Abstract
Current anti-aging strategies focusing on druggable targets have met with relatively limited success to date. Replacement of cells, tissues, and organs could provide an alternative means for targeting age-induced damage and potentially eliminating some of it. However, before this is a viable option, numerous challenges need to be addressed. Most notably, whether the brain, which defines our self-identity, is amenable to replacement therapies is unclear. Here, we consider whether progressive cell replacement is a potential approach to reverse brain aging without grossly altering function. We focus mainly on the neocortex, seat of our highest cognitive functions, because of abundant knowledge on neocortical development, plasticity, and how the neocortex can functionally incorporate new neurons. We outline the primary challenges for brain cell replacement, and key areas that require further investigation.
Keywords: neocortex, rejuvenation, neural stem cell, age-related damage, regeneration, transplant
Limitations to pharmacological approaches for slowing age-related damage
The research community and, more recently, biotech companies have begun taking the first steps towards identifying molecular targets for pharmaceuticals that could have, if not systemic, at least broad anti-aging benefits. Sometimes referred to as “geroscience”, this new area of research in aging originates, in part, from observations that dietary restriction (DR) or reduced somatotropic signaling result in improved lifespan and health-span [1–12]. The effects of dampening growth signaling can be mimicked by drugs, with several geroprotective compounds, for example rapamycin, demonstrated to have beneficial impact on multiple age-related phenotypes [13,14].
Some geroprotectors, including rapamycin, also appeared to increase maximal lifespan in mice [15]. However, while geroscience approaches are likely to improve health-span in elderly humans – and curtail certain life-shortening factors – an effect on human maximal lifespan is doubtful. It should be realized that results suggesting an effect on maximal lifespan all come from work with short-lived laboratory animals, and it’s not clear whether these effects can be extrapolated to humans – perhaps not even to the species being studied when in the wild. Indeed, DR’s effect on longevity in rodent studies depends, at least to some extent, on the control group being obese, and an increase in longevity is not shared by all genotypes, with no evidence that either DR or any geroprotective compound yet studied increases longevity beyond the maximum species lifespan [16]. Importantly, DR appears to have no effect, or only small effects, on lifespan in non-human primates, although there is no doubt about its positive effects on health-span [17,18]. The tremendous complexity of age-related damage may underlie the limitations of DR and mimetic drugs (Box 1).
Box 1. The complexity of age-related damage limits pharmacological approaches.
The molecular damage that occurs with age is very complex [117]. For proteins with slow turnover rates, large classes of damage include, for example, the products of glycation, oxidation, racemization, and deamidation. Lipid damage also increases over time, leading to the accumulation of metabolic byproducts such as lipofuscins. Diverse forms of DNA damage also increase with age, including breaks, crosslinks, depurination, depyrimidination, mutations (base pair changes, deletions, duplications), changes in methylation, various chromatin modifications, and chromosomal abnormalities including aneuploidy. Since molecular, cellular, and organ functions are interconnected, for pharmaceuticals to have a significant impact on maximal lifespan they may need to target many forms of damage, if not most of them. Given the enormous diversity in the forms of macromolecular damage, the irreversible nature of certain forms of damage such as DNA mutations, aneuploidy, and others, as well as the low turnover of the proteins that make up the bulk of our bodies, it is unclear whether endogenous repair systems exist that can be pharmacologically induced to slow or reverse enough of the damage that occurs to extend maximal lifespan. This implies the need for therapeutics that can directly repair damage on their own, which as of yet are lacking [115,118]. Hence, repair of age-related damage at the molecular level by pharmaceutical means, although worthwhile to pursue for increasing health and perhaps eventually maximal lifespan, seems at the moment overly ambitious.
In addition to drugs that might target metabolism, drugs that target the functionality of aged stem cells, such as “youthful” factors in the blood of young animals [19,20], while possibly improving health-span, will probably be unable to remove damage that accumulates in the stem cells themselves, in their niches, and in tissues with non-dividing cells. The same applies to senolytic drugs that ablate certain types of dysfunctional old cells [21]; they are unlikely to halt accumulation of damage. Thus far systematic ablation of senescent cells from the tissues of aging mice can significantly improve health-span but not maximum lifespan [22].
In conclusion, while extremely important for improving late-life health, current pharmacological geroscience approaches, we argue, seem unlikely to significantly increase human lifespan, which appears to have reached a plateau [23–25]. By contrast, the option of regularly replacing worn-out tissues, organs, and cells offers, at least in theory, the advantage of instantaneous reversion of the effects of aging (Box 2). Below we will critically review this option with a focus on the brain, which poses the most serious challenges for cell replacement therapy. Indeed, the need to preserve one’s individual self-identity rooted in the brain presents a formidable challenge to cell replacement as an approach to undo aging.
Box 2. Tissue and organ transplantation in non-neuronal tissues.
Transplanting artificial organs and body parts, e.g., knee and hip replacements, heart valves and more recently artificial hearts, has seen significant progress, and allogeneic organ and tissue transplantation have also become routine for many if not most organs and tissues (based on OPTN data as of 7/31/2017, https://optn.transplant.hrsa.gov/data/; [119]). Allogeneic organ and tissue transplants are used successfully to treat certain diseases, but their shortage precludes their use in replacing organs and tissues that have become dysfunctional with age. To circumvent this shortage, tremendous strides in tissue engineering are increasingly allowing the generation of replacement organs comprised of natural and transient structural scaffolds colonized with mixes of patient-derived cell types appropriate for each particular organ (e.g. [120–122]).
