Abstract
Objective
To evaluate changes in patient and graft survival for pediatric liver transplant recipients since 2002, and to determine if these outcomes vary by graft type (whole liver transplant (WLT), split liver transplant (SLT), and living-donor liver transplant (LDLT)).
Study design
We evaluated patient and graft survival among pediatric liver-only transplant recipients the PELD/MELD system was implemented using the Scientific Registry of Transplant Recipients.
Results
From 2002–2009 to 2010–2015, survival for SLT at 30 days improved (94% vs 98%; P < .001), and at one year improved for SLT (89% to 95%; P<0.001) and LDLT (93% to 98%; P=0.002). There was no change in survival for WLT at either 30 days (98% in both; P=0.7) or one year (94% vs 95%; P=0.2). Risk of early death with SLT was 2.14-fold higher in 2002–2009 (adjusted hazard ratio (aHR) vs WLT: 1.472.143.12) but this risk disappeared in 2010–2015 (aHR: 0.651.131.96), representing a significant improvement (P=0.04). Risk of late death following SLT was similar in both time periods (aHR 2002–2009: 0.871.141.48; aHR 2010–2015: 0.560.881.37). LDLT had similar risk of early death (aHR 2002–2009: 0.491.032.14; aHR 2010–2015: 0.260.742.10) and late death (aHR 2002–2009: 0.520.831.32; aHR 2010–2015: 0.170.441.11). Graft loss was similar for SLT (aHR: 0.931.091.28) and was actually lower for LDLT (aHR: 0.530.710.95).
Conclusion
In recent years, outcomes following use of technical variant grafts are comparable with whole grafts, and may be superior for LDLT. Greater use of technical variant grafts might provide an opportunity to increase organ supply without compromising post-transplant outcomes.
Keywords: pediatric, liver, transplant, allograft, split
Liver transplantation provides life-saving therapy for children with end-stage liver disease.(1) Unfortunately, successful pediatric transplantation is hindered by a scarcity of suitable livers.(2) Under the current PELD (Pediatric End-stage Liver Disease) and MELD (Model for End-stage Liver Disease) system, organs are allocated to patients based on their probability of death within 90 days while awaiting transplant. This strategy means: (1) the pre-transplant course for most individuals is associated with significant morbidity, hospitalization, and costs; (2) delays in transplantation exacerbate long-term impairments in cognition and growth; and (3) in some instances, children die on the waitlist.(3–5)
Use of technical variant donation, including split liver transplantation (SLT) and living-donor liver transplantation (LDLT), represents a potential solution to the organ shortage.(6) Given that approximately 6,000 whole livers are used for adult recipients each year, SLT for children represents an exciting opportunity to improve organ supply, shorten waitlist times, and decrease pre-transplant morbidity and mortality. Evidence from studies of adult recipients suggest that outcomes following SLT have improved in recent years and may have achieved parity with WLT.(7) However, reports on outcomes for pediatric recipients following technical variant donation, and in particular SLT, are conflicting.(8–10)
Given these inconsistent findings, the purpose of our analysis was to use a large national registry to better understand the impact of allograft type on patient and graft survival for pediatric liver transplant recipients in the most recent era. Furthermore, we sought to assess whether the association between allograft type and outcomes following transplantation have changed over time period and whether these effects vary by follow-up time. Finally, we wanted to better understand which factors are associated with graft failure and whether the causes of graft failure have changed in recent years.
METHODS
This study used data from the SRTR data system that includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN) and has been described elsewhere.(11) The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of, or interpretation by, the SRTR or the U.S. Government.
We identified 5715 pediatric (age <18 years), liver-only transplant recipients who received an organ between March 1, 2002 (ie, after implementation of the PELD/MELD system) and December 31, 2015. Additionally, people were excluded for the following reasons: listed as live donor whole liver (n = 10), missing weight (n = 1), missing cold ischemia time (n = 395; 6% of eligible individuals). Additionally, retransplanted indivuals were excluded (n = 735) because there was very low number of individuals retransplanted with technical variant grafts (n = 194 for split liver transplant; n = 35 for living donor liver transplant). No donor organs were obtained from executed prisoners or other institutionalized persons. Individuals were defined as having a split liver transplant (SLT) if they received a portion of a deceased donor graft, irrespective of whether the organ was used by one or two recipients as evidence from other studies suggest comparable graft and patient survival, and even potentially comparable biliary strictures and vascular thromboses.(7,8) We compared demographic (e.g. age, sex, race, and insurance status) and clinical (e.g., weight, PELD/MELD, and diagnosis) characteristics between pediatric recipients of SLT, whole liver transplantation (WLT) and living donor liver transplantation (LDLT) using χ2 tests for categorical variables and analysis of variance (ANOVA) for continuous variables.
