Abstract
Objective
Fluorescence-guided surgery with protoporphyrin IX (PpIX) as a photodiagnostic marker is gaining acceptance for resection of malignant gliomas. Current wide-field imaging technologies do not have sufficient sensitivity to detect low PpIX concentrations. We evaluated a scanning fiber endoscope (SFE) for detection of PpIX fluorescence in gliomas and compared it with an operating microscope (OPMI) equipped with fluorescence module and a benchtop confocal laser scanning microscope (CLSM).
Methods
5-aminolevulinic acid (5-ALA)-induced PpIX fluorescence was assessed in GL261-Luc2 cells in vitro and in vivo after implantation in mouse brains, at an invading glioma growth stage, simulating residual tumor. Intraoperative fluorescence of high and low PpIX concentrations in normal brain and tumor regions with SFE, OPMI, CLSM, and histopathology were compared.
Results
SFE imaging of PpIX correlated to CLSM at the cellular level. PpIX accumulated in normal brain cells but significantly less than in glioma cells. SFE was more sensitive to accumulated PpIX in fluorescent brain areas than OPMI (P<.01) and dramatically increased imaging time (>6×) before tumor-to-background contrast was diminished because of photobleaching.
Conclusions
SFE provides new endoscopic capabilities to view PpIX-fluorescing tumor regions at cellular resolution. SFE may allow accurate 5-ALA application to gliomas and other tumor types when current detection techniques have failed to provide reliable visualization. SFE was significantly more sensitive than OPMI to low PpIX concentrations, which is relevant to identifying the leading edge or metastasizing cells of malignant glioma or to treating low-grade gliomas. This new application has the potential to benefit surgical outcomes.
Keywords: 5-aminolevulinic acid, fluorescence-guided surgery, glioma, microscopy, protoporphyrin IX, scanning fiber endoscope
INTRODUCTION
Surgical removal is the first-line treatment for brain gliomas, and increasing the extent of resection correlates with better survival and quality of life.[1] The theranostic implications of fluorescence-guided neurosurgery—increased surgical precision and improved completeness of resection during brain tumor surgery—have been reviewed.[2] Specifically, the extent of glioblastoma multiforme resection has substantially improved under aminolevulinic acid (5-ALA)–induced protoporphyrin IX (PpIX) fluorescence guidance.[3,4] When administered orally before surgery, 5-ALA metabolizes and accumulates as the photodiagnostic biomarker PpIX within neoplastic tissue. Although high-grade gliomas are usually highly fluorescent, low-grade gliomas show significantly lower PpIX fluorescence, which is detectable in only 9%–15.9% of cases.[5,6] Fluorescence in detectable low-grade diffuse infiltrative gliomas is not observed throughout the tumor mass, but rather mostly in the anaplastic foci, which correlates to increased cell proliferation and malignant potential.[5] Similarly, several studies suggest that PpIX detection using current operating microscopes is suboptimal and that PpIX accumulates in low-grade gliomas at levels below the detection limit of current systems.[7–10] Therefore, improved PpIX detection offers considerable promise for PpIX-guided surgery.
In this study, we assess a scanning fiber endoscope (SFE) for PpIX-guided glioma removal in an animal model of malignant glioma. We also describe the features of in vivo PpIX cellular-level imaging in tumor using SFE technology to identify the leading margin and metastasizing cells in a malignant glioma, and its imaging of tissue in normal brain.
MATERIALS AND METHODS
SFE Probe
We used an SFE imaging platform integrated with low-power 405-nm diode laser for illumination (see Figure, Supplemental Digital Content 1, showing the SFE).[11,12] A piezoelectric actuator sweeps the laser-delivery fiberoptic element in a spiral pattern resulting in a 50°–80° field of view. A concentric ring of 6 light-collection fiberoptics (0.5-mm diameter) relays reflected and fluoresce light to a 3-channel (red, green, and blue [RGB]), wavelength separation, light-detection system. The SFE probe has a 30-Hz imaging frame rate and 20–35 μm optical resolution for probe-to-target distances of 3–8 mm. A real-time image processing algorithm mitigated the autofluorescence level in the red fluorescence channel.[11] Images were recorded as a single frame of the red channel or as a color image (8-bit RGB, 608×608 pixels). RGB images with exposure times of 0.2–2.0 seconds were collected using a Canon EOS camera focused on the SFE display screen.
Operating Microscope
OPMI Pentero 900 (Carl Zeiss Meditec, AG, Oberkochen, Germany) with a BLUE-400 mode was used for imaging (8-bit RGB, 1920×1080 pixels) at a 20-cm distance from the target.
Cell Culture
GL261-Luc2 cells were rendered bioluminescent as described previously.[13] Cells were grown in Dulbecco’s modified Eagle’s medium, containing 10% fetal calf serum, and maintained at 37°C with 5% CO2. Cells were harvested by trypsinization, washed, and resuspended at a concentration of 1–2×107 cells/mL in medium without fetal calf serum before implantation in mice.
Animal Tumor Model
C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine) were used in accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals and with approval from the Institutional Animal Care and Use Committee of St. Joseph’s Hospital and Medical Center. Intracranial implantation was performed by intraperitoneal injection of xylazine (80 mg/kg) and ketamine (10 mg/kg) in anesthetized 10-week-old female mice (n=15) following a previously established protocol, [14] and 2 mice were left without tumors for controls. A GL261-Luc2 cell solution (2 μl of 2.11×107 cells/mL) was injected 3 mm below the brain surface. Confirmatory in vivo tumor growth imaging was performed 14 days later with an IVIS Spectrum System (PerkinElmer, Inc., Waltham, MA)[13] (see Figure, Supplemental Digital Content 2, showing IVIS imaging results).
