Introduction
It is becoming increasing clear that within a given microbial sample, be it from a lake, soil or human effluvia, most of the microbes present are uncultivatable and therefore their identity and function remain unknown. Yet genetic sequencing mapped to globally accumulating databases can not only identify the species of microbes present, but also their community structure and with applied metabolomics, their function. This holistic approach has led to the term “microbiome” to encompass not only which species of microbes are present in a given sample, but how they relate to one another, the genes they express and the small metabolites they secrete that allow them to communicate with one another and interact with other living systems. As such, we are now witnessing the drafting of a large “connectome” that has the potential to uncover not only how and why complications develop in surgical patients, but also why disease develops in the first place. Common problems that have eluded explanation such as ileus, surgical site infections and anastomotic leak may be highly influenced by a patient’s microbiome. Microbiome science applied to medicine has the potential to change everything, the way we diagnose our patients, our approach to prepare them for surgery, the way we operate and the way we feed and rehabilitate them postoperatively.
The great Argentinian poet, Jorge Luis Borges (1899-1986) translator and essayist, arguably one of the greatest writers of all time, was once heard whispering to a colleague during a lecture that “everything touches everything.” Ever since uttering that phase, which is nowhere to be found in his literature, it has been attributed to Borges across various contexts, biology, ecology, sociology and politics. The phrase refers to a virtual connectome at the extreme of all living things such as imagining that soil microbes, working across various living interfaces, can influence human behavior and vice versa. Of course this is no stretch to ecologists and evolutionary biologists who spend their careers creating methods to understand and measure the functional biogeography of such interfaces. In fact they are the ones responsible for our ability to sequence microbes and develop technologies to track and define, in real time, changes in the genetic and functional structure of living microbial communities across various ecological environments (1). Simple culture and identification of microbes at the species level tells us very little of their life history and behavior. As Jacque Monod, the discover of RNA stated, “all living organisms are changed by experience” and this suggests that no two species of bacteria remain genetically identical as they pass through various environments, encounter predatory pathogens and horizontally transfer genes to one another as needed (2). As microbial ecologists genetically and functionally trace the life history of bacterial communities to understand the factors that shape their community behavior, surgeons can imagine leveraging genomic sequencing to map the movement of potentially harmful pathogens across space and time as they operate on patients with complex diseases. This notion, coupled with advances in mass spectrometry, allows for the measurement of all elements within a given sample from pH to bacterial metabolites with which microbial community function can be inferred and mapped to the metagenome (all the genetic material across all living organisms in a given sample) (3).
Given the advances in molecular and genetic analysis, it is not surprising that microbiome sciences have taken center stage in human health and disease. One only needs to consider that the processed and pesticide exposed food we eat including the meat from animals exposed to antibiotics, has its first pass through our microbiota which process these foodstuff and release thousands of unknown metabolites that interact with our tissues. Similarly, all ingested xenobiotics, including medications, are metabolized and processed by the intestinal microbiome (4). When we consider all of this in the context of operating on a given patient, the status of their microbiome relative to the foods they eat, the antibiotics to which they have recently been exposed, the medication they take, their recent travel history and the microbiomes of their living partners and home, it becomes evident that we routinely dismiss this information as relevant to patient outcome. As surgeons operating in a technologically advanced arena, when we consider our experience at the whole population level, we observe complications following surgery to be at an all-time low reinforcing our bias that the use of antiseptics, minimally invasive surgery, sterile technique and antibiotics to have achieved a stable low level incidence of complications. We now need only to standardize and apply best practices in surgery across all institutions so that all outliers can benefit from our collective success and achieve the best level of performance for their patients. Based on retrospective analyses of large databases accumulating in real time, bundles and protocols are being standardized and implemented at the national level. It seems after all of our advances, a “one size fits all” strategy is being heralded as the most effective way to reduce infection-related complications though data transparency and adherence to protocols (5,6).
In this lecture I will argue that a major deficiency of this approach, which involves massive digital efforts to count adverse events and apply probabilistic predictors, is the lack of any mechanistic or molecular detail to explain “within-group” differences in one treatment protocol versus the other. For example when a bundle such as use of a full bowel preparation (purgatives, oral and intravenous antibiotics), hyperoxygenation, avoidance of opioids, elimination of the nasogastric tube and early resumption of oral feeding is implemented, a statistically significant reduction (i.e P <0.05) in the incidence of infection-related complications (ileus, surgical site infections, anastomotic leak) can be realized from 20% (considered an outlier institution) to 7% (considered within range) (7) (8). This “between-group” difference may be statistically validated in such a retrospective analysis of large accumulated database, but without any attempt at a molecular level mechanistic analysis, the within-group differences (i.e why did 80% of the non-intervention group do well and why did 7% of the bundle applied group develop infections?) cannot be reconciled. Without the application of molecular level analysis of a given patient’s “biome,” personalized medicine cannot be applied so that not only is every patient’s success or failure counted, but there is an attempt to understand why there is an outcome difference in the first place. Weighted risk-level analyses will not yield actionable data as they are probabilistic not deterministic. In the context of the microbiome, I will advance the argument that most major life-threatening surgical complications are still due to infections, and although considered to be “rare adverse events” they remain disabling and lethal. Evidence is accumulation to suggest that the great majority of our microbiota actually drive and enhance our state of wellbeing (9). Yet, because we cannot distinguish who is friend or foe among our microbiota, we apply protocols to eliminate as many microbes as possible. The microbiome of a given host carries its own life-history of exposure to multiple environments, foods and zenobiotics and the genetic makeup of a host not only reflects its inherited genes, but also how those genes and their expression patterns have been changed in response to the unique experience they have had and continue to have with the world surrounding them. This complex network of interactions at the individual level cannot simply be manipulated by a one-size-fits-all strategy of antibiotics, oxygen, oral intake etc. Here I will argue that if every complication is to count as potentially disabling and life-threatening, then we need to do more than just count them.