Neural plasticity in the aging brain
Unlike the brain, the rest of the body is amenable to larger-scale – whole tissue and organ – replacements (Box 2). However, when it comes to treating the age-related decline in brain function, organ or large-scale tissue replacements are not an option, given the obvious loss of self-identity that would occur, particularly for areas such as the neocortex. Yet the plasticity that exists in neocortical neural networks suggests that cell transplants, if done progressively over years, could rejuvenate the neocortex biologically and functionally without greatly disrupting ongoing functions. Cortical plasticity, defined as the ability to modify circuits by changing the synapses, neurons, and/or cortical maps underlying a particular function, is a remarkable feature of the neocortex and well-documented in several experimental paradigms (reviewed for example by [26–28]). In fact, even under normal conditions the neocortex is constantly reorganizing its functional circuits in response to new experiences as it allocates and re-allocates cortical areas based on use. A classic example, conserved across mammalian species, is the re-allocation of areas in the somatotopic map of the sensorimotor cortex that occurs over the course of weeks, months, or years in response to digit or limb amputations; areas previously responsive to the limb or digit become responsive instead to inputs from surrounding tactile skin areas. Studies of the other sensory cortices reveal similar plasticity [26–28]. Even higher order functions such as language can progressively relocate to new neocortical areas when the original eloquent areas are destroyed due to slow growing gliomas, for instance (Box 3).
Box 3. Relocation of language in the injured adult neocortex.
One of the most remarkable examples of cortical plasticity over long time-scales comes from comparing patients of similar age with similar-sized lesions in the eloquent cortex that are due to either stroke or slow-growing low-grade gliomas [123]. While stroke patients often exhibit major permanent deficits in speech and movement, patients with low-grade gliomas, even after massive resections of the eloquent areas, can remain with nearly no detectable functional consequences. This is, at least in part, because, while there is no time for plasticity to compensate for the catastrophic loss of tissue from a stroke, the slow growth of a benign tumor that progressively destroys the original eloquent area allows the time necessary for plasticity to re-establish eloquent function around the tumor area, in more distant areas of the ipsilateral hemisphere, and even in the contralateral hemisphere [123,124]. Serial surgeries with both direct electrostimulation mapping and fMRI mapping in the same patients performed years apart due to tumor regrowth confirm the plastic re-allocation of functional areas over time [124].
As we get old, neuronal complexity declines. Dendritic arborization, length, synapse number and spine density decrease to variable degrees in cortical areas accompanied by reductions in most aspects of cognitive performance, including memory, awareness, and intellectual abilities [29–33]. In degenerative diseases, a threshold is reached when degeneration overwhelms plasticity so that the cortex can no longer adequately compensate for disrupted circuits. Likewise, in healthy aging adults, mechanisms of plastic compensation probably operate to offset the effects of aging, until at some point aging again wins out [35–39]. Together with recent studies revealing an ability of transplant-derived neurons to integrate into adult cortical networks (discussed below), the innate plasticity of cortical synapses, neurons, and circuits, which underlie our thought patterns and memories, bode well for regenerative approaches that would involve the progressive introduction of new neurons over time into the neocortex to curb or reverse the effects of aging. However, formidable challenges lie ahead.
Cell replacement to treat brain aging – promises and pitfalls
Although the innate plasticity of the neocortex and its ability to accommodate new neurons offer hope for cell replacement strategies, several major questions must be addressed and hurdles overcome before we can consider cell replacement as a viable means of reversing age-related damage in the brain. Below, prompted by recent reports relevant to introducing new cells in the cerebrum, we outline key open questions related to cell sources, types, numbers, dispersion, integration, and the extracellular space. We discuss existing approaches to potentially overcoming some of these obstacles in determining if cell replacement could become a viable option to rejuvenate the aged brain.
Can endogenous cells be used as sources of new cortical neurons?
A potential alternative to transplanting new neurons into the aging cortex is to cajole endogenous precursors into generating new cortical neurons. Although evidence is mixed as to the existence of endogenous neural stem cells in the adult human forebrain, the activity of such cells appears at best limited to the hippocampal dentate gyrus (DG) where they would generate glutamatergic granule neurons, and the anterior subventricular zone (SVZ) where they would generate striatal GABAergic neurons (rather than olfactory bulb neurons as in rodents for example; [40–44]. Understanding the mechanistic details underlying how new neurons integrate into existing adult circuits in the DG and striatum in some mammals could provide useful clues for enhancing the integration of experimentally generated new neurons in the neocortex. It has also been suggested that endogenous neural stem cells themselves could be manipulated into populating the neocortex with new neurons [45,46].
However, endogenous neural stem cells and their niches also undergo aging, leading to loss of function and cell fate alterations [47]. While there is some evidence that damage accumulation in stem cells is slower than in normal cells [48–49], using one’s own stem cell pools in reversing aging would be limited in scope. In addition, the human DG is proportionately small compared with the neocortex (a ~1:1000 volume ratio; [50,51]), making it difficult for the limited DG stem cells to populate the neocortex, especially given its location outside of this much larger tissue. Moreover, the neural stem cells of the DG and SVZ have defined fates (to generate dentate granule and striatal neurons, respectively), which would need to be reprogrammed to neocortical fates while somehow still maintaining their normal hippocampal and striatal functions. As alternatives to DG and SVZ progenitors, the conversion of non-neuronal cell types already present in the neocortical parenchyma has also been proposed as a means of generating new neurons to repair local damage (Box 4), but such approaches may be difficult to apply to the repair of broad damage that occurs with degenerative diseases or aging.
Box 4. Reprogramming endogenous cells to neurons.
The conversion of non-neuronal cells located throughout the neocortex could in theory be used to generate new neocortical neurons. Astrocytes, pericytes, and oligodendrocyte progenitors have all been converted to neurons via forced overexpression of one or two transcription factors (Figure I; [86,125,126] or using small molecules [88,127]). These findings lay important groundwork for using non-neuronal cells to generate neurons in the adult neocortex without the need for cell transplantation. The potential consequences of usurping the normal fate and function of astrocytes, pericytes, or oligodendroglial cells would nevertheless need to be considered, as would their age-associated damage that would essentially constrain any attempt to completely reverse aging. In addition, the conversion of cells in broad areas of the normal aging neocortex or in neurodegenerative diseases remains a technically unaddressed challenge because of the difficulty of genetically manipulating specific cell types across broad areas in vivo.
