Abstract
Anaplastic lymphoma kinase (ALK) activation has been associated with many types of human cancer. Significant efforts have been devoted to development of ALK inhibitors to antagonize the kinase activity of ALK. Four ALK inhibitors have been approved by the FDA to date for treating patients with ALK-positive non-small cell lung cancers (NSCLC). However, drug resistance has been observed in the majority of patients treated with these inhibitors. New therapeutic strategies (e.g., compounds with novel mechanisms of action) are needed to overcome the drug resistance issue. The emerging PROTAC (Proteolysis Targeting Chimera) technology has been successfully applied to selective degradation of multiple protein targets, but not ALK. Since ALK protein levels are not important for viability in mammals, ALK PROTACs could lead to novel therapeutics with minimal toxicity. Here we report the design, synthesis and biological evaluation of novel PROTACs (degraders) of ALK. MS4077 (5) and MS4078 (6) potently decreased cellular levels of oncogenic active ALK fusion proteins in a concentration- and time-dependent manner in SU-DHL-1 lymphoma and NCI-H2228 lung cancer cells. The ALK protein degradation induced by compounds 5 and 6 was cereblon and proteasome dependent. In addition, compounds 5 and 6 potently inhibited proliferation of SU-DHL-1 cells. Furthermore, compound 6 displayed good plasma exposure in a mouse pharmacokinetic study, thus is suitable for in vivo efficacy studies. We also developed MS4748 (7) and MS4740 (8), very close analogs of 5 and 6 respectively, which are incapable to degrade the ALK fusion proteins, as negative controls. Compounds 5 – 8 are valuable chemical tools for investigating effects of ALK pharmacological degradation. Our study paved the way for developing the next generation of ALK PROTACs.
Keywords: PROTAC, Protein degrader, Anaplastic lymphoma kinase, Lymphoma, Lung cancer
Graphical Abstract

1. Introduction
Anaplastic lymphoma kinase (ALK), a receptor tyrosine kinase, belongs to the insulin receptor kinase subfamily [1]. Oncogenic activation of ALK is associated with the initiation and progression of multiple human cancer types [2, 3], including anaplastic large-cell non-Hodgkin's lymphoma (ALCL) [1], non-small-cell lung cancer (NSCLC) [4], inflammatory myofibroblastic tumour (IMT) [5], diffuse large B cell lymphoma (DLBCL) [6], squamous cell carcinoma (SCC) [7], renal cell carcinoma (RCC) [8], thyroid cancer [9], breast cancer [10], colon carcinoma [10], ovarian cancer [11], and neuroblastoma [12–15], Three different mechanisms have been reported to active the ALK kinase [2]. The most common genetic alterations in this gene are chromosomal translocations, which result in constitutive activation of the ALK kinase. Nearly 30 different ALK fusion proteins have been identified [3]. Two of them, NPM-ALK and EML4-ALK, have been extensively studied in ALCL [1] and NSCLC [4], respectively. The second mechanism for ALK activation is through substitution mutations. Hotspot mutations at residues, F1174 and R1275, at the kinase domain are most commonly observed in patients with neuroblastoma [16]. The third ALK activation mechanism involves gene amplification and overexpression, which has also been reported in different human cancers [17–20]. For example, co-amplification of ALK and MYCN has been reported as the oncogenic driver in neuroblastoma [16, 21].
Significant efforts have been devoted to the development of therapeutics that inhibit the kinase catalytic activity of ALK. To date, four ALK inhibitors have been approved by the FDA for treating patients with ALK-positive NSCLC, including 1 (crizotinib), 2 (ceritinib), 3 (alectinib), and 4 (brigatinib) (Figure 1) [22]. Various clinical trials are ongoing to investigate potential applications of these drugs in the treatment of other diseases linked to ALK alterations [22]. Clinical results have demonstrated that patients with ALK-positive lung cancer showed remarkable responses and increased progression-free survival when treated with ALK inhibitor 1 [23, 24], 2 [25], and 3 [26, 27]. Despite the initial response to such treatments, however, the majority of these patients eventually develop resistance to these drugs within 1 – 2 years [22, 28]. Hence, developing new therapeutic approaches to delay or overcome drug resistance is needed.
Figure 1.
Chemical structures of FDA-approved ALK inhibitors.
Proteolysis-targeted chimeras (PROTACs) are hetero-bifunctional small molecules that are aimed at achieving selective degradation of the target proteins [29–32]. Typically, PROTACs (also known as degraders) include one moiety that binds the protein target of interest, another that binds an E3 ubiquitin ligase, and a linker that connects these two moieties. Simultaneous binding of the target protein and an E3 ubiquitin ligase by a PROTAC brings the target protein close enough to the E3 ligase, enabling ubiquitination and subsequent 26S proteasome-dependent degradation [30]. While traditional enzyme inhibitors only inhibit the catalytic activity of the target enzyme, PROTACs bind and induce degradation of the enzyme, thus eliminating potential scaffolding functions of the protein, in addition to inhibiting the enzymatic activity [29]. The PROTAC technology has been successfully applied to selective degradation of multiple targets, including kinases. Besides a couple of pan kinase PROTACs [33, 34], most of kinase PROTACs reported to date target specific kinases, such as RIPK2 [35], BCR-ABL [36, 37], CDK9 [38, 39], TBK1 [40], FLT3 [33], BTK [33], EGFR [41], HER2 [41], and c-Met [41].
ALK is an ideal target for developing PROTACs. While ALK activation is found in multiple types of human cancer, ALK is predominantly expressed throughout the nervous system during embryogenesis and its expression is very low in normal adult tissues [2, 42]. In addition, ALK is not required for viability in mammals, as mice with ALK deletion are viable with only mild behavior phenotypes, such as antidepressant profile, enhanced performance in novel object-recognition, and enhanced spatial memory [43, 44]. Therefore, pharmacological degradation of ALK is expected to display minimal toxicity in the clinic and could provide a novel, potential therapeutic strategy for patients with ALK-positive cancers.
In this study, we designed and synthesized two ALK PROTACs (degraders), 5 (MS4077) and 6 (MS4078), by linking ceritinib and pomalidomide [45] through two different linkers. Using human ALCL and NSCLC cells, we characterized both compounds in a battery of assays to demonstrate their effects on reducing fused ALK proteins, inhibiting ALK downstream signaling, and inhibiting cancer cell proliferation. In addition, we studied the mechanism of ALK degradation induced by both compounds through a series of rescue assays. We also evaluated compound 6 in a mouse pharmacokinetic (PK) study. Moreover, we developed 7 (MS4748) and 8 (MS4740), close analogs of 5 and 6, respectively, which are incapable of degrading ALK and can serve as negative controls in cellular studies.