Animal Surgeries
Animals underwent surgery under anesthesia (80 g/kg xylazine and 10 g/kg ketamine) on days 16–20 post-injection during invading-growth tumor stage as shown on IVIS imaging. Craniotomy, in vivo, and rapid ex vivo imaging with OPMI Pentero and SFE was performed at 2 hours (n=9 implanted, n=2 controls), 3 hours (n=2), 8 hours (n=2), and 24 hours (n=2) after 5-ALA administration (2.5-mg intraperitoneally). The 2-hour post–5-ALA injection start time corresponded to the 2- to 4-hour window used clinically.[15] After surgery, the animals were euthanized according to institutional guidelines. Brains were rapidly removed, sliced in the coronal plane, then imaged ex vivo and further processed.
Brain Specimen Preparation and Histopathologic Imaging
Brains were fixed in 10% formalin, embedded in paraffin, sliced, stained (hematoxylin and eosin [H&E] and Luxol fast blue for myelin) and imaged with an upright Aperio microscopy system (Leica Biosystems, Nussloch, Germany).
For confocal imaging, the brains were fixed in 4% paraformaldehyde and processed using CUBIC protocol.[16] All reagents were from Sigma-Aldrich Co., LLC (St. Louis, Missouri).
Confocal Laser Scanning Microscopy (CLSM)
Live-cell imaging was performed in 35-mm glass-bottom dishes (MatTek Corp., Ashland, Massachusetts) in 5% CO2 at 37°C after 4 mM 5-ALA (NIOPIK, Moscow, Russia) treatment in culture medium. A 405-nm laser was used for PpIX excitation, along with a 598- to 740-nm detection window. Next, cells were fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and stained with Alexa Fluor 633 Phalloidin (Thermo Fischer Scientific, Waltham, Massachusetts) and 4′,6-diamidino-2-phenylindole (DAPI) following manufacturer recommendations.
Coronal mouse brain slices were stained with acridine orange (AO) and imaged on glass-bottom dishes using 405-nm laser and 635–750-nm detection for PpIX, and 488-nm laser with 493- to 593-nm detection for AO.[14]
Confocal imaging was performed on an LSM 710 DUO (Carl Zeiss AG, Oberkochen, Germany) using a Plan-Apochromat 20×/0.8 M27 objective and on a Nikon A1+ (Nikon Instruments Inc., NY) using Apo 40×/1.25 water immersion objective (CFI Apo Lambda S 40X WI).
Image Analysis
Image analysis was performed with Fiji software.[17] The fluorescence tumor-to-background and tumor-to-normal brain ratios were calculated from the red channel. Heat-map histograms were displayed using a 16-color linear look-up table of the red channel. Specular reflections in SFE images were removed by subtracting coregistered regions in the green channel from the red channel to avoid false-positive visual interpretation of the PpIX signal. Quantification and analysis of 3-dimensional CLSM images were performed using Imaris (Bitplane, Inc., Belfast, Northern Ireland).
Statistical Analysis
Statistical analysis was performed using Statistica (Dell, Round Rock, Texas). P<.05 was selected as a minimal significance value.
RESULTS
SFE is Capable of Visualizing PpIX in Individual Tumor Cells in a Monolayer Culture
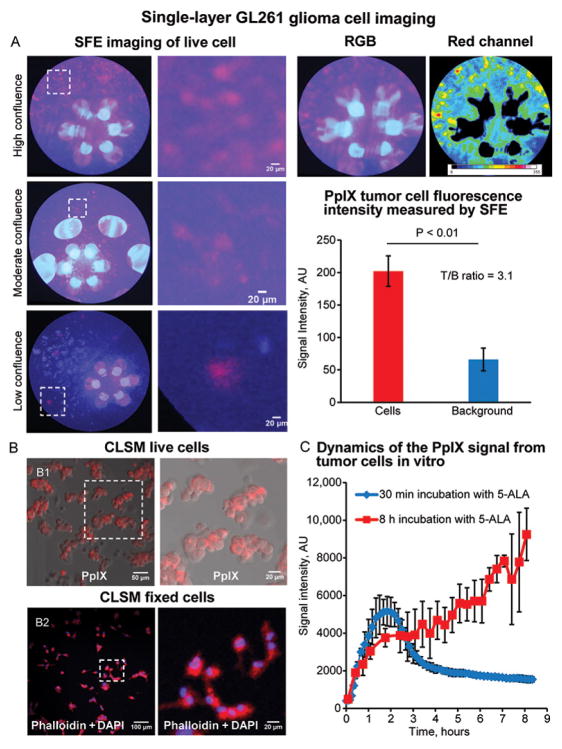
After 1 hour of incubation with 5-ALA, SFE imaging detected PpIX fluorescence from individual glioma cells in a monolayer GL261-Luc2 culture (Figure 1). Measured PpIX fluorescence intensity was 202.1±23.4 arbitrary units (AU) from the cells and 66.0±17.5 AU from the background region (P<.01, Mann-Whitney 2-sided test). Tumor-to-background (T/B) ratio was calculated as 3.1 and matched reported T/B ratio using automatic detection in SFE images.[18] Background fluorescence intensity was higher with SFE than with the CLSM. However, background noise in SFE images can be further reduced by applying a median filter and integrating multiple frames. Correlative CLSM of live cells incubated with 5-ALA and fixed cells stained for nuclei and cytoskeleton suggested that individual groups of cells and separate single cells were visualized with SFE. Live-cell CLSM showed a rapid increase of PpIX fluorescence intensity within 20–40 minutes after incubation with 5-ALA and a subsequent linear increase in values within 8 hours of observation. PpIX fluorescence from a single layer of glioma cells was not visible in OPMI Pentero (see Figure, Supplemental Digital Content 3, operative microscope images).