Figure I. Generating neurons through cell reprogramming.
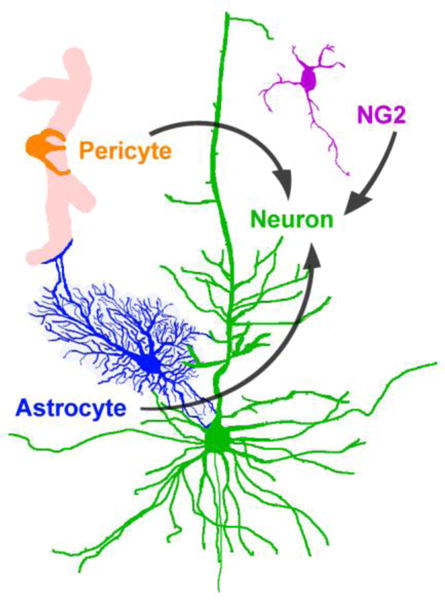
Cell types that are distributed throughout the neocortex, including astrocytes, pericytes, and NG2+ oligodendrocyte precursors, have been reprogrammed to neurons in previous studies via forced overexpression of one or two transcription factors (see text for details).
Transplantation of cortical precursor cells – what we know so far
Immune rejection is always a concern when cell transplants are used to treat an individual. Nevertheless, allogeneic grafts of human embryonic midbrain tissue into the adult striatum of Parkinson patients have an impressive track record of survival (up to 24 years so far; [52–54]). These early transplant trials along with the potential use of neural cells derived from patient-specific induced pluripotent stem (iPS) cells bode well, one could argue, for the circumvention of immune rejection in brain cell transplants.
Over the past decade or so, the transplantation of embryonic neocortical precursor cells in mice has revealed their innate ability to survive, differentiate into cortical excitatory neurons, and integrate – at least to some degree – into existing adult neocortical circuits (e.g. [55–60]). Similar results have been obtained with embryonic stem cell-derived neurons [61,62]. In the visual cortex, neurons generated from transplanted embryonic cortical tissue can project to appropriate targets and respond electrophysiologically to light [57]. More recently, Falkner et al. performed an extensive characterization of the dendritic arborization, axon projection patterns, pre-synaptic connectome, and orientation and directional selectivity for visual input of transplant-derived neurons in V1 derived from dissociated embryonic cortical cells [59]. Their results suggest that these transplant-derived neurons acquire over time functional features of their endogenous neighbors.
These initial studies illustrate the potential of embryonic cortical precursor cells to generate functionally integrated neurons in the adult neocortex. Nevertheless, much more research is needed to demonstrate their usefulness in rejuvenating neocortical function in the aging human brain. The observed synaptic connectivity, electrophysiological activity, and area-appropriate function of transplant-derived cortical neurons do not necessarily equate with normal function. As we continue to learn about cortical circuits, their remarkable complexity becomes only more apparent.
The classical, but over-simplistic, view of information processing in the neocortex is that information generally flows through the layers of excitatory projection neurons. Namely, input enters, for example, from the thalamus onto layer 4 neurons, which then project to layer 2/3 neurons, which in turn project among themselves and to layer 5 and 6 neurons, prior to information flow exiting the neocortex. However, not only are there other excitatory connections not mentioned in this canonical pathway, but there are also numerous superimposed forms of modulation, not the least of which comes from the complex classes of inhibitory interneurons [63]. Cortical interneurons, which are interspersed among the layers of excitatory neurons, regulate network activity in several ways, including feed-forward inhibition, feedback inhibition, disinhibition, and volume inhibition. Since improper circuitry of interneurons probably underlies several cognitive or psychiatric disorders, their use in cell-based transplant therapies is actively being explored [64]. In addition to local cortical interneurons, modulation of the cortical excitatory neurons can come from other parts of the brain, for example through direct inputs to the cortex from cholinergic, dopaminergic, and serotonergic neurons [65–67]. Hence, the extent to which transplant-derived cortical excitatory neurons can receive proper local and long distance inhibitory and modulatory inputs, which are necessary for them to participate in normal network activity, remains to be determined.
It is possible that, along with cortical precursors, the co-transplantation of young interneurons (which greatly increase plasticity; [68]) will be necessary to achieve a more normal integration of the principle cortical neurons. It is interesting to speculate that the maturation, orientation and directional selectivity observed by Falkner et al. in their transplant-derived V1 neurons is in part due to the presence of co-transplanted interneurons, since their source of cells was unpurified dissociated neocortex from E18.5 mouse embryos [59], a stage at which interneurons have already migrated into the neocortex. To further assess the functionality of transplant-derived neurons, in addition to testing their circuit activity, it would be instructive to determine whether they can contribute to behavior in young and old mice. This could be attempted, for example, by replacing neurons in a layer of a neocortical sensory area with transplant-derived neurons, training the recipient mice to respond to an appropriate sensory stimulation, and then optogenetically silencing the transplant-derived neurons to test whether task performance depends on their function. Nevertheless, despite these open questions, initial studies suggest a certain innate aptitude of young transplant-derived neurons to receive and process information from the host circuitry [57–60].