2. Results and Discussion
2.1. Designs and Synthesis
We chose ceritinib as the ALK binding moiety, mainly due to its high potency and selectivity for ALK [46]. Analysis of the X-ray crystal structure of ALK in complex with ceritinib (PDB ID: 4MKC) revealed that the piperidinyl group is solvent exposed (Supporting Figure S1) [47]. We therefore hypothesized that an extended linker attached at the piperidinyl nitrogen atom would have minimal effects on binding to ALK. Indeed, the published structure-activity relationship (SAR) studies revealed that a few substituents, including an acetamide group, were well tolerated at this position [46]. Thus, we used the acetamide group as a short bridge to connect ceritinib with the linker portion (Scheme 1). It has been documented that linker type and length are critical for the successful development of effective PROTACs [30]. Therefore, we explored a small set of linkers with various linker length and type. From this study, we identified two promising linkers: one with a long PEG linker, and the other with a short carbon linker (Scheme 1). For the cereblon (CRBN)-DDB1-CUL4-ROC1 E3 ligase (CRL4CRBN) binding moiety, we used pomalidomide since PROTACs based on it have shown good degradation capability for ABL and BCR-ABL [36]. Based on the X-ray crystal structures of DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide, pomalidomide, or lenalidomide, the glutarimide NH group acts as a hydrogen donor and forms an important hydrogen bond with the backbone carbonyl group of CRBN His380 residue [45]. Methylation at the glutarimide nitrogen blocks this key interaction. In fact, methylated pomalidomide and lenalidomide derivatives have been utilized as non-CRBN-binding control compounds [48, 49]. Therefore, the methylated pomalidomide moiety was incorporated into 7 and 8, which were designed as potential negative control compounds (Scheme 1).
Scheme 1. Synthesis of ALK PROTACs and controls 5 – 8.
Reagents and conditions: (a) K2CO3, DMF, rt, overnight; (b) TFA, DCM, rt, 5 h; (c) NMP, DIPEA, microwave, 90 °C, 50 min; (d) TFA, DCM, rt, 30 min; (e) EDCI, HOAt, NMM, DMSO, rt, overnight.
We developed a convergent synthetic route to prepare the designed ALK degraders and their control compounds (Scheme 1). Alkylation of the piperidinyl nitrogen of ceritinib with bromoacetate 9, followed by t-butyl ester deprotection provided the common intermediate 10, which contains the ALK binding moiety. The linker attached pomalidomide analogs 14 and 15 were synthesized using nucleophilic aromatic substitution of the fluoro-substituted thalidomide 11a and its N-methyl analog 11b with mono-Boc protected diamines 12 and 13, followed by BOC deprotection.[48] Amide coupling of 14 or 15 with 10 provided the desired compounds 5 – 8.
2.2. Compounds 5 – 8 bind ALK with high affinity
The binding affinities of the synthesized compounds were assessed by a competitive binding assay using DNA tag labeled ALK, which quantitatively measures the ability of the test compounds to compete with an immobilized, active-site directed ligand [50]. As shown in Figure 2, comparing to the ALK inhibitor ceritinib (Kd = 1.3 ± 0.2 nM), the degrader compounds 5 and 6 showed weaker binding affinity to ALK (5: Kd = 37 ± 4 nM; 6: Kd = 19 ± 3 nM). The control compounds 7 and 8 had slightly reduced binding affinities (7: Kd = 54 ± 5 nM; 8: Kd = 78 ± 9 nM) comparing to the corresponding degraders. Although these ceritinib derivatives showed decreased binding affinities, they are still high affinity ALK binders. Therefore, these compounds were subsequently evaluated in cellular assays to determine their effects on ALK protein degradation.
Figure 2.
ALK binding affinities of ceritinib and compounds 5 – 8. ALK binding affinities were determined using a competitive binding assay in duplicate. The lowest concentration points represent the DMSO control points. Error bars represent ± SEM in duplicated independent experiments.
2.3. Compounds 5 and 6 effectively reduced ALK fusion protein levels and inhibited the ALK downstream signaling in cancer cells
We chose two tumor cell lines, SU-DHL-1 and NCI-H2228, expressing two different ALK fusion proteins to test cellular activity of compounds 5 – 8. SU-DHL-1 is a human ALCL cell line expressing nucleophosmin (NPM)-ALK fusion protein resulting from t(2;5)(p23;q35) translocation [1]. NCI-H2228 is a human NSCLC cell line expressing echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion protein variant 3 resulting from an inversion within chromosome 2p [4]. We confirmed the expression of CRBN in both cell lines (Supporting Figure S2).
We found that compounds 5 and 6 potently reduced the ALK fusion protein levels and inhibited the ALK auto phosphorylation and down-steam STAT3 phosphorylation in both SU-DHL-1 (Figure 3A) and NCI-H2228 cells (Figure 3B) in a concentration-dependent manner. In SU-DHL-1 cells, compounds 5 and 6 reduced the NPM-ALK protein levels with impressive DC50 (50% degradation) values: DC50 = 3 ± 1 nM for compound 5 and DC50 = 11 ± 2 nM for compound 6, after 16-hour treatment. Over 90% reduction of the ALK fusion protein levels were achieved at 100 nM compound concentration. With up to 100 nM degrader concentrations, we did not observe the “hook effect” [41]. We further determined the functional consequence of the ALK-fusion protein degradation by assaying tyrosine 1507 (Y1507) phosphorylation in ALK (Y527 in NPM-ALK) which is located at the carboxyl terminal region of ALK and is a direct docking site for SH2 domain-containing transforming protein (SHC1), and tyrosine 705 (Y705) phosphorylation of signal transducer and activator of transcription 3 (STAT3) which is activated following activation of ALK. Over 90% of inhibition of both ALK Y1507 and STAT3 Y705 phosphorylation was achieved at the 100 nM concentration (Figure 3A). At concentrations above 100 nM, these degraders led to significant cell death. Therefore, higher compound concentrations were not performed in the blots. The control compounds 7 and 8 did not reduce fused ALK protein levels at 30 nM, suggesting that the ALK degradation is mediated by the CRL4CRBN E3 ligase (Figure 3A). Furthermore, these control compounds at 30 nM were over 10-fold less active than their corresponding degraders in the signaling pathway inhibition (Figure 3A). The parental ALK inhibitor ceritinib did not significantly change the ALK protein levels at 30 nM, but were more potent at inhibiting the downstream signaling than the degraders (Figure 3A), presumably due to its higher ALK binding affinity (Figure 2). Similarly, compounds 5 and 6 potently reduced the EML4-ALK protein levels in NCI-H2228 cells with similar DC50 values: DC50 = 34 ± 9 nM for 5 and DC50 = 59 ± 16 nM for 6, after 16-hour treatment (Figure 3B). At the 100 nM concentration, compounds 5 and 6 were able to reduce more than 90% of EML4-ALK protein levels (Figure 3B). In addition, compounds 5 and 6 potently and concentration-dependently inhibited the ALK auto phosphorylation (Figure 3B). As expected, the control compounds 7 and 8, and the parent ALK inhibitor ceritinib did not significantly affect the EML4-ALK protein levels in NCI-H2228 cells at the100 nM compound concentration (Figure 3B).
Figure 3.
Compounds 5 and 6 significantly reduced ALK fusion protein levels and inhibited the ALK down-stream signaling in a concentration-dependent manner. SU-DHL-1 (A) and NCI-H2228 (B) cells were treated with DMSO or serial dilutions of compounds for 16 hours. The ALK fusion protein levels were determined by western blot and normalized against GAPDH (lower panels). Error bars represent ± SD in three independent experiments.
