Abstract
CD40L signaling occurs in several diseases with inflammatory components, including ocular and retinal diseases. However, it has never been evaluated as a pathogenic mechanism in age-related macular degeneration (AMD) or as an inducer of inflammasome formation in any cell type. mRNA and protein levels of CD40, IL-1β, NALP1, NALP3, caspase-1, and caspase-5 were determined by RT-PCR, qPCR, and Western blot. CD40L receptor (CD40, α5β1, and CD11b) expression was determined by Western and immunofluorescent staining. IL-1β, IL-18, and MCP-1 secretions were determined by ELISA. NALP1 and NALP3 inflammasome formation were determined by Co-IP. Experiments were conducted on primary human retinal pigment epithelial (hRPE) cells from four different donors. Human umbilical vein endothelial (HUVEC) and monocytic leukemia (THP-1) cells demonstrated the general applicability of our findings. In hRPE cells, CD40L-induced NALP1 and NALP3 inflammasome activation, cleavage of caspase-1 and caspase-5, and IL-1β and IL-18 secretion. Interestingly, neutralizing CD11b and α5β1 antibodies, but not CD40, reduced CD40L-induced IL-1β secretion in hRPE cells. Similarly, CD40L treatment also induced HUVEC and THP-1 cells to secret IL-1β through CD11b and α5β1. Additionally, the CD40L-induced IL-1β secretion acted in an autocrine/paracrine manner to feed back and induce hRPE cells to secrete MCP-1. This study is the first to show that CD40L induces inflammasome activation in any cell type, including hRPE cells, and that this induction is through CD11b and α5β1 cell-surface receptors. These mechanisms likely play an important role in many retinal and non-retinal diseases and provide compelling drug targets that may help reduce pro-inflammatory processes.
Keywords: CD40L, inflammasome, IL-1β, MCP-1, retinal pigment epithelium, RPE, age-related macular degeneration, AMD
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment and blindness in patients over 60 years old (Klein et al., 2011). Various inflammatory processes have been closely linked to the pathogenesis of AMD, including complement activation, macrophage infiltration, pro-inflammatory cytokine release, and oxidative injury (Bian et al., 2011; Kinnunen et al., 2012; Telander, 2011). Inflammasome complex assembly was recently shown to be elevated in the hRPE of AMD eyes with geographic atrophy and in neovascular AMD (Doyle et al., 2012; Tarallo et al., 2012; Tseng et al., 2013). The inflammatory mediator CD40L (CD154, TNFSF5) and its receptor, CD40, have been implicated in promoting pathogenic inflammation in various ocular and neurodegenerative diseases, including ischemic and diabetic retinopathy (Portillo et al., 2008; Portillo et al., 2016), thyroid-associated ophthalmopathy (Hwang et al., 2009), and Alzheimer’s disease (Togo et al., 2000). Alternatively, CD40L has been implicated in promoting choroidal neovascularization through macrophage infiltration and release of anti-inflammatory, pro-angiogenic factors (Marneros, 2013). However, CD40L has never been evaluated as a pro-inflammatory mediator in AMD or hRPE cells or as an inducer of inflammasome activation in any cell type or tissue.
Initially found on the surface of activated T-cells, CD40L expression has now been identified on a wide variety of cells, including platelets, B-cells, mast cells, macrophages, natural killer, endothelial, and epithelial cells (Elgueta et al., 2009; Schonbeck and Libby, 2001). Originally CD40L was thought to only bind to CD40, a co-stimulatory protein found on antigen presenting cells that is essential for their activation. However, since then, three more CD40L receptors (Hassan et al., 2012) have been found: αIIβ3 (Andre et al., 2002), α5β1 (Leveille et al., 2007), and CD11b (αMβ2 or MAC-1) (Zirlik et al., 2007), all of which are integrin proteins. Of these CD40L receptors, CD40, α5β1, and CD11b have all been detected on hRPE cells (Elner et al., 2003; Elner et al., 1981; Robbins et al., 1994; Willermain et al., 2000). The CD40L/CD40 dyad is well characterized, being implicated in various immune and inflammatory diseases (Elgueta et al., 2009; Hassan et al., 2012), including several ocular diseases (Bagenstose et al., 2005; Brignole et al., 2000; Zhao et al., 2010). The other CD40L receptors in the eye, α5β1 and CD11b, are associated with cell migration, adhesion, proliferation and metastasis (Elner and Elner, 1996). CD40L is a 32–39 kDa, type II transmembrane glycoprotein that belongs to the TNF superfamily. It can be cleaved into an 18 kDa, soluble, truncated form (sCD40L) with similar biological functions and is released from activated platelets, macrophages and T cells (Hassan et al., 2012; Kilmon et al., 2007; Lee et al., 2010; Pietravalle et al., 1996; Verma et al., 2016).
Inflammasomes contain Nod-like receptors (NLR) that recognize a wide spectrum of danger- and pathogen-associated molecular patterns (DAMPs and PAMPs), respectively (Kanneganti et al., 2007). Of the 22 members of the NLR family, NALP1 and NALP3 represent two inflammasomes that are well recognized to play important roles in innate immunity and inflammation. Assembly of these inflammasomes leads to activation of caspase-1 and caspase-5, which we have recently shown to occur in hRPE cells in response to DAMPs and PAMPs (Bian et al., 2011). The NALP1 inflammasome consists of NALP1, caspase-1, caspase-5, and an adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC or PYCARD). The NALP3 inflammasome lacks caspase-5 but has caspase-1 and ASC. These inflammasomes mediate the proteolytic cleavage of the pro-forms of interleukin-1β (IL-1β) and IL-18 to their mature, active forms for secretion into the extracellular environment (Lamkanfi and Dixit, 2012), playing a major role in inflammasome-mediated immune responses (Vyleta et al., 2012).
In this study, we found that CD40L induces inflammasome activation and cleavage and secretion of mature IL-1β and IL-18. To our knowledge, this is the first study to show that CD40L induces inflammasome activation in any cell type. Interestingly, without IFN-γ priming, CD40L-induced MCP-1 secretion was CD40 receptor independent and occurred due to autocrine/paracrine effects of CD40L-induced IL-1β secretion through α5β1- and CD11b receptor signaling. These novel mechanisms likely play an important role in the pathogenesis of AMD, as well as other retinal and non-retinal diseases.
2. Material and methods
2.1. Materials
Recombinant human IL-1β, IFN-γ, IL-4 and CD40L were purchased from R&D Systems (Minneapolis, MN). The recombinant CD40L was an 18 kDa monomer composed of the amino acids present in the soluble form (amino acids 108–261). The caspase-1 inhibitor Z-YVAD-FMK and caspase-1 colorimetric assay kit were from Clontech and BioVision (Mountain View, CA and Milpitas, CA), respectively. The rabbit polyclonal antibody against CD40 was from Santa Cruz (Santa Cruz, CA). QIAshredder and RNeasy mini kit were purchased from Qiagen (Valencia, CA). The monoclonal anti-MCP-1 and biotinylated anti-MCP-1 antibodies were purchased from R&D Systems. All other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Pittsburgh, PA). The CD40, α5β1, CD11b, and IL-1β antibodies have all been previously validated in several studies (Hanissian and Geha, 1997; Hirakawa et al., 2006; Jadhav et al., 2001; Nakamori et al., 1993; Symons et al., 1987; Trompouki et al., 2003). All solutions without LPS specifically added tested negative for LPS using the limulus amebocyte lysate assay from Lonza (Basel, Switzerland).
2.2. Cell Isolation and Culture
In accordance with the Helsinki agreement, the hRPE cells were isolated and cultured as previously described (Elner et al., 1992; Elner et al., 1990, Bian et al, 2004). Briefly, hRPE cells were isolated within 24 hr of death from donor eyes obtained from the Midwest Eye Bank from 4 individuals aged 40–55 years, with no known history of ophthalmic disease. The neurosensory retina was gently separated from the RPE monolayer, and the RPE cells were removed from Bruch’s membrane with papain (5 U/ml). The RPE cells were cultured in Dulbecco’s modified essential medium (DMEM/F12) containing 15% fetal bovine serum, penicillin G (100 u/ml), streptomycin sulfate (100µg/ml), and amphotericin B (0.25 µg/ml) in Falcon Primaria culture plates to inhibit fibroblast growth. The human RPE monolayers exhibited typical hexagonal arrays with uniform immunohistochemical staining for cytokeratin 8/18 and for fibronectin, laminin, and type IV collagen in chicken-wire distributions characteristic of these epithelial cells. The identity of RPE cells in the culture was also confirmed by apical immunohistochemical staining of Na+- K+-ATPase (Bian et al, 2004). Cells were subcultured up to 3 passages, grown to confluence, and maintained for approximately 1 month before seeding for experiments. At each passage, all 4 cell lines grown to confluence exhibited polygonal arrays and 3 lines exhibited intracellular pigment. For experiments other than immunostaining, cultured hRPE cells were seeded from the subcultures at a density of 2 × 104 cells/cm2 and grown to near confluence 5–6 days before beginning experiment protocols. For immunostaining, cells were seeded at a density of 4 × 104 in each chamber of 4-chamber slide chambers 2 days before experiments.
