Abstract
Mobility and memory declines with aging can limit independence. Several single nucleotide polymorphisms (SNPs) have been associated with cognitive performance, but studies investigating motor function are scant. We examined four SNPs involved in dopaminergic metabolism: BDNF (Val66Met), DRD3 (Ser9Gly), DBH (C>T), and COMT (Val158Met) for their relationship to motor and cognitive function in healthy older adults (n = 4,605 and n = 7,331) who participated in the U.S. Health and Retirement Study. Individuals with genotypes associated with reduced dopamine metabolism exhibited poorer balance and memory. We found the most pronounced effects in the oldest participants (85+ years old), supporting the notion that age-related declines in dopamine availability contribute to magnified genotype effects with advancing age. Moreover, males demonstrated stronger associations than did females between number of beneficial dopamine alleles and cognitive scores, suggesting that sex differences in dopaminergic transmission interact with genotype to influence performance. These findings point to common genetic variants related to dopaminergic metabolism that characterize individual differences in motor and cognitive function in older adults.
Keywords: gait, memory, BDNF, COMT, DRD3, DBH
1. Introduction
It is well established that age-related declines in mobility (Hirvensalo et al., 2000) and cognitive function (Bassett and Folstein, 1991; Warren et al., 1989) limit older adults’ independence. Diminished functional independence is strongly associated with fall risk (Deandrea et al., 2010; Holtzer et al., 2007; Muir et al., 2012), disability status (Kuh et al., 2014), likelihood of institutionalization (Kuh et al., 2014), poorer overall health (Roos and Havens, 1991) and greater mortality (Roos and Havens, 1991). Thus as the general population continues to age (Vincent and Velkoff, 2010), it is critical to understand the underlying mechanisms of why some individuals age more successfully than others.
The dopaminergic (DA) system declines with age, and these declines are associated with poorer motor and cognitive performance (Backman et al., 2000; Backman et al., 2006; Volkow et al., 1998). DA declines with age occur against a backdrop of “baseline” genetic differences in DA transmission capacity. Certain single nucleotide polymorphisms (SNPs) are associated with more effective DA transmission and consequently better motor and cognitive performance, while other SNPs are associated with depressed DA functioning and resulting impairments in motor and cognitive behaviors, especially in older age (Nagel et al., 2008; Noohi et al., 2016; Papenberg et al., 2013).
The influential work of several groups has identified multiple SNPs associated with cognitive performance, but studies investigating motor function are scant. For instance, Met alleles at the catechol-O-methyltransferase (COMT) Val158Met polymorphism have been associated with greater DA availability in the prefrontal cortex (Lotta et al., 1995; Tunbridge et al., 2006) and better cognitive performance in young (Bellander et al., 2015; Sheldrick et al., 2008) and older adults (de Frias et al., 2004; Nagel et al., 2008; Witte and Floel, 2012). However, effects on motor behaviors are conflicting. We previously reported that those with the “low” prefrontal DA genotype (Val/Val) showed slower reaction times and slower adaptation to a visuomotor perturbation than those with “high” prefrontal DA alleles (Met) (Noohi et al., 2014), but another study found that older adults with the “intermediate” DA genotype (Val/Met) showed faster gait speed than high DA carriers (Met/Met) (Holtzer et al., 2010). Thus while high DA alleles are associated with better cognitive performance, further research is necessary to better understand associations between DA genotype and motor behaviors. Moreover, as the majority of recent candidate gene studies have included only about 50 (McHughen and Cramer, 2013; McHughen et al., 2010) to a few hundred subjects at most (Ghisletta et al., 2014; Nagel et al., 2008), investigation in a large sample is clearly needed.
To address these substantial gaps in the literature, here we analyzed a very large sample of older adults from the Health and Retirement Study (HRS), a representative sample of Americans age 50 or older. We selected four genes with SNPs known to influence DA function: brain-derived neurotrophic factor (BDNF, Val66Met), COMT (Val158Met), dopamine D3 receptor (DRD3, Ser9Gly), and dopamine beta-hydroxylase (DBH, C>T). We then tested for associations between genetic data and motor performance of over 4,500 older adults and cognitive performance of over 7,000 older adults.
Each of these SNPs is clearly linked to individual differences in brain DA availability and thus has a biologically plausible influence on motor and cognitive behaviors (Table A1). For instance, in human brains, COMT Val alleles have been associated with 40 percent higher COMT enzyme activity than Met alleles (Chen et al., 2004). As COMT is directly implicated in the DA metabolism pathway (i.e., COMT enzyme catabolizes DA), Val carriers show decreased DA availability as a direct result of this SNP. Previous literature has demonstrated the role of each of these SNPs in cognitive performance (Table A2); however, associations with motor behaviors have not been well investigated and thus warrant further examination. Thus, these four SNPs were selected with the a priori hypothesis that, through their influence on brain DA transmission, each SNP would be related to individual differences in motor and cognitive performance in older adults.
In addition to the large sample size and a priori selection of dopaminergic candidate SNPs, this work includes examination of a very old age group (≥85 years) and evaluation of sex differences. Most candidate gene studies have not included participants older than 75 years (Li et al., 2010; Nagel et al., 2008; Papenberg et al., 2013; Papenberg et al., 2016). Much older age groups are severely underrepresented among all human research (Bayer and Tadd, 2000; Davies et al., 2010); the current study presents novel data on DA genotype associations for very old adults (≥85 years, up to 101 years). The level of DA decline that has occurred by this point may be sufficient to unmask genetic differences due to reduced efficiency of compensatory processes (Lindenberger et al., 2008). Additionally, although there are well-documented sex differences in DA transmission (Becker, 1990) and exploration of sex differences in public health research has been an NIH priority since the 1990s (Mazure and Jones, 2015), only a limited number of studies have tested for sex differences in associations between DA SNPs and behavior. Most of these studies had small sample sizes and conflicting results (Holtzer et al., 2010; Soeiro-De-Souza et al., 2013). The larger sample size here allows for a more conclusive investigation of whether males and females differ in how DA genotype may influence their performance.
In the present study we examine whether and how BDNF and DA genotypes relate to mobility and memory performance in the largest and oldest sample yet reported. We hypothesized that individuals with more of the low DA transmission alleles would perform more poorly on both motor (i.e., walk time, balance time for various stances, and grip strength) and cognitive measures (i.e., recall memory, delayed memory, and serial subtraction) and that older age and sex would interact with these DA genotypes to influence behavior. Neurocognitive declines with aging are surely mediated by multiple factors. If our hypothesis is supported it would indicate however that DA genotypes may be a significant predictor of successful aging and could flag individuals who would benefit from more frequent monitoring in advancing years.
2. Methods
Data were obtained from the HRS, which is sponsored by the National Institute on Aging (NIA U01AG009740, RC2AG036495, and RC4AG039029) and is conducted by the University of Michigan. This work was approved and classified as “exempt” by the University of Michigan IRB: Health Sciences and Behavioral Sciences (HUM00119891). All behavioral data are available for download from: http://hrsonline.isr.umich.edu. Access to genetic data requires authorization from the Database of Genotypes and Phenotypes (dbGaP) and HRS. This paper provides a cross-sectional analysis of participants’ cognitive test scores in 2006 and their motor skill performance in either 2006 or 2008, depending on which year they were chosen to complete the motor tasks.
2.1 Genotyping
The HRS obtained saliva DNA samples from 12,507 participants in 2006 and 2008. The Center for Inherited Disease Research genotyped these samples for over 2.5 million SNPs using the Illumina HumanOmni2.5-4v1 array. Further details regarding data collection and processing are provided in the quality control (Weir et al., 2012), imputation (CIDR, 2012), and candidate gene (Faul et al., 2014a, b) reports. Briefly, before imputation, standard quality control filters set forth by the Genetics Coordinating Center at the University of Washington were implemented (Weir et al., 2012). IMPUTE2 was used to impute data to the 1000 Genomes Project phase I integrated variant set (v3, released March 2012). The HRS extracted various candidate SNPs from the imputed data, as described in Section 2.2. As recommended by HRS, the four candidate SNPs included in this work had a minor allele frequency > 0.05 and an “INFO” score (i.e., a measure of SNP imputation quality) of > 0.80, which is a conservative threshold (Faul et al., 2014b).