Readmission rates, infection-related complications and surgical quality
For all stakeholders involved in the process of surgery, surgeon, patient, hospital administrator, insurer and quality and safety officer, readmission rates are now the new proxy for surgical quality. Multiple studies now demonstrate that the most common reason for readmission on a surgical service is infection (10). This spans across all specialties and it alone can be the major criteria for exclusion from becoming a center of excellence. For general surgeons operating on the intestinal track, infection rates remain unacceptable high and are the most common cause of readmission with ileus being the second most common cause. Considering infection and ileus together, they represent well over 50% of the readmissions following surgery. Surgical infections are also the most costly complication especially when they involve deep spaces and are linked to major organ complications such as anastomotic leak. Yet within the confines of both surgical morbidity and mortality conferences and in the mind of most surgeons, infection, ileus and anastomotic leak are considered to be major breaches in technique and are often reported as such. More importantly, position statements from protocols and bundles make the bold assertion that these complications can be avoided with proper infection control measures, adherence to the imposed protocols and bundles and application of more standardized and rigorous attention to technique in the case of anastomotic surgery in high risk areas such as in the esophagus, pancreaticojejunostomy and rectum. Yet to consider surgical site infections (SSIs), ileus and anastomotic leak as preventable breaches in technique, the following assumptions must be made: 1. The vast majority of wound infections occur as a result of intraoperative microbial contamination, 2. ileus is due to excessive and inappropriate handling of the bowel during surgery and the inappropriate use of opioids, 3. when a high volume, well trained and highly experienced surgeon’s patient develops an anastomotic leak, it is due to a technical error (ischemia, tension, poor technique). Here I will advance the argument that, despite 50 years of steady improvements in surgical outcomes, none of the above assumptions/explanations has been formally tested nor mechanistically confirmed. Furthermore, the lack of any attempt by surgical investigators to generate the molecular level detail needed to confirm/refute the above assertions is problematic given their impact on patient morbidity and readmission rates.
To expose potential pitfalls in this thinking and emphasize the need to apply precision medicine in surgery, I will again refer to the issue of the “between-group comparison fallacy” which plagues clinical studies that lack mechanistic level detail. For example, application of enhanced recovery after surgery programs (ERAS) consistently demonstrate a reduction in complication rates, which here we will arbitrarily indicate as decreasing from 1X to .5X (11). As a result of applied bundles and protocols lacking any mechanistic detail to account for the positive/negative changes, the conclusion that all positive changes are due to the intervention (s) and all negative changes (i.e lack of an effect) are either related to breaches in the protocol or patient related factors remains problematic (12). Multiple variables (time, use of anesthetics, pain control, previous exposure to antibiotics, previous healthcare encounters etc) remain unaccounted for in these studies. Here I propose that to further drive down complication rates, it will be necessary to depart from probabilistic-based studies and embrace molecular level technology that can yield a more deterministic understanding of outcome at the individual patient level. This is rapidly occurring in the cancer field and must now be applied to surgical complications in order for us to know precisely why things happen to some patients and not others. Many of the elements applied to patient care in ERAS programs have a major influence on the intestinal microbiome (Figure 1). It is now time to embrace next generation technology in these programs so that within-group differences can be understood at the molecular level and every patient can benefit.
Figure 1.
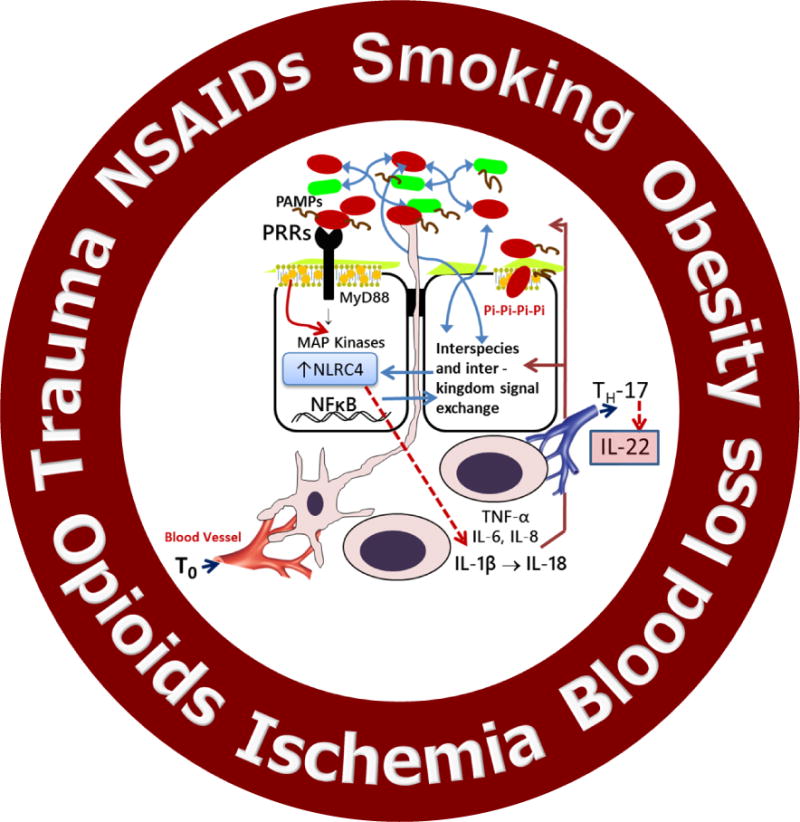
Multiple life-style inputs into the microbiome-host interactome that can shape an individual’s immune response to surgical injury. MAP, mitogen-activated protein; NSAIDs, non-steroidal anti-inflammatory drugs; PAMPs, pathogen associated molecular patterns; PRR, pathogen related receptor; TNF, tumor necrosis factor.