Human umbilical vein endothelial cells (HUVEC) and respective culture supplies were purchased from Invitrogen (Carlsbad, CA) and cultured according to American Type Culture Collection (ATCC) recommended protocols. HUVEC were maintained in a humidified atmosphere with 5 % CO2 at 37 °C. The human monocytic leukemia cell line (THP-1) was obtained from ATCC (Manassas, VA.) and grown in ATCC RPMI 1640 culture medium supplemented with 10% fetal bovine serum, penicillin G (100 U/ml) and streptomycin sulfate (100µg/ml).
2.3. RT-PCR and qPCR
The total cellular RNA was isolated from hRPE cells by QIAshredder and RNeasy mini kit according to manufacturer's protocol. The cDNA synthesis was set up according to the protocol for a reverse transcription system. Briefly, 5 µg of RNA was added to the reaction mixture with 1µl of Superscript III reverse transcriptase (200 U/µl), 1µl of Oligo(dT)20 (0.5 µg µl), and 1µl of dNTP mix in a total volume of 20 µl. PCR for each product was performed as described previously (Bian et al., 2003). Briefly, PCR was performed with three different cycles (15, 25 and 35) in order to choose unsaturated cycles. PCR was accepted as semi-quantitative, when individual amplifications were within the mid-linear portion of the response curve. Specific cDNA was amplified by 35, and 20 cycles for MCP-1 and beta-actin, respectively (Bian et al., 2003). The condition for caspase-5 PCR was as described by Lin et al (Lin et al., 2000) and confirmed by examining three cycles (15, 25 and 35) first and then cycle 32 was selected for CD40, IL-1β, IL-18, Caspase-1, NALP1, and NALP3. The reaction was initiated by adding 0.15 µl of Taq DNA polymerase (5u/ml) to a final volume of 20 µl. Each PCR product was analyzed by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The intensity of the ethidium bromide luminescence was measured by image sensor with a computer-controlled display. Primers are shown in Table 1. β-actin was used as a control with the following forward and reverse primers for both RT-PCR and qPCR: 5’-GTGGGGCGCCCCAGGCACCA-3’ and 5’-GCTCGGCCGTGGTGGTGAAGC-3’. qPCR was performed by using the CFX96 qPCR detection system (Bio-Rad, Hercules, CA) to measure SYBR Green I dye (Molecular Probes, Eugene, OR). PCR reactions were performed in triplicate. PCR without cDNA was used as a negative control. Primers for IL-1β were 5′-ACAGATGAAGTGCTCCTTCCA-3′ and 5′-GTCGGAGATTCGTAGCTGGAT-3′. Thermal cycling conditions were: 3 min at 95 °C, followed by 40 cycles at 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s. All PCR reaction products were verified by melting curve analysis and agarose gel electrophoresis. The IL-1β mRNA expression levels were quantified by calculating the average value of triplicate reactions, normalized by the average value of triplicate reactions for the housekeeping β-actin gene.
Table 1.
Primer sequences used for RT-PCR.
| Forward | Reverse | |
|---|---|---|
| CD40 | 5'-CAGAGTTCACTGAAACGGAATGCC-3' | 5'-TGCCTGCCTGTTGCACAACC-3' |
| IL-1β | 5'-CTTATTACAGTGGCAATGAGGATG-3' | 5'-CTTTCAACACGCAGGACAGGTACA-3' |
| IL-18 | 5'-CCAGTAGAAGACAATTGCATCAAC-3' | 5'-TCTTATTATCATGTCCTGGGACAT-3' |
| Caspase-1 | 5'-CACCACGGCAGGCCTGGATGATGAT-3' | 5'-TCAAACATCTGGAAATTACCTTAATAT-3' |
| Caspase-5 | 5'-TAGACTCTTTGCGAAAGAATCGCGTGGCTCAT-3' | 5'-CACCTCTGCAGGCCTGGACAATGATGAC-3' |
| NALP1 | 5'-CAGCTGCCTGACACATCTGGACGC-3' | 5'-GACTATGCGAGGTTCTTGGGTATC-3' |
| NALP3 | 5'-AACAGCCACCTCACTTCCAG-3' | 5'-GACGTAAGGCCAGAATTCAC-3' |
| MCP-1 | 5'-GCTCATAGCAGCCACCTTCATTC-3' | 5'-GTCTTCGGAGTTTGGGTTTGC-3' |
2.4. ELISA
The levels of immunoreactive MCP-1 in the hRPE conditioned media, collected after 24 hr incubations of hRPE cells seeded into 24-well plates, were determined by modification of a double ligand ELISA method as previously described (Bian et al., 2004). Briefly, 200ul of non-concentrated conditioned medium from each culture well were distributed in 50ul aliquots in 4 wells of a 96-well ELISA plate and diluted 1:1 with buffer as per manufacturer eBioscience (San Diego, CA; Ray Biotech Norcross, GA; Pierce Biotechnology, Inc., Rockford, IL) instructions. For IL-1β, a commercial human IL-1β high sensitivity ELISA kit from eBioscience was used to detect IL-1β from 0 to 10pg/ml in conditioned media. For IL-18, human IL-18 ELISA kit from Ray Biotech was used to detect IL-18 from 0.5 to 75 pg/ml in the conditioned media. For low concentrations of MCP-1, the Endogen Human MCP-1 ELISA kit from Pierce Biotechnology, Inc. was used. In all assays the samples used were conditioned media without any pooling or concentrating. Standards included half-log dilutions of corresponding chemokines at concentrations from 1 pg to 100ng/well. Production of each cytokine per cell/per hr can be calculated by normalizing the reported cytokine values by dividing by 1 × 105 (cell seeding density per well)/24hr.
2.5. Western Blotting
Cell lysis buffer (product code# MCL1) was purchased from Sigma-Aldrich (St. Louis, MO). The buffer was used to perform cellular extraction of hRPE cell cultures and the cell lysates were used for Western blots that were processed according to the manufacturer's protocol (Sigma-Aldrich). Briefly, 20–50µg of protein/sample was analyzed by SDS-PAGE, protein was electro-transferred to a nitrocellulose membrane, blocked with a solution of TBS containing 5% non-fat milk and 0.1% Tween-20 (TBST) for 1 hr, probed with primary antibodies overnight, and then washed three times with TBST. Next, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hr at room temperature, and washed three additional times with TBST. The membrane was then visualized using an enhanced chemiluminescent technique.
2.6. Co-Immunoprecipitation (Co-IP)
Live cells were treated with dithiobissuccinimidylpropionate (DSP) to stabilize transient enzyme/substrate interactions as has been performed in other studies of inflammasome assembly. DSP contains an amine-reactive N-hydroxysuccinimide (NHS) ester at each end of an 8-carbon spacer arm. At pH 7–9, the NHS ester reacts with primary amines, forming stable amide bonds. In general, proteins, including antibodies, have several primary amines in their side chain of lysine (K) residues and the N-terminus of each polypeptide which are targets for NHS-ester crosslinking reagents. When two or more proteins have specific affinity to one another, it brings them to come together, thus this bioconjugation technology can be a useful tool for capturing or freezing these momentary contacts. The crosslinker allows even transient interactions to be frozen in place or weakly interacting molecules to be seized in a complex stable enough for isolation and characterization. This method has been widely used, especially in the study of inflammasome (Huang SX et al, 2013; Cummins TD et al, 2017; Chen YJ et al, 2018; Khare S et al, 2016).
Briefly, after removing the medium and washing hRPE cells with PBS, DSP from Thermo Scientific (Rockford, IL) was used for intra-cellular crosslinking followed by the Pierce classic IP Kit (Thermo Scientific). Briefly, cell lysates were generated using ice-cold non-denaturing lysis buffer in the presence of Halt™ proteinase and phosphatase inhibitor cocktail (Thermo Scientific). The total protein of the lysates was measured by BCA protein assay kit (Sigma-Aldrich). A total of more than 100 µg of protein per sample were used for Co-IP experiments. All lysates were pre-cleared using the control agarose resin. Following the pre-clear step, 2 µg of affinity purified rabbit anti-human caspase-1 antibody (BioVision) and 4 µg of affinity purified rabbit anti-human NALP1 antibody from Abcam (Cambridge, UK) were combined with each pre-cleared cell lysate for overnight incubation. Next, the antibody/lysate samples were added to protein A/G plus agarose in the spin column with gentle end-over-end mixing for 1 hr. The resin was washed and the protein complexes bound to the antibody were eluted. Western blot analyses were performed as described above. Proteins were separated by 4–15% gradient SDS-PAGE and transferred onto nitrocellulose membranes using a mini Trans Blot Cell from Bio-Rad Laboratories (Berkeley, CA). NALP3 and NALP1 were detected by mouse anti-human NALP3 (Abcam) and rabbit anti-human NALP1 antibodies (Abcam), respectively. Caspase-1 and caspase-5 were detected by mouse anti-human caspase-1 (Santa Cruz) and mouse anti-human caspase-5 antibodies (Thermo Scientific), respectively.