Roughly twenty billion neocortical neurons - the problem of numbers and size
A major unaddressed issue when considering the rejuvenation of the neocortex using cell transplantation is the number of its neurons. The human neocortex is comprised of roughly 15–20 billion neurons [50,69], which raises unanswered questions. Can such a large number of cells be replaced progressively over time? What proportion of new neurons is needed to begin observing positive effects? As a useful comparison, the adult mouse dentate gyrus, comprised of approximately 3×105 neurons, incorporates roughly 15,000 new neurons in 6 months [70,71]. If we scale these numbers up to the human neocortex, and for the sake of the calculation use 2×1010 (20 billion) as an estimate of the number of cortical neurons, the rate of incorporating new neurons would need to be 1×109 new neurons in 6 months, or 2×1010 (the equivalent of all neocortical neurons) in 10 years. Given a means of progressively eliminating old neurons (discussed below) and given that not all neurons would necessarily need to be replaced within 10 years to potentially begin reversing age-related dysfunction, then in theory at least the number of neurons needed for noticeable effects, if one extrapolates from the analogy of adult-neurogenesis in the hippocampus, is not in itself insurmountable. However, the optimal rate at which cells should be transplanted over time is not obvious. Ultimately, protocols would need to determine a rate that maximizes connectivity of newly introduced neurons while supporting or bolstering existing neuronal networks rather than ‘muddying’ them, so to say.
Another issue relates to neocortical size. Although the thickness of all the cellular layers of the human neocortex together totals only ~2 mm, its surface area covers roughly 2000–2500 cm2 folded into sulci and gyri [72,73]. This underscores a need to develop approaches in which cells disperse upon transplantation, given that arrayed injections of cells throughout the neocortex is difficult practically and might carry various adverse side effects. However, the neurons derived from transplanted embryonic neocortical precursors essentially remain at the transplant site (e.g. [59]), making them useful for repairing local damage but limiting their applicability to the more widespread damage that occurs with degenerative diseases and aging. Therefore, developing approaches for dispersion of transplanted cells is perhaps one of the most formidable challenges for achieving wide-scale neuronal integration and rejuvenation of the aging neocortex [74].
One approach that could enhance implanted cell dispersion involves cell fate conversion. In this approach, transplanted cells with an inherent ability to disperse in the mature neocortex, such as embryonic interneuron precursors, microglia, or oligodendrocyte precursors [75–80], could be induced to convert to a neocortical projection neuron fate after they have migrated into the surrounding host parenchyma (Figure 1). This could be accomplished, for example, by modifying the migratory donor cells with DNA constructs carrying inducible promoters that drive expression of reprogramming transcription factors, so that once cells have migrated they can be reprogrammed. Direct reprogramming of several cell types, including fibroblasts [81,82], astrocytes [83–85], pericytes [86], oligodendrocyte precursors [85,87,88], cord blood-derived stem cells [82], and hepatocytes [89] into cortical neurons has been achieved with efficiencies in some cases as high as 65–98% of cells converted [82,84,85,88] and without the need for cell division during the reprogramming process [84]. These previous studies suggest that reprogramming should be achievable for candidate migratory donor cells such as inhibitory neuron precursors, microglia, or oligodendrocyte precursors.
Figure 1. Cartoon illustration of two key challenges facing cortical cell transplantation.
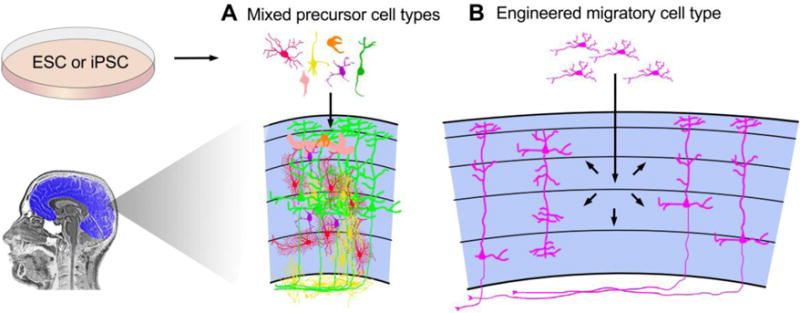
A. Multiple cortical cell types accumulate damage with age and need replacement. The co-transplantation of mixed precursor cell types, including vascular, glial, and neuronal precursors, could serve to replace cells of multiple types while enhancing the survival and function of transplant-derived neurons. B. Engineered cells that migrate prior to differentiating into neurons could be used to disperse new cells and circumvent the damage that would otherwise occur from closely arrayed injections of cells throughout the cortex.
Would co-transplantations improve functional connectivity?
Neurons transplanted into a single location of the adult visual neocortex can develop appropriately-looking dendritic arbors, axon projection patterns, pre-synaptic inputs from local and distant targets as well as among themselves, and electrophysiological responses to visual stimuli [57,59]. It is worth noting, however, that during development, neocortical neurons mature at a time when some of their pre- and post-synaptic partners in other cortical areas and other brain regions are also maturing. With this perspective in mind, it would be instructive to assess how simultaneous transplants into multiple cortical areas (and even other brain regions) impacts overall connectivity of transplant-to-transplant neurons (compared for instance to transplant-to-host connectivity) to determine the pros and cons of simultaneous versus sequential co-transplantations. In addition, cortical precursor cells as they differentiate become restricted to area-specific fates, generating neurons with different subcortical axonal targets [57,90]. Therefore, when performing transplants or co-transplants, matching the subtypes of cortical precursors with their functional areas may be required for proper connectivity.
Although perhaps not directly relevant to neocortical transplants, fetal dopaminergic precursors co-transplanted in the striatum and substantia nigra of human patients have thus far revealed encouraging results, as evidenced by post-mortem analyses. Specifically, transplant-derived fiber tracts running between the striatum and substantia nigra were observed years after the transplants [91]. In comparison, however, projections to and from the neocortex are considerably more complex. Therefore, in addition to further studies showing the ability of newly introduced cortical neurons to project to normal targets, future studies would also need to determine the potential of transplant-derived neurons in other parts of the brain to project to normal targets including the neocortex and how their connectivity might be affected by co-transplantations in source and target regions.