2.2 Rationale for Selection of Candidate SNPs
In addition to the clear associations with brain DA availability (Table A1) and cognitive behaviors (Table A2), these four SNPs were selected because of their classification by the HRS as biologically promising candidate genes. These four SNPs were selected from the HRS “Candidate Genes for Cognition/Behavior” data package (Faul et al., 2014b), which contains over fifty candidate genes described as a “selection of the most biologically promising candidate genes” for cognition and behavior phenotypes. Only these four SNPs were selected for analysis because of their role in DA metabolism. Thus, here we used a hypothesis-driven approach to examine the association between four dopaminergic SNPs selected a priori and motor and cognitive performance.
2.3 Study Participants
As SNP frequencies are known to vary between racial and ethnic groups (Price et al., 2006), analyses included only the 8,562 participants who were classified as European Americans based on self-report and principal component analysis (PCA) of genetic data. These individuals were unrelated, self-identified as White, and had relatively homogenous ancestry, defined as falling within 1 standard deviation of all other self-identified non-Hispanic White individuals for eigenvectors 1 and 2 of the PCA of all unrelated study subjects. The quality control report includes further details regarding the PCA (Weir et al., 2012).
Of this sample, our analyses excluded those with a known memory (n = 63) or neurological disorder (n = 176). Memory disorders included any past diagnosis of a “memory-related disease” (HRS, 2007). Neurological disorders included conditions such as multiple sclerosis, Parkinson’s disease and others; a comprehensive list of conditions is found in the data description (HRS, 2014). For the motor tasks, analyses included only those individuals who were age 65 or older at the time of motor testing and who successfully completed each of the three primary motor tasks: timed walk, balance time, and grip strength (n = 4,605 total). Younger participants were excluded because only those ≥ 65 years old completed the timed walk. We separately analyzed cognitive task scores for this sample combined with participants who did not complete the motor tasks in 2006 or 2008. As participants of all ages complete the cognitive tasks, this sample included a fourth age group of those aged 50 to 65 years old. Participants were removed from this sample if they failed to complete any of the three primary cognitive tasks: immediate and delayed word recall and serial subtraction, or if they failed to report their years of education; these exclusions left a total cognitive task sample of n = 7,331. Allelic distribution across age and sex groups and detailed demographic information is provided for these samples in Tables 1 and B1–B2.
Table 1.
Allelic distribution for the motor and cognitive tasks
| Motor Tasks (n = 4,605) | ||||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Genotype | Total | 65–74 years | 75–84 years | 85+ years | |||
| M | F | M | F | M | F | |||
| BDNF (rs6265) | Val/Val* | 2952 | 758 | 894 | 470 | 564 | 106 | 160 |
| Val/Met | 1484 | 385 | 504 | 222 | 259 | 35 | 79 | |
| Met/Met | 169 | 39 | 44 | 32 | 34 | 6 | 14 | |
| COMT (rs4680) | Met/Met* | 1236 | 309 | 366 | 202 | 248 | 37 | 74 |
| Val/Met | 2331 | 617 | 728 | 357 | 435 | 69 | 125 | |
| Val/Val | 1038 | 256 | 348 | 165 | 174 | 41 | 54 | |
| DRD3 (rs6280) | Ser/Ser* | 2124 | 547 | 670 | 353 | 383 | 63 | 108 |
| Gly/Ser | 1972 | 523 | 607 | 296 | 363 | 71 | 112 | |
| Gly/Gly | 509 | 112 | 165 | 75 | 111 | 13 | 33 | |
| DBH (rs1611115) | CC* | 2896 | 718 | 910 | 481 | 538 | 95 | 154 |
| TC | 1516 | 423 | 471 | 210 | 283 | 43 | 86 | |
| TT | 193 | 41 | 61 | 33 | 36 | 9 | 13 | |
| Cognitive Tasks (n = 7,731) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Genotype | Total | < 65 years | 65–74 years | 75–84 years | 85+ years | ||||
| M | F | M | F | M | F | M | F | |||
| BDNF (rs6265) | Val/Val* | 4990 | 746 | 1098 | 820 | 942 | 473 | 612 | 107 | 192 |
| Val/Met | 2472 | 358 | 548 | 414 | 529 | 222 | 279 | 38 | 84 | |
| Met/Met | 269 | 32 | 55 | 42 | 53 | 32 | 38 | 6 | 11 | |
| COMT (rs4680) | Met/Met* | 2090 | 303 | 466 | 342 | 392 | 197 | 267 | 38 | 85 |
| Val/Met | 3901 | 597 | 839 | 639 | 784 | 366 | 462 | 76 | 138 | |
| Val/Val | 1740 | 236 | 396 | 295 | 348 | 164 | 200 | 37 | 64 | |
| DRD3 (rs6280) | Ser/Ser* | 3509 | 488 | 781 | 592 | 705 | 353 | 400 | 63 | 127 |
| Gly/Ser | 3366 | 525 | 725 | 565 | 648 | 300 | 411 | 68 | 124 | |
| Gly/Gly | 1376 | 195 | 318 | 171 | 290 | 118 | 192 | 36 | 56 | |
| DBH (rs1611115) | CC* | 4860 | 692 | 1084 | 781 | 971 | 482 | 583 | 97 | 170 |
| TC | 2543 | 396 | 544 | 448 | 486 | 215 | 302 | 44 | 108 | |
| TT | 328 | 48 | 73 | 47 | 67 | 30 | 44 | 10 | 9 | |
Allelic distribution for four SNPs in a mixed sex/age sample of Caucasian ethnicity for the motor (n = 4,605) and cognitive (n = 7,731) tasks. The motor task sample includes only those individuals who completed all of the motor tasks (i.e., timed walk, grip strength, and semi-tandem balance) in 2006 or 2008. The cognitive task sample includes only those individuals who completed all of the cognitive tasks (i.e., immediate and delayed recall and serial subtraction) in 2006. Motor task ages represent subject age at the time that they completed the motor tasks; cognitive task ages represent subjects’ ages in 2006. Motor task ages ranged from 65 to 99 years (Mean = 74.23 years ± 6.65 SD); cognitive task ages ranged from 50 to 101 years (Mean = 68.25 years ± 9.82 SD). All distributions were in Hardy-Weinberg equilibrium and consistent with the literature. Note: for models in which there were no differences between males and females, the samples were pooled across sex; for the models in which there were no differences between age groups, the samples were pooled across age.
Indicates the purportedly beneficial allele for each SNP
2.4 Motor Tasks
The HRS motor tasks have been described in detail elsewhere (Crimmins et al., 2008). To assess gait speed, participants walked unassisted at a normal pace for 2.5 meters; the average of two trials was calculated. Each participant completed two balance tasks. All participants attempted a 10-second semi-tandem stance. Those unable to hold the semi-tandem stance then attempted a 10-second side-by-side stance (n = 232), while the remainder of the participants attempted a full tandem stance for 30 seconds (≥70 years) or 60 seconds (< 70 years). Using a hand dynamometer, participants completed two dominant and two non-dominant maximum grip strength trials; the mean of four trials was computed.
2.5 Cognitive Tasks
Cognitive tasks have also been described in detail elsewhere (Wallace and Herzog, 1995). Briefly, participants were read a list of 10 words and then asked to recall these words immediately and after about 5 minutes of interference tasks. The actual delay time varied between participants depending on how long they required to perform the intermediate cognitive tasks. Participants completed a serial subtraction task in which they were asked to mentally compute five subtractions starting with “100 minus 7.” These measures were adapted for use by the HRS from the extensively validated “Telephone Interview of Cognitive Status” (Brandt et al., 1988), which was based on the Mini-Mental State Exam (Folstein et al., 1975).
2.6 Data Analyses
Data were analyzed using IBM® SPSS® Version 24 (IBM, Chicago, IL) and StataSE Version 14 (StataCorp, College Station, TX). SPSS was used to obtain summary measures of demographic variables. As is common practice in data collection for representative surveys, minority groups and certain geographical areas were oversampled, causing households and individuals to have different probabilities of being selected into the sample (Ofstedal et al., 2011). To account for this, survey weights were incorporated into all analyses using the Stata svyset command, and F statistics were adjusted for this complex sampling design. Denominator degrees of freedom (DF) reported for F statistics represent the survey design DF (i.e., 52 DF for the motor task sample and 56 DF for the cognitive task sample). These weighting procedures are described in further detail elsewhere (Ofstedal et al., 2011).