2.7. Immunofluorescence Analysis of CD40 in hRPE Cells
hRPE cells (4 × 104/chamber) were seeded into Lab-Tek four-chamber glass slides (Thermo Fisher Scientific, Rockford, IL) in the medium as described above. After incubation for 48 h at 37 °C, media were aspirated and adherent cells were fixed for 15 min with 4% phosphate-buffered paraformaldehyde solution, calibrated to pH 7.0 with 2 NaOH. Fixed cells were blocked in PBS solution containing 0.1% Triton X-100, 10% sheep serum and 5% BSA at room temperature for 60 min. After three washes with 1% normal goat serum, the cells were either incubated with or without primary anti-CD40 (Santa Cruz), -CD11b (BioLegend; San Diego, CA), -isotype control mouse IgG1 (BioLegend), -α5β1 (Millipore; Billerica, MA), or -non-immune rabbit IgG (Abcam) antibody overnight. The following day, cells were treated with secondary fluorescein isothiocyanate (FITC)-conjugated antibody and diluted in PBS solution containing 2% sheep serum and 1% BSA at room temperature for 60 min in a humidified dark chamber, which was followed by two washes with PBS solution. Finally, the cells were incubated with 1:10,000 bisBenzimide Hoechst 33258 for 2 min and washed with PBS. The slides were mounted with prolong anti-fade kit mount from Molecular Probe, Inc (Eugene, OR), sealed, and examined under a fluorescence microscope equipped with an argon-krypton laser with blue light for FITC excitation under 400× magnification.
2.8. Statistical Analysis
Each experiment was confirmed on hRPE cell lines cultured from 4 donors, thus results were representative of at least 4 independent experiments. For ELISA and functional assays, the results were representative of at least 4 independent experiments with similar results obtained for hRPE cultures from each of the 4 donors. For each figure, at least 4, but often 5, replicates were used to calculate means and error bars for each value from a representative experiment from cells cultured from a single donor. Various assay conditions were compared using ANOVA and t-test by StatView software, and p<0.05 was considered to be statistically significant. For ELISA and functional assays, displayed values of results represent mean ± SEM. For Western blots and RT-PCR data, a representative example was selected for the figures.
3. Results
3.1. CD40L Receptor Expression in hRPE
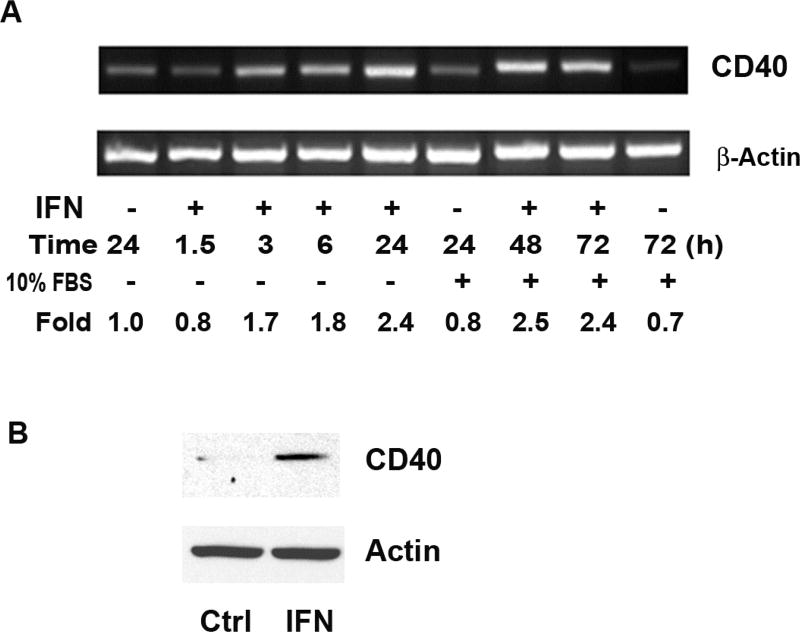
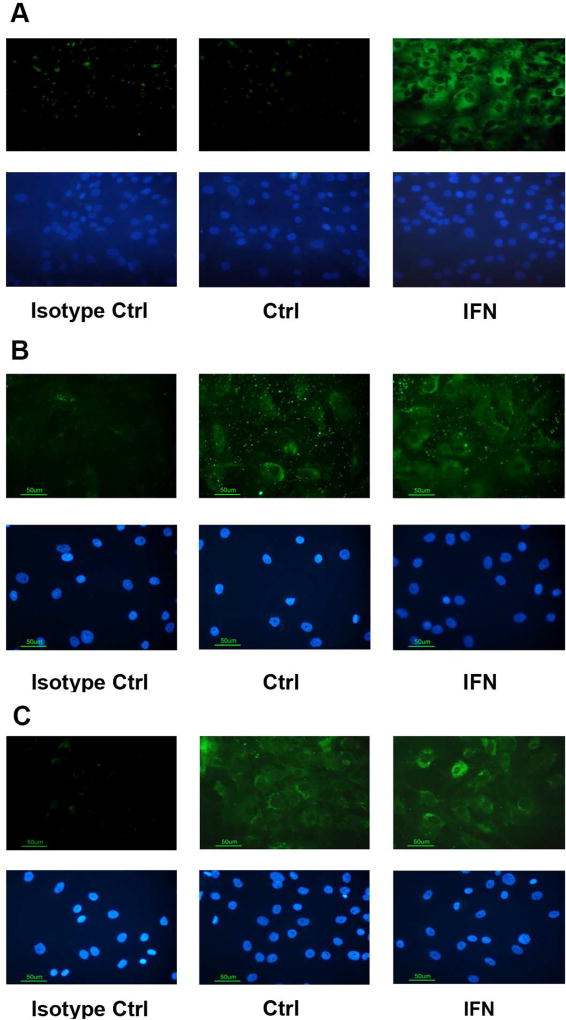
First, we sought to validate the expression of the three CD40L receptors that have been described on hRPE cells: CD40, α5β1, and CD11b (Elner et al., 2003; Elner et al., 1981; Robbins et al., 1994; Willermain et al., 2000). Unstimulated hRPE cells showed low levels of CD40 mRNA expression and no staining by Western blot or immunofluorescence in the hRPE subcultures at 48 hr (Fig. 1A–B, Fig. 2A), a finding we also observed for hRPE cultures at longer time points up to 1 week (data not shown). Because IFN-γ is known to be an inducer of CD40 (Gelbmann et al., 2003), we tested whether it would stimulate CD40 expression in hRPE cells and found that IFN-γ increased CD40 mRNA expression incrementally over time (Fig. 1A). The time course revealed that hRPE CD40 mRNA induction was detectable by 3hr of exposure and was sustained at maximal levels from 24 to 72 hr. FBS did not affect the CD40 mRNA expression. Western blot analysis showed that IFN-γ induced increased levels of CD40 protein expression (Fig. 1B), which was further confirmed by immunofluorescence microscopy (Fig. 2A). IFN-γ-induced CD40 expression contrasted with absent or very weak expression in unstimulated hRPE cells. Unlike CD40, α5β1 and CD11b, the two other CD40L receptors in unstimulated hRPE cells (Hassan et al., 2012; Zirlik et al., 2007), showed high constitutive protein expression (Fig. 2B–C), which was only mildly increased by IFN-γ treatment.
Fig 1.
Stimulation of CD40 mRNA synthesis and protein production in hRPE cells. CD40 mRNA expression in hRPE cells, unstimulated or with IFN-γ (IFN) for 1.5, 3, 6, 24, 48, and 72 hours (A). FBS had no effect on stimulation. Western blot analysis of CD40 protein expression in hRPE cells at 24 hr of stimulation with IFN-γ (B). Fold changes in mRNA levels were calculated by comparison with unstimulated control after normalization against β-actin. An antibody against actin was used in the Western blot analysis as a loading control. The concentration of IFN-γ used was 500 U/ml. FBS, fetal bovine serum.
Fig 2.
CD40L receptor protein expression in hRPE cells. Immunofluorescent protein expression (green) in hRPE cells of CD40 (A), α5β1 (B) and CD11b (C) with and without exposure to IFN-γ (IFN) for 24 hr. The nuclei were stained with bisBenzimide Hoechst 33258 (blue). For CD40, hRPE cells were unstimulated as control (Ctrl) or immunostained with replacement of primary antibody with isotypic control antibody (isotype Ctrl), and omission of primary antibody. For hRPE α5β1 and CD11b immunostaining, cells were left unstimulated (Ctrl) whereas isotypic control antibody (isotype Ctrl), were used in immunostaining of unstimulated and IFN-γ stimulated cells. All images, original magnification of 400×.