Contingent on context-“There are no units, only interactive systems”- James Shapiro PhD (13)
When considering the critical function of the intestinal microbiome to our health, it is important to recognize that its function as a highly organized and complex community with well-established networks of communication allowing for social group behavior (13). The ability of bacteria to rapidly process information in response to local environmental cues and nutrient availability is a key mechanism by which they maintain their fitness (reproductive success) and, if needed, express virulence (harmfulness). Through the well described quorum sensing signaling system, bacteria have developed cell-to-cell communication systems where they can “sense” changes in their environment and “respond” accordingly (14). Host cells, on the other hand can sense the presence and state of virulence of its colonizing microbes via pathogen recognition receptors and secrete mucus to keep pathogens at bay from triggering a host response, and if needed secreted antimicrobial peptides (15). As a counter response, bacteria can “sense” the release of these host signals and mount a counter-counter response by expressing virulence products that subvert the host immune system (16). This bidirectional iterative loop of host-microbial, microbial-microbial signaling indicates an evolved interspecies, intraspecies and interkingdom network of ongoing information exchange between a host and its microbial partners (Figure 2A). Under conditions of health and minimal environmental perturbations, this ongoing signal exchange is a system of “trust with verification,” maintaining a healthy state of molecular détente. Logically, it is in the best interest of both parties to participate in each other’s wellbeing, the host benefits by having its nutrients metabolized and ingested zenobiotics detoxified, while bacteria obtain a steady supply of nutrients by feeding off a healthy host (17). In this scenario, game theory can be applied and we can begin to understand how contingencies are adjudicated based on context (i.e availability of resources in a biologic marketplace) (18). When considering the evolved complexity of the host-microbial signal exchange, it is remarkable that modern medicine has been so successful in navigating along this molecular Sylla and Charybdis when exposing patients to solid organ transplantation, chemotherapy and radiation and rescue from severe traumatic injury (19). Do we successfully navigate these fiery waters by our ability to stay one step ahead of a small group of renegade microbes from killing its host in order to jump to a new healthy one (20)? Or occasionally does the ship sink (i.e multiple organ failure) and we often never really understand why? Here I posit that digging into the molecular weeds of the host-microbe exchange will not only elucidate the mechanisms by which our patients recover (or not) from the evolutionarily unprecedented insults we impose upon them, but it will also tell us what we are doing right and what we are doing wrong. And as we will see, predicting surgical complications at the individual level remains at inaccurate because we have not appreciated that the host-microbial interaction is “a matchless web of dense dynamic interactions (21).” Yet next generation technology is now fully capable of delivering precision medicine in a way that has the potential to predict which patients are truly at risk of developing life-threatening and disabling complications by interrogating, at high resolution, the microbiome-host interactome.
Figure 2.
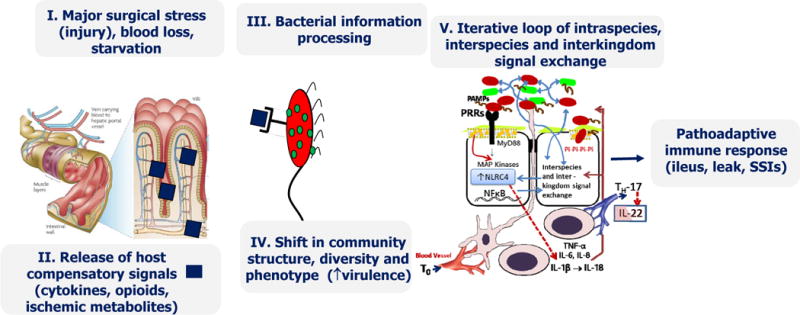
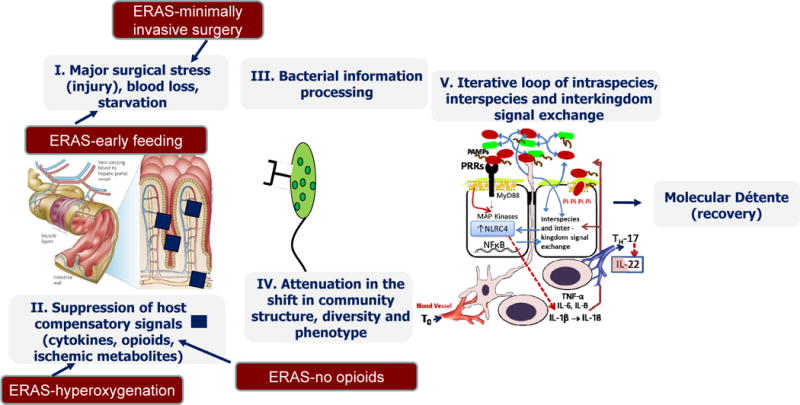
Proposed mechanism by which the intestinal microbiome influences the host response to surgical injury. (A) Sequence of events by which surgical injury leads to a pathoadaptive response of the microbiome adversely affecting the recovery process. (B) Potential mechanisms by which enhanced recovery programs after surgery (ERAS) lead to a state of molecular détente between the host and its microbiome and promote recovery. MAP, mitogen-activated protein; PAMPs, pathogen associated molecular patterns; PRR, pathogen related receptor, SSI, surgical site infection; TNF, tumor necrosis factor.
Disruptive thinking on the pathophysiology of the three most common causes of readmission following gastrointestinal surgery: surgical site infections, ileus, and anastomotic leak
Surgical Site Infections
If Shakespeare were an academic surgeon studying wound infections, he would likely declare: “the first thing we do is kill all the bacteria.” Major advances have been made over the last 50 years to achieve a wound infection rate under 3% for all wounds, 5-15% for colorectal surgery and ∼1% for major orthopedic surgery. The assumption is that efforts to sterile the operative field both preoperatively (chlorhexidine bathing), intraoperatively (use of the 3 minute rule, use of chlorhexidine and iodine solutions, timing and re-dosing of antibiotics) and postoperatively (sealants, etc) have had a major impact on wound contamination by bacteria. There is little doubt that these efforts have been useful. However to drive infection rates towards zero in elective surgery, we must break down the following assumption: in the current era of adherence to guidelines using aseptic technique, the majority of clinical wound infections in elective surgery, i.e. surgical site infections (SSIs), are due to direct intraoperative contamination of the wound. Here I will posit that at current best practices of asepsis for elective surgery, there is no level 1 evidence that SSIs, either superficial or deep, are a result of direct intraoperative contamination. In fact, there is overwhelming evidence to refute it. One might ask, if SSIs following elective surgery do not develop from intraoperative contamination of the wound, then how do the pathogens get there? The discussion below will be confined to wound infections that occur after elective surgery, when there is no gross contamination of the wound such as occurs following trauma and burn injury.