In Stata, the svy: glm procedure was used to compare performance on each motor and cognitive task by genotype for each SNP. Between-subjects factors included genotype, age, and sex; the cognitive task models also included years of education as a covariate. The model tested for each task-genotype combination and included all two-way interactions for these factors and the three-way genotype by age by sex interaction. Additionally, as is common practice in genetic association studies, in order to account for population structure and stratification, the first 10 principal components from the ancestry PCA were included as covariates in each model (Price et al., 2006; Weir et al., 2012). Models were separately recalculated when including each of the additional covariates detailed in Tables B1 and B2 (e.g., mental health status, BMI, height, blood pressure, etc.). The quality control report includes further details regarding the PCA; specifically see Section 9, Figures 11–16, and Table 8 within this report (Weir et al., 2012). Additionally, see Faul et al. (2014a) for further information and recommendations regarding use of these PCA results for sample selection.
Given the extensive literature support for genotype by age interactions in genetic association studies examining cognitive performance (Lindenberger et al., 2008), we hypothesized that genotype associations with behavior would vary by age group. Thus, we used a set of polynomial contrasts (−1, 0, 1 and 1, −2, 1) to examine potential linear and quadratic patterns in the age groups (collapsed across sex) for each genotype by age interaction. The alpha level was corrected to p = 0.025 to account for these two preplanned contrasts. Due to the less definitive literature support for genotype by sex interactions, we examined the two-way genotype by sex and the three-way genotype by age by sex interactions as exploratory analyses using the same set of polynomial contrasts. To remain conservative in these analyses, the alpha level was still corrected to p = 0.025 to account for the two contrast tests per interaction.
3. Results
Not surprisingly, mobility and memory scores declined with age on all tasks. Males performed better than females on all of the motor tasks. Females generally performed better on the word recall tasks, and males generally performed better on the subtraction task. We did not find any main effects of genotype, but as hypothesized, there were several significant two- and three-way interactions which we elaborate below.
3.1 Motor Measures
In general, results for the motor measures followed our hypotheses. Genotype-behavior associations were seen only among the oldest individuals for several SNPs for gait speed (BDNF and DRD3) and balance time (COMT). As expected, individuals with genotypes associated with more effective DA transmission (e.g., COMT Met/Met) performed better on several motor tasks (e.g., balance time). A few unexpected results were identified (e.g., a U-shaped function was noted for BDNF and walk time); these results are further explained in the Discussion.
3.1.1 Timed Walk
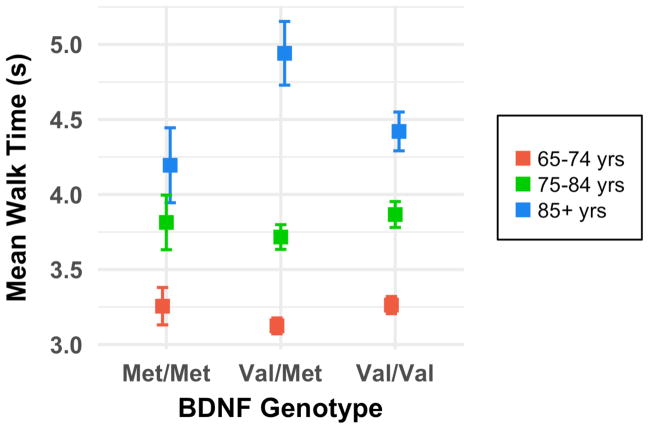
For the timed walk, planned comparisons indicated that 85+ year olds with either the BDNF Met/Met or Val/Val genotype outperformed those with the Val/Met genotype (F1,52 = 7.72; p = 0.008) (Fig. 1). Although this U-shaped function does not follow our prediction that a greater number of Val alleles (which are associated with greater BDNF protein activity and higher DA availability) would be associated with better performance, since several other measures produced this same pattern for BDNF, we propose an interpretation of these results in the discussion (Section 4.2).
Fig. 1.
Mean walk time by BDNF rs6265 genotype and age for a 2.5 meter unassisted timed walk (n = 4,605). Error bars represent standard error. Lower scores (i.e., indicative of faster gait speed) are desired. Among the oldest age group, those with the Val/Val and Met/Met genotypes had faster walk times than those with the intermediate Val/Met genotype (p < 0.01). The Val/Val genotype is associated with the highest BDNF and DA availability; thus Val/Val individuals were anticipated to perform best, and the initial hypothesis was partially supported.
For COMT, among the youngest females (65 – 74 years old), the linear contrast indicated that Val/Val individuals had the fastest gait speed (F1,52 = 5.69; p = 0.021). Among the youngest males, a quadratic pattern emerged in which Val/Met outperformed Val/Val and Met/Met individuals (F1,52 = 5.82; p = 0.019). Although these results for females do not fit with our hypothesis, as younger females with the low DA genotype demonstrated the fastest walk times, results for younger males match those of the only previous report of COMT-gait speed associations, which similarly identified the best performance among Val/Met males (Holtzer et al., 2010); these results are further discussed in Section 4.4.
Planned contrasts indicated an association between DRD3 genotype and walk time for the oldest males. In this case, a linear pattern emerged with Gly/Gly outperforming Gly/Ser and Ser/Ser individuals (F1,52 = 11.47; p = 0.001). These effects were somewhat unanticipated, as those with the genotype associated with lower tonic DA (Gly/Gly) had the fastest gait speed. However the findings do follow the prediction of magnified genotype effects in the oldest age group. DBH genotype was not significantly associated with gait speed.
3.1.2 Grip Strength
Genotype was not associated with grip strength. Age by sex interactions emerged for grip strength in all statistical models (Fig. C1); males tended to decline more with age in grip strength than females.
3.1.3 Balance Time
As 94.74% of participants (n = 4,363) held the semi-tandem stance for the full time (10 seconds), the data were not normally distributed due to a ceiling effect and we did not assess genotype associations with this task. Genotype was not associated with balance time for the 232 participants who completed the side-by-side stance. Of the 4,038 participants who completed the full tandem stance, 69.76% (n = 2,817) held this stance for the maximum time, so the data were negatively skewed. Square root and log transformations did not alleviate this issue. Although simulation data suggest that in large samples (n > 500) linear regression results are valid even for extremely non-normal data (Lumley et al., 2002), results for the full tandem stance should be interpreted with caution.
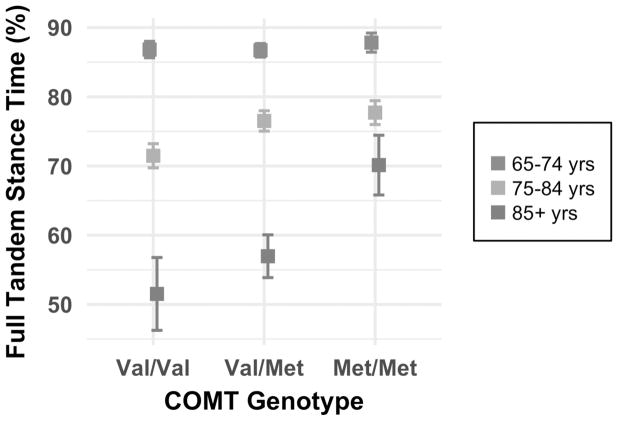
Linear contrasts revealed that COMT Met/Met individuals in the middle (F1,52 = 8.39; p = 0.006) and oldest (F1,52 = 8.47; p = 0.005) age groups had the highest full tandem scores (Fig. 2). These results followed our predictions, as those with two high DA alleles in the older age groups demonstrated the best balance abilities. When collapsed over age, females showed a linear association between COMT genotype and balance time (F1,52 = 6.21; p = 0.016) while males did not, with Met/Met still performing best. In particular, the linear contrast on the three-way genotype by age by sex interaction revealed the strongest association for 74–84 year old females (F1,52 = 6.68; p = 0.013) in which, as predicted, Met/Met scored higher than Val/Met and Val/Val. The other three SNPs were not significantly associated with full tandem balance time.
Fig. 2.
Full tandem stance time by COMT rs4680 genotype and age (n = 4,038). Error bars represent standard error. Balance time is shown as the percentage of total prescribed time (i.e., 30 or 60 seconds) that participants were able to maintain the full tandem stance. Higher scores (i.e., longer balance time) are desired. Met/Met individuals performed best in the middle (75–84 years) and oldest (85+ years) age groups. The Met/Met genotype is associated with high DA availability, so this result supports our hypothesis.