3.2. CD40L-Induced Inflammasome Activation
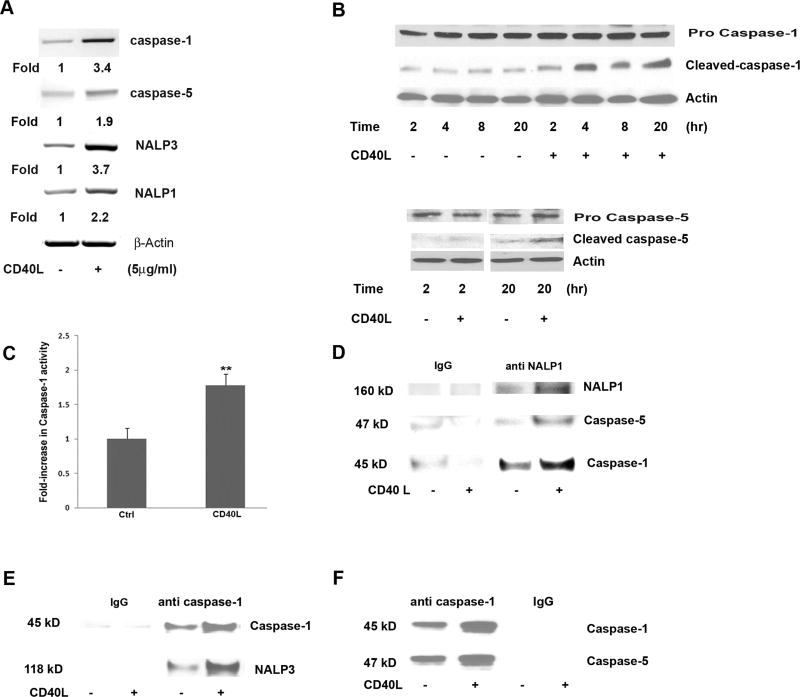
CD40L increased mRNA expression of caspase-1, caspase-5, NALP3 and NALP1 by 3.4, 1.9, 3.7 and 2.2 fold (Fig. 3A), respectively, and induced cleavage of caspase-1 and caspase-5 in hRPE cells (Fig. 3B). Pro-caspase-1 was cleaved as early as 2hr following stimulation by CD40L and the cleaved product remained elevated up to 20hr after stimulation (Fig. 3B). Similarly, active caspase-5 was detected in untreated cells, but cleaved caspase-5 increased after CD40L treatment, though not as potently as activated caspase-1 (Fig. 3B). Accordingly, catalytic activity of caspase-1 was significantly increased in whole cell lysate (WCL) by 78% after exposure to CD40L for 20hr (Fig. 3C). In contrast to cleaved caspases-1 and -5, the bands of the pro-caspases did not show noticeable changes following CD40L exposure, suggesting that considerable pools of non-activated caspases-1 and -5 remained in the hRPE cells.
Fig 3.
CD40L-induced inflammasome activation in hRPE cells. mRNA levels of inflammasome components caspase-1, caspase-5, NALP1 and NALP3 were determined by RT-PCR in hRPE cells treated with or without CD40L for 6 hr (A). The fold changes were calculated by normalization against β actin and comparison with untreated control mRNA levels. Caspase-1 and -5 protein cleavage in hRPE cells was analyzed by Western blot with or without CD40L for 2, 4, 8, or 20 hr (B). An antibody against actin was used in each Western blot analysis as a loading control. Untreated (Ctrl) and CD40L treated hRPE cell lysate were examined for caspase-1 activation by a caspase-1 assay kit (C). Co-IP pulldown of anti-NALP1 (D) or anti-caspase-1 (E, F) followed by Western blotting using antibodies against NALP3, caspase-1, NALP1 or caspase-5. Anti-IgG antibody was used as a control for immunoprecipitation. All concentrations of CD40L in these experiments were 5µg/ml. **P<0.01 as compared with untreated control (C).
To confirm the formation of NALP3 and NALP1 inflammasome complexes in the CD40L-stimulated hRPE cells, co-IP was performed. NALP1 co-precipitated with caspase-1 and -5 (Fig. 3D), and caspase-1 co-precipitated with NALP-3 (Fig. 3E) and caspase-5 (Fig. 3F) at greater levels after hRPE cells were challenged with CD40L. These results clearly indicate that CD40L results in inflammasome complex formation, a condition necessary for inflammasome activation in hRPE cells.
3.3. CD40L-Induced IL-1β and IL-18 Secretion and CD40L Receptor Involvement
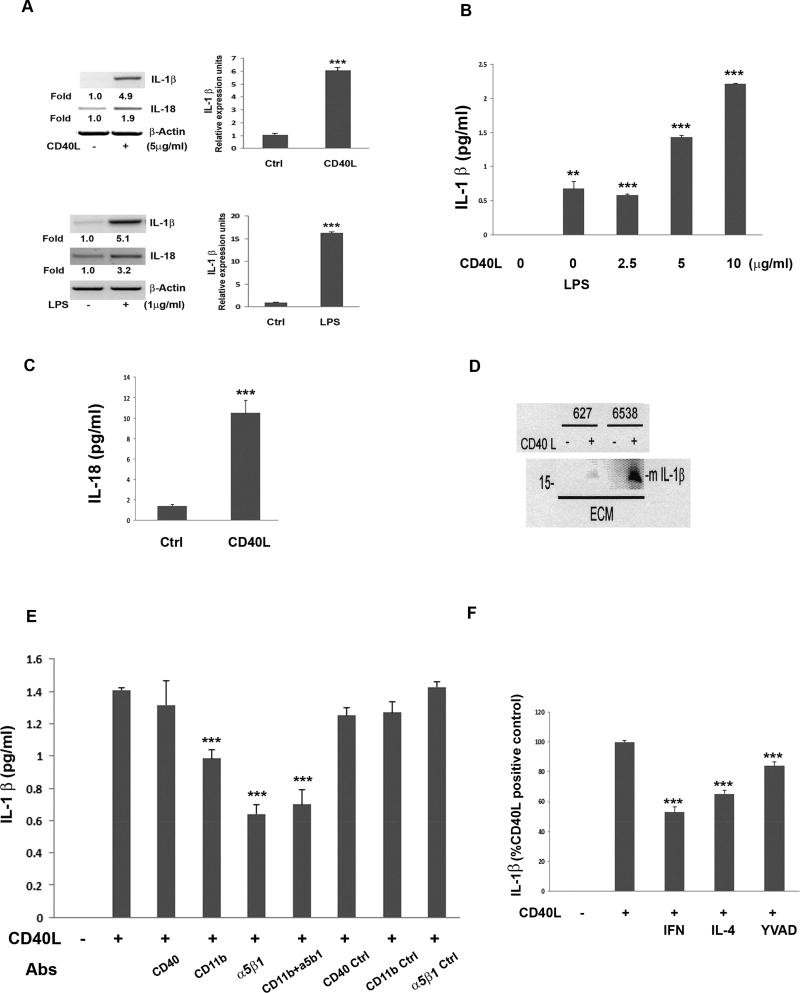
It has been reported that hRPE cells have the capacity to produce IL-1β mRNA (Fukuoka et al., 2003; Jaffe et al., 1992; Planck et al., 1993) and secrete IL-1β and IL-18 proteins in response to oxidative stress (Kauppinen et al., 2012). Thus, as inflammasome activation leads to cleavage of pro-IL-1β and pro-IL-18 into their mature forms and inflammasome activation occurs in hRPE cells in response to CD40L stimulation (shown here), we next evaluated the effect of CD40L on IL-1β and IL-18 expression and activation in hRPE cells. Post challenge with 5µg/ml of CD40L for 6 hr, IL-1β and IL-18 mRNA expression was increased 4.9 and 1.9 fold, respectively (Fig. 4A).
Fig 4.
Detection of CD40L-induced cleaved, mature IL-1β from hRPE cells through CD40L receptors CD11b and α5β1. mRNA levels measured by RT-PCR and qPCR of IL-1β and IL-18 with or without CD40L (5 ug/ml) or LPS (1 µg/ml) for 6 hr (A). IL-1β secretion from hRPE cells cultured with CD40L (0, 2.5, 5, 10 µg/ml) or LPS (1 µg/ml) for 24 hr (B). CD40L-induced hRPE secretion of IL-18 (C). hRPE cells cultured from 2 donors (627 and 6538) were left unstimulated or stimulated with CD40L followed by Western blot analysis for cleaved IL-1β in the extracellular media (ECM)(D). IL-1β ELISA of conditioned media from hRPE cells cultured with or without CD40L for 24 hr in the presence or absence of antibodies (Abs) against CD40 (5 µg/ml), CD11b (20 µg/ml) or α5β1 (10 µl/m), as well as the corresponding isotype control (E). hRPE cells were cultured with or without CD40L in the presence or absence of IFN-γ (IFN, 500 U/ml), IL-4 (100 ng/ml), or Z-YVAD-FMK (YVAD, 2µM) for 24 hr (F). The concentrations of CD40L were 5 µg/ml (C–F). *p<0.05; **p<0.01; ***p<0.001, as compared with untreated control (A–C) or CD40L-stimulated control (E, F). Ctrl, Control; LPS, lipopolysaccharide; Abs, antibodies.