It is unclear at the moment why both a long linker (compound 5) and a short linker (compound 6) can lead to effective degradation of ALK fusion proteins. It is possible that the long linker of compound 5 adapts a turn conformation, which mimics the distance of the short linker in compound 6. Consequently, both linkers can lead to formation of productive ternary complexes, resulting in effective degradation of ALK fusion proteins. It is also possible that compounds 5 and 6 recruit the CRL4CRBN E3 ligase to target different lysine residues of ALK fusion proteins. To shed light on why both linkers can lead to formation of productive ternary complexes and effective protein degradation, we are currently attempting to obtain crystal structures of ternary complexes of ALK–compound 5–CRBN and ALK–compound 6–CRBN, which will be reported in due course.
We next conducted time-course studies to assess the temporal degradation of ALK fusing proteins and inhibition of signaling pathways by compounds 5 and 6 in both SU-DHL-1 (Figure 4A) and NCI-H2228 cells (Figure 4B). In SU-DHL-1 cells, while the p-ALK and p-STAT3 were significantly inhibited after 2-hour treatment, significant amount (>50%) of NPM-ALK degradation was not achieved until 4-hour post compound treatments at the 30 nM concentration. The maximum degradation of NPM-ALK was observed after 16-hour treatment. The NMP-ALK degradation and ALK signaling inhibition by both degraders sustained for at least 24 hours. In NCI-H2228 cells, the degrader induced EML4-ALK degradation and p-ALK and p-STAT3 signaling inhibition occurred at later time-points. After 8-hour treatment, significant NMP-ALK degradation and ALK signaling inhibition were observed at a fixed degrader concentration of 60 nM. Similar to the SU-DHL-1 cells, the maximum degradation of EML4-ALK was observed after 16-hour treatment, and the EML4-ALK degradation and signaling pathway inhibition sustained for at least 24 hours.
Figure 4.
Compounds 5 and 6 time-dependently reduced ALK fusion protein levels and inhibited the ALK down-stream signaling. SU-DHL-1 (A) and NCI-H2228 (B) cells were treated with DMSO or compounds for the indicated length of time.
2.4. Compounds 5- and 6-induced ALK fusion protein degradation is mediated by the CRL4CRBN E3 ligase
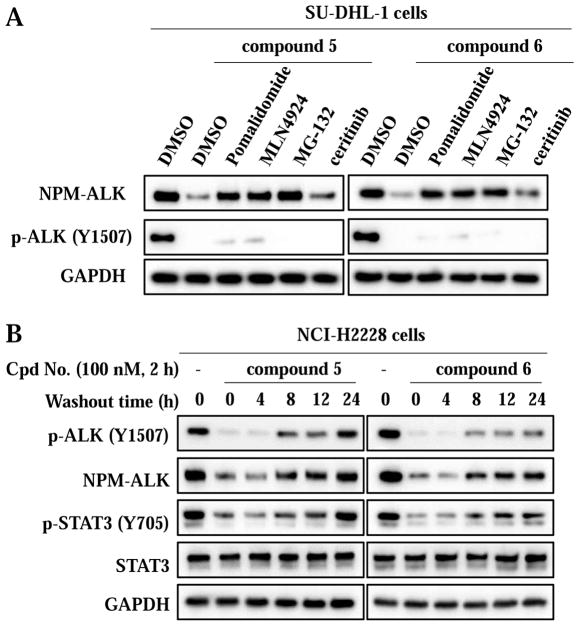
To confirm the mechanism of action of ALK degradation by compounds 5 and 6, we performed a series of rescue experiments using SU-DHL-1 cells (Figure 5A). As expected, pretreatment with an excess amount of the CRBN ligand pomalidomide (10 μM), which competes with the degraders to the same binding site of CRBN, for 2 hours, significantly increased the NPM-AKL protein levels, confirming that intracellular CRBN target engagement of 5 and 6 is required for the NPM-ALK degradation. MLN4924 [51] is an inhibitor of the NEDD8-activating enzyme (NAE), thus blocking cullins neddylation and inactivating cullin RING ligases (CRLs). Pretreatment of cells with MLN4924 (1 μM) for 2 hours also drastically diminished the degradation effect of both 5 and 6. This result has demonstrated that the activated CRL complex is involved in the PROTAC induced NPM-ALK protein degradation. Similarly, pretreatment with a proteasome inhibitor MG-132 [52] (20 μM) for 2 hours significantly rescued the NPM-ALK degradation, indicating the requirement of the proteasome function for the ALK fusion protein degradation. Overall, these results, together with the data from the control compounds 7 and 8 (Figure 3), have demonstrated that compounds 5 and 6 degraded ALK fusion proteins through a CRBN and proteasome dependent mechanism.
Figure 5.
Compounds 5 and 6 induced ALK fusion protein degradation is mediated by hijacking the E3 ubiquitin ligase CRBN. (A) Compounds 5 and 6 induced ALK fusion protein degradation can be rescued. Cells were pre-treated with DMSO, Pomalidomide (10 μM), MLN4924 (1 μM), MG-132 (20 μM) or ceritinib (100 nM) for 2 h, before being treated with the 100 nM compounds for 6 h. (B) Compounds 5 and 6 induced ALK fusion protein degradation is reversible. Cells were treated with DMSO or 100 nM compounds for 2 h, before being washed with PBS and incubated for the indicated length of time in fresh medium.
Interestingly, different from the NPM-ALK protein degradation, pretreatment with pomalidomide, MLN4924, or MG-132 only slightly rescued the ALK phosphorylation (Figure 5A), suggesting that the downstream signaling inhibition by 5 and 6 may be the result of both ALK kinase activity inhibition and ALK protein degradation.
We also attempted to rescue the NPM-ALK protein degradation with 100 nM of the parental ALK inhibitor ceritinib (Figure 5A). Pretreatment with ceritinib for 2 hours only slightly rescued the NPM-ALK protein degradation. Although higher concentrations of ceritinib could be more effective at rescuing the NPM-ALK protein degradation, ceritinib at concentrations above 100 nM were toxic to SU-DHL-1 cells.
We also found that the effect of compounds 5 and 6 on NPM-ALK protein degradation and inhibition of ALK downstream signaling started to diminish at 8 hours after removal of the degraders (Figure 5B). This result confirmed the reversible nature of the PROTAC-mediated NPM-ALK protein degradation and signaling pathway inhibition [35]. Compared with compound 5, compound 6 has slightly prolonged effect in the down-regulation of NMP-ALK and inhibition of the down-stream signaling (Figure 5B), suggesting that 6 may be more potent in inhibiting cell proliferation.
2.5. Compounds 5 and 6 inhibited proliferation of cancer cells
We next evaluated effects of compounds 5 and 6 on cell growth using SU-DHL-1 (Figure 6) and NCI-H2228 cells (Supporting Figure S3). Both 5 and 6 concentration-dependently inhibited proliferation of SU-DHL-1 cells with similar potencies (IC50 = 46 ± 4 nM and 33 ± 1 nM, respectively). On the other hand, the control compounds 7 and 8 were much less active at inhibiting cell growth (incomplete inhibition (Figure 6)), which is consistent to their weaker potencies in the ALK down-stream signaling inhibition (Figure 3). Interestingly, compounds 5 and 6 were only 3- and 2- fold less potent than ceritnib (IC50 = 15 ± 1 nM) at inhibiting cell growth, even though the binding affinities of both compounds were much lower than that of ceritnib (Figure 2). In comparison with SU-DHL-1 cells, the proliferation of NCI-H2228 cells was less sensitive to degraders 5 and 6, control compounds 7 and 8, and ceritnib (Supporting Figure S3).