We calculated patient and graft survival at 30-days and 1-year following SLT, WLT, or LDLT, and compared survival between allograft types using Kaplan-Meier curves and log-rank tests. Patient death was identified using the SRTR, which is linked to the Social Security Master File and confirmed through clinician report. Graft failure was identified as any reported graft failure or death (i.e., “all cause graft loss”). Recipients were censored upon re-transplantation or multi-organ transplantation (e.g., liver-kidney).
We used Cox proportional hazards models to characterize the association between allograft type and graft and patient survival after adjustment for recipient weight at transplant, recipient age at transplant, sex, race/ethnicity, underlying disease, allocation PELD/MELD at transplant, status 1 designation, donor age, cold ischemia time (CIT), and insurance type; sensitivity analysis with laboratory PELD/MELD and exception status were also performed and did not influence findings. Additional sensitivity analysis with transplant region was assessed using shared frailty modelling were reported.(12) The decision to include these specific variables in the final model for multivariable regression was derived from associations between covariates with risk factors and the outcome in both the published literature as well as statistical tests (e.g., chi-square, ANOVA) within this cohort.
To characterize changes in unadjusted patient and graft survival over time, 30-day and 1-year survival between allograft types were further stratified by time period of transplantation (i.e., 2002–2009 vs 2010–2015). We used an interaction term analysis to determine whether the association between allograft type and adjusted patient and graft survival varied over time period.
We tested whether the hazard associated with patient survival following SLT, LDLT, and WLT varied over follow-up time using a time-binned analysis and estimated the hazard associated with each allograft type within the first 30 days post-transplant (i.e., early) and after the first 30 days post-transplant (i.e., late).
Statistical Analyses
All statistical tests used a 2-sided α of 0.05. Categorical variables were compared using a chi-square test and continuous variables were compared using ANOVA. Confidence intervals are reported using the method of Louis and Zeger, as previously reported.(13) All analyses were performed using STATA 14.0 (College Station, Texas). This study was approved by the Institutional Review Board of Johns Hopkins University School of Medicine.
RESULTS
Among the 5715 children who underwent liver transplantation, 3428 (60%) received a WLT, 1626 (28.5%) a SLT, and 661 (11.6%) an LDLT (Table I; available at www.jpeds.com). SLT and LDLT recipients were more likely to be under 2 years and 10 kg (P < 0.001 for both age and weight). African Americans were less likely than Caucasians to undergo LDLT, and more likely to receive a whole graft (P < 0.001). LDLT recipients were more likely to have had biliary atresia (P < 0.001). The donor age for nearly all individuals receiving a living donor was 18–50 years of age, whereas WLT recipients were more likely to have donors age 0–17 years (P < 0.001). PELD/MELD score at transplant was lower in LDLT (P < 0.001), and SLT recipients were more likely to be status 1 than WLT and LDLT recipients (P < 0.001). The mean cold ischemia time (CIT) was shortest for LDLT (P < 0.001). LDLT recipients were more likely to have private insurance and SLT recipients were more likely to have public insurance (P < 0.001).
Table 1.
Demographic and Clinical Characteristics by Transplant Type
| Characteristic | WLT | SLT | LDLT | P |
|---|---|---|---|---|
| Total | 3428 (60.0) | 1626 (28.5) | 661 (11.6) | |
| Recipient Age | ||||
| <2 years | 1328 (38.7) | 983 (60.5) | 418 (63.2) | <0.001 |
| 2–5 years | 592 (17.3) | 355 (21.8) | 95 (14.4) | |
| 5–12 years | 709 (20.7) | 225 (13.8) | 87 (13.2) | |
| 12–18 years | 799 (23.3) | 63 (3.9) | 61 (9.2) | |
| Recipient Weight | ||||
| <10 kg | 1119 (32.6) | 832 (51.2) | 361 (54.6) | <0.001 |
| 10–35 kg | 1296 (37.8) | 691 (42.5) | 218 (33.0) | |
| >35 kg | 1013 (29.6) | 103 (6.3) | 82 (12.4) | |