Figure 1.
In vivo protoporphyrin IX (PpIX) imaging of monolayer GL261 cell culture. (A) Scanning fiber endoscope (SFE) images of individual tumor cells in high-, moderate-, and low-cell-density cell cultures. (B) Confocal laser scanning microscope (CLSM) image of the same culture. Live cells (B1) and cells stained with 4′,6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 633 Phalloidin (B2). (C) Graph of PpIX fluorescence measured during time-series live-cell imaging of GL261 cells incubated with 5-aminolevulinic acid (5-ALA). Group one was imaged continuously after treatment with 5-ALA. The second group was treated for 30 minutes, washed with phosphate-buffered saline 3 times, and incubated in media for 8 hours. N=3 for each time point at each group. Data are presented as mean and standard deviation. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
SFE Imaging of PpIX Fluorescence in Normal Brain
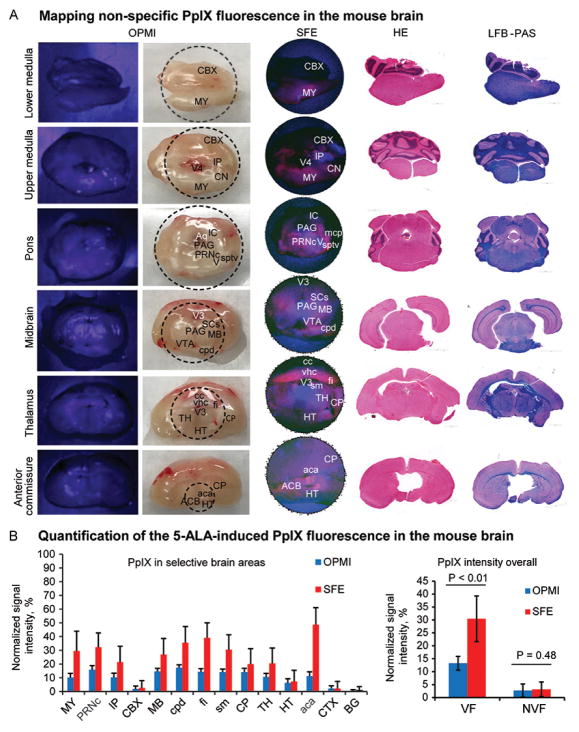
SFE imaging of fresh brain slices and correlative histology showed that nuclei of the medulla, pons, cerebellar peduncles, thalamus, and to a lesser degree the hypothalamic nuclei produced increased PpIX signal. Significant fluorescence signal was also detected from the fimbria of the hippocampus and anterior commissure (Figure 2). OPMI Pentero showed minimal red signal from the same structures, which was barely distinguishable through oculars. PpIX signal intensities obtained by SFE and the OPMI Pentero from the same areas were correlated (Spearman ρ = .82, P<.05). The relative PpIX fluorescence intensities from the cerebellar and cerebral cortex areas were low in readings from both SFE and the OPMI Pentero. Overall, SFE showed appreciably higher sensitivity to PpIX in the fluorescent brain areas (30.4±8.8%) compared to the OPMI Pentero (13.3±2.7%), P<.01.
Figure 2.
Rapid protoporphyrin IX (PpIX) fluorescence mapping in fresh coronal slices of normal mouse brain. (A) Correlative images obtained with the OPMI Pentero BLUE-400 and the scanning fiber endoscope (SFE), with the same sections shown after staining with hematoxylin and eosin and luxol fast blue–periodic acid-Schiff (LFB-PAS). (B) Quantification of the PpIX signal in various brain regions and comparison (Mann-Whitney U test) of the PpIX signal from areas with no visible fluorescence (NVF) (CBX, TH, CTX, BG areas selected for analysis) and areas with visible fluorescence (VF). Abbreviations: aca, anterior commissure; ACB, nucleus accumbens; Aq, cerebral aqueduct; BG, basal ganglia; CBX, cerebellar cortex; cc, corpus callosum; CN, cochlear nuclei; CP, caudoputamen; cpd, cerebral peduncle; CTX, cerebral cortex; fi, fimbria; HT, hypothalamus; IC, inferior colliculus; IP, interposed nucleus; MB, midbrain; mcp, middle cerebellar peduncle; MY, medulla; PAG, periaqueductal gray; PRNc, pontine reticular nucleus, caudal part; SCs, superior colliculus, sensory related; sm, stria medullaris; sptv, spinal tract of the trigeminal; TH, thalamus; V, nucleus of cranial nerve five; vhc, ventral hippocampal commissure; VTA, ventral tegmental area; V3, third ventricle; V4, fourth ventricle. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
SFE Imaging of PpIX in Brain Tumors In Vivo
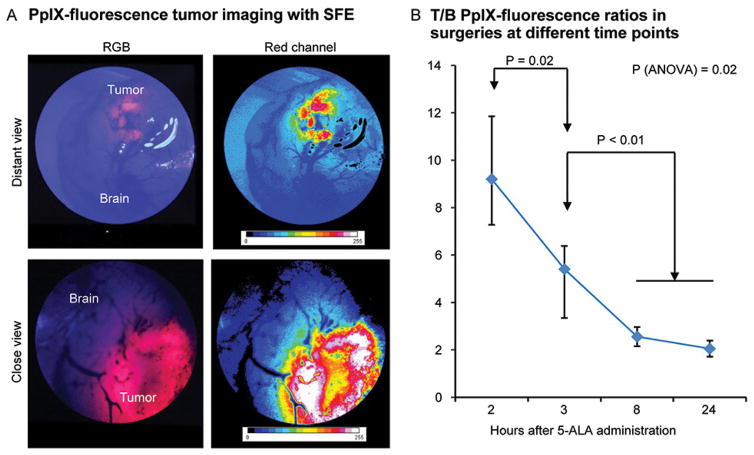
The optimal SFE imaging time period—when the tumor produced the brightest signal and a higher T/B ratio—was within 2 to 3 hours after 5-ALA administration (Figure 3).[14,19] Tumor fluorescence was significantly weaker at 8 and 12 hours, with suboptimal T/B ratio (T/B=2). Visible tumor fluorescence bleached faster when it was weak, especially at 8 and 12 hours after 5-ALA administration (see Figure, Supplemental Digital Content 4, demonstrating rapid bleaching of low PpIX signal). Bleaching was most pronounced at the peripheral tumor areas and occurred gradually over the entire tumor.
