM), CD45RA+ (TN, TEMRA), and CD4+ (TN, TCM, TEM) cells (20%–40%), whereas CD8+TEMRA cells are seen in higher frequency than CD4+T cells32. Our results in healthy humans (Group 3) were comparable (Figure 4, Table S3).
TEMRA cells (with the same function as TEM cells) are found mostly in peripheral inflamed tissues and can secrete effector molecules in response to antigens33. Our data show that, compared with the negative cPRA and healthy human groups, high cPRA patients had the highest frequency of TEMRA cells (among the total CD3+T cell population and in the CD8+T cell subset, but not in the CD4+T cell subset) (Figures 4C,D,E). This might be associated with previous exposure to alloantigens.
Our study supports the conclusion that human subjects with a high level of sensitization would not react immunologically to DKO (or presumably triple-KO) donor pig cells any more strongly than patients with a negative cPRA (as measured by antibody binding). Furthermore, their T cell responses would not be stronger than in patients with a negative cPRA.
Our observation that patients awaiting kidney allotransplantation (whether with a high or negative cPRA) had significantly lower levels of anti-Gal and anti-Neu5Gc antibodies (but not of total immunoglobulin) than healthy controls does not appear to have been reported previously. It is unlikely that hemodialysis directly removes antibodies since standard hemodialysis filters typically have a cut-off between 10–20 kDa, whereas an IgG molecule is about 150 kDa, and is thus not removed by hemodialysis34.
It is well-recognized that ESRD patients are to some extent immunocompromised, and are more susceptible to infections35–46. The clinical impact of ESRD on anti-pig immunity remains largely unknown. However, in the present study, we did not document major differences in T and B cell proliferation compared with healthy humans. The profound reduction in antibody levels to pig glycan antigens, however, was surprising, particularly as total immunoglobulin levels remained within the normal ranges.
Before 2004, studies of human anti-pig responses were limited by not having access to GTKO pigs. However, in 1998, two studies examined the reactivity of sera from HLA-highly-sensitized and nonsensitized patients19, 47. Taylor et al adsorbed natural anti-glycan antibodies on WT pRBCs (and on pooled human platelets), and found a decrease of reactivity in the nonsensitized patients, but not in the highly-sensitized patients, leading them to conclude that some reactivity was due to anti-HLA class I-specific antibodies (presumably cross-reacting to SLA)47. Naziruddin abd Varela independently came to similar conclusions19,22. Barreau used pig kidneys to adsorb xenoreactive antibodies from sera of HLA-highly-sensitized patients20. Eluted antibodies from the pig kidneys were mostly anti-Gal, but some anti-HLA class I antibodies were also detected. In 2002, Taylor’s group reported that sera (adsorbed as above) from HLA-sensitized patients (cPRA >64%) demonstrated a positive cross-match against CD55 (hDAF)-transgenic pig leukocytes, again indicating that some anti-HLA antibodies might cross-react with SLA21. Cross-reactivity between HLA and SLA was studied by Mulder et al23, who tested the binding of 68 human anti-HLA mAbs to haploidentical inbred miniature swine PBMCs. Of the 68 anti-HLA mAbs, 9 were reactive to pig cells, and 4 of these were specific to the 65QIS epitope on the SLA α-helix.
The availability of GTKO pigs was a breakthrough in xenotransplantation research48–50 by greatly reducing human serum antibody binding to pig cells16, 51, and reducing antibody-mediated injury to pig organs52–54. By 2006, two groups had studied antibody binding to cells from 2 different GTKO pigs with sera from patients awaiting kidney transplantation16, 17. Both studies showed that sera from HLA-highly-sensitized patients showed no greater binding to GTKO pig cells than nonsensitized sera.
Interestingly, a reduced level of anti-pig antibody in patients with ESRD was not reported in any of these studies.
Investigators have identified 2 other glycans that are targets for human anti-pig antibodies55–57. These are Neu5Gc and Sda, suggesting that KO of CMAH (coding for Neu5Gc) and of β4GalNT2 (coding for Sda) in GTKO pig cells would reduce antibody binding further. This proved to be the case when investigators showed that human serum antibodies bound less to double-KO (lacking Gal and Sda or Gal and Neu5Gc) and triple-KO (lacking Gal, Neu5Gc, and Sda) pig cells than to WT or GTKO cells58, 59. In 2016, this group studied sera from 820 wait-listed patients using triple-KO pig cells24. Their conclusion was that, if all anti-pig glycan antibodies have been removed from the serum or no longer bind to the genetically-engineered pig cells, some patients can be demonstrated to have anti-SLA class I antibodies.