In addition to co-transplantations, it is worth considering activity-dependent processes that may enhance the integration of new neurons into existing networks. During development and adulthood, neuronal connections of existing as well as new neurons are made and lost, in part based on activity-dependent process. Activity can guide connectivity changes for instance via the Hebbian principle of coincident synaptic firing, as well as synaptic competition that is based on usage [92–95]. Therefore, optimal integration and functionality of transplant-derived neurons will probably also be facilitated by the active use of existing networks and integration of new neurons within them.
Finally, despite a considerable shrinkage in brain size with age, which is due predominantly to the loss of dendritic and axonal projections rather than neurons [34,50,96], it might be necessary at some point to create additional space for transplant-derived cells by progressively eliminating aged and dysfunctional neurons as well as other cell types, for example with senolytics or through non-proliferation-dependent cell competition [21,97].
Replacing neurons solely may not be sufficient to reverse age-related functional decline in the brain
Neuron survival and function rely on several non-neuronal cell types, namely astrocytes, oligodendrocytes, microglia, and vascular cells. In a cell-replacement framework for reversing aging, these cells will likely also need replacement with age. It is noteworthy that transplants in primates and mice using dissociated unpurified cells from fetuses or embryos fare considerably better than transplants that use purified neuronal precursors. In humans, bilateral transplants of fetal midbrain cells to the striatum of Parkinson’s Disease patients can survive for at least 24 years post-transplantation. The transplant-derived neurons show a generally healthy morphology and support continued therapeutic benefits, with – in some cases – no signs of the pathology that is found in the host neurons (e.g. [52–54,98]). In contrast, when cells for transplantation were obtained from monkey embryonic stem cell-derived dopaminergic precursors, only small grafts and low percentages of surviving dopamine neurons were observed after transplantation to either rat or monkey striata (e.g. [99,100]. Similarly, for transplants in mice, the use of cells derived from embryonic neocortex results in robust survival [59], whereas the use of purified neural progenitors leads to poor survival rates (e.g. [101–105]).
A likely reason for the better survival of transplants derived from embryonic tissue is the presence of mixed cell types, including neuronal precursors, vascular precursors, macroglia, and microglia. In particular, neovascularization may be critical for transplant cell survival [60,106,107]. As in the fetus, where vascularization must match the physiological demands of each growing tissue, vascularization of mixed cell transplants might occur to promote optimal neuron survival, differentiation, and function. Microglia may also play several critical roles by participating in blood vessel branching and fusion and in the maturation of transplant-derived neurons [108–113]. Astrocytes and oligodendrocytes are of course also required for normal neuron function. Within mixed-cell transplants, some cell types, such as vascular cells, microglia, oligodendrocyte precursors, and interneuron precursors, may to some extent self-organize as they do during development.
An overview of the cell types found in the adult neocortex is illustrated in Figure 2. Many of these cell types have previously been derived from human embryonic stem (ES) or iPS cells, which would serve as a likely source for transplantation (Figure 1) after appropriate “pan-omic” quality control tests as previously described [114].
Figure 2. Neocortical cell types.
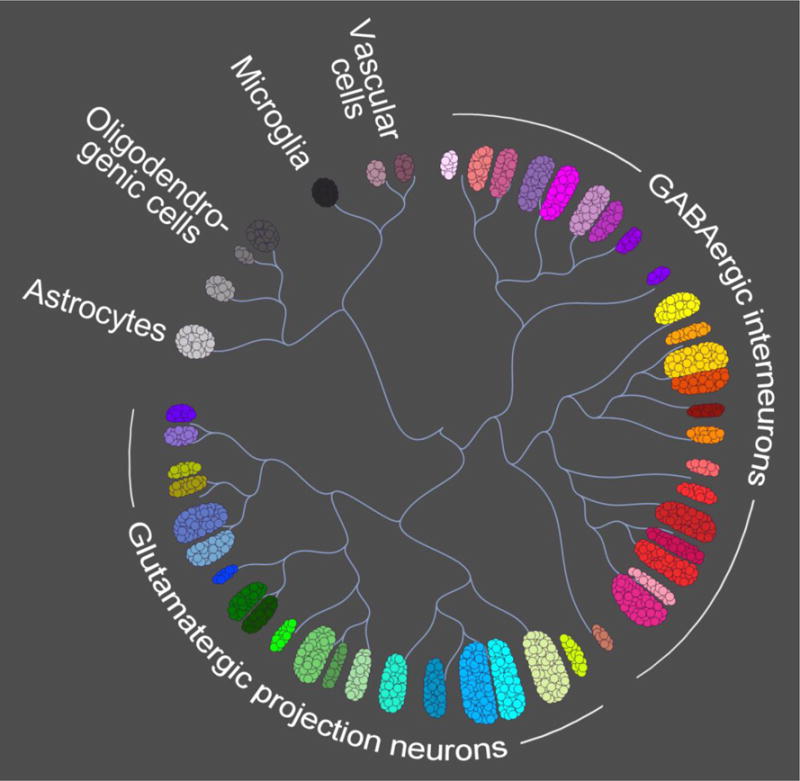
In mice, single cell transcript sequencing of unselected neocortical cells followed by unbiased clustering analyses revealed a total of 47–49 cell types, which included GABAergic interneurons, glutamatergic projection neurons, oligodendrocyte progenitors, oligodendrocytes, astrocytes, microglia, vascular endothelial cells, vascular smooth muscle cells, pericytes, and ependymal cells [128,129] (illustration adapted from the Allen Brain Atlas, http://casestudies.brain-map.org/celltax). Using similar approaches, corresponding cellular subtypes were observed in the human neocortex [130], although the specific number of cell types (in humans and other species) is debated
In addition to cells, the extracellular matrix (ECM) is a prime locus of age-related damage because of its limited turnover. It must be considered in any brain rejuvenation strategy, including transplant-based approaches. Transplant-derived cells will probably produce some of their own ECM, perhaps mitigating to some extent the accumulated overall damage. However, molecular repair or replacement strategies for the ECM will undoubtedly also be beneficial. Repair strategies have thus far met with very limited success [115], underscoring a need for new approaches in this area [116].