Figure 3.
Protoporphyrin IX (PpIX) detection in tumor with scanning fiber endoscope (SFE). (A) SFE image of mouse brain with tumor visible below cortex. (B) Quantification of tumor PpIX fluorescence at different times after 5-aminolevulinic acid injection (n=3 at 2 h, n=6 at 3 h, n=2 at 8 h, and n=2 at 24 h). Kruskal-Wallis analysis of variance (ANOVA) and Mann-Whitney U tests used for comparisons. Data presented as mean and range. RGB, red, green, blue; T/B, tumor-to-brain. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
SFE Detected Invasive Tumor Areas and Tumor Border
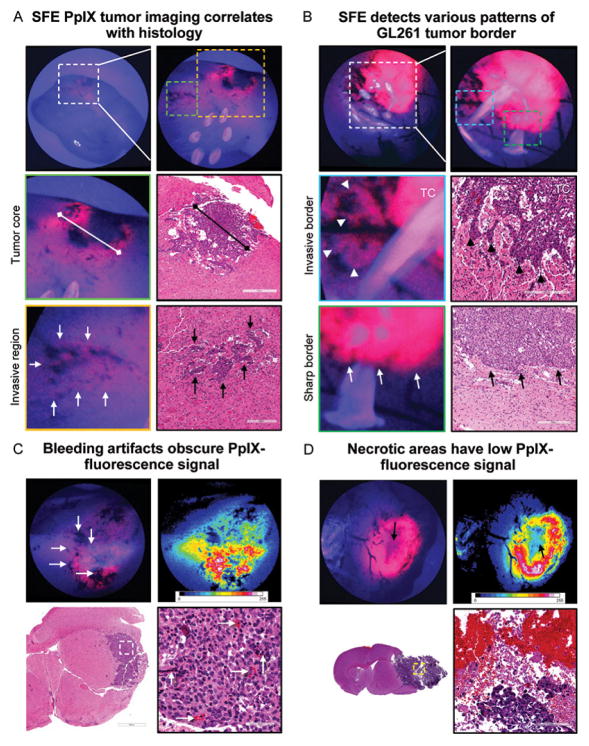
SFE imaging at the higher magnifications permitted visualization of the tumor core and various tumor border patterns (Figure 4A–B). Groups of cells, and some individual cells, were identifiable. Dark spots on the fluorescence images corresponded to bleeding points near injured vessels (Figure 4C). Necrotic areas exhibited diminished PpIX fluorescence (Figure 4D).
Figure 4.
Tumor border visualized with scanning fiber endoscope (SFE). (A) Representative SFE images of coronal brain cut through tumor core (TC) and correlative hematoxylin-and-eosin (HE)–stained sections. TC size measured on SFE and histological sections matched. Regions of invasion (arrows) are visible as patches of increased protoporphyrin IX (PpIX) signal close to areas of bleeding from abnormal vasculature. The 6 bright circles on the top right-side image are retroreflections from SFE light-collection fibers. (B) Invasive border regions with flames of glioma cells penetrating normal brain (arrowheads) and noninvasive tumor border regions (arrows). (C) HE-stained section demonstrates bleeding points associated with tumor microvasculature (arrows) corresponding to dark spots on SFE. (D) Correlative images illustrate diminished PpIX signal (arrow) at necrotic TC. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Quantification of PpIX Fluorescence at the Tumor Border
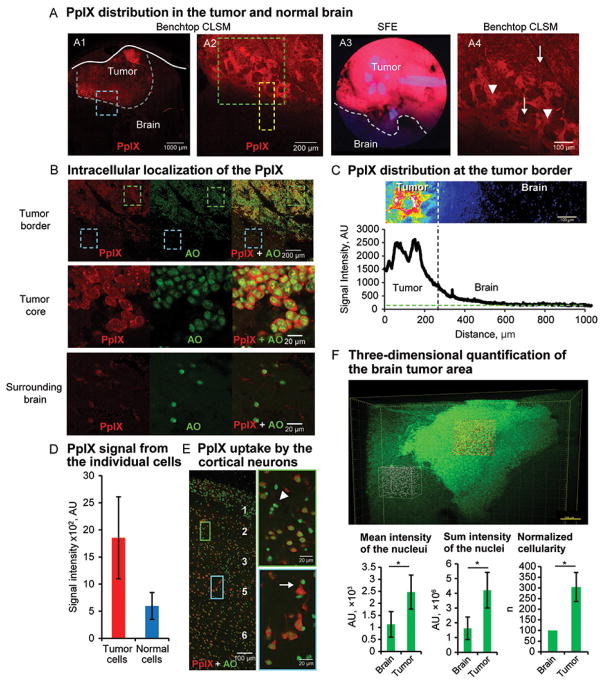
We further studied PpIX distribution at the tumor border on fresh and stained mouse brain tissue (Figure 5). Most of the strong PpIX fluorescence was detected inside tumor cells. PpIX was also detected extracellularly near the tumor border. Particularly, single-channel CLSM imaging confirmed the presence of the PpIX diffusion gradient from the tumor about 200 μm into the normal brain. This may be accounted for by the gradual signal decrease observed with SFE at the tumor border. Normal brain cells, especially pyramidal-shaped neurons, had accumulated PpIX, but to a significantly lesser degree than tumor cells (Figure 5D–E). Further CLSM investigation of cleared specimens stained with AO and imaged up to 400 μm deep confirmed the complex tumor shape associated with invasive tumor fronts and perivascular spread, similar to in vivo SFE imaging.