Some investigators therefore demonstrated a potential increased risk of rejecting a pig graft in HLA-highly-sensitized patients (through cross-reactivity between anti-HLA and anti-SLA antibodies), but others did not. It is possible that these varying results are associated with differences in the SLA phenotype of the pigs from which the cells were derived. For example, there is convincing evidence that antibodies to certain HLA cross-react with specific SLA expressed in the pigs that one group has used for its studies (where the SLA type of the pigs is known)24. However, there has been no definite evidence for such cross-reactivity in studies using pigs from other sources. Wong used the Massachusetts General Hospital SLA-well-defined inbred miniature swine as sources of cells17, and Hara used the Revivicor pigs, which were also those used in the present study (in which the SLA typing is unknown)16. We suggest that SLA may be expressed in some pigs against which specific HLA-reactive antibodies may be directed, but not in other pigs. This important point needs to be clarified before clinical trials can be considered in patients with a high cPRA.
Another potential reason is that, in the present study, the target pig cells still expressed one of the known glycans, Sda, and antibody binding to Sda might have ‘masked’ the binding to other antigens, eg, SLA. However, this would suggest that binding to SLA is low.
A third possibility may be the differences in the number of sera tested, which was far higher (n=820) in the Martens study24. In this study, in a subset of patients with cPRA >80% (n=119), in which sera were depleted with WT pRBCs, only 13 samples contained SLA class I-specific IgG, thus demonstrating that some sensitized patients (but by no means all) may demonstrate cross-reactivity.
A fourth possibility is that the immunosuppressed state in ESRD patients reduces the production of antibodies to a point where it is difficult to identify anti-SLA antibodies, the incidence and avidity of which are low. However, this would not account for their identification in some studies, but not in others, as presumably all ESRD patients are relatively equally immunocompromised.
The present study, and most others, indicate that there are at least some ESRD patients with a high cPRA who would appear to be at no greater risk of undergoing pig organ transplantation than patients with a negative cPRA. Furthermore, when antibodies are present that cross-react with a specific SLA, it should be possible by genetic engineering of the pig to delete the offending antigen(s)60.
Supplementary Material
Acknowledgments
Funding: Zhongqiang Zhang was supported by the China Scholarship Council (File No. 201506370112). Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is, or has been, supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
We thank our colleague, Diana Metes, for her advice and support. We also thank Sheila Fedorek and Stephenie Dermont for identifying, consenting, and drawing the blood on all study subjects.
Abbreviations
- AECs
aortic endothelial cells
- β4GalNT2
beta-1,4-N-acety1-galactosaminyltransferase 2
- CFSE-MLR
carboxyfluorescein diacetate succinimidyl ester-mixed lymphocyte reaction
- CMAHKO
cytidine monophosphate-N-acetylneuraminic acid hydroxylase-knockout
- cPRA
calculated panel-reactive antibodies
- ESRD
end-stage renal disease
- Gal
galactose-α1–3 galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- HLA
human leukocyte antigens
- mAb
monoclonal antibody
- Neu5Gc
N-glycolylneuraminic acid
- PBMCs
peripheral blood mononuclear cells
- RBCs
red blood cells
- SLA
swine leukocyte antigens
Footnotes
Author Contributions: All authors participated in revising and approving the manuscript. ZZ, HQ, HH, MW, DKCC designed and initiated this study. ZZ, CL, IH, HH participated in laboratory assay. Data were collected and analyzed by ZZ, CL, HH, CM, MM, AZ, ME, MW, DKCC. The manuscript was prepared by ZZ, HH, ME, CM, AZ, MW, DKCC. Genetically-engineered pig cells were provided by DA.
Conflicts of interest: David Ayares is an employee of Revivicor, Inc. No other author has a conflict of interest.