Figure 6.
Compounds 5 and 6 inhibited proliferation of SU-DHL-1 cells. Cells were seeded in 96-well plates at a density of 5000 cells per well, in triplicate. The cells were treated with DMSO or indicated serial dilutions of compounds for 3 days. Cell growth was measured using the CellTiter-Glo luminescent cell viability assay. Data were analyzed using the GraphPad Prism. Error bars represent ± SD in three independent experiments.
The basis for the differential sensitivities of ALK-positive cells to ALK degraders or inhibitors is unclear at the moment. This could be caused by the differences in the nature of the fusion proteins (e.g., NPM versus EML4), in the subcellular localizations (predominant nuclear localization of NPM [1] versus co-localized with microtubules in the cytoplasm of EML4 [53]), in the expression levels of ALK fusion proteins and/or CRBN protein (Supporting Figure S2), or in the dependency on activated ALK. These cellular proliferation results suggest that different cell types may have different sensitivity to the novel degraders. A wide range of cell lines will be tested to probe potential applications of these PROTACs.
2.6. Compound 6 is bioavailable in mice
Finally, we assessed in vivo pharmacokinetic (PK) properties of compound 6 in mice. Following a single intraperitoneal (IP) injection of compound 6, good plasma exposure of the compound was observed over 12 h (Figure 7). The highest plasma concentration (3,000 nM) was achieved at 2 h post dosing and the plasma level of 340 nM was achieved at 12 h post dosing, which is about 10-fold higher than the IC50 value of compound 6 (anti-proliferative activity in SU-DHL-1 cells). Furthermore, compound 6 at 50 mg/kg was well tolerated by the test mice and no clinical signs were observed. Taken together, these results suggest that compound 6 is a valuable chemical tool for investigating effects of pharmacological degradation of ALK in vivo.
Figure 7.
Plasma concentrations of compound 6 following a single 50 mg/kg IP injection over 12 hours in male Swiss Albino mice.
3. Conclusions
We discovered compounds 5 and 6 as novel, potent and cell-active ALK PROTACs. Compounds 5 and 6 displayed very similar potencies in cellular assays even though they have two different types of linkers. In a competitive binding assay, compounds 5 and 6 exhibited high affinity for ALK with Kd values of 37 ± 4 nM and 19 ± 3 nM, respectively. In cellular assays, compounds 5 and 6 were able to degrade two different ALK fusion proteins and inhibit phosphorylation of ALK and STAT3 in a concentration- and time-dependent manner in both SU-DHL-1 cells (5: DC50 = 3 ± 1 nM; 6: DC50 = 11 ± 2 nM) and NCI-H2228 cells (5: DC50 = 34 ± 9 nM; 6: DC50 = 59 ± 16 nM). To study the ALK fusion protein degradation mechanisms, we designed and synthesized compounds 7 and 8 as negative controls and performed a series of rescue assays. These experiments have demonstrated that the ALK fusion protein degradation induced by compounds 5 and 6 is CRBN and proteasome dependent. Our washout experiments revealed that the PROTAC-mediated ALK fusion protein degradation is reversible. Finally, compounds 5 and 6 potently inhibited proliferation of SU-DHL-1 cells (5: IC50 = 46 ± 4 nM; 6: IC50 = 33 ± 1 nM). Taken together, these results suggest that compounds 5 and 6 along with control compounds 7 and 8 are valuable tools for the research community to study cellular effects of the PROTAC-induced ALK fusion protein degradation. Furthermore, compound 6 displayed good plasma exposure in mice. Thus, this compound can be a useful chemical tool for in vivo efficacy studies. Our study laid the foundation for developing the next generation of ALK PROTACs.
4. Experimental section
4.1. Synthesis of compounds 5 – 8
HPLC spectra for all compounds were acquired using an Agilent 1200 Series system with DAD detector. Chromatography was performed on a 2.1×150 mm Zorbax 300SB-C18 5 μm column with water containing 0.1% formic acid as solvent A and acetonitrile containing 0.1% formic acid as solvent B at a flow rate of 0.4 mL/min. The gradient program was as follows: 1% B (0–1 min), 1–99% B (1–4 min), and 99% B (4–8 min). High-resolution mass spectra (HRMS) data were acquired in positive ion mode using an Agilent G1969A API-TOF with an electrospray ionization (ESI) source. Nuclear Magnetic Resonance (NMR) spectra were acquired on a Bruker DRX-600 spectrometer with 600 MHz for proton (1H NMR) and 150 MHz for carbon (13C NMR); chemical shifts are reported in ppm (δ). Preparative HPLC was performed on Agilent Prep 1200 series with UV detector set to 254 nm. Samples were injected onto a Phenomenex Luna 250 x 30 mm, 5 μm, C18 column at room temperature. The flow rate was 40 mL/min. A linear gradient was used with 10% (or 50%) of MeOH (A) in H2O (with 0.1 % TFA) (B) to 100% of MeOH (A). HPLC was used to establish the purity of target compounds. All final compounds had > 95% purity using the HPLC methods described above.
2-(4-(4-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2-methylphenyl)piperidin-1-yl)acetic acid (10)
To a solution of ceritinib (500 mg, 0.896 mmol) and potassium carbonate (247 mg, 1.79 mmol) in DMF (5 mL) was added tert-butyl 2-bromoacetate (0.17 mL, 1.16 mmol). After being stirred at room temperature overnight, the reaction was quenched with water (15 mL) and extracted with dichloromethane (3 x 20 mL). The combined organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced vacuum to give the Boc protected product as sticky oil. The resulting residue was dissolved in dichloromethane (5 mL) and trifluoroacetic acid (5 mL). After stirred at room temperature for 5 h, the solvents were removed under reduced pressure. The mixture was purified by reverse phase ISCO to give the title compound as gray solid (414 mg, 75% yield over two steps). 1H NMR (600 MHz, CD3OD) δ 8.32 (d, J = 8.4 Hz, 1H), 8.21 (s, 1H), 7.98 (dd, J = 7.8, 1.8 Hz, 1H), 7.71 (t, J = 7.8 Hz, 1H), 7.50 – 7.47 (m, 2H), 6.89 (s, 1H), 4.67 – 4.61 (m, 1H), 4.12 (s, 2H), 3.78 (t, J = 9.6 Hz, 2H), 3.39 – 3.34 (m, 1H), 3.27 (t, J = 9.6 Hz, 2H), 3.14 – 3.09 (m, 1H), 2.18 (s, 3H), 2.11 – 2.04 (m, 4H), 1.31 (d, J = 6.0 Hz, 6H), 1.25 (d, J = 7.2 Hz, 6H); 13C NMR (150 MHz, CD3OD) δ 166.6, 160.2, 157.4, 153.8, 147.9, 146.0, 139.3, 136.5, 134.7, 131.2, 127.6, 127.5, 125.6, 117.0, 115.1, 111.2, 105.5, 71.3, 55.9, 55.4, 53.8, 34.9, 29.4, 20.9, 17.4, 14.0; HRMS calcd for C30H39ClN5O5S [M + H+] 616.2355, found 616.2354.
