Abstract
We report the results from a phase I study of buparlisib, an oral pan-class I phosphotidyinositol-3-kinase inhibitor, combined with capecitabine in patients with metastatic breast cancer. The maximum tolerated dose of the combination was buparlisib 100 mg daily and capecitabine 1000 mg/m2 twice daily. A complete response was seen in 1 patient with a basal-like tumor. Pharmacokinetic analysis suggested that a pharmacokinetic interaction might exist between the 2 agents.
Background
Buparlisib is an oral pan-class I phosphotidyinositol-3-kinase (PI3K) inhibitor. The present phase I study evaluated the safety, pharmacokinetics, and efficacy of buparlisib with capecitabine in patients with metastatic breast cancer.
Patients and Methods
Patients received buparlisib once daily (range, 50 to 100 mg) for 3 weeks with capecitabine twice daily (range, 1000 to 1250 mg/m2) for 2 weeks with a 1-week break. Dose escalation used a traditional “3 + 3” design with standard definitions of dose-limiting toxicity (DLT) and maximum tolerated dose.
Results
Of the 25 patients enrolled, 23 were evaluable for DLT and 17 were evaluable for response. The maximum tolerated dose of the combination was buparlisib 100 mg daily and capecitabine 1000 mg/m2 twice daily. DLTs included grade 3 hyperglycemia and grade 3 confusion. The most common grade 3 toxicities were diarrhea and elevation of aspartate aminotransferase and alanine transaminase. One patient exhibited a complete response to treatment and four had a confirmed partial response. In cohorts 3 and 4, in which the buparlisib dose remained constant but the capecitabine dose was increased, significant increases in the buparlisib plasma concentration were noted.
Conclusion
The combination of buparlisib with capecitabine in patients with metastatic breast cancer was generally well-tolerated, with several patients demonstrating prolonged responses. Unexpectedly low rates of PIK3CA mutations (3 of 17) were seen, and only 2 of 7 tumors with subtyping were luminal, making exploration of these putative predictive markers impossible. Further study of the combination is not unreasonable, with expanded pharmacokinetics and sequencing analysis to better elucidate potential drug–drug interactions and more accurate predictive biomarkers of response.
Keywords: Breast cancer, Buparlisib, Capecitabine, PI3K, Phase I
Introduction
Constitutive activation of the phosphatidylinositol-3-kinase (PI3K) pathway has been observed in a variety of human malignancies, including breast cancer, in which PIK3CA mutations are the most common genetic abnormality.1 Molecular changes can include (1) gain-of-function mutations encoding positive regulators of PI3K; (2) loss-of-function mutations affecting negative regulators (eg, PTEN); and (3) mutations of genes encoding downstream effectors.2 Thus, pathway-mutated tumors might be particularly sensitive to treatment with PI3K inhibitors.
Physicians have long observed considerable heterogeneity in the prognosis and behavior of breast cancer. The results from gene expression profiling studies have supported this heterogeneity and underscore the importance of developing more effective treatment strategies for advanced disease by the identification of driver mutations or pathway addiction.3 It has become increasingly clear that gene expression profiling can be used to more accurately classify and predict the behavior of breast cancer. Using DNA microarray technology, Sorlie et al4 have identified and validated 5 major subtypes of breast tumors, including basal-like, HER2 overexpressing, luminal-like (including luminal A and B), and normal breast tissue-like. Somatic mutation rates of PIK3CA vary according to molecular subtype, with luminal-like (A and B), HER2-enriched, and basal-like harboring 45%, 29%, 39%, and 9%, respectively.1
Luminal B subtype tumors represent 10% to 18% of human breast cancer cases and have a poor prognosis relative to other estrogen receptor-positive (ER+) breast cancers, with significantly lower survival in both locally advanced and early-stage settings.5 Creighton et al,6 who developed a genetic signature comprehensively examining the PI3K pathway in hormone receptor-positive breast cancer, found a high correlation between their signature and the luminal B subtype with a worse prognosis. PIK3CA mutations have also been detected at a high rate in HER2-enriched tumors and are thought to contribute to resistance to HER2 targeted therapies.7 Knowing the intrinsic subtype of a patient’s tumor might, therefore, provide a better selection process for patients who might benefit from PI3K targeted therapy.