References
- 1.Lloveras J, Arcos E, Comas J, Crespo M, Pascual J. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation. 2015;99:991–996. doi: 10.1097/TP.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 2.Santos AH, Casey MJ, Wen XR, et al. Survival with dialysis versus kidney transplantation in adult hemolytic uremic syndrome patients: a fifteen-year study of the waiting list. Transplantation. 2015;99:2608–2616. doi: 10.1097/TP.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 4.Gebel HM, Kasiske BL, Gustafson SK, et al. Allocating deceased donor kidneys to candidates with high panel-reactive antibodies. Clin J Am Soc Nephro. 2016;11:505–511. doi: 10.2215/CJN.07720715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield RR, Scalea JR, Zens TJ, et al. The mode of sensitization and its influence on allograft outcomes in highly sensitized kidney transplant recipients. Nephrol Dial Transpl. 2016;31:1746–1753. doi: 10.1093/ndt/gfw099. [DOI] [PubMed] [Google Scholar]
- 6.Schinstock C, Cheungpasitporn W, Cosio F, et al. Kidney transplant with low level DSA and low positive b-flow crossmatch: an underappreciated option for highly sensitized transplant candidates. Am J Transplant. 2016;16:700–701. doi: 10.1097/TP.0000000000001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16:1834–1847. doi: 10.1111/ajt.13770. [DOI] [PubMed] [Google Scholar]
- 8.Baxter-Lowe LA, Kucheryavaya A, Tyan D, Reinsmoen N. CPRA for allocation of kidneys in the US: more candidates >/=98% cPRA, lower positive crossmatch rates and improved transplant rates for sensitized patients. Hum Immunol. 2016;77:395–402. doi: 10.1016/j.humimm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Scornik JC, Bromberg JS, Norman DJ, Bhanderi M, Gitlin M, Petersen J. An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. Bmc Nephrol. 2013;14:217. doi: 10.1186/1471-2369-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg. 2015;23:217–222. doi: 10.1016/j.ijsu.2015.07.684. [DOI] [PubMed] [Google Scholar]
- 11.Butler JR, Wang ZY, Martens GR, et al. Modified glycan models of pig-to-human xenotransplantation do not enhance the human-anti-pig T cell response. Transpl Immunol. 2016;35:47–51. doi: 10.1016/j.trim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijkstrom M, Iwase H, Paris W, Hara H, Ezzelarab M, Cooper DK. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017;91:790–796. doi: 10.1016/j.kint.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DK, Tseng YL, Saidman SL. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 16.Hara H, Ezzelarab M, Rood PPM, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to alpha 1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 18.Albritton A, Leonard DA, Barone AL, et al. Lack of cross-sensitization between alpha-1, 3-Galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation. 2014;97:1209–1215. doi: 10.1097/TP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naziruddin B, Durriya S, Phelan D, et al. HLA antibodies present in the sera of sensitized patients awaiting renal transplant are also reactive to swine leukocyte antigens. Transplantation. 1998;66:1074–1080. doi: 10.1097/00007890-199810270-00018. [DOI] [PubMed] [Google Scholar]
- 20.Barreau N, Godfrin Y, Bouiiours JF, et al. Interaction of anti-HLA antibodies with pig xenoantigens. Transplantation. 2000;69:148–156. doi: 10.1097/00007890-200001150-00025. [DOI] [PubMed] [Google Scholar]
- 21.Oostingh GJ, Davies HFS, Tang KCG, Bradley JA, Taylor CJ. Sensitisation to swine leukocyte antigens in patients with broadly reactive HLA specific antibodies. Am J Transplant. 2002;2:267–273. doi: 10.1034/j.1600-6143.2002.20312.x. [DOI] [PubMed] [Google Scholar]
- 22.Varela ID, Mozo PS, Cortes AC, Blanco CA, Canedo FV. Cross-reactivity between swine leukocyte antigen and human anti-HLA-specific antibodies in sensitized patients awaiting renal transplantation. J Am Soc Nephrol. 2003;14:2677–2683. doi: 10.1097/01.asn.0000088723.07259.cf. [DOI] [PubMed] [Google Scholar]
- 23.Mulder A, Kardol MJ, Arn JS, et al. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol Immunol. 2010;47:809–815. doi: 10.1016/j.molimm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101:E86–E92. doi: 10.1097/TP.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popma SH, Krasinskas AM, Szeto WY, et al. Allosensitization increases human anti-pig cellular xenoreactivity. Transplant P. 2000;32:954–955. doi: 10.1016/s0041-1345(00)01057-5. [DOI] [PubMed] [Google Scholar]
- 26.Oostingh GJ, Davies HF, Bradley JA, Taylor CJ. Comparison of allogeneic and xenogeneic in vitro T-cell proliferative responses in sensitized patients awaiting kidney transplantation. Xenotransplantation. 2003;10:545–551. doi: 10.1034/j.1399-3089.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–1462. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh P, Ezzelarab M, Bovin N, et al. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010;17:197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee W, Long C, Ramsoondar J, et al. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration? Xenotransplantation. 2016;23:370–380. doi: 10.1111/xen.12254. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Andreyev O, Chen M, et al. Human T cells upregulate CD69 after coculture with xenogeneic genetically-modified pig mesenchymal stromal cells. Cell Immunol. 2013;285:23–30. doi: 10.1016/j.cellimm.