Next, we evaluated the effects of LPS, a known strong inducer of the inflammasome in many cell types, with those of CD40L on IL-1β induction in hRPE cells. As with CD40L, LPS also induced IL-1β and IL-18 mRNA expression by 5.1 and 3.2 fold. qPCR further confirmed that CD40L and LPS increased hRPE IL-1β mRNA synthesis by 6.0- and 16.3-fold, respectively (Fig. 4A). CD40L-induced IL-1β secretion into the extracellular environment in a concentration-dependent manner (Fig. 4B) without any observable evidence of alterations to cell morphology at any concentration used (data not shown). LPS alone only mildly, but statistically significantly increased IL-1β secretion (Fig. 4B). As with IL-1β, a 6-fold increase in IL-18 secretion was also observed post CD40L stimulation (Fig. 4C). Furthermore, we used Western blotting to demonstrate that CD40L induced the formation of the 17 kD mature form of IL-1β in the extracellular media in the two hRPE cell lines (Fig. 4D). These results clearly indicate that CD40L induces inflammasome activation, which leads to the induction and secretion of mature IL-1β and IL-18 from hRPE cells.
To characterize possible involvement of the three known hRPE CD40L receptors in this process, we used neutralizing antibodies against CD40, CD11b, and α5β1. Blocking CD40L receptors by either anti-CD11b or anti-α5β1 reduced the induced IL-1β secretion by 40–50% (Fig. 4E). In clear contrast, anti-CD40 did not inhibit the CD40L-induced IL-1β secretion. Concurrent use of blocking antibodies against α5β1 and CD11b did not further inhibit IL-1β compared to using each alone (Fig. 4E). To further delineate involvement of the inflammasome in CD40L-induced IL-1β expression, we used caspase-1 inhibitor, Z-YVAD-FMK, to test its role in IL-1β secretion by CD40L. As shown in Fig. 4F, Z-YVAD-FMK reduced by 20% the amount of IL-1β induced by CD40L above control levels. Additionally, as CD40L exhibits pro-inflammatory effects, it is possible that some immune regulatory cytokines, such as IFN-γ and IL-4, might block the CD40L-mediated pro-inflammatory responses by hRPE. Indeed, IFN-γ and IL-4 inhibited CD40L-induced hRPE IL-1β secretion by 47 and 35%, respectively (Fig. 4F).
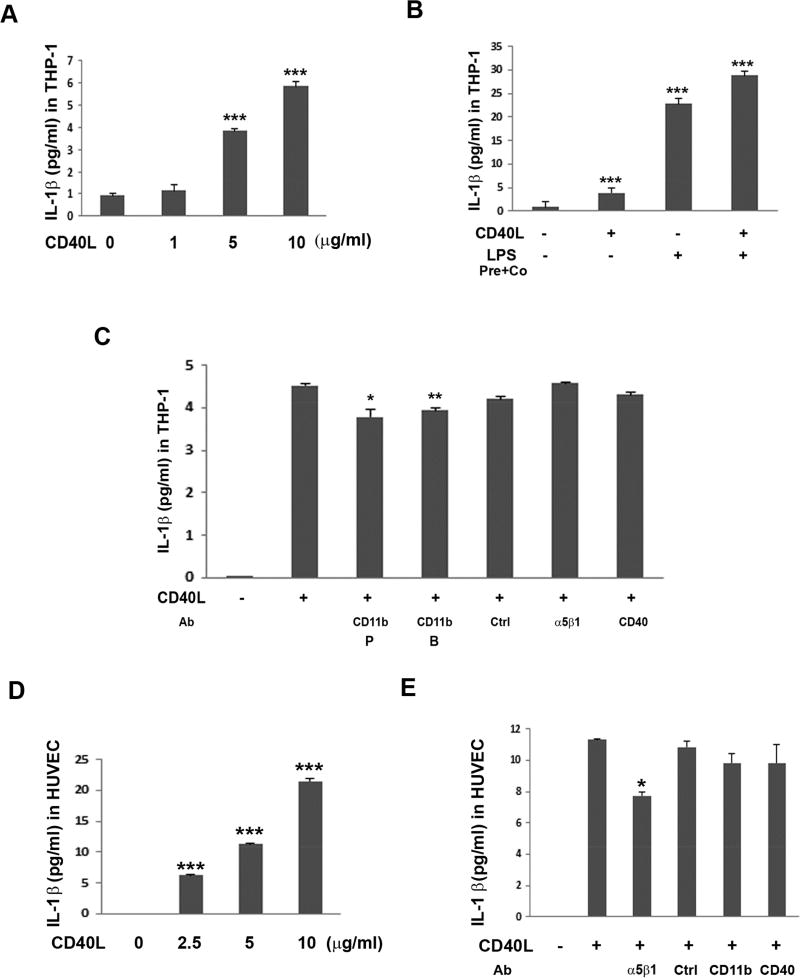
3.4. CD40L-Induced IL-1β Secretion in THP-1 and HUVEC Cells
To test whether CD40L induces IL-1β secretion in other cell types, in addition to hRPE, we evaluated a monocytic cell line, THP-1, and an endothelial cell line, HUVEC. Results showed that CD40L significantly induced concentration-dependent IL-1β secretion in both cell types (Fig. 5A and D), with the relative levels of IL-1β induced in hRPE cells averaging 33% compared to THP-1 cells. The induction by similar concentrations of CD40L in these two cell types was stronger than that in hRPE cells. Moreover, LPS treatment induced IL-1β secretion about 6 times more than that by CD40L in THP-1 cells (Fig. 5B). Co-incubation of THP-1 cells with LPS and CD40L showed additive increases in IL-1β secretion. Interestingly, based on neutralizing antibody studies, CD11b may be involved in IL-1β secretion by CD40L in THP-1 cells, CD11b antibodies having reduced CD40L IL-1β induction by an average of 15%. In contrast, integrin α5β1 may be involved in IL-1β secretion by CD40L in HUVEC cells (Fig. 5C and E). This is in contrast to hRPE where both CD11b and α5β1 seem to be involved in CD40L-induced IL-1β secretion. Strikingly, similarly to as in hRPE, CD40 does not seem to be involved in CD40L-induced IL-1β secretion in either THP-1 or HUVEC cells (Fig. 5C and E).
Fig 5.
CD40L induced IL-1β secretion in THP-1 and HUVEC cells through CD40L receptors CD11b and α5β1. THP-1 (A–C) and HUVEC cells (D–E) were challenged by CD40L (1, 2.5, 5 or 10 µg/ml) for 24 hr. Concentration-dependent CD40L-induced IL-1β secretion in THP-1 (A) and HUVEC (D) cells, respectively. Effect of LPS priming plus co-culture (pre-co) on CD40L-induced IL-1β secretion (B). Effects of CD40L-induced IL-1β secretion following treatment with neutralizing antibodies targeting CD40L receptors in THP-1 (C) and HUVEC (E) cells. The antibodies used were anti-CD11b P (from Pharmingen), anti-CD11b B (from Biolegend), anti-α5β1, anti-CD40, and anti-isotype IgG1k control for anti-CD11b (Ctrl) and anti-α5β1. In all experiments, IL-1β protein was detected by ELISA. CD40L concentration was 5 µg/ml. *p<0.05; **p<0.01; ***p<0.001, as compared with untreated control (A, B, D) or between presence and absence of antibodies (C, E). HUVEC, human umbilical vein endothelial cells; LPS, lipopolysaccharide; Ab, antibody.
3.5. CD40L-Induced hRPE MCP-1 Secretion
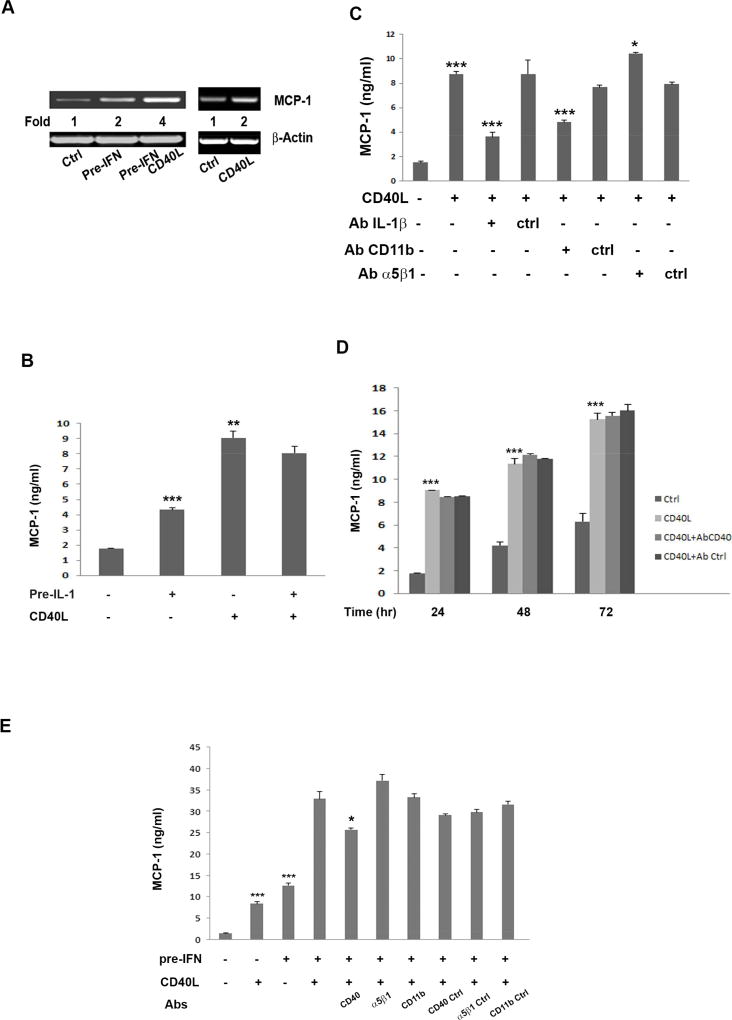
It has been known that CD40L induces secretion of MCP-1 in many cell types (D'Aversa et al., 2002; Gelbmann et al., 2003; Koumas et al., 2001; Sugiura et al., 2000), including retinal endothelial cells (Greene et al., 2015). In the present study, we examined effects of CD40L on MCP-1 expression in unstimulated and IFN-γ- or IL-1β-primed hRPE cells. Untreated hRPE cells exhibited low levels of MCP-1 mRNA (Fig. 6A), a result consistent with our previous studies (Elner et al., 1997; Willermain et al., 2000). Pretreatment with IFN-γ or treatment with CD40L alone each doubled hRPE MCP-1 mRNA synthesis. The level of secreted MCP-1 protein was significantly increased by CD40L or pre-IL-1β treatment (Fig. 6B).