| Female | 1769 (51.6) | 812 (49.9) | 331 (50.1) | 0.5 |
| Race/ethnicity | ||||
| Caucasian, non-Hispanic | 1793 (52.3) | 796 (49.0) | 389 (58.9) | <0.001 |
| African American | 594 (17.3) | 243 (15.4) | 74 (11.2) | |
| Hispanic | 736 (21.5) | 431 (26.5) | 142 (21.5) | |
| Asian | 193 (5.6) | 99 (6.1) | 43 (6.5) | |
| mixed/other | 112 (3.3) | 50 (3.1) | 13 (2.0) | |
| Disease | ||||
| biliary atresia | 1195 (34.9) | 710 (43.7) | 341 (51.6) | <0.001 |
| metabolic disease | 578 (16.9) | 231 (14.2) | 50 (7.6) | |
| acute hepatic necrosis | 451 (13.2) | 222 (13.7) | 81 (12.3) | |
| tumor | 314 (9.2) | 159 (9.8) | 31 (4.7) | |
| miscellaneous | 890 (26.0) | 304 (18.7) | 158 (23.9) | |
| Donor Age | ||||
| 0–17 years | 2890 (84.3) | 968 (59.5) | 1 (0.2) | <0.001 |
| 18–50 years | 468 (13.7) | 628 (38.6) | 642 (97.1) | |
| >50 years | 70 (2.0) | 30 (1.8) | 18 (2.7) | |
| PELD/MELD at transplant* | 23.6 (8.9) | 24.9 (8.6) | 21.0 (11.8) | <0.001 |
| Status 1 | 1089 (31.8) | 614 (37.8) | 144 (21.8) | <0.001 |
| Cold Ischemia Time* (hour) | 7.3 (3.5) | 7.3 (2.9) | 2.8 (5.3) | <0.001 |
| Insurance | ||||
| public | 1697 (46.9) | 898 (55.2) | 217 (32.8) | <0.001 |
| private | 1608 (46.9) | 685 (42.1) | 413 (62.5) | |
| mixed/other | 123 (3.6) | 43 (2.6) | 31 (4.7) | |
| Time Period | ||||
| 2002–2009 | 1842 (53.7) | 890 (54.7) | 346 (52.3) | 0.6 |
| 2010–2015 | 1586 (46.3) | 736 (45.3) | 315 (47.7) | |
mean (SD)
From 2002–2009 to 2010–2015, the frequency of transplants was similar for WLT (60% for both), SLT (29% and 28%) and LDLT (11% and 12%) (P = 0.6). Frequency of reduced SLT (i.e., “cut-down”), where only one portion was used, was the same for both periods (13.2% and 13.6%). Among 104 centers performing any type of liver transplant, 66 (63%) centers performed at least one SLT over the entire study period, and 8 (8%) performed at least one SLT each year. Fifty-seven (55%) centers performed at least one LDLT over the entire study period, and 4 (4%) centers performed at least one LDLT per year.
Short-term Patient Survival
Since the PELD/MELD system was implemented in 2002, the unadjusted 30-day patient survival across all allograft types was 97%, and was significantly lower in SLT compared with WLT (96% vs 98%; P < 0.001), and survival for LDLT and WLT was similar (98% for both; P = 0.4; Table 2). The relative impact of allograft type on short-term survival varied by time period (Figure 1). From 2002–2009, survival following SLT was worse than WLT (94% vs 98%; P < 0.001) whereas from 2010–2015, no significant difference was observed (98% for both; P = 0.96). Outcomes following LDLT were similar to WLT in both 2002–2009 (97% vs 98% P = 0.96) and 2010–2015 (99% vs 98%; P = 0.2). Only SLT demonstrated an improvement in short-term survival (94% in 2002–2009 vs 98% in 2010–2015; P < 0.001). In an adjusted model, SLT was associated with a 2.14-fold higher risk of early death (i.e., within 30 days) from 2002–2009 (aHR: 1.472.143.12; Table 3) and there was no increased risk of early death from 2010–2015 (aHR: 0.651.131.96), representing a significant improvement (P = 0.04). Adjustment for transplant region did not change inferences. Short-term survival following LDLT was the same in both time periods (aHR vs WLT in 2002–2009: 0.491.032.14; aHR vs WLT in 2010–2015: 0.260.742.10).
Table 2.
30-day and 1-year Unadjusted Patient Survival
| Overall | 2002–2009 | 2010–2015 | |||||
|---|---|---|---|---|---|---|---|
| Survival | P | Survival | P | Survival | P | P* | |
| 30-day | |||||||
| all allografts | 0.97 | 0.96 | 0.98 | 0.004 | |||
| WLT | 0.98 | – | 0.98 | – | 0.98 | – | 0.7 |
| SLT | 0.96 | <0.001 | 0.94 | <0.001 | 0.98 | 0.96 | <0.001 |
| LDLT | 0.98 | 0.4 | 0.97 | 0.96 | 0.99 | 0.2 | 0.2 |
| 1-year | |||||||
| all allografts | 0.94 | 0.93 | 0.96 | <0.001 | |||
| WLT | 0.95 | – | 0.94 | – | 0.95 | – | 0.2 |
| SLT | 0.92 | 0.002 | 0.89 | <0.001 | 0.95 | 0.7 | <0.001 |
| LDLT | 0.96 | 0.2 | 0.93 | 0.6 | 0.98 | 0.01 | 0.002 |
WLT (whole liver transplantation); SLT (split liver transplantation); LDLT (living-donor liver transplantation)
P represents test of significance from log-rank tests for difference in survival for a specific allograft type from 2002–2009 to 2010–2015
Figure 1.