3.2 Cognitive Measures
Similar to the motor measures, results for the cognitive tasks generally followed our hypotheses. For multiple SNPs (BDNF, COMT, DBH) associations were only seen in the oldest age group. As expected, those with genotypes associated with more effective DA transmission performed better on the memory tasks for multiple SNPs (COMT, DRD3, DBH). In particular, males showed the strongest associations between cognitive performance and number of high DA alleles for several SNPs (COMT, DRD3, DBH). Again, a few unexpected results were identified (e.g., the same U-shaped function emerged for BDNF); we elaborate on these findings in the Discussion.
3.2.1 Immediate Recall
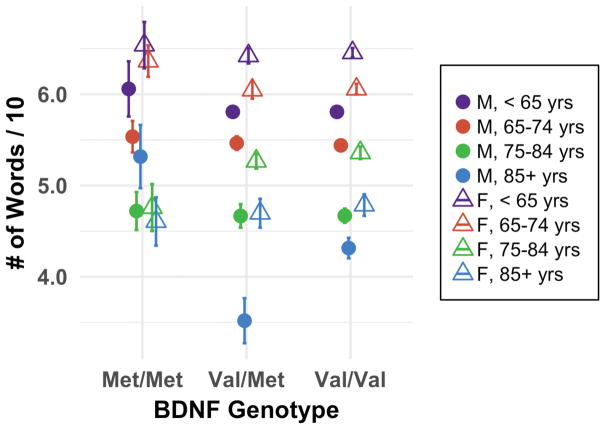
The quadratic contrast revealed an effect of BDNF genotype on immediate recall performance in the oldest age group (F1,56 = 12.41; p = 0.001), with Val/Val and Met/Met outperforming Val/Met individuals. Collapsed across age, this effect was evident in males (F1,56 = 14.33; p < 0.001) but not in females. The quadratic contrast for the three-way interaction again showed the poorest performance by Val/Met individuals only among the oldest males (F1,56 = 14.61 p < 0.001) (Fig. 3). As with BDNF associations for timed walk, this U-shaped function did follow our predictions; we will address this in detail later (Section 4.2).
Fig. 3.
Immediate word recall by BDNF rs6265 genotype (n = 7,331). Error bars represent standard error; M = male, F = female. Higher scores (i.e., more words recalled) are desired. Among the oldest males, Val/Val and Met/Met outperformed Val/Met individuals (p < 0.01). As the Val/Val genotype is associated with the highest BDNF and DA availability and thus Val/Val individuals were expected to perform best, these data partially support initial predictions. Contrasts did not indicate effects in females or in the younger age groups.
For DBH, regardless of age, males and females showed opposite linear patterns of performance across genotype; that is, as expected males with two high DA alleles (TT) performed best (F1,56 = 8.11; p = 0.006), but females with low DA alleles (CC) performed better than females with two high DA alleles (TT) (F1,56 = 12.39; p = 0.001) (Fig. 4A). In particular, among the oldest females, a negative linear pattern emerged in which CC females performed best (F1,56 = 7.15; p = 0.010) (Fig. 4B). For the oldest males, the same positive linear pattern remained, although the corrected p-value was not significant (F1,56 = 4.56; p = 0.037). Thus our hypothesis was generally supported only for males for immediate recall. COMT and DRD3 genotype were not associated with immediate recall scores.
Fig. 4.
Immediate and delayed word recall by DBH rs1611115 genotype and sex (n = 7,331). Error bars represent standard error; M = male, F = female. Higher scores (i.e., more words recalled) are desired. A. Immediate Recall. Regardless of age, male performance followed a positive linear pattern (p < 0.01), with TT (high DA) males performing best. Females showed the opposite linear pattern, with CC (low DA) females performing best (p < 0.01). B. Immediate Recall. The oldest females had a negative linear pattern in which CC (low DA) females performed best (p < 0.01); the opposite linear pattern in which TT (high DA) males performed best was evident for the oldest males, but was not significant (p = 0.037). C. Delayed Recall. The same patterns emerged for delayed recall. TT (high DA) males (p < 0.01) and CC (low DA) females performed best (p < 0.025). D. Delayed Recall. The oldest males exhibited the strongest linear association (p < 0.01), with TT (high DA) males performing best and no associations in females. 75–84 year old males also showed a quadratic pattern with TT and CC performing better than TC (p < 0.025).
3.2.2 Delayed Recall
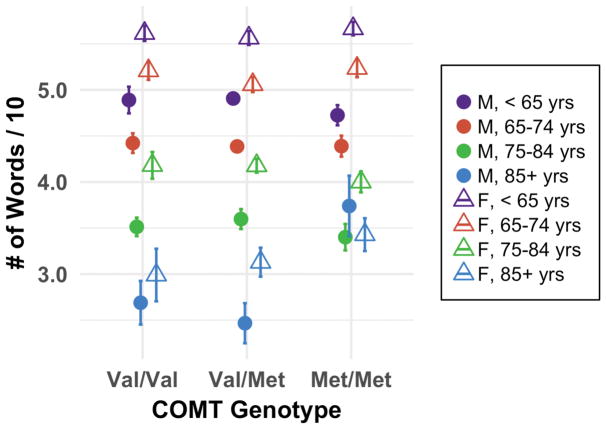
For COMT, a significant linear pattern emerged only in the oldest age group (F1,56 = 9.61, p = 0.003); that is among 85+ year olds, those with the Met/Met (i.e., the high DA availability) genotype outperformed others. In particular, the oldest males showed the strongest positive linear fit in which Met/Met individuals had the highest delayed recall scores (F1,56 = 6.69, p = 0.012) (Fig. 5).
Fig. 5.
Delayed word recall by COMT rs4680 genotype and age (n = 7,331). Error bars represent standard error. Higher scores (i.e., more words recalled) are desired. Collapsing across sex, a linear association emerged only for the oldest age group in which Met/Met performed best (p < 0.01). Among age and sex group, the oldest males showed the strongest genotype association, with Met/Met again performing best (p = 0.012). These results were as hypothesized, as Met/Met is associated with the highest DA availability.
DRD3 genotype was associated with delayed recall, but only for males in the youngest age group (< 65 years old) (F1,56 = 6.07, p = 0.017). Gly/Gly individuals had the lowest recall scores, which was expected as Gly/Gly is associated with lower tonic DA levels.
For DBH, overall, males showed a positive linear pattern (F1,56 = 11.54; p = 0.001) and females showed a negative linear pattern (F1,56 = 5.87; p = 0.019) (Fig. 4C); that is, regardless of age, TT performed better than CC males, while females showed opposite patterns. As the T allele is associated with the greatest DA availability, this was the expected pattern for males but not females. Contrasts for the three-way interaction revealed that the oldest males demonstrated the strongest linear pattern in which males with two high DA alleles had the highest recall scores (F1,56 = 8.94; p = 0.004) (Fig. 4D). Additionally, a quadratic pattern emerged for 75–84 year old males in which TT performed best, but CC performed slightly better than TC individuals (F1,56 = 5.87; p = 0.019). Among females, no DBH genotype associations specific to age group emerged. Thus, similar to immediate recall, our hypothesis was generally supported only in males (and particularly for the oldest males) for DBH associations with delayed recall. No associations emerged between BDNF and delayed recall performance.
3.2.3 Serial Subtraction
The serial subtraction data were negatively skewed. Square root and log transformations did not alleviate this issue. Again, although simulation data suggest that in large samples (n > 500) linear regression results are valid even for extremely non-normal data (Lumley et al., 2002), serial subtraction results should be interpreted with caution.
A quadratic pattern emerged for BDNF genotype for the 65–74 year old age group (F1,56 = 6.00; p = 0.017). Val/Met individuals scored slightly higher than those in the Val/Val and Met/Met groups. This result does not fit with the two other BDNF associations reported here and is discussed further in Section 4.4.
For COMT, when collapsing across age group, the linear contrast indicated that Met/Met females demonstrated the poorest performance (F1,56 = 7.10; p = 0.010), which was not expected as the Met/Met genotype is associated with greater DA availability. The linear contrast across age and sex groups revealed the same effect for females in the 65–74 year old age group, with Met/Met individuals again performing worst (F1,56 = 6.02; p = 0.017).