Fig 6.
CD40L-induced MCP-1 mRNA synthesis and protein secretion by hRPE cells. MCP-1 mRNA levels measured by RT-PCR in hRPE cells treated with or without IFN-γ pre-treatment (Pre-IFN) or control (Ctrl) for 24 hr and then switched to serum-free media containing 0 or 5 µg/ml of CD40L for 6 hr (A). The fold changes were calculated by normalization against β-actin and comparison with untreated control. MCP-1 ELISA of conditioned media from hRPE cells pre-treated with or without IL-1β (20 pg/ml) for 24 hr, then replaced with media with or without CD40L for another 24 hr (B). MCP-1 ELISA of conditioned media from hRPE cells treated with or without CD40L (5 µg/ml) in the presence or absence of anti-IL-1β, anti-CD11b, anti-α5β1 or corresponding isotype control antibody for 24 hr (C). MCP-1 ELISA of conditioned media from hRPE cells treated with 10% serum media containing 0 or 5 µg/ml of CD40L in the presence or absence of anti-CD40 or corresponding isotype control antibody for 24, 48 and 72 hr (D). MCP-1 ELISA of conditioned media from hRPE cells treated with 10% serum media with or without IFN-γ for 24 hr, then replaced with CD40L containing or CD40L-free media in the presence or absence of anti CD40, α5β1 or CD11b antibody or isotype controls for an additional 24 hr (E). The concentration of IFN-γ (IFN) was 500 U/ml. *p<0.05; **p<0.01; ***p<0.001, as compared with untreated control (CD40L-treated in C, pre-IL-1 or CD40L-treated in D, and pre-IFN or CD40L-treated in E) or between presence and absence of antibodies (B, CD40Ab in E).
Because IL-1β is a potent inducer of hRPE MCP-1 secretion (Elner et al., 1991), we hypothesized that CD40L-induced hRPE IL-1β secretion may regulate MCP-1 expression in an autocrine/paracrine manner. As CD40L receptors, CD11b and α5β1, were involved in hRPE IL-1β secretion (Fig. 4), we questioned whether both receptors also function in this process. To test this assumption, we tested blocking antibodies against IL-1β, CD11b, or α5β1. Our data showed that neutralizing IL-1β reduced the CD40L-induced MCP-1 secretion by 70% (Fig. 6C). Neutralizing CD11b reduced the CD40L-induced MCP-1 secretion by 45%. In contrast, neutralizing α5β1 did not inhibit, but weakly increased the CD40L-induced MCP-1 secretion (Fig. 6C). These observations suggest that the relative effects of signaling molecules are complex. It is possible that secreted hRPE IL-1β induced by CD40L results in hRPE MCP-1 secretion and is additive, or even synergistic, to the effect of CD40L binding to CD11b overwhelming the effect of α5β1 antibodies in the same pathways. Another possibility is the CD40L binding to α5β1 directly stimulates hRPE MCP1. Furthermore, the CD40L-induced MCP-1 secretion was insensitive to anti-CD40 antibody even when incubation time was extended to 72hr when extracellular MCP-1 levels reached about 15ng/ml, indicating that CD40L-induced MCP-1 secretion in unstimulated hRPE cells is via a CD40-independent mechanism (Fig. 6D).
A synergistic effect on MCP-1 secretion was observed when cells were primed by IFN-γ before CD40L induction. In contrast, IL-1β-priming did not have a similar effect (Fig. 6B). The stimulation combined with IFN-γ priming was sensitive to blockade by anti-CD40 antibody, which reduced MCP-1 secretion by about 36% in hRPE cells exposed to IFNγ and CD40L, whereas co-incubation with either anti-CD11b or anti-α5β1 antibody had no effect (Fig. 6E). The disparity in hRPE MCP-1 secretion seen in Figures 6C and 6E appears to be due to the fact that CD40L-stimulated hRPE cells produce a significant amount of MCP-1 that is CD40-independent and may be inhibited by antibody to IL-1β produced by the inflammasome or antibody to CD11b which may reduce the IL-1β produced through activation of the inflammasome, as shown in Figure 6C. In contrast, hRPE cells stimulated with IFNγ express CD40 which appears to mediate hRPE MCP-1 induction by CD40L in IFNγ-exposed cells, but not by hRPE cells not stimulated by IFNγ. Therefore, anti-α5β1 and anti-CD11b antibodies have no significant effect on MCP-1 secretion by IFNγ-stimulated hRPE cells driven by CD40 and other independent mechanisms.
4. Discussion
The hRPE monolayer forms the outer blood–retina barrier (BRB). Together with the inner BRB formed by the retinal vasculature, BRB limits the entry of inflammatory cells, pathogens, and some macromolecules from the circulation. However, the integration of the BRB can be dramatically disrupted due to disease conditions, including inflammatory diseases, diabetic retinopathy (Frank, 1984), AMD (Miller et al., 1986), hypertensive choroidopathy (Hayreh et al., 1986), and photocoagulation (Pollack et al., 1986). These pathological conditions change the external environment of hRPE cells, which can lead to leukocytes, platelets, and macromolecules encountering hRPE cells as they cross the BRB. First, hRPE cells may become exposed to circulating CD40L and platelets due to high permeability and/or retinal hemorrhage (Heeschen et al., 2003; Schonbeck and Libby, 2001). Second, the infiltrating CD40L-expressing leukocytes (activated T cells and macrophages) may bind hRPE cells, or they can release CD40L into the extracellular environment (Cherepanoff et al., 2010; Cruz-Guilloty et al., 2013; Kilmon et al., 2007; Mach et al., 1997a; Mach et al., 1997b; Pietravalle et al., 1996). Under these conditions, CD40L receptor pathways can be activated in hRPE cells (Bagenstose et al., 2005; Elner et al., 2003; Elner et al., 1981; Li et al., 2009).
CD40L plays an important role in cellular immune responses by activating T cells, macrophages, and neutrophils and inducing the release of pro-inflammatory cytokines and chemokines (Khan et al., 2006; Kilmon et al., 2007; Pietravalle et al., 1996). CD40L also exhibits a critical role in the humoral immune system by supporting T-cell-dependent B-cell differentiation and immunoglobulin isotype switching (Kroczek et al., 1994). Of the four known CD40L receptors: CD40, α5β1, CD11b, and αIIβ3, CD40 is the best studied in a variety of cell types (Michel et al, 2017). Previous reports on hRPE CD40 expression are conflicting. In all studies, immunohistochemistry and flow cytometry have demonstrated the absence of CD40 on unstimulated hRPE cells. However, with IFN-γ stimulation, fetal hRPE cells were reported to express CD40 in response to IFN-γ, while immortalized hRPE (ARPE-19) cells did not. One explanation for this discrepancy may be that transformed hRPE cells that are extensively passaged due not maintain a normal physiological response to IFN-γ. To test this hypothesis, we repeated the above experiment using early-passage, primary hRPE cells. RT-PCR, Western blotting, and immunofluorescence all clearly demonstrated inducible expression of CD40 in hRPE cells from four separate donors. Thus, we conclude that normal hRPE cells do indeed have the capacity to upregulate CD40 protein expression in response to IFN-γ. We also demonstrated constitutive baseline and induced expression of α5β1 and CD11b in these hRPE cells from the same donors.
Although CD40L has been shown to activate IL-1β secretion through activating caspase-1, there has been no report linking this finding to inflammasome activation (Schonbeck et al., 1997). Instead, CD40L has been shown to inhibit, not activate NALP1 and NALP3 inflammasomes resulting from cell-cell contact of activated T cells (Guarda et al., 2009). Our results are the first to show that CD40L is involved in inflammasome activation and secretion of mature IL-1β and IL-18 in any cell type. Our results also demonstrate that a single, circulating endogenous ligand (CD40L) is sufficient to induce inflammasome activation in hRPE cells. The secretion of mature IL-1β by hRPE cells was dependent on CD40L binding to α5β1 or CD11b, but not CD40, demonstrating that alternative CD40L receptors may be responsible for downstream inflammasome activation. Our findings strongly suggest that CD40 is not necessary for CD40L to exert its effects on the inflammasome in hRPE cells.