4-((17-Amino-3,6,9,12,15-pentaoxaheptadecyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (14a)
A mixture of 2-(2,6-dioxopiperidin-3-yl)-4-fluoroisoindoline-1,3-dione 11a (28 mg, 0.1 mmol), tert-butyl (17-amino-3,6,9,12,15-pentaoxaheptadecyl)carbamate 12 (38 mg, 0.1 mmol) and DIPEA (0.1 mL, 0.6 mmol) in NMP (1 mL) was heated by microwave irradiation at 90 °C for 50 min. The reaction mixture was purified by preparative HPLC to give the intermediate. The resulting intermediate was dissolved in dichloromethane (1 mL) and trifluoroacetic acid (1 mL). After being stirred at room temperature for 1 h, the solvents were removed under reduced pressure. The mixture was purified by reverse phase ISCO to give 14a as yellow oil in TFA salt form (27 mg, 49% yield over two steps). 1H NMR (600 MHz, CD3OD) δ 7.55 (dd, J = 9.0, 6.6 Hz, 1H), 7.08 (d, J = 9.0 Hz, 1H), 7.04 (d, J = 6.6 Hz, 1H), 5.05 (dd, J = 12.6, 5.4 Hz, 1H), 3.73 (t, J = 5.4 Hz, 2H), 3.70 (t, J = 5.4 Hz, 2H), 3.67 – 3.61 (m, 16H), 3.50 (t, J = 5.4 Hz, 2H), 3.12 (t, J = 5.4 Hz, 2H), 2.90 – 2.84 (m, 1H), 2.77 – 2.66 (m, 2H), 2.13 – 2.11 (m, 1H); 13C NMR (150 MHz, DMSO-d6) δ 173.3, 170.5, 169.3, 167.7, 146.8, 136.7, 132.5, 117.8, 111.1, 109.6, 70.2, 70.0, 69.3, 67.0, 49.0, 42.1, 38.9, 31.4, 22.5; HRMS calcd for C25H37N4O9 [M + H+] 537.2555, found 537.2559.
4-((17-Amino-3,6,9,12,15-pentaoxaheptadecyl)amino)-2-(1-methyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (14b)
The title compound (42% yield over two steps) was prepared according to the synthetic procedures for 14a. 1H NMR (600 MHz, CD3OD) δ 7.57 (dd, J = 9.0, 7.2 Hz, 1H), 7.10 (d, J = 9.0 Hz, 1H), 7.07 (d, J = 7.2 Hz, 1H), 5.08 (dd, J = 12.6, 5.4 Hz, 1H), 3.74 (t, J = 5.4 Hz, 2H), 3.69–3.60 (m, 18H), 3.52 (t, J = 5.4 Hz, 2H), 3.14 (s, 3H), 3.09 (t, J = 5.4 Hz, 2H), 2.90 – 2.87 (m, 2H), 2.72 – 2.65 (m, 1H), 2.11 – 2.07 (m, 1H); MS (ESI) m/z 551.2 [M+H]+.
4-((2-Aminoethyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (15a)
The title compound (66% yield over two steps) was prepared according to the synthetic procedures for 14a. 1H NMR (600 MHz, CD3OD) δ 7.62 (t, J = 7.8 Hz, 1H), 7.15 (t, J = 7.8 Hz, 2H), 5.08 (dd, J = 12.6, 5.4 Hz, 1H), 3.67 (t, J = 6.0 Hz, 2H), 3.19 (t, J = 6.0 Hz, 2H), 2.89 – 2.83 (m, 1H), 2.77 – 2.69 (m, 2H), 2.12 – 2.09 (m, 1H); 13C NMR (150 MHz, DMSO-d6) δ 173.3, 170.5, 169.0, 167.7, 146.2, 136.7, 132.7, 117.5, 111.4, 110.5, 48.9, 40.3, 38.2, 31.4, 22.6; HRMS calcd for C15H17N4O4 [M + H+] 317.1244, found 317.1240.
4-((2-Aminoethyl)amino)-2-(1-methyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (15b)
The title compound (81% yield over two steps) was prepared according to the synthetic procedures for 14a. 1H NMR (600 MHz, CD3OD) δ 7.63 (t, J = 7.8 Hz, 1H), 7.15 (t, J = 7.8 Hz, 2H), 5.10 (dd, J = 12.6, 5.4 Hz, 1H), 3.67 (t, J = 6.0 Hz, 2H), 3.19 (t, J = 6.0 Hz, 2H), 3.14 (s, 3H), 2.90 – 2.87 (m, 2H), 2.73 – 2.66 (m, 1H), 2.10 – 2.07 (m, 1H); 13C NMR (150 MHz, DMSO-d6) δ 172.2, 170.2, 169.0, 167.6, 146.2, 136.8, 132.7, 117.6, 111.5, 110.4, 49.6, 40.3, 38.2, 31.5, 23.6, 21.8; HRMS calcd for C16H19N4O4 [M + H+] 331.1401, found 331.1398.
2-(4-(4-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2-methylphenyl)piperidin-1-yl)-N-(17-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-3,6,9,12,15-pentaoxaheptadecyl)acetamide (5, MS4077)
To a solution of 10 (12 mg, 0.02 mmol) and 14a (13 mg, 0.02 mmol) in DMSO (1.0 mL) were added NMM (11 μL, 0.10 mmol), EDCI (6 mg, 0.03 mmol) and HOAt (4 mg, 0.03 mmol). The reaction mixture was stirred at room temperature overnight and purified by preparative HPLC to give the desired product MS4077 as yellow solid (13 mg, 59% yield). 1H NMR (600 MHz, CD3OD) δ 8.36 (d, J = 7.8 Hz, 1H), 8.20 (s, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.69 (t, J = 7.8 Hz, 1H), 7.63 (s, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 8.4 Hz, 1H), 7.04 (d, J = 7.2 Hz, 1H), 6.85 (s, 1H), 5.05 (dd, J = 12.6, 5.4 Hz, 1H), 4.62– 4.58 (m, 1H), 3.96 (s, 2H), 3.71– 3.62 (m, 20H), 3.58 (t, J = 5.4 Hz, 2H), 3.48 – 3.45 (m, 4H), 3.37 – 3.33 (m, 1H), 3.24 (t, J = 12.0 Hz, 2H), 3.09 – 3.05 (m, 1H), 2.88 – 2.82 (m, 1H), 2.75 – 2.66 (m, 2H), 2.14 (s, 3H), 2.11 – 1.97 (m, 5H), 1.32 (d, J = 6.0 Hz, 6H), 1.24 (d, J = 7.2 Hz, 6H); 13C NMR (150 MHz, CD3OD) δ 173.2, 170.2, 169.3, 167.8, 163.9, 157.3, 155.7, 153.9, 146.7, 145.8, 137.9, 136.0, 135.9, 134.7, 132.4, 131.0, 127.6, 127.3, 125.8, 124.4, 123.8, 122.3, 116.9, 110.9, 110.7, 109.8, 105.2, 71.6, 70.2, 70.1, 70.0, 69.8, 69.1, 68.7, 57.3, 55.4, 54.0, 48.8, 41.8, 39.1, 34.8, 30.8, 29.6, 22.4, 21.0, 17.7, 14.1; HRMS calcd for C55H73ClN9O13S [M + H+] 1134.4732, found 1134.4739.