Figure 5.
Protoporphyrin IX (PpIX) distribution at tumor border. (A) Confocal laser scanning microscope (CLSM) findings for fresh samples correlate to SFE findings. The image in A1 shows PpIX in tumor Blue box denotes tumor border area shown at higher magnification on A2. Comparison of tumor border visualized by the SFE (A3) and CLSM (A4). Green box in A2 denotes area shown in A4; yellow box denotes area used for quantification in part C. Increased PpIX accumulation is shown in A4 in tumor cells (arrows) and in extracellular regions at border (arrowheads). (B) CLSM images of fresh sample counterstained with acridine orange (AO) confirm intracellular localization of PpIX and significantly lower but detectable accumulation of PpIX in surrounding normal brain cells. Boxes denote areas shown at a higher magnification (green box = tumor core; blue box = surrounding brain). (C) Quantification of the PpIX at tumor border. Green line indicates level of the noise; vertical line delineates tumor border. (D) Quantification of PpIX fluorescence intensity in tumor and normal cells (P<.01 Mann-Whitney U test, n=10 per group). (E) CSLM image of brain cortex 2 hours after 5-aminolevulinic acid administration. Boxes denote cortical layers 2 (green box) and 5 (blue box) shown at higher magnification on right. Neurons, especially large pyramidal cells in layer 5, show a higher PpIX signal than glial cells (arrowhead). (F) Volume image of cleared and AO-stained brain tumor. Boxes denote examples of volumes outlined for quantification shown in graphs (n=3, *P<.01 with Mann-Whitney U test). AU, arbitrary units. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Comparison of SFE and Operating Microscope for PpIX Detection
In the small tumors modeling lower-grade tumors, the OPMI Pentero photobleached PpIX before the tumor could be located and brought into focus (~30 seconds to 1 minute) in 3 trial animals. Doubling the 5-ALA dose to 5 mg produced bright lava-like red fluorescence detectable and localizable by both SFE and the OPMI Pentero. To investigate the quantitative difference in PpIX detection, we separately analyzed time-series images of mouse brain tumors with SFE and the OPMI Pentero. In this experiment, initially highly fluorescent tumors, as well as initially red-pink “average” and low-fluorescent tumors, had significantly longer imaging time (Table 1) without noticeable photobleaching under SFE illumination than with OPMI Pentero light exposure (see Figure, Supplemental Digital Content 5, showing dynamic PpIX bleaching).
Table 1.
Imaging time before loss of PpIX tumor-to-brain contrast.*
| Technique | Signal Intensity
|
||
|---|---|---|---|
| Low | Moderate | High | |
| OPMI Pentero | <3 min | No data | 10–15 min |
| SFE | 20 min | 50–60 min | >60 min |
Abbreviations: PpIX, protoporphyrin IX; SFE, scanning fiber endoscope.
Data extrapolated from graphical results of time imaging experiments (see Supplemental Digital Content 5, showing changes of PpIX signal intensity during imaging).
SFE as a Tool for Surgical Visualization
We performed brain tumor removal under PpIX fluorescence guidance with SFE in 2 mice. The SFE probe was fixed in a constant position with a Greenberg retractor and provided sufficient working distance to perform manipulations with microsurgical instruments and a suction device. Surgical access to the main mass, and to border regions of the tumor, was well visualized and guided using SFE imaging of the PpIX signal. Histologic investigation of the samples confirmed complete tumor removal (see Figure, Supplemental Digital Content 6, demonstrating brain histology after tumor removal). SFE provided visualization for microsurgical manipulations comparable to that of other endoscopes used in clinical neurosurgery.[20] Decreasing the SFE imaging distance from about 2 cm to 3 mm provided wide-field to magnified views for efficient detection of areas harboring only a few tumor cells with low PpIX signal for relatively long durations.
DISCUSSION
PpIX in the Normal Brain
PpIX distribution in normal rabbit brain was previously mapped with a benchtop CLSM[21]; however, the study provided only small field images that do not characterize PpIX signal gradient across large brain areas. Here, we mapped PpIX signal distribution in a whole mouse brain using a clinical tool. We observed that PpIX is produced by normal brain cells in the mouse model, but to a lesser extent than by tumor cells. Previous clinical trials found that weak PpIX signal may be visible in non-tumor reactive tissue.[3,22,23] Additionally, the operating microscope was limited in detecting low PpIX signal. Moreover, we demonstrated that SFE overcame such limitations and offered a surgically useful visualization tool capable of detecting significantly lower concentrations of PpIX from non-tumor normal brain areas and of successfully differentiating them from PpIX signal obtained from an experimental glioma (see Figures, Supplemental Digital Content 4 and 5 showing correlative SFE and operative microscope images).