One study recently reported that CD40L did not induce IL-1β secretion in human retinal endothelial or Müller cells; however, the limit of detection of the assay used was 3.9 pg/ml (Portillo et al., 2016). It is worth noting that the IL-1β secretion of hRPE cells in our study was below this limit of detection and required a high sensitivity ELISA kit and a Western blot of pooled, concentrated extracellular media, opening the possibility that these other cells might also secrete IL-1β in response to CD40L at lower levels. One vital question is whether the CD40L-induced IL-1β secretion from the hRPE could be involved in the low-grade autocrine/paracrine inflammation that occurs in many human neurodegenerative diseases such as AMD (Medzhitov, 2008) and whether CD40L or other ligands for those receptors are present in human lesions.
Another critical question is whether this pathway is broadly applicable to many human diseases with inflammatory components. The cultured hRPE cells used in our experiments had distinctive morphologic and biochemical similarities to the native hRPE, but significant structural and functional differences inherent in the cultured cells are undoubtedly present, especially in the experimental subcultures when compared with native hRPE cells in-situ. Here, we showed that CD40L treatment induced human THP-1 and HUVEC cells to secrete IL-1β and that this was through the same CD40L receptors as in hRPE: CD11b for THP-1 and α5β1 for HUVEC. This is particularly important for atherosclerosis, in which both platelets and monocytes/macrophages provide a readily available supply of CD40L that can stimulate CD40L receptors on endothelial cells. This is something that should be carefully explored in future studies.
Next, we found that CD40L-stimulated hRPE cells produce a significant amount of MCP-1 that is CD40-independent and subject to inhibition by anti-IL-1β neutralizing antibody. This finding suggests that an autocrine/paracrine mechanism exists in which CD40L-induced hRPE IL-1β feeds back on hRPE cells and induces them to also secrete MCP-1. In vivo such a mechanism would serve to chemoattract monocytes with MCP-1 expression and stimulate them via IL-1β. On the other hand, a greatly enhanced MCP-1 secretion by CD40L was observed in IFN-γ, but not IL-1β primed cells. This induced MCP-1 secretion was subject to inhibition by anti-CD40, but not anti-α5β1 or anti–CD11b antibodies, implicating that the induced MCP-1 secretion was largely through the CD40L-CD40 pathway. In fact, in retinal endothelial cells that express low levels of CD40, CD40L significantly induces MCP-1 secretion, which is completely blocked by retroviral knockdown of CD40 (Greene et al., 2015).
IFN-γ is a multi-function cytokine that often is involved in a complex immunopathologic network involving other pro-inflammatory cytokines, including TNF-α, IL-1, IL- 2 and IL-6, as well as immunosuppressive cytokines, including TGF-β, IL-4 and IL-10 (De Vos et al., 1992; Wakefield and Lloyd, 1992; Nussenblatt and Whitcup, 2004). Secreted by T-lymphocytes and macrophages, both locally within the eye and systemically, INFγ has been shown to be present in significant concentrations in the eye in overtly inflammatory and non-clinically inflammatory human retinal diseases and animal models of human retinal disease. (Deschenes et al., 1988; Limb et al., 1991; Franks et al., 1992). The ability of IFN-γ to synergize or antagonize the effects of cytokines, growth factors, and PAMP-signaling pathways is particularly important in hRPE cells, as hRPE cells constantly receive multiple signals and integrate them to generate responses appropriate to the extracellular milieu. Our study showed that IFN-γ, as with IL-4 (a Th2 anti-inflammatory cytokine), reduced CD40L-stimulated IL-1β secretion. When primed with IFN-γ, we found that CD40L caused strong stimulation of MCP-1 expression in a CD40-dependent manner. The IFN-γ priming-dependent CD40L stimulation of MCP-1 production in hRPE cells appears to be IFN-γ-specific because we showed that IL-1β did not have a similar effect. Further investigation on the molecular mechanism by which IFN-γ primes unstimulated hRPE cells for activation by CD40L-CD40 binding is warranted, but the results in this study improve our understanding of the mechanisms by which IFN-γ coordinates its pleiotropic effects. It is also important to mention that we cannot rule out the existence of other yet to be identified CD40L receptor pathway(s).
In conclusion, we show that CD40L promotes inflammasome assembly and activation via CD40L receptors α5β1 and CD11b, which leads to secretion of mature IL-1β and IL-18. CD40L both promotes MCP-1 secretion independent of CD40 via IL-1β secretion followed by autocrine/paracrine signaling, as well as through CD40 with IFN-γ priming. The CD40L-induced, but relatively low, IL-1β and MCP-1 secretion observed in primary hRPE cells is consistent with the chronic, low-grade inflammation that is characteristic of AMD, atherosclerosis and other age-related and inflammatory conditions (Buschini et al., 2011; Chaurasia et al., 2009; Xu et al., 2009). In addition, both CD40L/α5β1 and CD40L/CD11b dyads represent potential new drug targets. Further delineation of the CD40L receptor pathways and better understanding of their functional roles in hRPE and other cells will shed more light into therapeutic strategies for hRPE-related retinal diseases, including AMD, as well as non-retinal conditions.
Highlights.
CD40L-induces inflammasome activation and secretion of IL-1β and IL-18.
This mechanism occurs through the CD11b and α5β1 cell-surface receptors.
Secreted IL-1β acts in an autocrine/paracrine manner to induce MCP-1 secretion.
Acknowledgments
Funding: This study was supported by NIH Grants EY-09441, N007361, EY007003, and Research to Prevent Blindness-Senior Scientific Award (VME).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- Bagenstose LM, Agarwal RK, Silver PB, Harlan DM, Hoffmann SC, Kampen RL, Chan CC, Caspi RR. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol. 2005;175:124–130. doi: 10.4049/jimmunol.175.1.124. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Khanna H, Murga-Zamalloa CA, Patil S, Elner VM. Expression and functional roles of caspase-5 in inflammatory responses of human retinal pigment epithelial cells. Investigative ophthalmology & visual science. 2011;52:8646–8656. doi: 10.1167/iovs.11-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Elner VM. Human RPE-monocyte co-culture induces chemokine gene expression through activation of MAPK and NIK cascade. Experimental eye research. 2003;76:573–583. doi: 10.1016/s0014-4835(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Elner VM. Differential involvement of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression. Investigative ophthalmology & visual science. 2004;45:1887–1896. doi: 10.1167/iovs.03-0608. [DOI] [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Investigative ophthalmology & visual science. 2000;41:1356–1363. [PubMed] [Google Scholar]
- Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011;95:14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Chaurasia P, Mezei M, Zhou MM, Ossowski L. Computer aided identification of small molecules disrupting uPAR/alpha5beta1--integrin interaction: a new paradigm for metastasis prevention. PloS one. 2009;4:e4617. doi: 10.1371/journal.pone.0004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YJ, Wang SF, Wang IC, Hong MH, Lo TH, Jan JT, Hau LC, Chen HY, Liu FT. Galectin-3 enhances avian H5N1 influenza A virus-induced pulmonary inflammation by promoting NLRP3 inflammasome activation. Am J Pathol. 2018 doi: 10.1016/j.ajpath.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. The British journal of ophthalmology. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TD, Sapkota GP. Characterization of protein complexes using chemical crosslinking coupled electrospray mass spectometry. 2018 doi: 10.1007/7651_2017_85. Accepted manuscript. [DOI] [PubMed] [Google Scholar]
- D'Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos AF, Hoekzema R, Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992:11581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- Deschênes J, Char DH, Kaleta S. Activated T lymphocytes in uveitis. Br J Ophthalmol. 1988;72:83–87. doi: 10.1136/bjo.72.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O'Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner SG, Elner VM. The integrin superfamily and the eye. Investigative ophthalmology & visual science. 1996;37:696–701. [PubMed] [Google Scholar]
- Elner SG, Elner VM, Kindzelskii AL, Horino K, Davis HR, Todd RF, 3rd, Glagov S, Petty HR. Human RPE cell lysis of extracellular matrix: functional urokinase plasminogen activator receptor (uPAR), collagenase and elastase. Experimental eye research. 2003;76:585–595. doi: 10.1016/s0014-4835(03)00028-9. [DOI] [PubMed] [Google Scholar]
- Elner SG, Elner VM, Pavilack MA, Todd RF, 3rd, Mayo-Bond L, Franklin WA, Strieter RM, Kunkel SL, Huber AR. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Laboratory investigation; a journal of technical methods and pathology. 1992;66:200–211. [PubMed] [Google Scholar]
- Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Laboratory investigation; a journal of technical methods and pathology. 1991;64:819–825. [PubMed] [Google Scholar]
- Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Experimental eye research. 1997;65:781–789. doi: 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- Elner VM, Schaffner T, Taylor K, Glagov S. Immunophagocytic properties of retinal pigment epithelium cells. Science. 1981;211:74–76. doi: 10.1126/science.7444450. [DOI] [PubMed] [Google Scholar]
- Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–750. [PMC free article] [PubMed] [Google Scholar]