It is worth revisiting at this point the idea of using cortical tissue transplants, rather than dissociated cells – or, as a more practical source of tissue, using neocortical organoids composed of multiple cell types. Despite the possible greater difficulty for neurons from a cortical tissue graft compared to neurons from dissociated cells to integrate into existing circuits and maintain their function, there are several potential advantages to transplanting embryonic-like neocortical tissue. First, transplantation of embryonic neocortical tissue composed of multiple cell types grafted into the adult neocortex exhibits excellent survival and electrophysiological integration of neurons [56,60]. Second, the spatial organization of subtypes of neocortical neurons is likely to be better preserved in such grafts. Third, the issue of removing or repairing damaged ECM and dysfunctional cells is alleviated within the transplant. Overall, given the plasticity of the neocortex described above, in which functions can relocate progressively to new neocortical areas over time, and the ability of neurons within neocortical tissue grafts to connect to their host [56,60], it is not unreasonable to expect such grafts to be of some potential benefit in neocortical rejuvenation. On the other hand, to remedy the widespread changes that occur during aging, transplants of small cortical-like tissue would need to be spaced throughout the neocortex, possibly limiting the practicality of this approach.
Concluding remarks
Extending human lifespan has been a fantasy almost since the origin of human consciousness. However, with the enormous progress in medical technology since the 19th century, including more recently a deeper understanding of why and how we age, extending human lifespan while preserving health has become plausible. Cell replacement as a strategy for reversing age-related damage has been frequently discussed in the context of body tissues and organs but less so in the context of the brain. Unlike the brain, body organs do not determine our consciousness and self-identity, making the cell replacement strategy easier to envision, at least conceptually. In this Opinion, we explore the idea of cell replacement as an approach possibly applicable to brain rejuvenation. While one can see promise in the approach, key questions remain that would need to be addressed before progressive cell replacement could be considered as a means of reversing brain aging while retaining self identity (Box 5 and Outstanding Questions). Yet promising early reports of transplant-based cell replacement in the neocortex are providing a basis for exploring the potential of using such strategies for brain rejuvenation.
Box 5. Present limitations of cell transplantation approaches in neural tissues.
The extent to which transplant-derived neurons receive normal excitatory, inhibitory, and modulatory inputs has not been fully characterized.
Whether transplant-derived neurons can participate in a range of area-appropriate behaviors requires further testing.
The optimal numbers and rate with which new neurons should be introduced to bolster rather than undermine existing function is unknown.
Dispersing transplanted cells remain a major challenge for addressing wide-spread changes in the brain.
Transplanting vascular cells and glia alongside with neuron precursors may enhance effectiveness but this approach has not yet been tested.
Other than the production of new ECM by transplanted cells, there is currently no approach to replacing (or repairing) old and damaged ECM.
Outstanding Questions.
Cortical neurons receive modulatory inputs, for example from GABAergic, dopaminergic, cholinergic, and serotonergic neurons. Do transplant-derived neurons acquire such modulatory inputs from the host, and will co-transplantation of different neuron types facilitate appropriately modulated neuron activity?
Can transplant-derived neurons slow or reverse declines in behavioral performance affected by age?
Can cell types be engineered to disperse within the neocortical environment prior to differentiating, to facilitate their widespread integration into existing networks?
What mix of cell types, including neuronal, glial, and vascular precursors, will provide optimal survival and function of transplanted cells?
To what extent will the old ECM need to be repaired or removed given the production of new ECM from transplant-derived cells?
What would be the best age to first apply replacement therapies (both for the brain and for the body as a whole) and what ultimately will be their impact on mortality rates?
At this time, there is no evidence that aging can in fact be reversed through cell transplantation. Nonetheless, it is worth contemplating how extending maximum life span could in principle be accomplished by cell, tissue, and organ replacement applied to the whole body, which would erase the multitude of accumulated molecular defects. Whether this would truly delay or even halt the increased mortality observed with age is something only time can tell. Here we provided arguments that among the many organs and tissues that may someday become subject to replacement therapy, the brain does not need to be the exception.
Highlights.
Research on aging has recently surged, with a primary focus on developing druggable targets for interventions. To date, drug-based approaches have shown evidence of enhancing health in old age, but without clear evidence for extending maximal lifespan.
Progress in cell, tissue, and organ replacements is providing – at least in principle – an alternative to pharmacological approaches that could undo age-related damage. However, whether cell replacement is also applicable to the brain, due to possible loss of self-identity, is an open question.
Recent breakthroughs in brain-cell transplant studies show that new neurons can integrate in the adult neocortex, suggesting that, combined with normal plasticity, progressive cell replacements are possible.
While major challenges remain, rejuvenation of the brain via cell replacement to reverse age-related damage and functional decline appears to be as valid an approach as it is for most other organs and tissues.
Acknowledgments
We thank laboratory members for discussions and critical reading of the manuscript. This work was supported by the Brain Research Foundation, Hirschl/Weill-Caulier Foundation, and NIH NS088943 (JMH) and by NIH AG056278, AG047200, and AG017242 (JV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflicts of interest.