Planned contrasts for DRD3 revealed a positive linear pattern among the youngest males (F1,56 = 7.10; p = 0.010). Ser/Ser males performed better than Gly/Gly males; that is, as expected, those with the genotype associated with higher tonic DA (Ser/Ser) had the highest scores. A quadratic pattern emerged for 75–84 year old females in which Gly/Ser participants scored lower than both Ser/Ser and Gly/Gly (F1,56 = 6.19; p = 0.016). In this case, results did not fit with predictions, as Ser/Ser individuals were hypothesized to perform best.
No associations emerged for the other SNPs with serial subtraction performance. However, we did obtain significant age by sex interactions for most SNPs in the combined sample for the serial subtraction task (Fig. C2). These interactions showed that, in general, females tended to decline faster with age than males in serial subtraction performance.
3.3 Effects of Covariates
For the motor task sample, analyses were repeated including the following covariates in the model: mental health score, total health conditions, arthritis, stroke history, BMI, height, systolic and diastolic blood pressure, history of falls in the last two years, and use of an assistive walking device. When adding each of these covariates to the model, results were essentially identical to those described previously, except for the few instances illustrated in Table D1. For the cognitive task sample, covariates included: mental health score, total health conditions, and stroke history (in addition to years of education, which was kept in every model). Again, results remained essentially the same, with exceptions listed in Table D1.
3.4 Analysis of Unrelated SNP
We examined one additional SNP, rs12670850, on the Cytochrome P450 Family 3 Subfamily A Member 4 (CPY3A4) gene. The purpose of this was to determine whether other SNPs that are unrelated to DA metabolism and the hypotheses of this paper do not also show spurious associations with the motor and cognitive behaviors that we examine here. The CPY3A4 gene encodes an enzyme involved in drug metabolism and lipid synthesis in the liver and small intestine and does not have any known role in brain DA transmission or metabolism. Thus we would not expect to find any effects of this SNP on motor or cognitive behaviors. This SNP was selected from the same data package, passed the same quality checks as the other SNPs we examine here, and showed a reasonable distribution across the three genotypes in the motor (AA, n = 1,455; AG, n = 2,261; GG, n = 889) and cognitive task samples (AA, n = 2,461; AG, n = 3,785; GG, n = 1,485). When running all analyses with this SNP, we did not find any associations between this SNP and any of the behaviors of interest, with the exception of one random association; serial subtraction performance for those under 65 years old showed a significant linear trend (F1,56 = 5.36; p = 0.024). However, this association did not follow the specific genotype by age interaction pattern that we predicted a priori and observed for BDNF, COMT, and DBH; thus this association can likely be discounted. The lack of other associations between the CPY3A4 SNP and motor and cognitive behaviors suggests that the effects we present here are indeed specific to the DA-related genes under investigation.
4. Discussion
In the largest and oldest sample yet described, we have identified multiple associations between DA genotype, age, and sex on mobility, balance, and cognition. As hypothesized, those with more low DA alleles at the COMT, DRD3, and DBH SNPs performed worse on the balance and memory tasks, especially in the oldest age group (85+ years). Additionally, males, particularly those 85+ years old, showed stronger associations than did females between number of high DA alleles and cognitive performance (for COMT, DRD3, and DBH); that is, males with more high DA alleles tended to outperform others on the memory tasks. While the oldest males showed the strongest association between BDNF genotype and recall memory, surprisingly, those with the intermediate genotype performed worst; this relationship, which we elaborate below, also held for BDNF and timed walk among the oldest participants. Taken together, these results shed light on how declines in DA metabolism and BDNF across the lifespan interact with common genetic polymorphisms to explain a notable portion of individual differences in successful aging—especially in very old age and in males.
4.1 Support for the Resource Modulation Hypothesis
The oldest of the older adults (those 85+ years old), who are often unnecessarily excluded from research studies (Bayer and Tadd, 2000; Davies et al., 2010), showed the most pronounced genotype effects. This finding follows Lindenberger and colleagues’ resource modulation hypothesis, which suggests that genetic variability is more likely to influence performance when brain resources become limited, as in older age (Lindenberger et al., 2008). Here the associations of COMT, DBH, BDNF, and DRD3 genotypes with motor and cognitive performance provide support for this hypothesis. As balance time and delayed recall performance improved with increasing number of high DA (Met) alleles for COMT, these results follow the additive assumption of the resource modulation hypothesis (i.e., more high DA alleles are associated with more “brain resources” and slower declines in performance with age) (Lindenberger et al., 2008). These results fit with the extant literature and validate prior findings in the largest sample to date; several studies have noted benefits of high DA COMT Met alleles that were evident only in older adults, including on memory tasks (Papenberg et al., 2014; Sambataro et al., 2009), measures of white matter integrity (Papenberg et al., 2015), and longitudinal changes in executive function (de Frias et al., 2004) and episodic memory (Josefsson et al., 2012). No previous work has examined effects of the interaction between age and COMT genotype on motor behaviors in older adults; thus the association between COMT and balance time identified here only in the oldest age group is novel and warrants further exploration in future studies.
Additionally, DBH effects on delayed recall were evident only among the oldest individuals. Although fewer studies have examined DBH genotype effects on cognition, our results corroborate the findings of Greenwood et al. (2014), who found that older but not younger individuals homozygous for the low DA genotype for both COMT (Val/Val) and DBH (CC) had poorer working memory performance under the most difficult discrimination conditions for the task. Here, we show that those with the high DA genotype (DBH TT) performed best on the delayed recall task, with effects limited to males in the oldest age group.
Similarly, BDNF effects on gait speed and immediate recall were only evident in the oldest age group, providing further support for the resource modulation hypothesis (Lindenberger et al., 2008) and the idea that some genotype effects might be unmasked in older age. However, instead of a linear pattern, BDNF associations with both walk time and word recall yielded a U-shaped performance curve; that is, those with the intermediate Val/Met genotype performed the worst, while the Val/Val and Met/Met BDNF genotypes performed similarly to each other. While the majority of the literature suggests that Met alleles (which are associated with less BDNF and DA availability) are detrimental for both motor behaviors (Cheeran et al., 2008; Kleim et al., 2006; McHughen et al., 2011; McHughen et al., 2010) and memory (Egan et al., 2003; Hariri et al., 2003; Li et al., 2010; Miyajima et al., 2008), a few previous studies have actually identified neuroprotective effects of Met alleles for cognitive tasks in older age (Erickson et al., 2008; Harris et al., 2006). These effects have not before been examined for motor behaviors among older adults. Thus the relationship between genotype, availability of BDNF and DA, and performance may be more complex than for the other SNPs that we examined.
BDNF Val alleles are typically associated with higher BDNF protein secretion (Egan et al., 2003) and increased corticostriatal DA neuron survival and function (Baquet et al., 2005; Hyman and Hofer, 1991); however, elevated BDNF levels have also been associated with poorer working memory in older rats (Bimonte et al., 2003; Bimonte-Nelson et al., 2003), supporting that number of Val alleles and cognitive performance may not be positively associated in older adults. A proposed mechanism is that concentrations of tissue plasminogen activator (tPA) decline with age (Cacquevel et al., 2007), and tPA converts the precursor form of BDNF (pro-BDNF) into mature BDNF (mBDNF). While mBDNF is associated with long-term potentiation and synapse growth, pro-BDNF is related to long-term depression, synaptic retraction, and cell death (Lu et al., 2005). If tPA is not present in high enough concentration in older adults, the greater BDNF secretion associated with Val alleles may actually be detrimental to cognitive function, while Met alleles may exert neuroprotective effects later in life (Lu et al., 2005; Pang et al., 2004). Given the nonlinear relationship of BDNF genotype with cognitive performance in older age that we observed, we propose a modification of the resource modulation hypothesis specific to BDNF, the Neurotrophin Surplus Hypothesis (Fig. E1).