- Frank RN. On the pathogenesis of diabetic retinopathy. Ophthalmology. 1984;91:626–634. doi: 10.1016/s0161-6420(84)34258-0. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y, Strainic M, Medof ME. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol. 2003;131:248–253. doi: 10.1046/j.1365-2249.2003.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chignell AH, Dumonde DC. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11(Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- Gelbmann CM, Leeb SN, Vogl D, Maendel M, Herfarth H, Scholmerich J, Falk W, Rogler G. Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut. 2003;52:1448–1456. doi: 10.1136/gut.52.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JA, Portillo JA, Lopez Corcino Y, Subauste CS. CD40-TRAF Signaling Upregulates CX3CL1 and TNF-alpha in Human Aortic Endothelial Cells but Not in Retinal Endothelial Cells. PloS one. 2015;10:e0144133. doi: 10.1371/journal.pone.0144133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- Hanissian SH, Geha RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Merhi Y, Mourad W. CD40 ligand: a neo-inflammatory molecule in vascular diseases. Immunobiology. 2012;217:521–532. doi: 10.1016/j.imbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignant hypertension. VI. Hypertensive choroidopathy. Ophthalmology. 1986;93:1383–1400. doi: 10.1016/s0161-6420(86)33554-1. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML, Investigators CS. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, Oike M, Watanabe M, Karashima Y, Ito Y. Pivotal role of integrin alpha5beta1 in hypotonic stress-induced responses of human endothelium. FASEB J. 2006;20:1992–1999. doi: 10.1096/fj.05-5580com. [DOI] [PubMed] [Google Scholar]
- Huang BX, Kim HY. Effective identification of Akt interacting proteins by tw-step chemical crosslinking, co-immunoprecipitation and mass spectometry. PLoS ONE. 2013;8(4):1–11. doi: 10.1371/journal.pone.0061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, Chen B, Phipps RP, Goldberg RA, Smith TJ, Douglas RS. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Investigative ophthalmology & visual science. 2009;50:2262–2268. doi: 10.1167/iovs.08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S, Bochner BS, Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol. 2001;167:5986–5993. doi: 10.4049/jimmunol.167.10.5986. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Van Le L, Valea F, Haskill S, Roberts W, Arend WP, Stuart A, Peters WP. Expression of interleukin-1 alpha, interleukin-1 beta, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Experimental eye research. 1992;55:325–335. doi: 10.1016/0014-4835(92)90197-z. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells--implications for age-related macular degeneration (AMD) Immunol Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Radlan AD, Dorfleutner A, Stenhik C. Methods to measure NLR oligomerization: size exclusion chromatography, co-immunoprecipitation and crosslinking. Methods Mol Biol. 2016;1417:131–143. doi: 10.1007/978-1-4939-3566-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, Vilen BJ. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Archives of ophthalmology. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 2001;159:925–935. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczek RA, Graf D, Brugnoni D, Giliani S, Korthuer U, Ugazio A, Senger G, Mages HW, Villa A, Notarangelo LD. Defective expression of CD40 ligand on T cells causes "X-linked immunodeficiency with hyper-IgM (HIGM1)". Immunol Rev. 1994;138:39–59. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lee VW, Qin X, Wang Y, Zheng G, Wang Y, Wang Y, Ince J, Tan TK, Kairaitis LK, Alexander SI, Harris DC. The CD40–CD154 co-stimulation pathway mediates innate immune injury in adriamycin nephrosis. Nephrol Dial Transplant. 2010;25:717–730. doi: 10.1093/ndt/gfp569. [DOI] [PubMed] [Google Scholar]
- Leveille C, Bouillon M, Guo W, Bolduc J, Sharif-Askari E, El-Fakhry Y, Reyes-Moreno C, Lapointe R, Merhi Y, Wilkins JA, Mourad W. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. The Journal of biological chemistry. 2007;282:5143–5151. doi: 10.1074/jbc.M608342200. [DOI] [PubMed] [Google Scholar]
- Li R, Maminishkis A, Zahn G, Vossmeyer D, Miller SS. Integrin alpha5beta1 mediates attachment, migration, and proliferation in human retinal pigment epithelium: relevance for proliferative retinal disease. Investigative ophthalmology & visual science. 2009;50:5988–5996. doi: 10.1167/iovs.09-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb GA, Little BC, Meager A, Ogilvie JA, Wolstencroft RA, Franks WA, Chignell AH, Dumonde DC. Cytokines in proliferative vitreoretinopathy. Eye. 1991;5:686–693. doi: 10.1038/eye.1991.126. [DOI] [PubMed] [Google Scholar]
- Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. The Journal of biological chemistry. 2000;275:39920–39926. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997a;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997b;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros AG. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 2013;4:945–958. doi: 10.1016/j.celrep.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Michel NA, Zirlik A, Wolf D. CD40L and its receptors in athrothrombosis- an update. Front Cardiovasc Med. 2017;4:40. doi: 10.3389/fcvm.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Miller B, Ryan SJ. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Investigative ophthalmology & visual science. 1986;27:1644–1652. [PubMed] [Google Scholar]
- Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- Pietravalle F, Lecoanet-Henchoz S, Blasey H, Aubry JP, Elson G, Edgerton MD, Bonnefoy JY, Gauchat JF. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. The Journal of biological chemistry. 1996;271:5965–5967. doi: 10.1074/jbc.271.11.5965. [DOI] [PubMed] [Google Scholar]
- Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993;12:205–212. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- Pollack A, Korte GE, Heriot WJ, Henkind P. Ultrastructure of Bruch's membrane after krypton laser photocoagulation. II. Repair of Bruch's membrane and the role of macrophages. Archives of ophthalmology. 1986;104:1377–1382. doi: 10.1001/archopht.1986.01050210131040. [DOI] [PubMed] [Google Scholar]
- Portillo JA, Van Grol J, Zheng L, Okenka G, Gentil K, Garland A, Carlson EC, Kern TS, Subauste CS. CD40 mediates retinal inflammation and neurovascular degeneration. J Immunol. 2008;181:8719–8726. doi: 10.4049/jimmunol.181.12.8719. [DOI] [PubMed] [Google Scholar]
- Portillo JC, Corcino YL, Miao Y, Tang J, Sheibani N, Kern TS, Dubyak GR, Subauste CS. CD40 in Retinal Muller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes. 2016 doi: 10.2337/db16-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SG, Brem RB, Wilson DJ, O'Rourke LM, Robertson JE, Westra I, Planck SR, Rosenbaum JT. Immunolocalization of integrins in proliferative retinal membranes. Investigative ophthalmology & visual science. 1994;35:3475–3485. [PubMed] [Google Scholar]
- Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Bonnefoy JY, Loppnow H, Flad HD, Libby P. Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes elaboration of active interleukin 1beta. The Journal of biological chemistry. 1997;272:19569–19574. doi: 10.1074/jbc.272.31.19569. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kawaguchi Y, Harigai M, Takagi K, Ohta S, Fukasawa C, Hara M, Kamatani N. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J Immunol. 2000;164:6593–6600. doi: 10.4049/jimmunol.164.12.6593. [DOI] [PubMed] [Google Scholar]
- Symons JA, Bundick RV, Suckling AJ, Rumsby MG. Cerebrospinal fluid interleukin 1 like activity during chronic relapsing experimental allergic encephalomyelitis. Clin Exp Immunol. 1987;68:648–654. [PMC free article] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telander DG. Inflammation and age-related macular degeneration (AMD) Semin Ophthalmol. 2011;26:192–197. doi: 10.3109/08820538.2011.570849. [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer's disease and other neurological diseases. Brain Res. 2000;885:117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D'Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Investigative ophthalmology & visual science. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyleta ML, Wong J, Magun BE. Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome. PloS one. 2012;7:e36044. doi: 10.1371/journal.pone.0036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield D, Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992;4:1–5. doi: 10.1016/1043-4666(92)90028-p. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Nussenblatt RB, Lightman SL, Hollander DA. Inflammation in retinal disease. Int J Inflam. 2013:724648. doi: 10.1155/2013/724648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willermain F, Caspers-Velu L, Baudson N, Dubois C, Hamdane M, Willems F, Velu T, Bruyns C. Role and expression of CD40 on human retinal pigment epithelial cells. Investigative ophthalmology & visual science. 2000;41:3485–3491. [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Progress in retinal and eye research. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Zhao LQ, Wei RL, Cheng JW, Cai JP, Li Y. The expression of intercellular adhesion molecule-1 induced by CD40-CD40L ligand signaling in orbital fibroblasts in patients with Graves' ophthalmopathy. Investigative ophthalmology & visual science. 2010;51:4652–4660. doi: 10.1167/iovs.09-3789. [DOI] [PubMed] [Google Scholar]
- Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, Ahrens I, Ernst S, Bassler N, Missiou A, Patko Z, Aikawa M, Schonbeck U, Bode C, Libby P, Peter K. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115:1571–1580. doi: 10.1161/CIRCULATIONAHA.106.683201. [DOI] [PubMed] [Google Scholar]