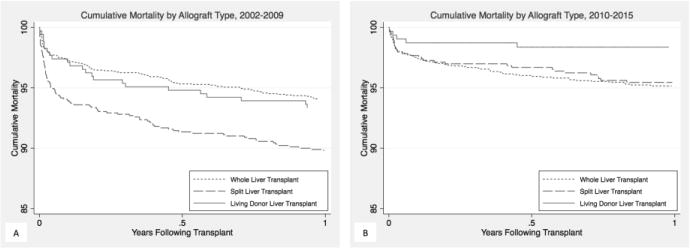
Kaplan-Meier curve of patient survival by allograft type in the first year after transplant from (A) 2002–2009; and (B) 2010–2015
Table 3.
Adjusted Hazard Ratio (aHR) for Risk of Death
| 2002–2009 | 2010–2015 | ||||
|---|---|---|---|---|---|
| aHR (95% CI) | P | aHR (95% CI) | P | P* | |
| Within 30 days | |||||
| WLT | – | – | – | – | – |
| SLT | 1.472.143.12 | <0.001 | 0.651.131.96 | 0.7 | 0.04 |
| LDLT | 0.491.032.14 | 0.9 | 0.260.742.10 | 0.65 | 0.6 |
| After 30 days | |||||
| WLT | – | – | – | – | – |
| SLT | 0.871.141.48 | 0.4 | 0.560.881.37 | 0.6 | 0.3 |
| LDLT | 0.520.831.32 | 0.4 | 0.170.441.11 | 0.08 | 0.2 |
CI (confidence interval); WLT (whole liver transplantation); SLT (split liver transplantation); LDLT (living-donor liver transplantation)
P tests whether the aHR for SLT and LDLT, each compared to WLT, varies by time period (i.e., interaction).
Long-term Patient Survival
Patient survival at 1 year was 94% for all pediatric recipients over the study period and significantly lower for SLT compared with WLT (92% vs 95%; P = 0.002), but similar for LDLT and WLT (96% vs 95%; P = 0.2). The relative impact of allograft type on short-term survival varied by time period. From 2002–2009, survival following SLT was worse than WLT (89% vs 94%; P < 0.001), whereas no significant difference was observed from 2010–2015 (95% for both; P = 0.2). From 2002–2009, survival following LDLT was similar to WLT (93% vs 94%, P = 0.6), but from 2010–2015, survival was higher for LDLT (98% vs 95%; P = 0.01). Survival at 1 year improved for both SLT (89% in 2002–2009 and 95% in 2010–2015; P < 0.001) and LDLT (93% in 2002–2009 and 98% in 2010–2015; P = 0.002) but did not improve for WLT (94% in 2002–2009 and 95% in 2010–2015; P = 0.2). Compared with WLT, the long-term (i.e., after 30 days) risk of death was similar in both time periods for SLT (aHR in 2002–2009: 0.871.141.48; aHR in 2010–2015: 0.560.881.37) and LDLT (aHR in 2002–2009: 0.520.831.32; aHR in 2010–2015: 0.170.441.11).
Graft Survival
Overall unadjusted 30-day graft survival was 93% (Table 4 and Figure 2; available at www.jpeds.com). Only SLT showed significant improvement from 2002–2009 to 2010–2015 (90% vs 93%; P = 0.01) whereas no improvement was seen in WLT (92% vs 93%; P = 0.1) or LDLT (94% vs 95%; P = 0.6). Graft survival at 1 year improved for SLT (85% vs 90%; P = 0.002) and LDLT (89% vs 94%; P = 0.03) but not WLT 89% vs 90%; P = 0.14). In an adjusted model, the association between allograft type and graft survival did not vary by time period (P > 0.05 for interaction coefficients) and thus, overall estimates are reported instead. Additionally, the hazard of graft failure was proportionally constant throughout the follow-up period and therefore early and late graft failure were not evaluated separately. Compared with WLT, SLT was not associated with an increased risk of graft failure (aHR: 0.931.091.28), although LDLT was associated with a lower risk of graft failure (aHR: 0.530.710.95; Table 5). Overall graft survival improved from 2002–2009 to 2010–2015 (aHR: 0.650.740.86).
Table 4.
30-day and 1-year Unadjusted Graft Survival
| Overall | 2002–2009 | 2010–2015 | |||||
|---|---|---|---|---|---|---|---|
| Survival | P | Survival | P | Survival | P | P* | |
| 30-day | |||||||
| all allografts | 0.93 | 0.92 | 0.93 | 0.1 | |||
| WLT | 0.93 | – | 0.93 | – | 0.93 | – | 0.9 |
| SLT | 0.92 | 0.06 | 0.90 | 0.005 | 0.93 | 0.7 | 0.01 |
| LDLT | 0.94 | 0.2 | 0.94 | 0.6 | 0.95 | 0.2 | 0.6 |
| 1-year | |||||||
| all allografts | 0.89 | 0.87 | 0.91 | <0.001 | |||
| WLT | 0.89 | – | 0.89 | – | 0.90 | – | 0.14 |
| SLT | 0.87 | 0.02 | 0.85 | 0.004 | 0.90 | 0.9 | 0.002 |
| LDLT | 0.91 | 0.2 | 0.89 | 0.9 | 0.94 | 0.05 | 0.03 |
WLT (whole liver transplantation); SLT (split liver transplantation); LDLT (living-donor liver transplantation)
P represents test of significance using log-rank test of difference in survival for a specific allograft type from 2002–2009 to 2010–2015
Figure 2.