Buparlisib is an oral pan-class I PI3K inhibitor that targets the catalytic subunits encoded by the PI3KCA gene. The first in-human phase I trial of buparlisib in patients with advanced refractory solid tumors established the maximum tolerated dose (MTD) of buparlisib to be 100 mg daily.8 The most frequent adverse events (AEs) in that study were decreased appetite, nausea, constipation, diarrhea, fatigue, rash, and hyperglycemia. Subsequent randomized studies of buparlisib plus the selective ER downregulator fulvestrant for patients with advanced breast cancer (BELLE-2 [buparlisib plus fulvestrant vs. placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer] and BELLE-3 [a phase III study of BKM120 with fulvestrant in patients with HR+ (hormone receptor positive), HER2−, AI (aromatase inhibitor) treated, locally advanced or metastatic breast cancer who progressed on or after mTORi (mammalian target of rapamycin inhibitor)]) have been reported, with modest improvements in progression-free survival (PFS) for the combination compared with hormonal therapy alone.9,10 Other clinical trials evaluating the safety of buparlisib combined with chemotherapy (including paclitaxel as a single agent and carboplatin plus paclitaxel) have recently been reported.11,12
Capecitabine is an oral fluoropyrimidine that was approved in 1998 for the treatment of metastatic breast cancer. Capecitabine is among the preferred single agents recommended by the National Comprehensive Cancer Network Oncology Practice Guidelines for treatment of metastatic breast cancer and has also demonstrated safety and activity combined with biologic agents, including trastuzumab, lapatinib, and bevacizumab.13–15 As a single agent, capecitabine has demonstrated efficacy and a favorable side effect profile.16,17 In the present trial, we evaluated capecitabine combined with buparlisib in a phase I trial of patients with metastatic breast cancer to determine the safety, dose-limiting toxicity (DLT), and MTD of the combination. Our rationale was that this all-oral regimen, if tolerable, might be an attractive and effective therapy, especially for patients with luminal B breast cancer refractory to endocrine therapy, and could be further tested in that subgroup. Exploratory analyses aimed at evaluating the differential response to treatment according to intrinsic molecular subtypes and PIK3CA mutational analysis were performed.
Patients and Methods
Patient Eligibility
Patients with a histologic diagnosis of metastatic breast cancer for which capecitabine was deemed a reasonable treatment option were eligible for enrollment. The eligibility criteria also included age ≥ 18 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and life expectancy of ≥ 12 weeks. The patients were required to have either measurable or nonmeasurable, but evaluable, disease as defined using Response Evaluation Criteria In Solid Tumors, version 1.1. Normal bone marrow function was defined as follows: absolute neutrophil count ≥ 1500/µL, platelet count ≥ 100,000/µL, and hemoglobin ≥ 8.5 g/dL. Organ function was required to be within normal limits, with the following caveats: total bilirubin ≤ 1.5 times the upper limit of normal and aspartate aminotransferase (AST)/alanine transaminase (ALT) ≤ 3 times the upper limit of normal, if liver metastases were present. The fasting plasma glucose level was required to be < 120 mg/dL. The patients had to have recovered from all toxicities related to their previous treatment (other than alopecia) to grade ≤ 1 or baseline. Brain metastases were allowed if central nervous system-directed treatment had been given ≥ 3 months before enrollment with clinically and radiographically stable disease for ≥ 8 weeks.
The pertinent exclusion criteria included previous treatment with capecitabine or a PI3K inhibitor; concurrent use of an endocrine, a cytotoxic, or a biologic agent; major surgery ≤ 2 weeks before starting buparlisib; and chronic systemic treatment with steroids or other immunosuppressive agents. Patients with known dihydropyrimidine dehydrogenase deficiency were excluded, as were patients with known coagulopathies resulting from warfarin-derived anticoagulant agents. Other comorbid conditions that prohibited enrollment included acute or chronic liver disease, renal disease, pancreatitis, poorly controlled diabetes mellitus, a known diagnosis of human immunodeficiency virus infection, inadequately controlled hypertension, and clinically significant active cardiac disease. Patients with documented mood or anxiety disorders and those with gastrointestinal dysfunction resulting in impaired absorption were also excluded.
The institutional review board of the University of North Carolina at Chapel Hill approved the present study. It was registered with the US National Institutes of Health (ClinicalTrials.gov identifier, NCT01300962). All patients provided written informed consent before the initiation of any study-related procedures or treatments.