That skin microorganisms (i.e Staphylococcal sp) are the most common causative pathogens involved in SSIs following clean cases, has resulted in a legacy of thought that all SSIs in such cases must be due to skin organisms, from the patient or staff, migrating to the wound during or immediately after surgery. Similarly, that enteric organisms (i.e E. coli, B. fragilis) are commonly found in postoperative SSIs following intestinal surgery, maintains the presumption that there is a causative link between intraoperative contamination and clinical SSIs. Yet today, in the era of proper skin decontamination, adherence to aseptic technique, routine use of antibiotics, SSIs following elective surgery continue and there is some evidence they are increasing (22). To explain this, risk factor analyses have again made the repeated declaration that patient-related factors, obesity, blood loss, length of surgery, ASA classification, frailty etc can increase wound infection rates up to 8 fold in such clean cases. Unfortunately, culture results and within-group analyses are missing from these studies, rendering the vast majority to be subject to the “between-group comparison fallacy.” If we apply this line of reasoning to spine surgery, where the reported infection rate is 1-3%, up to a 10 fold increase (odds ratio) in SSIs can be observed in patients when patient related risk factors are present (23), (24). Not only are these SSIs highly disabling, but the reported mortality rate can be as high as 25% (23). Yet herein lies the incongruence: if patients with clearly identified risk factors (i.e lengthy surgery, blood loss, frailty, ASA score) have a 10 fold increase in SSIs (i.e 10%–30% incidence of infection), how do we account for the fact that the remaining 70-90% within the high risk group do NOT develop an SSI? The easy answer is that within the high risk group, the rate of intraoperative wound contamination is probabilistically higher among those that developed infections versus those that do not yet the risk factors themselves are not deterministic. A sobering fact in this argument is that, among available studies, even when intraoperative cultures are positive the end of an operative case, they are do not predict a subsequent wound infection (25). Taken together, these studies suggest that neither risk factors nor wound cultures are deterministic of an SSI at the individual patient level. How can this be?
A common posited explanation for lack of predictability of wound culture is that current culture techniques do not capture all bacteria present within a wound. One might consider that were advanced culturomics (culturing approaches that use multiple culture conditions and matrix-assisted laser desorption/ionization-time of flight and 16S rRNA for identification) applied (26), intraoperative wound contamination would bear out as highly predictive and deterministic of postoperative SSIs. While this latter assertion remains to be formally tested in clinical studies, the failure of the use of space suits, laminar flow, wound lavage, wound irrigation with antiseptics and antibiotics, continuation of postoperative antibiotics, etc, make a strong case against intraoperative contamination as a major cause of SSIs (27),(28). Perhaps the most convincing evidence against intraoperative wound contamination as a deterministic cause of postoperative wound infections following elective surgery however is the consistent observation that even when wound cultures are positive, in the vast majority of such cases, wound infections do not develop (29), (30). Of course the counterargument to this observation is that while intraoperative contamination might occur, only when highly virulent and resistant pathogens are present at high concentrations and enter the wound during surgery, do wound infections develop. Unfortunately even this latter assertion remains untenable in view of the consistent findings that when wound cultures are performed both intraoperatively and when patients present with a clinical wound infection, invariably there is discordance between culture results (25). The continued efforts by both commercial and non-commercial stakeholders in keeping the operating theater ever more sterile with space suits, hepafilters, laminar flow, infection control committees, etc, while laudable, is in desperate need of rigorous science to support its claims. Application of next generation technology to such studies could finally prove or disprove causality between air quality, wound contamination and postoperative clinical wound infection.
To illustrate the near universal acceptance that all SSIs are due to intraoperative contamination, again I will refer to spinal surgery as postoperative SSIs following adult reconstructive surgery of the spine are, in general, of low incidence and of high morbidity and mortality. The most common organism recovered from these wound infections, is Staphylococcus aureus. Studies have verified that even when wound cultures at the end of spinal cases are positive, wound infections do not develop in these patients (30). Yet there remains a major effort to decrease spinal infections with the use of vancomycin powder placed directly into the wound at the end of the case (31,32). Studies demonstrate that wound application of vancomycin powder following spine surgery does not result in its systemic absorption and, based on levels in drains, remains in the wound for up to 3 days. While many prospective controlled studies suggest that vancomycin powder may indeed reduce S. aureus wound infection rates, the findings remain controversial due to irregularities in study design and patient selection (33). More importantly, several studies have verified that vancomycin powder wound application following spinal surgery actually increases infections due to gram-negative enteric pathogens (Serratia marcescens, Enterobacter aerogenes, Bacteroides fragilis, Enterobacter cloacae, Pseudomonas aeruginosa) (34). The presumption here is that use of vancomycin powder has provided opportunism for gram-negative bacteria that have contaminated the wound during surgery to grow now unimpeded by the absence of S. aureus. Investigators are now suggesting the antibiotics with a broader spectrum be applied to the wound following spinal surgery. This line of reasoning is a result of the the near universal acceptance by all stakeholders involved in operating room infection control that postoperative wound infections occur from direct intraoperative contamination of the wound.