4.2 Neurotrophin Surplus Hypothesis
Our Neurotrophin Surplus Hypothesis posits that BDNF Val alleles (which are associated with higher BDNF availability) and performance are linearly related in young adults, such that Met/Met individuals (who also have the lowest BDNF production) are poorer performers earlier in life. However, we suggest that by older adulthood, BDNF Met alleles become neuroprotective, and this linear relation transitions to a U-shaped function. Consequently, while Met/Met individuals only marginally decline in cognitive performance with age, Val/Val individuals may encounter significant cognitive declines because decreasing tPA concentrations with age cause them to accumulate a “surplus” of BDNF (which becomes detrimental to cognitive performance). Intermediate Val/Met individuals are ultimately the worst performers in old age because their cognitive performance was intermediate to the two homozygous genotypes in younger age. In older age, they too accumulate some surplus of BDNF, which is detrimental to their cognitive performance. We suggest that the BDNF interaction with timed walk that we observed here also follows our Neurotrophin Surplus Hypothesis. Although supported by the present results, this hypothesis requires further testing, as other factors such as interactions between compensatory processes and age may also contribute to these effects.
4.3 Sex Differences in Genotype Associations
Males were more likely than females to show associations between number of high DA alleles and cognition for all SNPs. Moreover, associations between genotype and performance were in opposing directions for the two groups in some cases. Although examination of sex differences in public health research has been an NIH priority for multiple decades (Mazure and Jones, 2015), only a limited number of studies have accomplished this. For instance, Li and colleagues noted that, in a sample of older Chinese adults, only females showed an association between BDNF and Alzheimer’s disease risk (Li et al., 2017); the authors cited that estrogen upregulates BDNF mRNA in cortical areas (Solum and Handa, 2002) and thus age-related estrogen declines induce female-specific memory deficits in older age. However, our findings conflict with their report of enhanced genotype effects in females. This may be because the previous work only assessed disease risk rather than performance on specific memory tasks such as in the current study; additionally, as discussed previously, declines in cortical BDNF concentration may actually be neuroprotective in older age.
For DBH, on both recall tasks, the high DA genotype (TT) was associated with the best performance in males. Similar to BDNF, one study identified relationships between DBH genotype and behavior (harm avoidance) in young females, but not males (Kamata et al., 2009), citing that estrogen also upregulates DBH expression (Sabban, 2007). Again, as the females in the present sample are all postmenopausal and any estrogen effects on DBH expression are likely minimal, DBH genotype might contribute less overall to individual differences in cognitive performance in these older females. Thus, it follows that the older females did not exhibit a strong association between DBH genotype and delayed recall performance.
COMT genotype showed associations with recall performance in males but not females and specifically in the oldest males. Others have identified COMT genotype associations limited to males for inhibitory control (White et al., 2014), personality traits (Chen et al., 2011), and schizophrenia (Hoenicka et al., 2010). Additionally, estrogen downregulates COMT mRNA expression (Jiang et al., 2003; Xie et al., 1999); that is, women with higher estrogen levels have lower COMT activity. It would follow then that estrogen levels would interact with genotype to influence COMT activity, creating greater individual differences in COMT activity and resulting DA availability among females. However, in this postmenopausal sample, this interaction might weaken or disappear, resulting in reduced individual differences in COMT activity among the females in this sample (Tunbridge and Harrison, 2010). Further, as post-mortem work has revealed a significant interaction between sex and COMT genotype in brain lymphocytes and has shown that females have 17% less COMT protein activity in the prefrontal cortex than males, COMT genotype effects are likely to be more pronounced in males than in females (Chen et al., 2004). Thus these mechanisms likely contributed to the male-specific COMT genotype effect on memory seen here.
Fewer studies have examined sex differences for DRD3 genotype; one study noted an association between DRD3 genotype and schizophrenia age of onset in males but not females (Renou et al., 2007). Our results here again revealed male-specific effects of DRD3 genotype on behavior, which fits with this past work. Of note is that in all cases here there were more females than males, yet we still identified effects specific to males. This suggests that the present study was adequately powered to detect effects in both sexes, but only associations among males emerged. Thus while it is well established that there are sex differences in DA transmission (Becker, 1990; Munro et al., 2006) and that these sex differences might vary as a function of age (Wong et al., 2012), the potential interactions of these effects with DA polymorphisms have not been well studied. Although our findings suggest that sex differences interact with dopaminergic polymorphisms to influence cognitive performance in older adults, future replication studies are definitely warranted.
4.4 SNPs with Unanticipated Results
A few results did not fit with our initial predictions. COMT Val/Met males performed better than Met/Met and Val/Val males, even though Met/Met is associated with the greatest DA availability. This Val/Met advantage among males for gait speed was also identified by Holtzer et al. (2010), who suggested that the Met/Met genotype, which is associated with the highest tonic DA levels, might contribute to superior cognitive performance (as was the case for delayed recall performance here). For gait, however, the intermediate tonic DA levels associated with the Val/Met genotype might contribute to an optimal level of tonic DA signaling in the striatum and result in faster gait speed (Holtzer et al., 2010). As females have greater tonic striatal DA levels than men (Munro et al., 2006; Tunbridge and Harrison, 2010), Val/Val (low DA) females might be closer to the DA levels required for optimal functional efficiency for gait and therefore demonstrate the fastest walk time (which was shown here for the youngest females).
The direction of DRD3 genotype effects on timed walk performance did not fit with our hypotheses, as the oldest Gly/Gly males demonstrated the fastest walk times. However, as genotype differences here were evident only in the oldest age group, these results do provide further support for the resource modulation hypothesis (Lindenberger et al., 2008). As previous studies examining DRD3 associations with motor behaviors are scant, and most have only considered disease risk or age of disease onset (Hassan et al., 2016; Jeanneteau et al., 2006), optimal DRD3 activity for gait might vary from initial expectations and a DRD3 genotype associated with different D3 receptor efficacy or tonic DA availability might be most beneficial for gait, so further work is needed.
4.5 Limitations
A primary limitation of the present work is that these results were not replicated in an independent cohort. As extensive literature has demonstrated associations between these SNPs and cognitive behaviors (Table A2), these results partially replicate previously published results but in a significantly larger and older sample. Nonetheless, future replication studies are warranted. As expected based on population distributions, we observed unequal numbers of individuals in groups assigned by their genotype (Table 1); however, as with most genetic association studies, the minor allele groups are hypothesized to display the effects of interest, so analyzing these groups separately is necessary. Further, as we noted effects for some behaviors but not for others, this provides additional support for the notion that we had sufficient group sizes for detecting effects. Nevertheless, future studies would benefit from larger sample sizes that would provide even greater statistical power to detect genotype–behavior associations.
As many of the individuals who completed the cognitive tasks chose not to complete the motor tasks, this could indicate a potential selection bias (i.e., that the motor task sample represents a higher functioning subset of the cognitive task sample). However, reasons for refusal to complete the motor tasks appeared to be more random than simple self-selected refusal by the potential poorest performers. For instance, more individuals failed to complete the timed walk due to lack of space than those who failed to complete the timed walk due to safety concerns (HRS, 2006, 2008). This suggests a more random pattern of refusal rather than a systematic selection bias. Additionally, as the average age across the genotype groups was slightly different for BDNF genotype for the motor tasks (Table B1), this could have influenced the inverted U-shaped pattern that emerged for BDNF and gait speed. However, as no age differences persisted across the BDNF genotype groups for the cognitive tasks and the same inverted U-shaped pattern was observed, we suspect that these minimal age differences did not substantially influence the timed walk results. As only self-reported Caucasian individuals (corroborated with PCA) were included in this sample, the generalizability of these results is limited to this subset of the population.
As this work analyzed a retrospective dataset, verification of differences in brain DA concentration based on genotype was not possible. While cell, rodent, and human studies provide clear support that the SNPs included here do indeed influence brain DA concentrations (Table A1), the collection of measures such as cerebrospinal fluid would be a valuable addition to future work to confirm these differences. Further, as these SNPs, particularly BDNF, contribute to other neural processes (e.g., differentiation of neurons), we cannot discount the possibility of pleiotropy effects. That is, these SNPs could be affecting motor and cognitive phenotypes through other mechanisms unrelated to DA. However, since these SNPs are each related to DA transmission and mostly demonstrated the predicted effects, we think this is unlikely. Finally, although we have used a correction of p = 0.025 for all analyses, there is still some possibility of type I error.