References
- 1.Kenyon C, et al. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 2.Kimura KD, et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 3.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 4.Bluher M, et al. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 5.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 6.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Bartke A. Growth hormone, insulin and aging: the benefits of endocrine defects. Exp Gerontol. 2011;46:108–111. doi: 10.1016/j.exger.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perice L, et al. Lower circulating insulin-like growth factor-I is associated with better cognition in females with exceptional longevity without compromise to muscle mass and function. Aging. 2016;8:2414–2424. doi: 10.18632/aging.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCay CM, et al. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 12.Fontana L, et al. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadon NL, et al. NIA Interventions Testing Program: Investigating Putative Aging Intervention Agents in a Genetically Heterogeneous Mouse Model. EBioMedicine. 2017;21:3–4. doi: 10.1016/j.ebiom.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blagosklonny MV. From rapalogs to anti-aging formula. Oncotarget. 2017;8:35492–35507. doi: 10.18632/oncotarget.18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohal RS, Forster MJ. Caloric restriction and the aging process: a critique. Free Radic. Biol Med. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rando TA, Wyss-Coray T. Stem cells as vehicles for youthful regeneration of aged tissues. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S39–42. doi: 10.1093/gerona/glu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellano JM, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker DJ, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X, et al. Evidence for a limit to human lifespan. Nature. 2016;538:257–259. doi: 10.1038/nature19793. [DOI] [PubMed] [Google Scholar]
- 24.Modig K, et al. How long do centenarians survive? Life expectancy and maximum lifespan. J Intern Med. 2017;282:156–163. doi: 10.1111/joim.12627. [DOI] [PubMed] [Google Scholar]
- 25.Gbari S, et al. Extreme Value Analysis of Mortality at the Oldest Ages: A Case Study Based on Individual Ages at Death. North American Actuarial Journal. 2017;21:397–416. [Google Scholar]
- 26.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 27.Pascual-Leone A, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 28.Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80:729–741. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 29.de Brabander JM, et al. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S, et al. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol. 1985;65:281–284. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- 31.Scheibel ME, et al. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 32.Craik FI, Salthouse TA. Handbook of aging and cognition. 2nd. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- 33.Dickstein DL, et al. Dendritic spine changes associated with normal aging. Neurosci. 2013;251:21–32. doi: 10.1016/j.neuroscience.2012.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 35.Reuter-Lorenz PA, et al. Neural recruitment and cognitive aging: Two hemispheres are better than one, especially as you age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- 36.Cabeza R. Functional neuroimaging of cognitive aging. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge: MIT Press; 2001. pp. 331–77. [Google Scholar]
- 37.Cabeza R, et al. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cer cor. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 38.Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- 39.Eyler LT, et al. A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry. 2011;70:115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonçalves JT, et al. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst A, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Sorrells SF, et al. Neurogenesis in the human hippocampus declines sharply during infancy to extremely low levels in children and undetectable levels in the adult. Presentation 272.02, Society for Neuroscience 2017; Washington D.C.. 2017. [Google Scholar]
- 45.Bordey A. Endogenous stem cells for enhancing cognition in the diseased brain. Front Neurosci. 2014;8:98. doi: 10.3389/fnins.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellenchi GC, et al. Adult neural stem cells: an endogenous tool to repair brain injury? J Neurochem. 2013;124:159–167. doi: 10.1111/jnc.12084. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193:257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cervantes RB, et al. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc Natl Acad Sci U S A. 2002;99:3586–3590. doi: 10.1073/pnas.062527199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouhani FJ, et al. Mutational History of a Human Cell Lineage from Somatic to Induced Pluripotent Stem Cells. PLoS Genet. 2016;12:e1005932. doi: 10.1371/journal.pgen.1005932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of age and sex. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 51.Boldrini M, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallett PJ, et al. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 2014;7:1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kefalopoulou Z, et al. Longterm clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci U S A. 2016;113:6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fricker-Gates RA, et al. Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J Neurosci. 2002;22:4045–4056. doi: 10.1523/JNEUROSCI.22-10-04045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaillard A, et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 57.Michelsen KA, et al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85:982–997. doi: 10.1016/j.neuron.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Ballout N, et al. Development and Maturation of Embryonic Cortical Neurons Grafted into the Damaged Adult Motor Cortex. Front Neural Circuits. 2016;10:55. doi: 10.3389/fncir.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falkner S, et al. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016;539:248–253. doi: 10.1038/nature20113. [DOI] [PubMed] [Google Scholar]
- 60.Peron S, et al. A delay between motor cortex lesions and neuronal transplantation enhances graft integration and improves repair and recovery. J Neurosci. 2017;37:1820–1834. doi: 10.1523/JNEUROSCI.2936-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinbeck JA, et al. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell Mol Life Sci. 2012;69:461–470. doi: 10.1007/s00018-011-0759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Espuny-Camacho I, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–56. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci. 2017;18:299–309. doi: 10.1038/nrn.2017.30. [DOI] [PubMed] [Google Scholar]