An alternative explanation for postoperative wound infections could be that, in selected cases, wounds become contaminated and subsequently infected by bacteria present at remote sites of colonization (35). My lab has recently proposed a Trojan Horse hypothesis for wound infection whereby pathogens such as MRSA remaining as a dormant colonizers in the intestinal tract, can be taken up by neutrophils and then are silently delivered to a remote operative wound site resulting in a clinical wound infection (36). This was modeled in mice and appears to be a plausible explanation for MRSA infections when wound cultures are negative at the end of an operation. This same mechanism has been recently proposed for brain infections involving macrophages as silently carriers of dormant strains of microbes capable of co-existing intracellularly while traveling to a fresh tissue site and causing gross infection (37). Given these findings, it is plausible that among patients identified in outcomes studies to be at risk for SSIs, i.e, those with lengthy operations, increased blood loss, re-operative surgery in the same wound site, poor nutritional status, smoking, alcohol/opioid use, etc. (Figure 2), that these patients have an intestinal microbiome that harbors highly subversive pathogens (i.e MRSA, E. faecalis) whose low abundance and pathogenic potential remains “contained” by the abundance and diversity of the normal microbiota. However during surgery, low abundance pathogens can become unleashed by the very process of preparing and treating the patient beginning with non-per os (NPO) after midnight, application of broad spectrum antibiotics, physiologic stress and its attendant loss of intestinal permselectivity, postoperative provision of clear liquid highly processed foods and exposure to hospital pathogens, which are known to rapidly colonize the intestinal track of surgical patients (38). Importantly, whether these pathogens are those that cause SSIs can be finally elucidated by genetically tracking the intestinal microbiota before, during and after surgery and mapping, at the strain level, the identified intestinal microbes to the pathogens that eventually are responsible for the SSI. Recall that genetically sequencing bacteria, in contrast to culture, not only allows bacteria to be identified at the species level, but also at the strain level. It is most important to recognize that no two strains of bacteria are alike, even those of the same species that originated from the same parental strain. Yet next generation sequencing technology can open the door for dynamic mapping of how pathogens travel from one space to the next, including within the hospital environment, as we recently have shown (39). Next generation microbial sequencing technology will allow us to move from the vague probabilistic nature of outcome studies to a more mechanistic and deterministic understanding, at the individual patient level, why some patients develop SSIs and others do not (40). It is time we recognize that any given individual’s disease is biologically unique and involves a dynamic and bidirectional signal exchange between the host and its microbiota, between the microbiota themselves and between the environment and the microbiota. As such, how a given microbiome reacts within an individual’s body during the preparation and over course of surgery and in a given hospital environment should be considered as an emergent property. Under this conceptual framework, a one-size-fits all approach to prevent infectious-related complications in surgery via standardized bundles and protocols will always be insufficient. Also a one-size-fits all approach to prevent infectious-related complications in surgery does not consider that microbes continually adapt to, and survive from antibiotics, antiseptics and environments. Bacteria can promiscuously swap genes and pivot between being a commensal one minute and a pathogen the next. As such we are not providing medicine that is in synch with the dynamic and adaptable ecologic nature of microbes within and around our patients. The future suggest that the moment that microbiome medicine can be applied at the point-of-care to surgical patients, it will change everything.
Postoperative ileus and anastomotic leak
Among the most contentious discussions at weekly morbidity and mortality conferences is the complication of anastomotic leak. Invariably, technique is invoked as the putative cause, given the low incidence of this dreaded complication and the idea that an operation’s success has much to do with the ability of a surgeon to be a master tissue engineer. The legacy of this reasoning is continuously reinforced by the logic of high volume expert surgeons who conclude, “if we can get it right more than 95% of the time, the rare complication of an anastomotic leak must somehow be due to a breach in technique.” The less self-accusatory of us might reason that “patient-related factors” such as a soft pancreatic glad, small duct, frail patient, preoperative radiation, etc is the cause of the occasional leak. Yet the tendency to rationalize cannot be denied: when your anastomosis leaks it is reasoned to be from patient-related factors; when a colleague’s leaks, it must be due to a technical error.
Yet compelling evidence dating back to 1954 demonstrates that anastomotic leak is most likely a result of an infectious process initiated and driven by intestinal bacteria. A seminal study performed in dogs in 1954 by the iconic American surgeon Isadore Cohen demonstrated that microbes, not technique play a key and causative role in anastomotic leak (41). This was demonstrated by repeatedly delivering antibiotics via an indwelling catheter to the anastomotic lumen to directly decontaminate the intestine of bacteria. This approach completely prevented anastomotic leak in the dog colon rendered visibly ischemic by segmental devascularization. Remarkably, this study demonstrated that even when an anastomosis is technically inadequate (i.e ischemic), elimination of its microbial content not only prevented anastomotic leak, but it reversed ischemia. Multiple studies followed in animals by Schardey and others demonstrating the key and contributory role of microbes on the mechanism of anastomotic leak (42). Yet despite these studies and randomized controlled trials (RCTs) in humans with and without oral antibiotics demonstrating the key contributory role of microbes in anastomotic leak, technique prevailed and continues to prevail as the primary explanation for the persistence of anastomotic leak in modern surgical practice (43). This legacy of thought is best exemplified in two discussion points from studies that demonstrated the value of using oral antibiotics in colon surgery in which, unexpectedly, use of oral antibiotics resulted in a statistically significant reduction in clinical anastomotic leak rates. The first quotation is from the landmark randomized placebo controlled trail of oral antibiotics in colon surgery performed in 1977 (44).
“Since anastomotic disruption may be viewed as a technical fault adversely affection the incidence of postoperative septic complications, we also have analyzed our data with such patients excluded.”
The second quotation is from a recent large retrospective report from 2016 mining the NSQIP data base (8).
“The effect of antibiotics on leak rates can be explained by fewer clinically evident leaks as opposed to actual leak”
What is intriguing about these conjectural conclusions, which to date remain unconfirmed, is the idea that the authors must apologize for any data that might suggest that something other than technique is responsible for anastomotic leak. Here I make the bold assertion that, despite decades of descriptive work on anastomotic leak, there is no evidence to support the claim that anastomotic leaks are caused by technique. There is no doubt that poor technique can lead to leak, however here I assert that there are no clinical studies analyzing the tissues or images of patients who have leaked that has verified technique to be the primary cause.