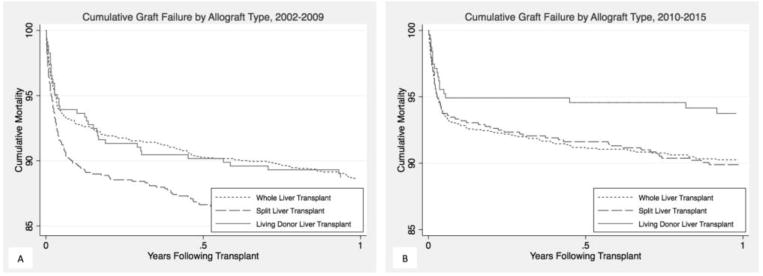
Kaplan-Meier curve of graft survival by allograft type in the first year after transplant from (A) 2002–2009; (B) 2010–2015
Table 5.
Adjusted Hazard Ratio (aHR) for Graft Failure
| Graft Failure | ||
|---|---|---|
| Allograft | aHR (95% CI) | P |
| WLT | – | – |
| SLT | 0.931.091.28 | 0.3 |
| LDLT | 0.530.710.95 | 0.02 |
CI (confidence interval); WLT (whole liver transplantation); SLT (split liver transplantation); LDLT (living-donor liver transplantation)
Additional Risk Factors for Death and Graft Failure
In a multivariable model, several other characteristics were associated with both death and graft failure (Table 6; available at www.jpeds.com). Acute hepatic necrosis, malignancy, and status 1 designation were associated with increased death and graft loss, as was public insurance and donor age >50 years. Recipient race/ethnicity, weight, and allocation score at transplant were not associated with death or graft failure. Although recipient age was not associated with death, children 2–12 years had lower graft loss than children <2 years. Years 2010–2015 was overall associated with lower death and graft loss.
Table 6.
Additional Risk Factors for Death and Graft Failure
| Death | Graft Failure | |||
|---|---|---|---|---|
| Characteristic | aHR | P | aHR | P |
| Disease | ||||
| Biliary Atresia | – | – | – | – |
| Metabolic Disease | 0.851.171.61 | 0.3 | 0.891.121.41 | 0.3 |
| Acute Hepatic Necrosis | 1.201.6 72.31 | 0.002 | 1.181.511.93 | 0.001 |
| Malignancy | 2.202.974.02 | <0.001 | 1.491.892.41 | <0.001 |
| Other/unknown | 1.511.922.45 | <0.001 | 1.301.561.86 | <0.001 |
| Recipient race/ethnicity | ||||
| Caucasian, non-Hispanic | – | – | – | – |
| African American | 0.961.211.52 | 0.1 | 0.921.101.30 | 0.3 |
| Hispanic | 0.780.961.20 | 0.7 | 0.740.871.03 | 0.1 |
| Asian | 0.610.911.35 | 0.6 | 0.570.781.06 | 0.1 |
| Mixed/other | 0.580.951.57 | 0.8 | 0.610.891.29 | 0.5 |
| Recipient weight | ||||
| <10 kg | – | – | – | – |
| 10–35 kg | 0.690.931.26 | 0.7 | 0.730.921.15 | 0.5 |
| >35 kg | 0.580.951.57 | 0.8 | 0.620.911.33 | 0.6 |
| Recipient age | ||||
| <2 years | – | – | – | – |
| 2–5 years | 0.530.751.04 | 0.09 | 0.600.770.99 | 0.04 |
| 5–12 years | 0.581.851.23 | 0.4 | 0.540.710.95 | 0.02 |
| 12–18 years | 0.701.171.95 | 0.5 | 0.660.971.44 | 0.9 |
| Insurance | ||||
| Private | – | – | – | – |
| Public | 1.201.441.72 | <0.001 | 1.081.241.41 | 0.002 |
| Other/missing | 0.540.941.61 | 0.8 | 0.520.791.20 | 0.3 |
| Allocation score at transplant | 0.991.001.01 | 0.5 | 0.991.001.00 | 0.3 |
| Status 1 | 1.001.231.51 | 0.05 | 1.021.191.61 | 0.03 |
| Cold ischemia time (hour) | 0.991.011.03 | 0.2 | 1.001.011.03 | 0.07 |
| Donor age | ||||
| <18 years | – | – | – | – |
| 18–50 years | 0.911.151.44 | 0.2 | 0.981.181.41 | 0.07 |
| >50 years | 1.171.791.2.73 | 0.007 | 1.742.383.24 | <0.001 |
| Era (versus 2002–2009) | 0.580.740.95 | 0.02 | 0.650.740.86 | <0.001 |
Regional Variation
Transplant region was associated with allograft type in both 2002–2009 (P < 0.001) and 2010–2015 (P < 0.001). Inclusion of transplant region into the model did not affect inferences on patient. For example, similar to the model without region, the 30-day risk of death for SLT was increased in 2002–2009 (aHR: 1.482.163.13) but not increased in 2010–2015 (aHR: 0.681.182.04) representing a significant improvement (P = 0.04). Similarly, inclusion of region into the model did not influence risk of graft loss (aHR for SLT: 0.941.101.29; aHR for LDLT: 0.550.741.00).