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZY, Li P, Butler JR, et al. Immunogenicity of renal microvascular endothelial cells from genetically modified pigs. Transplantation. 2016;100:533–537. doi: 10.1097/TP.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 32.Sathaliyawala T, Kubota M, Yudanin N, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appay V, van Lier RAW, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytom Part A. 2008;73a:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 34.Gondouin B, Hutchison CA. High cut-off dialysis membranes: current uses and future potential. Adv Chronic Kidney Dis. 2011;18:180–187. doi: 10.1053/j.ackd.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- 36.Hauser AB, Stinghen AEM, Kato S, et al. Characteristics and causes of immune dysfunction related to uremia and dialysis. Periton Dialysis Int. 2008;28:S183–S187. [PubMed] [Google Scholar]
- 37.Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dialysis. 2013;26:416–426. doi: 10.1111/sdi.12101. [DOI] [PubMed] [Google Scholar]
- 38.Xiang FF, Zhu JM, Cao XS, et al. Lymphocyte depletion and subset alteration correlate to renal function in chronic kidney disease patients. Renal Failure. 2016;38:7–14. doi: 10.3109/0886022X.2015.1106871. [DOI] [PubMed] [Google Scholar]
- 39.Litjens NHR, van Druningen CJ, Betjes MGH. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol. 2006;118:83–91. doi: 10.1016/j.clim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–212. doi: 10.1681/ASN.V131204. [DOI] [PubMed] [Google Scholar]
- 42.Moser B, Roth G, Brunner M, et al. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: a comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem Bioph Res Co. 2003;308:581–585. doi: 10.1016/s0006-291x(03)01389-5. [DOI] [PubMed] [Google Scholar]
- 43.Libetta C, Rampino T, Dal Canton A. Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. Am J Kidney Dis. 2001;38:286–295. doi: 10.1053/ajkd.2001.26092. [DOI] [PubMed] [Google Scholar]
- 44.Sester U, Sester M, Hauk M, Kaul H, Kohler H, Girndt M. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transpl. 2000;15:1217–1223. doi: 10.1093/ndt/15.8.1217. [DOI] [PubMed] [Google Scholar]
- 45.Litjens NH, Boer K, Zuijderwijk JM, et al. Natural regulatory T cells from patients with end-stage renal disease can be used for large-scale generation of highly suppressive alloantigen-specific Tregs. Kidney Int. 2017;91:1203–1213. doi: 10.1016/j.kint.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 46.Mathew RO, Mason DL, Song RJ, Tryniszewski T, Kennedy JS. Role of T-regulatory cells in the response to hepatitis B vaccine in hemodialysis patients. Hemodial Int. 2016;20:242–252. doi: 10.1111/hdi.12326. [DOI] [PubMed] [Google Scholar]
- 47.Taylor CJ, Tang KGC, Smith SI, White DJG, Davies HFS. HLA-specific antibodies in highly sensitized patients can cause a positive crossmatch against pig lymphocytes. Transplantation. 1998;65:1634–1641. doi: 10.1097/00007890-199806270-00016. [DOI] [PubMed] [Google Scholar]
- 48.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsoondar JJ, Machaty Z, Costa C, Williams BL, Fodor WL, Bondioli KR. Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod. 2003;69:437–445. doi: 10.1095/biolreprod.102.014647. [DOI] [PubMed] [Google Scholar]
- 50.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 52.Kuwaki K, Tseng YL, Dor FJMF, et al. Heart transplantation in baboons using 1,3-galactosyl transferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 53.Tseng YL, Kuwaki K, Dor FJ, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 54.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha 1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 55.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1–3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 56.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 57.Byrne GW, Du ZJ, Stalboerger P, Kogelberg H, McGregor CGA. Cloning and expression of porcine beta 1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burlak C, Paris LL, Lutz AJ, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14:1895–1900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193:5751–5757. doi: 10.4049/jimmunol.1402059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim EC, Chia D, Gjertson DW, Koka P, Terasaki PI. In vitro studies to explain high renal allograft survival in IgA nephropathy patients. Transplantation. 1993;55:996–9. doi: 10.1097/00007890-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Koka P, Chia D, Terasaki PI, et al. The role of IgA anti-HLA class I antibodies in kidney transplant survival. Transplantation. 1993;56:207–11. doi: 10.1097/00007890-199307000-00038. [DOI] [PubMed] [Google Scholar]
- 63.Regan J, Monteiro F, Speiser D, Kalil J, Pouletty P, Buelow R. Pretransplant rejection risk assessment through enzyme-linked immunosorbent assay analysis of anti-HLA class I antibodies. Am J Kidney Dis. 1996;28:92–8. doi: 10.1016/s0272-6386(96)90136-5. [DOI] [PubMed] [Google Scholar]
- 64.Süsal C, Döhler B, Opelz G. Graft protective role of high pretransplantation IgA-anti-Fab autoantibodies: confimatory evidence obtained in more than 4000 kidney transplants. the collaborative transplant study. Transplantation. 2000;15:1337–40. doi: 10.1097/00007890-200004150-00021. [DOI] [PubMed] [Google Scholar]
- 65.Serrano A, Garcia F, Serrano M, et al. IgA antibodies against β2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012;81:1239–44. doi: 10.1038/ki.2011.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.