The invaluable and unreplaceable role of the surgeon-scientist to solve complex problems in surgery
The major focus of my laboratory over the last 20 year has been to understand how the intestinal microbiome affects host physiology during surgical injury. We made the novel observation that intestinal microbes can “sense” host injury and “respond” with enhanced virulence resulting in a state of gut-derived septic-like physiology. Over the years we identified the host molecules released into the gut during physiologic stress (cytokines, opioids, ischemic end-products) and the bacterial receptors to which they bind that leads to the expression of enhanced virulence among the colonizing flora. By applying this conceptual framework to anastomotic leak pathogenesis, we made the novel observation that when collagenase producing microbes (i.e E faecalis, P. aeruginosa, S marscens) are “in vivo expressed”, by soluble elements released during host stress, they are able to colonize anastomotic tissues and produced collagenase. During this provoked state of “in vivo expression” not only does the bacterial collagenase break down anastomotic collagen, but it also degrades select host tissue proteases (i.e MMP9) such that they are converted from their inactive to their active form. As a result, there is over-amplification of collagen breakdown leading to pathoadaptive healing at the site of the anastomotic construction. Under this dynamic model, we are able to conclude that collagenolytic pathogens are required for an anastomotic leak to occur (no collagenase producing bacteria-no leak), however their presence alone is not sufficient to cause leak. In order for collagenase producing bacteria to cause leak, the following contingencies need to be fulfilled: 1. the normally diverse and abundant microbiome that provides colonization resistance to competitively exclude harmful pathogens must be sufficiently disrupted. 2. anastomotic tissues must undergo a sufficient release of inflammatory mediators to provide the proper environmental “cues” and chemoattractants such that bacteria are “activated,” leading to their binding to injured tissues and their production of collagenase and 3. Bacteria must be able to subvert innate defense mechanism such as epithelial defensins, mucus, IgA, peristalsis, etc to bind and invade anastomotic tissues. Thus under the scientific lexicon typically used to map a molecule to a specific disease phenotype, it is appropriate to state that collagenase producing bacteria are necessary but not sufficient to cause leak. To clarify, for example when a single host gene is responsible for the full expression of a disease, such as cystic fibrosis, the gene is deemed both “necessary and sufficient” to cause the disease, because in both mice and humans, the mere presence of the gene is both necessary and alone sufficient for the full expression of the phenotype. However with bacteria, which co-exist in complex heterogeneous colonies and can dynamically switch from one phenotype to the next depending on the environmental context, the previously applied Koch’s postulates no longer suffice and application of the “molecular” Koch’s postulates are needed (14). In the case of anastomotic leak, the environmental context can be very fluid (long difficult surgery, postoperative hypoxia, excessive use of opioids), exposure to microbes across various environments can be stochastic (antiseptics, antibiotics, staff, hospital environment) and manipulation of the background microbiome by a given bowel preparation regimen unpredictable. As such, the microbial pathogenesis of anastomotic leak becomes difficult to blame on a single pathogen and host pathway. It may be for this reason that surgeons today continue to abjure at the possibility that something other than ischemia, tension or misconstruction can cause anastomotic leak. Yet one compelling observation remains undeniable; the use of oral antibiotics in gastrointestinal surgery reduces the incidence of both ileus and anastomotic leak and neither the pathogen nor the mechanism has been fully identified. Reconciling the emerging molecular basis of anastomotic leak with the traditional bias of the high volume clinical surgeon that is due to technique requires a scientific thinking surgeon to provide proof that informs the clinical path forward.
Why is the incidence of Ileus and anastomotic leak decreased with the use of oral antibiotics?
Postoperative ileus represents another complication in which surgeons remain trapped in the legacy of thought that many, if not all surgical complications can be explained from the standpoint of the mechanics of surgical technique. Yet most recently, compelling molecular level evidence has emerged demonstrating a key causative role for the microbiome in postoperative ileus (45). Most surgeons accept that, in the absence of a documented mechanical cause, postoperative ileus is an inflammatory disorder of the intestine that most often resolves with conservative management. While the first report of immune-mediated ileus was initiated by surgeons (46), recent work by investigative gastroenterologists has been centered on the intestinal macrophage whose activation converges on the intestinal nervous system leading to peristaltic dysfunction that can spread far beyond the site of the initial trauma (45). Given that the intestinal microbiome has emerged as being involved in virtually all immune-mediated activation of intestinal inflammation, it stands to reason that the intestinal microbiome plays a key causative role in postoperative ileus development. Elegant studies by the same team have now demonstrated that the microbiome initiates and sustains postoperative ileus via its action on intestinal macrophages (47). Yet this should come as no surprise to surgeons who routinely give oral non-absorbable antibiotics to patients and repeatedly observe that ileus rates are statistically significantly decreased when compared to patients that do not receive oral non-absorbable antibiotics (8). Yet precisely which bacteria drive the pathogenesis of ileus and which are eliminated versus preserved when standard bowel preparation oral antibiotics are used is unknown. In fact there are no studies examining the microbiome of patients with ileus. Ileus is yet another reason to think about how we are preparing the bowel for surgery, its direct effect on postoperative complications and how microbiome sciences can deliver precision medicine to our patients.
Along this same line of reasoning, studies examining the use of oral antibiotics in colon surgery, from the first prospective randomized placebo controlled trial in 1977 to more recent retrospective large database analyses, consistently demonstrate that non-absorbable oral antibiotics decrease the incidence of anastomotic leak. These studies, individually, in the aggregate and in meta-analyses, all demonstrate the same thing-oral antibiotics decrease anastomotic leak rates. Unfortunately none of these studies has provided any information on the mechanisms and all lack any culture based or genetic based information on the bacteria responsible for this clinical observation.
Why is the incidence of SSIs decreased with the use of oral antibiotics?
It would seem axiomatic to most gastrointestinal surgeons that oral antibiotics decrease postoperative SSIs because they reduce the burden of potentially inoculating pathogens within the intestine and thus minimize intraoperative wound contamination. With little doubt, efforts from the early 1970’s to reduce intestinal microbial burden with purgative cleansing and oral antibiotics have had a major impact on wound infection rates following gastrointestinal surgery. Yet it is important to acknowledge that without simultaneous genetic tracking of intestinal bacteria and the pathogens that cause the subsequent postoperative SSI, at the strain level, it cannot be definitively concluded that the majority of bacteria that cause SSIs following intestinal surgery are a result of direct intraoperative contamination of the surgical site. The finding that wound cultures at the end of an operative case are not predictive of subsequent wound infections and the species that cause SSIs are incongruent with the cultures at the end of an operation, still does not distinguish between the possibility of direct versus indirect (i.e Trojan Horse) mechanisms of SSIs. We must figure a way to reconcile our confirmation bias in this regard by first acknowledging that an invariable observation in these studies is that even when intraoperative cultures are grossly positive, most patients do not develop a wound infection (30). Today, with protocol driven application of topical decontamination, checklist mandated prophylactic antibiotics, use of wound protectors, improvements in anesthesia delivery and pain control and early discharge from the hospital, wound infections are at a historical low. While it is important to consider the next steps to further reduce SSIs, it is equally important to recognize that the addition of more, more frequent, and broader antibiotic coverage is not an evolutionarily stable strategy. Today the most common pathogens cultured from clinical SSIs following gastrointestinal surgery in descending order of frequency are Enterococcus, Staph sp and Enterobactericiae, many of which are resistant to the antibiotics used for prophylaxis (48), (49). Most importantly, while it is presumed that common pathogens that cause SSIs, such as Staphylococcus species, originate from the skin, there is now compelling genomic-level evidence that intestinal carriage of methicillin resistant Staphylococcus aureus (MRSA) is the major source of autoinfection in nosocomial infections (50). Taken together, the above findings suggest that much work lies ahead in understanding the precise pathogenesis of SSIs and the vectors that influence bacteria movement from the site of colonization to the infected wound. Use of next generation sequencing technology can be highly informative to a more mechanism-based prophylaxis strategy which will likely include targeting pathogens that colonize the intestinal track.