DISCUSSION
In this national study examining trends in pediatric liver transplantation since the implementation of the PELD/MELD system, several important findings were evident with respect to the relationship between allograft type and patient/graft survival. First, although overall outcomes have improved, these can be largely attributed to improvements in early outcomes following SLT, as well as to improvements in long-term outcomes following SLT and LDLT; outcomes following WLT have been largely unchanged since the current PELD/MELD system was implemented. Second, poor outcomes for SLT were initially due to increased early death but this problem is no longer evident such that risk of early death in SLT has decreased, and is similar to, WLT. Finally, graft survival for LDLT was superior to WLT. Collectively, these findings suggest that the increasing experience with technical variant grafts such as SLT and LDLT have coincided with improved patient and graft survival.
Our analysis also identifies several important risk factors for death and graft failure in this large cohort including notable findings that better outcomes may be seen in biliary atresia, as well as lower rates of graft failure in children between 2–12 years. Additionally, although all race/ethnic groups had comparable outcomes, higher rates of death and graft loss were seen in individuals with public insurance. These findings are consistent with other challenges facing individuals in the pediatric liver transplant community with public insurance such as higher rates of waitlist mortality, and lower likelihood of obtaining exception points.(14,15)
Reports on the impact of allograft type in pediatric liver transplantation have been conflicting. The SPLIT consortium of 44 pediatric centers examined a range of outcomes on recipients from 1995–2006 and found increased graft failure in SLT, but not LDLT, when compared with WLT in an unadjusted model.(8) These authors also reported higher rates of complications requiring either surgical revision in both SLT and LDLT compared with WLT. A second study from the SPLIT consortium showed increased death and graft loss for both technical variant grafts.(16) A large single-center study of recipients between 1993–2006 similarly found a higher risk of mortality and graft failure in SLT, but not LDLT.(9) Other studies derived from the UNOS and SRTR registries prior to the implementation of PELD/MELD have shown a general tendency for SLT to have worse patient and graft survival, whereas LDLT may have superior or equivalent outcomes compared with WLT.(17,18) At the same time, some studies have suggested that allograft type does not affect outcomes. Austin et al looked at outcomes in UNOS from as early as 1987–2004 and showed no difference in patient and graft survival by allograft type, but this finding may be driven by relatively poor outcomes in this cohort from all transplants in the early years of the cohort. For example, the authors report an overall 1-year patient survival of 83% compared with 94% in our study.(19) Finally, in the most recent study from SRTR that evaluated patient and graft survival in a limited cohort of children under 12 years old from 2002–2004, there was no variability in outcomes by allograft type.(10)
Our finding that overall patient and graft survival following pediatric liver transplantation have improved over time is broadly consistent with other studies.(20,21) One large study derived from the United Network for Organ Sharing (UNOS) database from 1995–2010 of children showed that patient and graft survival improved from 1995–2000 to 2006–2010, but saw similar results between 2001–2005 and the end of the study period.(20) This study was limited to children under 2 years old that received a whole or deceased split graft, and because it spanned the implementation of PELD/MELD, it was not possible to adjust for the score or status 1 designation and authors used ICU and ventilator status instead. They also tested for interaction between eras and allograft status and showed a trend toward improved relative hazard following SLT compared with WLT, but the interaction between era and allograft type was not significant for patient or graft survival. Here, we demonstrated overall improvement since PELD/MELD was implemented and across all pediatric age groups. Furthermore, we showed significant improvement in the most recent transplant period (i.e., 2010–2015) such that outcomes following SLT are now similar to WLT.