2-(4-(4-((5-Chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2-methylphenyl)piperidin-1-yl)-N-(17-((2-(1-methyl-2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-3,6,9,12,15-pentaoxaheptadecyl)acetamide (7, MS4748)
The title compound (60% yield) was prepared according to the synthetic procedure for MS4077. 1H NMR (600 MHz, CD3OD) δ 8.38 (d, J = 8.4 Hz, 1H), 8.19 (s, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.69 (t, J = 7.8 Hz, 1H), 7.67 (s, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 7.04 (d, J = 7.2 Hz, 1H), 6.85 (s, 1H), 5.06 (dd, J = 13.2, 5.4 Hz, 1H), 4.62 – 4.58 (m, 1H), 3.96 (s, 2H), 3.72 – 3.61 (m, 20H), 3.58 (t, J = 5.4 Hz, 2H), 3.48 – 3.44 (m, 4H), 3.37 – 3.30 (m, 1H), 3.24 (t, J = 11.4 Hz, 2H), 3.13 (s, 3H), 3.10 – 3.06 (m, 1H), 2.88 – 2.84 (m, 2H), 2.70 – 2.63 (m, 1H), 2.15 (s, 3H), 2.08 – 1.98 (m, 5H), 1.32 (d, J = 6.0 Hz, 6H), 1.24 (d, J = 7.2 Hz, 6H); 13C NMR (150 MHz, CD3OD) δ 172.2, 170.0, 169.3, 167.8, 163.9, 161.0, 156.5, 155.5, 150.2, 146.7, 137.6, 137.2, 135.8, 134.7, 132.4, 131.2, 127.3, 126.5, 126.2, 124.7, 124.0, 123.1, 116.9, 110.8, 110.7, 109.8, 105.3, 71.4, 70.2, 70.1, 70.0, 69.8, 69.1, 68.7, 57.2, 55.4, 53.8, 49.4, 41.8, 39.1, 34.8, 31.1, 29.5, 26.0, 21.6, 20.9, 17.6, 14.0; HRMS calcd for C56H75ClN9O13S [M + H+] 1148.4888, found 1148.4880.
2-(4-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2-methylphenyl)piperidin-1-yl)-N-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)ethyl)acetamide (6, MS4078)
The title compound (80% yield) was prepared according to the synthetic procedure for MS4077. 1H NMR (600 MHz, CD3OD) δ 8.38 (d, J = 7.8 Hz, 1H), 8.19 (s, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.70 (t, J = 7.2 Hz, 1H), 7.67 (s, 1H), 7.58 (t, J = 7.8 Hz, 1H), 7.43 (t, J = 7.8 Hz, 1H), 7.16 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 7.2 Hz, 1H), 6.85 (s, 1H), 5.03 (dd, J = 12.6, 5.4 Hz, 1H), 4.62 – 4.59 (m, 1H), 3.91 (s, 2H), 3.65 (d, J = 10.2 Hz, 2H), 3.59 – 3.54 (m, 4H), 3.37 – 3.33 (m, 1H), 3.19 (t, J = 12.0 Hz, 2H), 3.09 – 3.05 (m, 1H), 2.84 – 2.78 (m, 1H), 2.71 – 2.65 (m, 2H), 2.16 (s, 3H), 2.07 – 1.96 (m, 5H), 1.33 (d, J = 6.0 Hz, 6H), 1.24 (d, J = 7.2 Hz, 6H). 13C NMR (150 MHz, CD3OD) δ 173.0, 170.1, 169.3, 167.6, 164.5, 157.3, 155.7, 154.0, 146.6, 145.8, 137.9, 136.0, 135.9, 134.6, 132.5, 131.0, 127.6, 127.3, 125.7, 124.3, 123.8, 122.3, 116.7, 110.87, 110.85, 110.1, 105.1, 71.6, 57.2, 55.3, 53.8, 48.7, 41.3, 38.2, 34.7, 30.7, 29.5, 22.3, 21.0, 17.7, 14.0. HRMS calcd for C45H53ClN9O8S [M + H+] 914.3421, found 914.3424.
2-(4-(4-((5-chloro-4-((2-(isopropylsulfonyl)phenyl)amino)pyrimidin-2-yl)amino)-5-isopropoxy-2-methylphenyl)piperidin-1-yl)-N-(2-((2-(1-methyl-2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)ethyl)acetamide (8, MS4740)
The title compound (85% yield) was prepared according to the synthetic procedure for MS4077. 1 H NMR (600 MHz, CD3OD) δ 8.37 (d, J = 8.4 Hz, 1H), 8.20 (s, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.70 (t, J = 7.2 Hz, 1H), 7.64 (s, 1H), 7.58 (t, J = 7.8 Hz, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.15 (d, J = 9.0 Hz, 1H), 7.08 (d, J = 6.6 Hz, 1H), 6.85 (s, 1H), 5.04 (dd, J = 12.6, 5.4 Hz, 1H), 4.62 – 4.58 (m, 1H), 3.92 (s, 2H), 3.64 (d, J = 10.2 Hz, 2H), 3.59 – 3.51 (m, 4H), 3.37 – 3.31 (m, 1H), 3.19 (t, J = 12.0 Hz, 2H), 3.09 – 3.05 (m, 1H), 3.10 (s, 3H), 2.83 – 2.79 (m, 2H), 2.69 – 2.61 (m, 1H), 2.15 (s, 3H), 2.07 – 1.97 (m, 5H), 1.32 (d, J = 6.0 Hz, 6H), 1.24 (d, J = 7.2 Hz, 6H); 13C NMR (150 MHz, CD3OD) δ 172.0, 169.9, 169.2, 167.7, 164.5, 160.8, 156.7, 154.9, 147.1, 146.6, 138.1, 137.0, 135.9, 134.7, 132.5, 131.2, 127.4, 126.9, 125.7, 125.1, 124.5, 124.0, 116.6, 111.0, 110.8, 110.0, 105.4, 71.4, 57.2, 55.4, 53.6, 49.4, 41.5, 38.2, 34.8, 31.0, 29.5, 25.7, 21.5, 20.9, 17.6, 13.9; HRMS calcd for C46H55ClN9O8S [M + H+] 928.3577, found 928.3584.
4.2. ALK binding affinity assays
ALK binding affinities were determined with KINOMEscan aasay by DiscoverX company. KINOMEscan™ is based on a competition binding assay that quantitatively measures the ability of a compound to compete with an immobilized, active-site directed ligand. The assay is performed by combining three components: DNA-tagged ALK; immobilized ligand; and a test compound. The ability of the test compound to compete with the immobilized ligand is measured via quantitative PCR of the DNA tag. Kds were determined using an 11-point 3-fold compound dilution series (top concentration = 3,000 nM in this case) with three DMSO control points in duplicates. Some outlier data points were subtracted. Cell Culture: SU-DHL-1 and NCI-H2228 cells were purchased from the American Type Culture Collection. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 μg/mL penicillin and streptomycin. Cells were cultured in a 37 °C incubator with 5% CO2.
4.3. Cell culture
SU-DHL-1 and NCI-H2228 cells were purchased from the American Type Culture Collection. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 50 μg/mL penicillin and streptomycin. Cells were cultured in a 37 °C incubator with 5% CO2.