4.6 Applications of Findings
The outcome metrics examined here carry substantial functional significance. Gait and balance disturbances are among the strongest risk factors for falls in older adults, and falls are the greatest cause of injury and death from injury in older adults; for review, see Ambrose et al. (2013). Similarly, the cognitive measures examined here were adapted from the Mini-Mental State Examination (MMSE), which is a well-utilized tool for measuring cognitive impairment and decline in older adults and shows moderate to high correlations with general intelligence, performance on complex memory tasks, and activities of daily living; for review, see Tombaugh and McIntyre (1992). Cognitive impairment among older adults is also associated with higher risk of falls (Alexander and Hausdorff, 2008; Muir et al., 2012). Taken together, this indicates the critical importance of identifying potential genetic markers for older adults who might be at the greatest risk of motor and cognitive decline.
We and others have examined similar dopaminergic SNPs in disease populations (e.g., Parkinson’s disease, PD), and found effects of DRD2 on gait function and medication responsiveness (Miller et al., 2017) and DRD3 rs6280 on overall PD symptoms and medication responsiveness (Liu et al., 2009). Such findings suggest that these SNPs may associate with motor and cognitive behaviors in PD patients in a similar manner to the healthy aging results we present here. However, given the differences between DA denervation in healthy aging and PD (Bohnen et al., 2006; Ofori et al., 2015), we caution overgeneralization from the current results. Instead, future work should examine the interaction between these SNPs, healthy aging, and PD.
5. Conclusions
In summary, we have identified various DA polymorphisms related to mobility and memory in a large population-based sample of older adults. For multiple SNPs, we have demonstrated that having more of the low DA alleles is associated with worse performance. We have also demonstrated that DA genotype associations with mobility, balance, and memory are enhanced with age (Lindenberger et al., 2008) and most pronounced in males. The observed COMT and DBH associations with balance and recall memory only in the oldest age group support previous literature (Lindenberger et al., 2008)—even when scaling up to a large, representative sample. The observed BDNF genotype associations with performance were more complex, as the oldest individuals with the intermediate BDNF genotype showed the most impaired performance. Thus we have proposed the novel Neurotrophin Surplus Hypothesis to explain these effects. As our findings showing the most pronounced associations between high DA genotype and behavior only partially support the scant literature on these interactions, we believe more studies are critically needed. Taken together, our findings provide strong support for the role of individual differences in DA transmission in the motor and cognitive declines seen with aging and the potential for genotyping to predict successful aging. These biomarkers could be used to anticipate which older individuals are at greater risk of falls or memory impairment and may lead to new interventions and personalized care approaches.
Supplementary Material
Highlights.
In general, older adults with more “low” DA alleles exhibited poorer performance
Some genotype associations were present only in the oldest age group (85+ years)
Males showed stronger associations between number of “high” DA alleles and memory
Older age and sex interact with DA genotype to influence motor/cognitive behavior
Acknowledgments
We thank Dr. Mary Beth Ofstedal and Mr. Kyle Latack for their input on this project, as well as those affiliated with the HRS and the RAND Center for the Study of Aging, without whom this project would not have been possible. RS is a member of The Life Course: Evolutionary and Ontogenetic Dynamics.
Funding
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1315138. We also thank support from R01 NS052318.
Footnotes
Disclosures
None of the authors has any conflicts of interest with the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander NB, Hausdorff JM. Guest editorial: linking thinking, walking, and falling. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(12):1325–8. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L. Age related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157(4):635–7. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25(26):6251–9. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Folstein MF. Cognitive impairment and functional disability in the absence of psychiatric diagnosis. Psychol Med. 1991;21(01):77–84. doi: 10.1017/s0033291700014677. [DOI] [PubMed] [Google Scholar]
- Bayer A, Tadd W. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: descriptive study. BMJ. 2000;321(7267):992–3. doi: 10.1136/bmj.321.7267.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990;5:157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Bellander M, Backman L, Liu T, Schjeide BM, Bertram L, Schmiedek F, Lindenberger U, Lovden M. Lower baseline performance but greater plasticity of working memory for carriers of the Val allele of the COMT Val158Met polymorphism. Neuropsychology. 2015;29(2):247–54. doi: 10.1037/neu0000088. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm A-CE. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiol Aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Experimental Neurology. 2003;181(2):301–12. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albm RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. Journal of Cerebral Blood Flow & Metabolism. 2006;26(9):1198–212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111–7. [Google Scholar]
- Cacquevel M, Launay S, Castel H, Benchenane K, Chéenne S, Buée L, Moons L, Delacourte A, Carmeliet P, Vivien D. Ageing and amyloid-beta peptide deposition contribute to an impaired brain tissue plasminogen activator activity by different mechanisms. Neurobiol Dis. 2007;27(2):164–73. doi: 10.1016/j.nbd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586(23):5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen C, Moyzis R, Dong Q, He Q, Zhu B, Li J, Li H, Li J, Lessard J. Sex modulates the associations between the COMT gene and personality traits. Neuropsychopharmacology. 2011;36(8):1593. doi: 10.1038/npp.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIDR. CIDR Health and Retirement Study: Imputation report-1000 genomes project reference panel. University of Washington; Seattle, WA: 2012. [Google Scholar]
- Crimmins E, Guyer H, Langa K, Ofstedal M, Wallace R, Weir D. Documentation of physical measures, anthropometrics and blood pressure in the Health and Retirement Study. HRS Documentation Report DR-011. 2008;14:47–59. [Google Scholar]
- Davies K, Collerton JC, Jagger C, Bond J, Barker SA, Edwards J, Hughes J, Hunt JM, Robinson L. Engaging the oldest old in research: lessons from the Newcastle 85+ study. BMC Geriatr. 2010;10(1):64. doi: 10.1186/1471-2318-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34(5):533–9. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: A systematic review and meta-analysis. Epidemiology. 2010;658:68. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: A 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci. 2008;2:11. doi: 10.3389/neuro.09.011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul J, Smith J, Zhao W. Health and Retirement Study: Candidate gene and SNP data description. University of Michigan; Ann Arbor, MI: 2014a. [Google Scholar]
- Faul J, Smith J, Zhao W. Health and Retirement Study: Candidate genes for cognition/behavior. University of Michigan; Ann Arbor, MI: 2014b. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Backman L, Bertram L, Brandmaier AM, Gerstorf D, Liu T, Lindenberger U. The Val/Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene predicts decline in perceptual speed in older adults. Psychol Aging. 2014;29(2):384–92. doi: 10.1037/a0035201. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lin MK, Sundararajan R, Fryxell KJ, Parasuraman R. Healthy aging increases the cognitive effects of two genes that influence extracellular dopamine. Psychol Aging. 2014;29(2):363–73. doi: 10.1037/a0036109. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-Derived Neurotrophic Factor Val66Met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry. 2006;11(5):505–13. doi: 10.1038/sj.mp.4001799. [DOI] [PubMed] [Google Scholar]
- Hassan A, Heckman MG, Ahlskog JE, Wszolek ZK, Serie DJ, Uitti RJ, van Gerpen JA, Okun MS, Rayaprolu S, Ross OA. Association of Parkinson Disease age of onset with DRD2, DRD3 and GRIN2B polymorphisms. Parkinsonism Relat Disord. 2016;22:102–5. doi: 10.1016/j.parkreldis.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48(5):493–8. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- Hoenicka J, Garrido E, Martínez I, Ponce G, Aragüés M, Rodríguez-Jiménez R, España-Serrano L, Alvira-Botero X, Santos JL, Rubio G. Gender - specific COMT Val158Met polymorphism association in Spanish schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2010;153(1):79–85. doi: 10.1002/ajmg.b.30957. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21(5):540. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31(3):523–31. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRS. Final Release Codebook, Section I: Physical Measures. University of Michigan; Ann Arbor, MI: 2006. [Google Scholar]
- HRS. HRS 2006-Section C: Health: Final Version 2. University of Michigan; Ann Arbor, MI: 2007. [Google Scholar]
- HRS. Final Release Codebook, Section I: Physical Measures. University of Michigan; Ann Arbor, MI: 2008. [Google Scholar]