To deliver quality, we need to count, but counting is not enough
Given the advances of next generation technology, we can now see much further into the depths of the microbes that colonize our patients. Each patient’s microbiome has its own life-history shaped by parental rearing, global travel (51), vaccination, antibiotic exposure, dietary choices and exposure to zenobiotics (smoking, alcohol, drug use, etc) (52). Gene sequencing at the strain level has revealed that, like humans, no two species of bacteria are alike (53). However a unique characteristic of bacteria, unlike humans, is that they are promiscuous gene swapping organisms that can exchange genetic material at a moment’s notice and adapt accordingly (54). In view of this, it seems implausible that a “one size fits all” decontamination strategy to prevent postoperative infections will work for all patients across place (cities, countries), space (one hospital to the next) and time (from the beginning of an illness to completion of treatment). Point of care diagnostics tracking the microbiome at high resolution are needed to inform how to apply precision medicine to our patients if we are to drive down postoperative infection rates. Just as when we board a plane and expect to land, our patients expect a safe return home. When a rare adverse event occurs in the airline industry, in-depth team investigations seek concrete answers. All patients deserve to know, at the highest level of precision, why they developed an anastomotic leak, an SSI or a prolonged ileus. Microbiome sciences are the path forward to provide these much needed answers and hold great promise for a more precise understanding, at the individual level, of the rare yet devastating and disabling complication of postoperative infections.
Acknowledgments
Support for this study: This work was supported by NIH grant 5R01GM062344-18 (JCA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosure outside the scope of this work: Dr Alverdy has stock and is a paid consultant to Applied Medical, and also receives royalty payments from Reshape Medical.
Presented at the American College of Surgeons 103rd Clinical Congress, San Diego, CA, October 2017.
References
- 1.Lane DJ, Pace B, Olsen GJ, et al. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6955–9. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gann A. Jacob and Monod: from operons to EvoDevo. Current biology: CB. 2010;20:R718–23. doi: 10.1016/j.cub.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Eckerle M, Ambroggio L, Puskarich MA, et al. Metabolomics as a Driver in Advancing Precision Medicine in Sepsis. Pharmacotherapy. 2017;37:1023–1032. doi: 10.1002/phar.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356:eaag2770. doi: 10.1126/science.aag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berian JR, Ban KA, Liu JB, et al. Association of an Enhanced Recovery Pilot With Length of Stay in the National Surgical Quality Improvement Program. JAMA surgery. 2017 doi: 10.1001/jamasurg.2017.4906. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berian JR, Ban KA, Liu JB, et al. Adherence to Enhanced Recovery Protocols in NSQIP and Association With Colectomy Outcomes. Annals of surgery. 2017 doi: 10.1097/SLA.0000000000002566. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Annals of surgery. 2015;262:331–7. doi: 10.1097/SLA.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 8.Kiran RP, Murray AC, Chiuzan C, et al. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Annals of surgery. 2015;262:416–25. doi: 10.1097/SLA.0000000000001416. discussion 423-5. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JA, Quinn RA, Debelius J, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 10.Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. Jama. 2015;313:483–95. doi: 10.1001/jama.2014.18614. [DOI] [PubMed] [Google Scholar]
- 11.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA surgery. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 12.Ban KA, Gibbons MM, Ko CY, Wick EC. Surgical Technical Evidence Review for Colorectal Surgery Conducted for the AHRQ Safety Program for Improving Surgical Care and Recovery. Journal of the American College of Surgeons. 2017;225:548–557 e3. doi: 10.1016/j.jamcollsurg.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro JA. Bacteria are small but not stupid: cognition, natural genetic engineering and socio-bacteriology. Studies in history and philosophy of biological and biomedical sciences. 2007;38:807–19. doi: 10.1016/j.shpsc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Seal JB, Morowitz M, Zaborina O, et al. The molecular Koch’s postulates and surgical infection: a view forward. Surgery. 2010;147:757–65. doi: 10.1016/j.surg.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Sellge G, Kufer TA. PRR-signaling pathways: Learning from microbial tactics. Seminars in immunology. 2015;27:75–84. doi: 10.1016/j.smim.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Duneau D, Luijckx P, Ben-Ami F, et al. Resolving the infection process reveals striking differences in the contribution of environment, genetics and phylogeny to host-parasite interactions. BMC biology. 2011;9:11. doi: 10.1186/1741-7007-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porcheron G, Schouler C, Dozois CM. Survival games at the dinner table: regulation of Enterobacterial virulence through nutrient sensing and acquisition. Current opinion in microbiology. 2016;30:98–106. doi: 10.1016/j.mib.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Kiers ET, Duhamel M, Beesetty Y, et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 2011;333:880–2. doi: 10.1126/science.1208473. [DOI] [PubMed] [Google Scholar]
- 19.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nature reviews Microbiology. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiese D, Rodriguez Escobar J, Hsu Y, et al. The fluidity of biosocial identity and the effects of place, space, and time. Social science & medicine. 2017;198:46–52. doi: 10.1016/j.socscimed.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. Journal of the American College of Surgeons. 2017;224:59–74. doi: 10.1016/j.jamcollsurg.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Gottschalk F, Wilke T, Mueller S, et al. Staphylococcus aureus Infections in German Patients with Type 2 Diabetes Mellitus after Orthopedic Surgery: Incidence, Risk Factors, and Clinical and Health-Economic Outcomes. Surgical infections. 2017;18:915–923. doi: 10.1089/sur.2017.063. [DOI] [PubMed] [Google Scholar]