4.3. Western blot assay
Cells were washed with cold PBS once and lysed in 1x Laemmli loading buffer directly. Lysates were heated at 99 °C for 10 min, and resolved on 4–15% precast SDS-PAGE (BIO-RAD) and transferred onto PVDF membrane. Membrane was blocked in 5% milk in Tris-buffered saline and Tween 20 (TBST) for 1 h at room temperature, followed by incubation with a primary antibody overnight at 4 °C, and a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. The membrane was imaged by a ChemiDoc MP Imaging system (BIO-RAD). Antibodies against GAPDH (abcam), Phospho-ALK (Tyr1507), ALK, STAT3 and Phospho-sTAT3 (TyrY705) (Cell Signaling Technology) were purchased commercially. 4.4. Cell viability assay Cells were seeded in 96-well plates at a density of 5000 cells per well in triplicate. Cells were treated with DMSO or indicated serial dilutions of compounds for 3 days. Cell growth/survival was measured by using the CellTiter-Glo luminescent cell viability assay following the manufacturer’s instructions (Promega). Data was analyzed using GraphPad Prism. Error bars represent ± SD for triplicate experiments.
4.5. Statistical analysis
Data were analyzed using GraphPad Prism. DC50 values (50% of degradation) were calculated using the nonlinear regression-“log(inhibitor) vs. response” analytical protocol. IC50 values (50% of cell growth inhibition) were calculated using the nonlinear regression-“ log(inhibitor) vs. response -- Variable slope” analytical protocol. All DC50 and IC50 value shown represent the results obtained from triplicated independent experiments with standard errors of the mean (MEAN ± SEM).
4.6. Mouse pharmacokinetic (PK) studies
Standard PK studies were conducted using male Swiss Albino mice by Sai Life Sciences. A single 50 mg/kg intraperitoneal (IP) injection of compound 6 was evaluated. Plasma concentrations of compound 6 reported at each of the 6 time points (30 min, 1 h, 2 h, 4 h, 8 h, and 12 h post dosing) are the average values from 3 test animals. Error bars represent + SD.
Supplementary Material
We discovered novel ALK PROTACs (MS4077 and MS4078) and their close analogs as negative controls.
MS4077 and MS4078 potently reduced cellular ALK protein levels in a cereblon and proteasome dependent manner.
MS4077 and MS4078 potently inhibited cellular ALK signaling.
MS4077 and MS4078 effectively inhibited cancer cell proliferation.
MS4078 displayed good plasma exposure in mice, thus is suitable for in vivo efficacy studies.
Acknowledgments
X-R.H. and Y.X. acknowledge the support by the grant R01GM067113 from the U. S. National Institutes of Health.
Appendix A. Supplementary data
Supplementary data related to this article can be found at 1H and 13C NMR spectra of 5 and 6; X-ray crystal structure analysis of ALK-ceritinib complex; ALK fusion protein levels and CRBN protein levels in cells; Anti-proliferation effect of compounds 5 and 6 in NCI-H2228 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- 3.Holla VR, Elamin YY, Bailey AM, Johnson AM, Litzenburger BC, Khotskaya YB, Sanchez NS, Zeng J, Shufean MA, Shaw KR, Mendelsohn J, Mills GB, Meric-Bernstam F, Simon GR. ALK: a tyrosine kinase target for cancer therapy. Cold Spring Harb Mol Case Stud. 2017;3:a001115. doi: 10.1101/mcs.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 5.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 6.De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, Poppe B, Laureys G, Brons P, Vandenberghe P, Speleman F, Praet M, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638–2641. doi: 10.1182/blood-2003-04-1050. [DOI] [PubMed] [Google Scholar]
- 7.Du XL, Hu H, Lin DC, Xia SH, Shen XM, Zhang Y, Luo ML, Feng YB, Cai Y, Xu X, Han YL, Zhan QM, Wang MR. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J Mol Med (Berl) 2007;85:863–875. doi: 10.1007/s00109-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 8.Debelenko LV, Raimondi SC, Daw N, Shivakumar BR, Huang D, Nelson M, Bridge JA. Renal cell carcinoma with novel VCL-ALK fusion: new representative of ALK-associated tumor spectrum. Mod Pathol. 2011;24:430–442. doi: 10.1038/modpathol.2010.213. [DOI] [PubMed] [Google Scholar]
- 9.Kelly LM, Barila G, Liu PY, Evdokimova VN, Trivedi S, Panebianco F, Gandhi M, Carty SE, Hodak SP, Luo JH, Dacic S, Yu YP, Nikiforova MN, Ferris RL, Altschuler DL, Nikiforov YE. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:4233–4238. doi: 10.1073/pnas.1321937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin E, Li L, Guan Y, Soriano R, Rivers CS, Mohan S, Pandita A, Tang J, Modrusan Z. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009;7:1466–1476. doi: 10.1158/1541-7786.MCR-08-0522. [DOI] [PubMed] [Google Scholar]
- 11.Ren H, Tan ZP, Zhu X, Crosby K, Haack H, Ren JM, Beausoleil S, Moritz A, Innocenti G, Rush J, Zhang Y, Zhou XM, Gu TL, Yang YF, Comb MJ. Identification of anaplastic lymphoma kinase as a potential therapeutic target in ovarian cancer. Cancer Res. 2012;72:3312–3323. doi: 10.1158/0008-5472.CAN-11-3931. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 13.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, Xue L, Zozulya S, Gregor VE, Webb TR, Gray NS, Gilliland DG, Diller L, Greulich H, Morris SW, Meyerson M, Look AT. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A, Schleiermacher G, Pierron G, Valteau-Couanet D, Frebourg T, Michon J, Lyonnet S, Amiel J, Delattre O. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 15.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou CP, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–U922. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM, Lakeman A, Vandesompele J, Hoebeeck J, Van Maerken T, De Paepe A, Laureys G, Schulte JH, Schramm A, Van Den Broecke C, Vermeulen J, Van Roy N, Beiske K, Renard M, Noguera R, Delattre O, Janoueix-Lerosey I, Kogner P, Martinsson T, Nakagawara A, Ohira M, Caron H, Eggert A, Cools J, Versteeg R, Speleman F. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [DOI] [PubMed] [Google Scholar]
- 17.Dirks WG, Fahnrich S, Lis Y, Becker E, MacLeod RA, Drexler HG. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int J Cancer. 2002;100:49–56. doi: 10.1002/ijc.10435. [DOI] [PubMed] [Google Scholar]
- 18.Lamant L, Pulford K, Bischof D, Morris SW, Mason DY, Delsol G, Mariame B. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passoni L, Longo L, Collini P, Coluccia AM, Bozzi F, Podda M, Gregorio A, Gambini C, Garaventa A, Pistoia V, Del Grosso F, Tonini GP, Cheng M, Gambacorti-Passerini C, Anichini A, Fossati-Bellani F, Di Nicola M, Luksch R. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69:7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 20.Salido M, Pijuan L, Martinez-Aviles L, Galvan AB, Canadas I, Rovira A, Zanui M, Martinez A, Longaron R, Sole F, Serrano S, Bellosillo B, Wynes MW, Albanell J, Hirsch FR, Arriola E. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol. 2011;6:21–27. doi: 10.1097/JTO.0b013e3181fb7cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janoueix-Lerosey I, Schleiermacher G, Michels E, Mosseri V, Ribeiro A, Lequin D, Vermeulen J, Couturier J, Peuchmaur M, Valent A, Plantaz D, Rubie H, Valteau-Couanet D, Thomas C, Combaret V, Rousseau R, Eggert A, Michon J, Speleman F, Delattre O. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27:1026–1033. doi: 10.1200/JCO.2008.16.0630. [DOI] [PubMed] [Google Scholar]