An important consideration when discussing increased adoption of SLT is the impact on the adult recipient who might otherwise get a whole graft, especially as split liver transplantation has been seen as contributing to a donor risk index in some, but not all, adult studies.(22,23) A recent study of the UNOS database looking at adult SLT recipients reported similar findings that patient and graft survival have improved over time and are now similar to WLT.(7) At the same time, increased vascular and biliary complications continue to be reported in technical variant donation relative to WLT and one important limitation of our study was that we were unable to explore these additional complications.(16,24,25) Although the use of these grafts will increase the organ supply, allow for earlier transplantation, and potentially decrease total costs, at the same time, complications from these grafts are likely to be associated with longer length of stay and greater cost in the perioperative period.(3,25,26) Given the current need to optimize outcomes and reduce costs (i.e., increase value), the decision to use these organs by transplant teams, and to advocate for greater use through policy, will require a better understanding of the frequency of these complications and how these complications impact care from a number of perspectives.(27) Nonetheless, some centers have incorporated practices where SLT is prioritized, and have been able to achieve the competing goal of good long-term outcomes alongside the benefits of increased organ supply.(28)
Another limitation of our study was that it was derived from an observational cohort as opposed to an experimental study. Although the finding that overall outcomes have improved over time should be expected, it is difficult to know whether the decision by the transplant team to perform a specific type of transplant is a reflection of their assessment of the patient’s disease severity, surgical experience, or some other factor; if SLT was only performed when the patient was perceived to be relatively stable, this decision might influence the observed outcomes. One advantage of our study is that it is conducted exclusively in the PELD/MELD era, and we adjusted for the score at transplant as well as exception status, a well-validated tool for assessing medical severity. Consequently, the relative effects that were seen in the multivariable model that adjusted for score and exception status should account for the impact of disease severity. Although it is possible that the improvement seen in SLT can be attributed to unmeasured or residual confounding of disease severity that coincides with a shift in clinical practice and decision making, our evidence nonetheless suggests that a group of children can do well with SLT, and that more research should be performed to identify the specific patient, donor, and surgical characteristics that yield good outcomes.
Finally, it should be noted that there can be errors in reporting from studies derived from national registries, such as patients being incorrectly classified as dead or with graft failure. However, these errors should be minimal, if they exist at all, given that SRTR verifies death with the Social Security Master File and that graft failure must be accurately identified in the registry in order for a patient to receive a new liver.
Given shortages in organ supply, there is continued interest and effort in identifying additional opportunities to expand the supply, including use of extended criteria donors, donation after cardiac death, and technical variant donation.(6,29–31) Children may be particularly vulnerable to decreased supply, with a recent report suggesting that nearly half of all children that died on the waitlist had not received a single offer of a liver, with a median offer number of one.(14) Size mismatch was identified in nearly one third of patients as a reasons offers were not accepted, though nearly half may have actually been an appropriate size, suggesting the potential for greater use of split transplantation in reducing waitlist mortality. Our national study of over 5000 pediatric liver transplant recipients provides strong evidence that allograft type no longer predicts patient and graft survival in this population. These findings have the potential to substantially influence policy for allocation of deceased organs to children in need. Given that children compose a relatively small percentage of people on the national waitlist, increased use of SLT might provide an optimal way to increase the supply for children, without placing them at risk for worse outcomes, so that pre-transplant mortality and morbidity can be minimized.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of, or interpretation by, the SRTR or the US Government.
Financial support
D.M. receives research support from the Agency for Healthcare Research and Quality (5K08HS023876-02). A.M. receives research support from the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK101677). D.S. receives research support from the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK101828). The other authors declare no conflicts of interest.
Abbreviations
- aHR
adjusted hazard ratio
- LDLT
living-donor liver transplant
- SLT
split liver transplant
- WLT
whole liver transplant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2015 Jan;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 2.Perera MTPR, Mirza DF, Elias E. Liver transplantation: Issues for the next 20 years. J Gastroenterol Hepatol. 2009 Oct;24(Suppl 3):S124–131. doi: 10.1111/j.1440-1746.2009.06081.x. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan P, Dzebisashvili N, Lentine KL, Axelrod DA, Schnitzler MA, Salvalaggio PR. Liver transplantation cost in the model for end-stage liver disease era: looking beyond the transplant admission. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009 Oct;15:1270–7. doi: 10.1002/lt.21802. [DOI] [PubMed] [Google Scholar]
- 4.Mohammad S, Alonso EM. Approach to optimizing growth, rehabilitation, and neurodevelopmental outcomes in children after solid-organ transplantation. Pediatr Clin North Am. 2010 Apr;57:539–557. doi: 10.1016/j.pcl.2010.01.014. table of contents. [DOI] [PubMed] [Google Scholar]