- 64.Southwell DG, et al. Interneurons from embryonic development to cell-based therapy. Science. 2014;344:1240622. doi: 10.1126/science.1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30:133–135. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- 66.Celada P, et al. Serotonin modulation of cortical neurons and networks. Front Int Neurosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neurosci. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Southwell DG, et al. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azevedo FA, et al. Equal numbers of neuronal and non-neuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 70.Dranovski A, et al. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 72.Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:125–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- 73.DeFelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders – time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wichterle H, et al. Young neurons from medial ganglionic eminence disperse in adult and embryonc brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 76.Daadi MM, et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 2009;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 77.De la Cruz E, et al. Interneuron progenitors attenuate the power of acute focal ictal discharges. Neurotherapeutics. 2011;8:763–773. doi: 10.1007/s13311-011-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Binamé F, et al. NG2 regulates directional migration of oligodendrocyte precursor cells via Rho GTPases and polarity complex proteins. J Neurosci. 2013;33:10858–10874. doi: 10.1523/JNEUROSCI.5010-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–97. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruttger J, et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 81.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ladewig J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblast. Nature Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 83.Berninger B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–64. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gascón S, et al. Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Guo Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–6. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Torper O, et al. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, et al. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell. 2015;17:735–747. doi: 10.1016/j.stem.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marro S, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Leary DD, Sahara S. Genetic regulation of arealization of theneocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mendez I, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hebb DO. Organization of behavior. John Willey and Sons; New York: 1949. [Google Scholar]
- 93.Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 94.Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron. 2002;35:1015–1017. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 95.Shors TJ, et al. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav Brain Res. 2012:450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merino MM, et al. Survival of the fittest: essential roles of cell competition in development, aging, and cancer. Trends Cell Biol. 2016;26:776–788. doi: 10.1016/j.tcb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Politis M, et al. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med. 2012;4:128ra141. doi: 10.1126/scitranslmed.3003391. [DOI] [PubMed] [Google Scholar]
- 99.Sánchez-Pernaute R, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferrari D, et al. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur J Neurosci. 2006;24:1885–1896. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim DE, et al. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006;241:822–830. doi: 10.1148/radiol.2413050466. [DOI] [PubMed] [Google Scholar]
- 102.Boehm-Sturm P, et al. A multi-modality platform to image stem cell graft survival in the naïve and stroke-damaged mouse brain. Biomaterials. 2014;35:2218–2226. doi: 10.1016/j.biomaterials.2013.11.085. [DOI] [PubMed] [Google Scholar]
- 103.Janowski M, et al. Survival of neural progenitors allografted into the CNS of immunocompetent recipients is highly dependent on transplantation site. Cell Transplant. 2014;23:253–62. doi: 10.3727/096368912X661328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sher F, et al. Bioluminescence imaging of Olig2-neural stem cells reveals improved engraftment in a demyelination mouse model. Stem Cells. 2009;27:1582–1591. doi: 10.1002/stem.76. [DOI] [PubMed] [Google Scholar]
- 105.Rockenstein E, et al. Neuro-peptide treatment with Cerebrolysin improves the survival of neural stem cell grafts in an APP transgenic model of Alzheimer disease. Stem Cell Res. 2015;15:54–67. doi: 10.1016/j.scr.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Broadwell RD, et al. Angiogenesis and the blood-brain barrier in solid and dissociated cell grafts within the CNS. Prog Brain Res. 1990;82:95–101. doi: 10.1016/s0079-6123(08)62595-9. [DOI] [PubMed] [Google Scholar]
- 107.Rosenstein JM. Why do neural transplants survive? An examination of some metabolic and pathophysiological considerations in neural transplantation. Exp Neurol. 1995;133:1–6. doi: 10.1006/exnr.1995.1001. [DOI] [PubMed] [Google Scholar]
- 108.Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013;5:4. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fantin A, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pennell NA, Streit WJ. Colonization of neural allografts by host microglial cells: relationship to graft neovascularization. Cell Transplant. 1997;6:221–230. doi: 10.1177/096368979700600305. [DOI] [PubMed] [Google Scholar]
- 111.Paolicelli RC, Ferretti MT. Function and Dysfunction of microglia during brain development: Consequences for Synapses and Neural Circuits. Front Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frost JL, Schafer DP. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016;26:587–97. doi: 10.1016/j.tcb.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mosser CA, et al. Microglia in CNS development: Shaping the brain for the future. Prog Neurobiol. 2017;149–150:1–20. doi: 10.1016/j.pneurobio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 114.French A, et al. Enabling consistency in pluripotent stem cell-derived products for research and development and clinical applications through material standards. Stem Cells Translat Med. 2015;4:217–223. doi: 10.5966/sctm.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuv Res. 2006;9:264–273. doi: 10.1089/rej.2006.9.264. [DOI] [PubMed] [Google Scholar]
- 116.Draghici C, et al. Concise total synthesis of glucosepane. Science. 2015;350:294–298. doi: 10.1126/science.aac9655. [DOI] [PubMed] [Google Scholar]
- 117.Finch CE. Longevity, senescence, and the genome. Chicago: The University of Chicago Press; 1990. [Google Scholar]
- 118.de Grey ADNJ. Challenging but essential targets for genuine anti-ageing drugs. Expert Opin Ther Targets. 2003;7:1–5. doi: 10.1517/14728222.7.1.1. [DOI] [PubMed] [Google Scholar]
- 119.Siemionow M. Impact of reconstructive transplantation on the future of plastic and reconstructive surgery. Clin Plastic Surg. 2012;39:425–434. doi: 10.1016/j.cps.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 120.Pedde RD, et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv Mater. 2017;29(19) doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- 121.Shafiee A, Atala A. Printing technologies for medical applications. Trends Mol Med. 2016;22:254–265. doi: 10.1016/j.molmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 122.Malchesky PS. Artificial organs 2016: a year in review. Artificial Organs. 2017;41:276–304. doi: 10.1111/aor.12931. [DOI] [PubMed] [Google Scholar]
- 123.Desmurget M, et al. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 124.Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Péron S, Berninger B. Reawakening the sleeping beauty in the adult brain: neurogenesis from parenchymal glia. Curr Opin Genet Dev. 2015;34:46–53. doi: 10.1016/j.gde.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Masserdoti G, et al. Direct neuronal reprogramming: learning from and for development. Development. 2016;143:2494–2510. doi: 10.1242/dev.092163. [DOI] [PubMed] [Google Scholar]
- 127.Gao L, et al. Direct Generation of Human Neuronal Cells from Adult Astrocytes by Small Molecules. Stem Cell Rep. 2017;8:538–547. doi: 10.1016/j.stemcr.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tasic B, et al. Adult mouse cortical cell taxonomy revealed by single celltranscriptomics. Nat Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeisel A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 130.Darmanis S, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]