- 22.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 24.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:2537–2539. doi: 10.1056/NEJMc1404894. [DOI] [PubMed] [Google Scholar]
- 26.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Perol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Wakihara T, Oshima K, Nishioka D, Hotta Y, Elangovan SP, Yanaba Y, Yoshikawa T, Chaikittisilp W, Matsuo T, Takewaki T, Okubo T. Widening synthesis bottlenecks: realization of ultrafast and continuous-flow synthesis of high-silica zeolite SSZ-13 for NOx removal. Angew Chem Int Ed Engl. 2015;54:5683–5687. doi: 10.1002/anie.201501160. [DOI] [PubMed] [Google Scholar]
- 28.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, Yatabe Y, Takeuchi K, Hamada T, Haruta H, Ishikawa Y, Kimura H, Mitsudomi T, Tanio Y, Mano H. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 29.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadd MS, Testa A, Lucas X, Chan KH, Chen W, Lamont DJ, Zengerle M, Ciulli A. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toure M, Crews CM. Small-molecule PROTACS: new approaches to protein degradation. Angew Chem Int Ed Engl. 2016;55:1966–1973. doi: 10.1002/anie.201507978. [DOI] [PubMed] [Google Scholar]
- 32.Ohoka N, Shibata N, Hattori T, Naito M. Protein knockdown technology: application of ubiquitin ligase to cancer therapy. Curr Cancer Drug Targets. 2016;16:136–146. doi: 10.2174/1568009616666151112122502. [DOI] [PubMed] [Google Scholar]
- 33.Huang HT, Dobrovolsky D, Paulk J, Yang G, Weisberg EL, Doctor ZM, Buckley DL, Cho JH, Ko E, Jang J, Shi K, Choi HG, Griffin JD, Li Y, Treon SP, Fischer ES, Bradner JE, Tan L, Gray NS. A chemoproteomic approach to query the degradable kinome using a multi-kinase degrader. Cell Chem Biol. 2017 doi: 10.1016/j.chembiol.2017.1010.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S, Wang J, Hamman BD, Ishchenko A, Crews CM. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem Biol. 2017 doi: 10.1016/j.chembiol.2017.1009.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondeson DP, Mares A, Smith IE, Ko E, Campos S, Miah AH, Mulholland KE, Routly N, Buckley DL, Gustafson JL, Zinn N, Grandi P, Shimamura S, Bergamini G, Faelth-Savitski M, Bantscheff M, Cox C, Gordon DA, Willard RR, Flanagan JJ, Casillas LN, Votta BJ, den Besten W, Famm K, Kruidenier L, Carter PS, Harling JD, Churcher I, Crews CM. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11:611–617. doi: 10.1038/nchembio.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai AC, Toure M, Hellerschmied D, Salami J, Jaime-Figueroa S, Ko E, Hines J, Crews CM. Modular PROTAC design for the degradation of oncogenic BCR-ABL. Angew Chem Int Ed Engl. 2016;55:807–810. doi: 10.1002/anie.201507634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demizu Y, Shibata N, Hattori T, Ohoka N, Motoi H, Misawa T, Shoda T, Naito M, Kurihara M. Development of BCR-ABL degradation inducers via the conjugation of an imatinib derivative and a cIAP1 ligand. Bioorg Med Chem Lett. 2016;26:4865–4869. doi: 10.1016/j.bmcl.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 38.Robb CM, Contreras JI, Kour S, Taylor MA, Abid M, Sonawane YA, Zahid M, Murry DJ, Natarajan A, Rana S. Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC) Chem Commun (Camb) 2017;53:7577–7580. doi: 10.1039/c7cc03879h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson CM, Jiang B, Erb MA, Liang Y, Doctor ZM, Zhang Z, Zhang T, Kwiatkowski N, Boukhali M, Green JL, Haas W, Nomanbhoy T, Fischer ES, Young RA, Bradner JE, Winter GE, Gray NS. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2017 doi: 10.1038/nchembio.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crew AP, Raina K, Dong H, Qian Y, Wang J, Vigil D, Serebrenik YV, Hamman BD, Morgan A, Ferraro C, Siu K, Neklesa TK, Winkler JD, Coleman KG, Crews CM. Identification and characterization of von hippel-lindau-recruiting proteolysis targeting chimeras (PROTACs) of TANK-binding kinase 1. J Med Chem. 2017 doi: 10.1021/acs.jmedchem.1027b00635. [DOI] [PubMed] [Google Scholar]
- 41.Burslem GM, Smith BE, Lai AC, Jaime-Figueroa S, McQuaid DC, Bondeson DP, Toure M, Dong H, Qian Y, Wang J, Crew AP, Hines J, Crews CM. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem Biol. 2017 doi: 10.1016/j.chembiol.2017.1009.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 43.Bilsland JG, Wheeldon A, Mead A, Znamenskiy P, Almond S, Waters KA, Thakur M, Beaumont V, Bonnert TP, Heavens R, Whiting P, McAllister G, Munoz-Sanjuan I. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JB, Xue C, Benice T, Xue L, Morris SW, Raber J. Anaplastic lymphoma kinase and leukocyte tyrosine kinase: functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol Biochem Behav. 2012;100:566–574. doi: 10.1016/j.pbb.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith RE, Harper JW, Jenkins JL, Thoma NH. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512:49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsilje TH, Pei W, Chen B, Lu W, Uno T, Jin Y, Jiang T, Kim S, Li N, Warmuth M, Sarkisova Y, Sun F, Steffy A, Pferdekamper AC, Li AG, Joseph SB, Kim Y, Liu B, Tuntland T, Cui X, Gray NS, Steensma R, Wan Y, Jiang J, Chopiuk G, Li J, Gordon WP, Richmond W, Johnson K, Chang J, Groessl T, He YQ, Phimister A, Aycinena A, Lee CC, Bursulaya B, Karanewsky DS, Seidel HM, Harris JL, Michellys PY. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 47.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S, Pferdekamper AC, Li J, Kasibhatla S, Sun F, Sun X, Hua S, McNamara P, Mahmood S, Lockerman EL, Fujita N, Nishio M, Harris JL, Shaw AT, Engelman JA. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou B, Hu J, Xu F, Chen Z, Bai L, Fernandez-Salas E, Lin M, Liu L, Yang CY, Zhao Y, McEachern D, Przybranowski S, Wen B, Sun D, Wang S. Discovery of a Small-Molecule Degrader of Bromodomain and Extra-Terminal (BET) Proteins with Picomolar Cellular Potencies and Capable of Achieving Tumor Regression. J Med Chem. 2018;61:462–481. doi: 10.1021/acs.jmedchem.6b01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, Hines J, Winkler JD, Crew AP, Coleman K, Crews CM. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22:755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 51.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 52.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 53.Richards MW, O'Regan L, Roth D, Montgomery JM, Straube A, Fry AM, Bayliss R. Microtubule association of EML proteins and the EML4-ALK variant 3 oncoprotein require an N-terminal trimerization domain. Biochem J. 2015;467:529–536. doi: 10.1042/BJ20150039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.