Timing of Imaging Relative to 5-ALA Administration
Although a maximum T/B PpIX fluorescence ratio of 85 was reportedly observed 24 hours after 5-ALA injection, [21] our study showed that the absolute values of tumor fluorescence intensity are much lower at 24 hours than at the earlier time points. Our in vivo observations demonstrated that tumor fluorescence was maximal at 2 hours post–5-ALA injection, whereas at 8 and 24 hours it was too low. In humans, the practically useful time for oral 5-ALA administration was defined as 3–4 hours before anesthesia.[3,24] This timing may vary in animal models because of the differences in metabolism and pharmacokinetics of 5-ALA. After oral administration in humans, plasma concentrations of 5-ALA start to increase after 3 hours, they peak at 7 hours, and they slowly decrease to about one-half their peak at 12 hours.[25] Concerns about variability in the time to peak fluorescence have previously been reported for non-brain malignancies.[25,26] It should also be noted that doses of 5-ALA used in experimental studies are usually higher (100 mg/kg in this study and in Swanson et al.[27]; 20, 100, and 200 mg/kg in Cho et al.[28]; and 62.5–125 mg/kg in Fisher et al.[29]) than the dose used in humans (20 mg/kg). Our observations showed that doses greater than 100 mg/kg result in bright fluorescence in animal tumors that is subjectively similar to the fluorescence observed in humans, whereas higher doses result in even brighter red fluorescence.
PpIX Detection Sensitivity
We demonstrated that SFE clearly detected PpIX signal from a single layer of tumor cells, which was not possible with the OPMI Pentero, a wide-field surgical visualization. SFE allowed visualization of the tumor border and detection of invading glioma fronts on the cellular level with a low PpIX signal. This unique capability of SFE may be practical for low-concentration 5-ALA clinical protocols (5 mg/kg)[30] used for high-grade tumors, as well as for the regular protocol (20 mg/kg) used for low-grade tumors, when detection sensitivity of operating microscopes is not sufficient.
Surgical Tools that Improve PpIX Detection
Several surgical tools have been studied to improve PpIX detection. The optical fiber spectrophotometer showed promise in detecting PpIX spectral signature in tumors, correlating to the OPMI Pentero visual PpIX signal intensity.[31,32] Confocal endomicroscopy was reported to provide real-time diagnostic imaging at the cellular level.[33,34] However, clinical-grade endomicroscopy systems, designed to work with 488-nm excitable fluorophores, [35] have not proven optimal for PpIX detection.[14] A common drawback of both spectrophotometer and endomicroscope is their limited field of view. These systems allow for intraoperative “optical biopsy” of a limited number of sites, but are impractical for examining a large resection cavity or an expansive tumor border region. In comparison, non-contact SFE is better for interrogation of large tissue surfaces with a viewing field in a 50 to 80-degree–range cone angle and increased probe-tissue separation distance. Furthermore, real-time quantitative spectral analysis is possible with the SFE platform.[36] SFE is a flexible endoscope that allows interrogation of brain areas behind in resection cavities—areas that are inaccessible to operating microscopes and current confocal endomicroscopy systems.[37] In this situation, the shorter working distance is practical to guide surgeries that are blocked from straight line-of-sight imaging with surgical microscopes. However, SFE is still an unsophisticated prototype. Large specular reflections can obscure much of the field of view and require adjustment of the SFE imaging angle or some additional optical filtering.
Photobleaching of PpIX
High-optical power density of the exciting light may result in a loss of fluorescence, which is relevant for PpIX-guided surgery (see supplemental text, Supplemental Digital Content 7, for further details). Our finding of rapid photobleaching of the weak PpIX signal from the low-grade tumor models that were illuminated by the OPMI Pentero BLUE-400 may provide an important clinical insight and explanation of why, besides their lower detection sensitivity, PpIX is rarely visible under operating microscopes in low-grade glial tumors.[6] Indeed, improvements in the operating microscopes’ signal detection increased the diagnostic accuracy.[7] Future clinical applications of SFE may benefit from the >6 times longer imaging without noticeable photobleaching, especially near the tumor border, where fluorescence is weak. This increased duration of imaging is attributed to the shorter time interval and smaller dose of light exposure and may be critical for interrogation of the resection cavity for residual tumor along the margins at the end of the surgical procedure. In the future, the procedure for residual tumor resection can be semiautomated under the surgeon’s supervision using a surgical robot with optical guidance from SFE (see Figure, Supplemental Digital Content 8, illustrating the concept of SFE guidance).
Study Limitations
Although the GL261 tumor model is an invasive syngeneic malignant glial tumor type, the extent of the tumor border observed in this study is less than that usually observed in human invasive gliomas, where the spread is more diffuse. However, no better larger models exist for investigating the PpIX signal in large-scale diffusive borders, except in clinical trials. Further clinical study is warranted to test the diagnostic utility of SFE in human brain tumor surgeries.
A cautionary note is that false-positive PpIX fluorescence in non-tumor abnormal reactive tissue has been reported, which is related to the intrinsic properties of 5-ALA, although healthy normal brain tissue visualized with the OPMI Pentero did not have visible fluorescence.[3] This is an expected limitation for any wide-field imaging system, including both SFE and the OPMI Pentero. Particularly, our findings demonstrate increased nonspecific PpIX fluorescence in brain areas characterized by increased cellularity, as observed with SFE and CLSM. Therefore, increased tumor cellularity and metabolic activity compared to that in the normal brain are two major factors contributing to the detectable high PpIX signal.