- 5.Alonso EM, Martz K, Wang D, Yi MS, Neighbors K, Varni JW, et al. Factors predicting health-related quality of life in pediatric liver transplant recipients in the functional outcomes group. Pediatr Transplant. 2013 Nov;17:605–11. doi: 10.1111/petr.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: A path toward improving outcomes and eliminating wait-list mortality. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017 Jan;23:86–95. doi: 10.1002/lt.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, et al. Deceased-donor split-liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg. 2013 Oct;217:672–684.e1. doi: 10.1016/j.jamcollsurg.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, et al. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT) Ann Surg. 2007 Aug;246:301–10. doi: 10.1097/SLA.0b013e3180caa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009 May;208(5):682–689. doi: 10.1016/j.jamcollsurg.2009.01.023. discusion 689-691. [DOI] [PubMed] [Google Scholar]
- 10.Becker NS, Barshes NR, Aloia TA, Nguyen T, Rojo J, Rodriguez JA, et al. Analysis of recent pediatric orthotopic liver transplantation outcomes indicates that allograft type is no longer a predictor of survivals. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2008 Aug;14:1125–32. doi: 10.1002/lt.21491. [DOI] [PubMed] [Google Scholar]
- 11.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014 Aug;14:1723–30. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 13.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostat Oxf Engl. 2009 Jan;10:1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu EK, Shaffer ML, Gao L, Sonnenday C, Volk ML, Bucuvalas J, et al. Analysis of Liver Offers to Pediatric Candidates on the Transplant Wait List. Gastroenterology. 2017 Oct;153:988–95. doi: 10.1053/j.gastro.2017.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perito ER, Braun HJ, Dodge JL, Rhee S, Roberts JP. Justifying Nonstandard Exception Requests for Pediatric Liver Transplant Candidates: An Analysis of Narratives Submitted to the United Network for Organ Sharing, 2009–2014. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2017 Aug;17:2144–54. doi: 10.1111/ajt.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011 Jul;254:145–54. doi: 10.1097/SLA.0b013e31821ad86a. [DOI] [PubMed] [Google Scholar]
- 17.Abt PL, Rapaport-Kelz R, Desai NM, Frank A, Sonnad S, Rand E, et al. Survival among pediatric liver transplant recipients: impact of segmental grafts. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2004 Oct;10:1287–93. doi: 10.1002/lt.20270. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JP, Hulbert-Shearon TE, Merion RM, Wolfe RA, Port FK. Influence of graft type on outcomes after pediatric liver transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2004 Mar;4:373–7. doi: 10.1111/j.1600-6143.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 19.Austin MT, Feurer ID, Chari RS, Gorden DL, Wright JK, Pinson CW. Survival after pediatric liver transplantation: why does living donation offer an advantage? Arch Surg Chic III 1960. 2005 May;140:465–470. doi: 10.1001/archsurg.140.5.465. discussion 470-471. [DOI] [PubMed] [Google Scholar]
- 20.Cauley RP, Vakili K, Potanos K, Fullington N, Graham DA, Finkelstein JA, et al. Deceased donor liver transplantation in infants and small children: are partial grafts riskier than whole organs? Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2013 Jul;19:721–9. doi: 10.1002/lt.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh A. An analysis of liver transplant survival rates from the UNOS registry. Clin Transpl. 2008:19–34. [PubMed] [Google Scholar]
- 22.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006 Apr;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 23.Avens Publishing Group. The Donor Risk Score: A Simpler Method to Grade Liver Allografts [Internet] [cited 2017 Oct 2]. Available from: http://www.avensonline.org/fulltextarticles/jsur-2332-4139-01-0012.html.
- 24.Mabrouk Mourad M, Liossis C, Kumar S, Gunson BK, Mergental H, Isaac J, et al. Vasculobiliary complications following adult right lobe split liver transplantation from the perspective of reconstruction techniques. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2015 Jan;21:63–71. doi: 10.1002/lt.24015. [DOI] [PubMed] [Google Scholar]
- 25.Axelrod DA, Dzebisashvili N, Lentine KL, Xiao H, Schnitzler M, Tuttle-Newhall JE, et al. Variation in biliary complication rates following liver transplantation: implications for cost and outcome. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2015 Jan;15:170–9. doi: 10.1111/ajt.12970. [DOI] [PubMed] [Google Scholar]
- 26.Axelrod DA, Gheorghian A, Schnitzler MA, Dzebisashvili N, Salvalaggio PR, Tuttle-Newhall J, et al. The economic implications of broader sharing of liver allografts. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2011 Apr;11:798–807. doi: 10.1111/j.1600-6143.2011.03443.x. [DOI] [PubMed] [Google Scholar]
- 27.Porter ME. What is value in health care? N Engl J Med. 2010 Dec 23;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 28.Battula NR, Platto M, Anbarasan R, Perera MTPR, Ong E, Roll GR, et al. Intention to Split Policy: A Successful Strategy in a Combined Pediatric and Adult Liver Transplant Center. Ann Surg. 2017 May;265:1009–15. doi: 10.1097/SLA.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 29.Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2007 May;7:1265–70. doi: 10.1111/j.1600-6143.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 30.Eren EA, Latchana N, Beal E, Hayes D, Whitson B, Black SM. Donations After Circulatory Death in Liver Transplant. Exp Clin Transplant Off J Middle East Soc Organ Transplant. 2016 Oct;14:463–70. [PMC free article] [PubMed] [Google Scholar]
- 31.San Juan F, Cortes M. Mortality on the waiting list for liver transplantation: management and prioritization criteria. Transplant Proc. 2011 Apr;43:687–9. doi: 10.1016/j.transproceed.2011.01.106. [DOI] [PubMed] [Google Scholar]