- HRS. Health and Retirement Study 2006 Core Final, Version 3.0: Data Description and Usage. University of Michigan; Ann Arbor, MI: 2014. [Google Scholar]
- Hyman C, Hofer M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Funalot B, Jankovic J, Deng H, Lagarde J-P, Lucotte G, Sokoloff P. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci. 2006;103(28):10753–8. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden D, Ho S. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45(7):1011–8. doi: 10.1016/s0028-3908(03)00286-7. [DOI] [PubMed] [Google Scholar]
- Josefsson M, Luna X, Pudas S, Nilsson LG, Nyberg L. Genetic and lifestyle predictors of 15 - year longitudinal change in episodic memory. J Am Geriatr Soc. 2012;60(12):2308–12. doi: 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- Kamata M, Suzuki A, Matsumoto Y, Shibuya N, Togashi H, Goto K, Otani K. Association study between the -1021C/T polymorphism of the dopamine-beta-hydroxylase gene promoter and personality traits in healthy subjects. Neurosci Lett. 2009;462(1):54–7. doi: 10.1016/j.neulet.2009.06.077. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF Val66Met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kuh D, Karunananthan S, Bergman H, Cooper R. A life-course approach to healthy ageing: maintaining physical capability. Proc Natl Acad Sci. 2014;73(02):237–48. doi: 10.1017/S0029665113003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GD, Bi R, Zhang DF, Xu M, Luo R, Wang D, Fang Y, Li T, Zhang C, Yao YG Alzheimer’s Disease Neuroimaging I. Female-specific effect of the BDNF gene on Alzheimer’s Disease. Neurobiol Aging. 2017 doi: 10.1016/j.neurobiolaging.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Li SC, Chicherio C, Nyberg L, von Oertzen T, Nagel IE, Papenberg G, Sander T, Heekeren HR, Lindenberger U, Bäckman L. Ebbinghaus revisited: Influences of the BDNF Val66Met polymorphism on backward serial recall are modulated by human aging. J Cognitive Neurosci. 2010;22(10):2164–73. doi: 10.1162/jocn.2009.21374. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2(2):234–44. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-Z, Tang B-S, Yan X-X, Liu J, Ouyang D-S, Nie L-N, Fan L, Li Z, Ji W, Hu D-L. Association of the DRD2 and DRD3 polymorphisms with response to pramipexole in Parkinson’s disease patients. European journal of clinical pharmacology. 2009;65(7):679–83. doi: 10.1007/s00228-009-0658-z. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature Rev Neurosci. 2005;6(8):603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23(1):151–69. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15(1):94. doi: 10.1186/s12905-015-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Cramer SC. The BDNF Val66Met polymorphism is not related to motor function or short-term cortical plasticity in elderly subjects. Brain Res. 2013;1495:1–10. doi: 10.1016/j.brainres.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Exp Brain Res. 2011;213(4):415–22. doi: 10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC. BDNF Val66Met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20(5):1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Chou KL, Bohnen NI, Müller ML, Seidler RD. Dopaminergic polymorphisms associated with medication responsiveness of gait in Parkinson’s disease. Parkinsonism & Related Disorders. 2017 doi: 10.1016/j.parkreldis.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Pendleton N, Horan M, Payton A. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7(4):411–7. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Muir SW, Gopaul K, Odasso MMM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age and Ageing. 2012;41(3):299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–74. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Backman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci. 2008;2:1. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noohi F, Boyden NB, Kwak Y, Humfleet J, Burke DT, Muller ML, Bohnen NI, Seidler RD. Association of COMT val158met and DRD2 G>T genetic polymorphisms with individual differences in motor learning and performance in female young adults. J Neurophysiol. 2014;111(3):628–40. doi: 10.1152/jn.00457.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noohi F, Boyden NB, Kwak Y, Humfleet J, Muller ML, Bohnen NI, Seidler RD. Interactive effects of age and multi-gene profile on motor learning and sensorimotor adaptation. Neuropsychologia. 2016;84:222–34. doi: 10.1016/j.neuropsychologia.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, Lai S, Okun MS, Vaillancourt DE. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain. 2015;138(8):2322–31. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofstedal M, Weir D, Chen K, Wagner J. Updates to the HRS sample weights. University of Michigan Survey Research Center; Ann Arbor, MI: 2011. [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung W-H, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–91. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Backman L, Nagel IE, Nietfeld W, Schroder J, Bertram L, Heekeren HR, Lindenberger U, Li SC. COMT polymorphism and memory dedifferentiation in old age. Psychol Aging. 2014;29(2):374–83. doi: 10.1037/a0033225. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schröder J, Bertram L, Heekeren HR, Lindenberger U, Li SC. Dopaminergic gene polymorphisms affect long-term forgetting in old age: Further support for the magnification hypothesis. J Cognitive Neurosci. 2013;25(4):571–9. doi: 10.1162/jocn_a_00359. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Becker N, Ferencz B, Naveh-Benjamin M, Laukka EJ, Backman L, Brehmer Y. Dopamine receptor genes modulate associative memory in old age. J Cogn Neurosci. 2016;29(2):245–53. doi: 10.1162/jocn_a_01048. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Lindenberger U, Backman L. Aging-related magnification of genetic effects on cognitive and brain integrity. Trends Cogn Sci. 2015;19(9):506–14. doi: 10.1016/j.tics.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genet. 2006;38(8):904. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Renou J, De Luca V, Zai CC, Bulgin N, Remington G, Meltzer HY, Lieberman JA, Le Foll B, Kennedy JL. Multiple variants of the DRD3, but not BDNF gene, influence age-at-onset of Schizophrenia. Mol Psychiatry. 2007;12:1058–60. doi: 10.1038/sj.mp.4002092. [DOI] [PubMed] [Google Scholar]
- Roos NP, Havens B. Predictors of successful aging: A twelve-year study of Manitoba elderly. Am J Public Health. 1991;81(1):63–8. doi: 10.2105/ajph.81.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban E. Catecholamines in stress: Molecular mechanisms of gene expression. Endocrine Regulations. 2007;41(2–3):61–73. [PubMed] [Google Scholar]
- Sambataro F, Reed JD, Murty VP, Das S, Tan HY, Callicott JH, Weinberger DR, Mattay VS. Catechol-O-methyltransferase valine158methionine polymorphism modulates brain networks underlying working memory across adulthood. Biol Psychiatry. 2009;66(6):540–8. doi: 10.1016/j.biopsych.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick AJ, Krug A, Markov V, Leube D, Michel TM, Zerres K, Eggermann T, Kircher T. Effect of COMT val158met genotype on cognition and personality. Eur Psychiatry. 2008;23(6):385–9. doi: 10.1016/j.eurpsy.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Soeiro-De-Souza MG, Bio DS, David DP, Missio G, Lima B, Fernandes F, Machado-Vieira R, Moreno RA. Gender effects of the COMT Val158Met genotype on verbal fluency in healthy adults. Mol Med Rep. 2013;8(3):837–44. doi: 10.3892/mmr.2013.1564. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22(7):2650–9. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Health and Retirement Study. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant numbers U01AG009740, RC2AG036495, and RC4AG039029) Ann Arbor, MI: [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini - mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ. Biological Basis of Sex Differences in Psychopharmacology. Springer; 2010. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders; pp. 119–40. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The next four decades: The older population in the United States: 2010 to 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- Volkow N, Gur R, Wang G, Fowler J, PJM, Ding Y, RH, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155(3):344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wallace RB, Herzog AR. Overview of the health measures in the Health and Retirement Study. J Hum Resour. 1995:S84–S107. [Google Scholar]
- Warren EJ, Grek A, Conn D, Herrmann N, Icyk E, Kohl J, Silberfeld M. A correlation between cognitive performance and daily functioning in elderly people. Topics in Geriatrics. 1989;2(2):96–100. doi: 10.1177/089198878900200209. [DOI] [PubMed] [Google Scholar]
- Weir D, Faul J, Kardia S, Smith J, Doheny K, Romm J. Quality control report for genotypic data. Health and Retirement Study. 2012 [Google Scholar]
- White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Paillere ML, Nees F, Paus T, Pausova Z, Rietschel M, Robbins T, Smolka MN, Shergill SS, Schumann G Consortium I. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology. 2014;39(11):2560–9. doi: 10.1038/npp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2012;88(5):418–28. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Wong KK, Muller ML, Kuwabara H, Studenski SA, Bohnen NI. Gender differences in nigrostriatal dopaminergic innervation are present at young-to-middle but not at older age in normal adults. J Clin Neurosci. 2012;19(1):183–4. doi: 10.1016/j.jocn.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho S-L, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56(1):31–8. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.