CONCLUSION
SFE has unique qualities compared to other tools intended to improve the overall efficiency of fluorescence-guided surgery. The significant aspects of this study are the high sensitivity and reduced photobleaching of PpIX with SFE compared to those of the OPMI Pentero operating microscope, which explains the lower effectiveness of fluorescence-guided resection of low-grade gliomas, and problems with using intraoperative guidance for full resection of residual tumor. SFE is the first wide-field surgical imaging tool capable of detecting PpIX fluorescence in individual cells. With SFE, we mapped PpIX fluorescence in the normal brain and identified tumor margins with low PpIX concentrations, not visible with current surgical microscope systems. Finally, SFE provides wide-field visualization that can be used to interrogate large areas of the resection cavity, including the hidden side-wall regions.
Supplementary Material
HIGHLIGHTS.
The scanning fiber endoscope (SFE) was developed and optimized for PpIX detection.
Unlike the OPMI, the SFE can visualize PpIX in individual tumor cells.
SFE has significantly higher PpIX fluorescence detection sensitivity than OPMI.
We mapped PpIX accumulation in glioma border and normal mouse brain regions.
Tumor PpIX bleached fast under OPMI, but imaging time increased >6 times with SFE.
Acknowledgments
FINANCIAL SUPPORT: The Barrow Neurological Foundation; the Newsome Chair in Neurosurgery Research to M.C.P.; and the National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering, R01 EB016457.
E.B. is thankful to Nikon Corp. and Carl Zeiss, AG, for fellowship support and to Dr. Simon Watkins for training at the Quantitative Microscopy Course at MDI Biological Laboratory, Bar Harbor, ME. We thank Carl Zeiss, AG, Oberkochen, Germany, for providing the LSM 710 confocal laser scanning microscope. The authors thank the staff of the Neuroscience Publications office at Barrow Neurological Institute for assistance with manuscript preparation.
ABBREVIATIONS
- 5-ALA
5-aminolevulinic acid
- AO
acridine orange
- AU
arbitrary unit
- CLSM
confocal laser scanning microscope
- CUBIC
clear, unobstructed brain/body imaging cocktails and computational analysis
- DAPI
4′,6-diamidino-2-phenylindole
- H&E
hematoxylin and eosin
- PBS
phosphate-buffered saline
- PpIX
protoporphyrin IX
- RGB
red, green, and blue
- SFE
scanning fiber endoscope
- T/B
tumor-to-background ratio
Footnotes
DISCLOSURES: Results of this study were presented at the 2017 American Association of Neurological Surgeons annual meeting, on April 22-26, 2017 in Los Angeles, CA. E.J.S. and L.Y.N. participate in the royalty-sharing program for inventors at the University of Washington, and they both hold no other relationship to private companies having a financial interest in scanning fiber endoscope technology.
References
- 1.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130:269–282. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 2.Belykh E, Martirosyan NL, Yagmurlu K, et al. Intraoperative Fluorescence Imaging for Personalized Brain Tumor Resection: Current State and Future Directions. Front Surg. 2016;3:55. doi: 10.3389/fsurg.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau D, Hervey-Jumper SL, Chang S, et al. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg. 2016;124:1300–1309. doi: 10.3171/2015.5.JNS1577. [DOI] [PubMed] [Google Scholar]
- 4.Nabavi A, Thurm H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65:1070–1076. doi: 10.1227/01.NEU.0000360128.03597.C7. discussion 1076–1077. [DOI] [PubMed] [Google Scholar]
- 5.Widhalm G, Kiesel B, Woehrer A, et al. 5-Aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. 2013;8:e76988. doi: 10.1371/journal.pone.0076988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaber M, Wolfer J, Ewelt C, et al. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery. 2016;78:401–411. doi: 10.1227/NEU.0000000000001020. discussion 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdes PA, Jacobs V, Harris BT, et al. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg. 2015;123:771–780. doi: 10.3171/2014.12.JNS14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdes PA, Leblond F, Jacobs VL, et al. Quantitative, spectrally-resolved intraoperative fluorescence imaging. Sci Rep. 2012;2:798. doi: 10.1038/srep00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meza D, Wang D, Wang Y, et al. Comparing high-resolution microscopy techniques for potential intraoperative use in guiding low-grade glioma resections. Lasers Surg Med. 2015;47:289–295. doi: 10.1002/lsm.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitai R, Takeuchi H, Miyoshi N, et al. Determining the tumor-cell density required for macroscopic observation of 5-ALA-induced fluorescence of protoporphyrin IX in cultured glioma cells and clinical cases. No Shinkei Geka. 2014;42:531–536. [PubMed] [Google Scholar]
- 11.Yang C, Hou VW, Girard EJ, et al. Target-to-background enhancement in multispectral endoscopy with background autofluorescence mitigation for quantitative molecular imaging. J Biomed Opt. 2014;19:76014. doi: 10.1117/1.JBO.19.7.076014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CM, Engelbrecht CJ, Soper TD, et al. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J Biophotonics. 2010;3:385–407. doi: 10.1002/jbio.200900087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelwahab MG, Sankar T, Preul MC, et al. Intracranial implantation with subsequent 3D in vivo bioluminescent imaging of murine gliomas. J Vis Exp. 2011:e3403. doi: 10.3791/3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martirosyan NL, Georges J, Eschbacher JM, et al. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg Focus. 2014;36:E16. doi: 10.3171/2013.11.FOCUS13486. [DOI] [PubMed] [Google Scholar]