- 24.Piper KF, Tomlinson SB, Santangelo G, et al. Risk factors for wound complications following spine surgery. Surgical neurology international. 2017;8:269. doi: 10.4103/sni.sni_306_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garibaldi RA, Cushing D, Lerer T. Predictors of intraoperative-acquired surgical wound infections. The Journal of hospital infection. 1991;18(Suppl A):289–98. doi: 10.1016/0195-6701(91)90035-7. [DOI] [PubMed] [Google Scholar]
- 26.Lagier JC, Hugon P, Khelaifia S, et al. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clinical microbiology reviews. 2015;28:237–64. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper GJ, Rothwell AG, Frampton C, Wyatt MC. Does the use of laminar flow and space suits reduce early deep infection after total hip and knee replacement?: the ten-year results of the New Zealand Joint Registry. The Journal of bone and joint surgery British volume. 2011;93:85–90. doi: 10.1302/0301-620X.93B1.24862. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff P, Kubilay NZ, Allegranzi B, et al. Effect of laminar airflow ventilation on surgical site infections: a systematic review and meta-analysis. The Lancet Infectious diseases. 2017;17:553–561. doi: 10.1016/S1473-3099(17)30059-2. [DOI] [PubMed] [Google Scholar]
- 29.Tayton ER, Frampton C, Hooper GJ, Young SW. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty: an analysis of 64,566 joints from the New Zealand Joint Registry. The bone & joint journal. 2016;98-B:334–40. doi: 10.1302/0301-620X.98B3.36775. [DOI] [PubMed] [Google Scholar]
- 30.Gelalis ID, Arnaoutoglou CM, Politis AN, et al. Bacterial wound contamination during simple and complex spinal procedures. A prospective clinical study. The spine journal: official journal of the North American Spine Society. 2011;11:1042–8. doi: 10.1016/j.spinee.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Ghobrial GM, Thakkar V, Singhal S, et al. Efficacy of intraoperative vancomycin powder use in intrathecal baclofen pump implantation procedures: single institutional series in a high risk population. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2014;21:1786–9. doi: 10.1016/j.jocn.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Ghobrial GM, Thakkar V, Andrews E, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine. 2014;39:550–5. doi: 10.1097/BRS.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 33.Bakhsheshian J, Dahdaleh NS, Lam SK, et al. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World neurosurgery. 2015;83:816–23. doi: 10.1016/j.wneu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Adogwa O, Elsamadicy AA, Sergesketter A, et al. Prophylactic use of intraoperative vancomycin powder and postoperative infection: an analysis of microbiological patterns in 1200 consecutive surgical cases. Journal of neurosurgery Spine. 2017;27:328–334. doi: 10.3171/2017.2.SPINE161310. [DOI] [PubMed] [Google Scholar]
- 35.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nature reviews Microbiology. 2011;9:215–22. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 36.Krezalek MA, Hyoju S, Zaborin A, et al. Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Annals of surgery. 2017 doi: 10.1097/SLA.0000000000002173. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Santiago-Tirado FH, Onken MD, Cooper JA, et al. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio. 2017;8:e02183–16. doi: 10.1128/mBio.02183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio. 2014;5:e01361–14. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lax S, Sangwan N, Smith D, et al. Bacterial colonization and succession in a newly opened hospital. Science translational medicine. 2017;9:eaah6500. doi: 10.1126/scitranslmed.aah6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escobar-Zepeda A, Vera-Ponce de Leon A, Sanchez-Flores A. The Road to Metagenomics: From Microbiology to DNA Sequencing Technologies and Bioinformatics. Frontiers in genetics. 2015;6:348. doi: 10.3389/fgene.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohn I, Jr, Rives JD. Antibiotic protection of colon anastomoses. Annals of surgery. 1955;141:707–17. doi: 10.1097/00000658-195505000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schardey HM, Kamps T, Rau HG, et al. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrobial agents and chemotherapy. 1994;38:2564–7. doi: 10.1128/aac.38.11.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shogan BD, An GC, Schardey HM, et al. Proceedings of the first international summit on intestinal anastomotic leak, Chicago, Illinois, October 4-5, 2012. Surgical infections. 2014;15:479–89. doi: 10.1089/sur.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Annals of surgery. 1977;186:251–9. doi: 10.1097/00000658-197709000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pohl JM, Gutweiler S, Thiebes S, et al. Irf4-dependent CD103(+)CD11b(+) dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut. 2017;66:2110–2120. doi: 10.1136/gutjnl-2017-313856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Annals of surgery. 1998;228:652–63. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engel DR, Koscielny A, Wehner S, et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nature medicine. 2010;16:1407–13. doi: 10.1038/nm.2255. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein EJ, Citron DM, Merriam CV, Abramson MA. Infection after elective colorectal surgery: bacteriological analysis of failures in a randomized trial of cefotetan vs. ertapenem prophylaxis. Surgical infections. 2009;10:111–8. doi: 10.1089/sur.2007.096. [DOI] [PubMed] [Google Scholar]
- 49.Kirby A, Santoni N. Antibiotic resistance in Enterobacteriaceae: what impact on the efficacy of antibiotic prophylaxis in colorectal surgery? The Journal of hospital infection. 2015;89:259–63. doi: 10.1016/j.jhin.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 50.van Belkum A. Hidden Staphylococcus aureus Carriage: Overrated or Underappreciated? mBio. 2016;7:e00079–16. doi: 10.1128/mBio.00079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu YG, Gillings M, Simonet P, et al. Microbial mass movements. Science. 2017;357:1099–1100. doi: 10.1126/science.aao3007. [DOI] [PubMed] [Google Scholar]
- 52.Samuelson DR, Shellito JE, Maffei VJ, et al. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS pathogens. 2017;13:e1006426. doi: 10.1371/journal.ppat.1006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herring CD, Raghunathan A, Honisch C, et al. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nature genetics. 2006;38:1406–12. doi: 10.1038/ng1906. [DOI] [PubMed] [Google Scholar]
- 54.Soucy SM, Huang J, Gogarten JP. Horizontal gene transfer: building the web of life. Nature reviews Genetics. 2015;16:472–82. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]