- 15.Ewelt C, Floeth FW, Felsberg J, et al. Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg. 2011;113:541–547. doi: 10.1016/j.clineuro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Susaki EA, Tainaka K, Perrin D, et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc. 2015;10:1709–1727. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- 17.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Jiang Y, Belykh E, et al. Toward real-time tumor margin identification in image-guided robotic brain tumor resection. Proc. SPIE 10135, Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling, 101350D; March 3, 2017; 2017. p. 101350D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sroka R, Beyer W, Gossner L, et al. Pharmacokinetics of 5-aminolevulinic-acid-induced porphyrins in tumour-bearing mice. J Photochem Photobiol B. 1996;34:13–19. doi: 10.1016/1011-1344(95)07265-9. [DOI] [PubMed] [Google Scholar]
- 20.Rapp M, Kamp M, Steiger HJ, et al. Endoscopic-assisted visualization of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery: a technical note. World Neurosurg. 2014;82:e277–279. doi: 10.1016/j.wneu.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Olivo M, Wilson BC. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int J Oncol. 2004;25:37–45. [PubMed] [Google Scholar]
- 22.Utsuki S, Oka H, Sato S, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo) 2007;47:210–213. doi: 10.2176/nmc.47.210. discussion 213–214. [DOI] [PubMed] [Google Scholar]
- 23.Stummer W, Novotny A, Stepp H, et al. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 24.Potapov AA, Goryaynov SA, Okhlopkov VA, et al. Clinical guidelines for the use of intraoperative fluorescence diagnosis in brain tumor surgery. Zh Vopr Neirokhir Im N N Burdenko. 2015;79:91–101. doi: 10.17116/neiro201579591-101. [DOI] [PubMed] [Google Scholar]
- 25.Webber J, Kessel D, Fromm D. On-line fluorescence of human tissues after oral administration of 5-aminolevulinic acid. J Photochem Photobiol B. 1997;38:209–214. doi: 10.1016/s1011-1344(96)07445-3. [DOI] [PubMed] [Google Scholar]
- 26.Regula J, MacRobert AJ, Gorchein A, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut. 1995;36:67–75. doi: 10.1136/gut.36.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson KI, Clark PA, Zhang RR, et al. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery. 2015;76:115–123. doi: 10.1227/NEU.0000000000000622. discussion 123–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho HR, Kim DH, Kim D, et al. Malignant glioma: MR imaging by using 5-aminolevulinic acid in an animal model. Radiology. 2014;272:720–730. doi: 10.1148/radiol.14131459. [DOI] [PubMed] [Google Scholar]
- 29.Fisher CJ, Niu C, Foltz W, et al. ALA-PpIX mediated photodynamic therapy of malignant gliomas augmented by hypothermia. PLoS One. 2017;12:e0181654. doi: 10.1371/journal.pone.0181654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haj-Hosseini N, Richter JC, Hallbeck M, et al. Low dose 5-aminolevulinic acid: Implications in spectroscopic measurements during brain tumor surgery. Photodiagnosis Photodyn Ther. 2015;12:209–214. doi: 10.1016/j.pdpdt.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Stummer W, Tonn JC, Goetz C, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74:310–319. doi: 10.1227/NEU.0000000000000267. discussion 319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potapov AA, Goriainov SA, Loshchenov VB, et al. Intraoperative combined spectroscopy (optical biopsy) of cerebral gliomas. Zh Vopr Neirokhir Im N N Burdenko. 2013;77:3–10. [PubMed] [Google Scholar]
- 33.Foersch S, Heimann A, Ayyad A, et al. Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. PLoS One. 2012;7:e41760. doi: 10.1371/journal.pone.0041760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sankar T, Delaney PM, Ryan RW, et al. Miniaturized handheld confocal microscopy for neurosurgery: results in an experimental glioblastoma model. Neurosurgery. 2010;66:410–417. doi: 10.1227/01.NEU.0000365772.66324.6F. discussion 417–418. [DOI] [PubMed] [Google Scholar]
- 35.Martirosyan NL, Eschbacher JM, Kalani MY, et al. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: experience with 74 cases. Neurosurg Focus. 2016;40:E11. doi: 10.3171/2016.1.FOCUS15559. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Kim AS, Ridge JS, et al. Trimodal detection of early childhood caries using laser light scanning and fluorescence spectroscopy: clinical prototype. J Biomed Opt. 2013;18:111412. doi: 10.1117/1.JBO.18.11.111412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JT, Meza D, Sanai N. Trends in fluorescence image-guided surgery for gliomas. Neurosurgery. 2014;75:61–71. doi: 10.1227/NEU.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konig K, Schneckenburger H, Ruck A, et al. In vivo photoproduct formation during PDT with ALA-induced endogenous porphyrins. J Photochem Photobiol B. 1993;18:287–290. doi: 10.1016/1011-1344(93)80077-m. [DOI] [PubMed] [Google Scholar]
- 39.Moan J, Streckyte G, Bagdonas S, et al. Photobleaching of protoporphyrin IX in cells incubated with 5-aminolevulinic acid. Int J Cancer. 1997;70:90–97. doi: 10.1002/(sici)1097-0215(19970106)70:1<90::aid-ijc14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Ericson MB, Grapengiesser S, Gudmundson F, et al. A spectroscopic study of the photobleaching of protoporphyrin IX in solution. Lasers Med Sci. 2003;18:56–62. doi: 10.1007/s10103-002-0254-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.