Treatment Plan
The present study was designed as a phase I trial to establish the safety, tolerability, and MTD of buparlisib combined with capecitabine in patients with metastatic breast cancer. The primary objective was to determine the DLT and MTD of the combination. The secondary objectives included characterizing the acute and chronic toxicities. As exploratory objectives, the pharmacokinetic (PK) profile of buparlisib with capecitabine was evaluated, and predictive biomarkers were correlated with the therapeutic response. The study consisted of 4 cohorts, outlined in Table 1. The patients received oral buparlisib at a starting dose of 50 mg once daily for 3 weeks combined with a starting dose of capecitabine at 1000 mg/m2 twice daily for 2 weeks followed by a 1-week break. The patients were instructed per protocol to take their morning dose of capecitabine at the same time as their daily dose of buparlisib 1 hour after a light breakfast. Any patient who received treatment in accordance with the protocol was evaluable for toxicity. Patients not experiencing DLTs were required to complete ≥ 1 cycle of therapy (3 weeks) to be evaluable for the MTD determination. Any patient who withdrew from study before day 14 of cycle 1 without experiencing a DLT was replaced.
Table 1.
Dose Escalation and Dose-limiting Toxicity
| Dose Level | Buparlisib Dose (mg/d) |
Capecitabine Dose (mg/m2) Twice Daily |
Total Patients (n) |
Patients Evaluable for DLT (n) |
Patients With DLT (n) |
Patients With Grade 3/4a Toxicity in Cycle 2 |
|---|---|---|---|---|---|---|
| 1 | 50 | 1000 | 4 | 3 | 0 | 1 |
| 2 | 80 | 1000 | 3 | 3 | 0 | 0 |
| 3 | 100 | 1000 | 13 | 12 | 1b | 2 |
| 4 | 100 | 1250 | 5 | 5 | 1c | 4a |
Abbreviation: DLT = dose-limiting toxicity.
Grade 3/4 adverse events included fatigue, photosensitivity/pruritus, rash, anxiety, depression, hallucinations, mania, psychosis and concentration impairment.
Hyperglycemia.
Confusion.
The study used a traditional “3 + 3” design. If none of the initial 3 patients enrolled experienced a DLT within the first 3 weeks, the subsequent cohort of patients was treated at the next dose level. If 1 of the 3 patients in any cohort experienced a DLT, 3 additional patients were enrolled into that same cohort. If only 1 of these 6 patients experienced DLT, subsequent patients were enrolled into the next cohort. If ≥ 2 of these 6 patients experienced a DLT, or 2 patients experienced a DLT in a cohort of 3 patients, the MTD was considered to have been exceeded. If 2 patients experienced a DLT in a cohort of 3 patients, 3 additional patients were assigned to the lower dose level, unless 6 patients had already been enrolled at that dose level, or if this had occurred in cohort 1.
Assessments
Toxicity Assessment and Definition of DLT
AEs and DLTs were defined using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4. The following were considered DLTs if they occurred with the first cycle of therapy (3 weeks) and were considered possibly related to study treatment: (1) any intolerable grade ≥ 2 nonhematologic or grade 3 hematologic toxicity that required withholding a dose for > 7 days or required a dose reduction; (2) any grade ≥ 4 hematologic toxicity; (3) other grade ≥ 3 nonhematologic toxicity (except for alopecia and nausea, vomiting, and diarrhea unless persistent for > 48 hours despite maximum supportive care); (4) grade ≥ 2 photosensitivity; and (4) ≥ 1 grade level increase in neurotoxicity. The MTD was defined as the highest dose at which ≤ 1 of 6 patients experienced a DLT. No intra-patient dose escalation was allowed.
PK Assessments
PK sampling was performed in cycle 1 on days 1 and 8 and in cycle 2 on day 1 (day 22 of treatment). A 3-mL blood sample was collected immediately before capecitabine administration and 2 hours after buparlisib administration on each day. The buparlisib plasma concentration was measured using a validated liquid chromatography–tandem mass spectrometry assay with a lower limit of quantification of 1 ng/mL.8 Buparlisib plasma concentrations were summarized at each measurement point.
PIK3CA Mutational Analysis
Mutational analysis for hotspot mutations within the PIK3CA gene was performed using Sanger dideoxy-sequencing of PIK3CA exons 9 and 20. After enrichment for tumor cells by macrodissection, genomic DNA was extracted from formalin-fixed paraffin-embedded (FFPE) slides and subjected to Sanger dideoxy-cycle sequencing using primers homologous to the relevant regions.
Gene Expression Profiling
RNA was extracted from archived, FFPE tumor samples (High Pure FFPE Kit; Roche Diagnostics, Indianapolis, IN). Nanostring analyses were performed at the Rapid Adoption Molecular Laboratory at the University of North Carolina. Nanostring probe sets included 110 genes, of which 50 were the PAM50 genes and 5 were housekeeping genes, as described previously.5 In brief, 100 ng of each purified total RNA was labeled with molecular barcodes representing genes. The labeled molecules were counted using the Nanostring nCounter (Nanostring Technologies, Seattle, WA) as gene expression raw data. Raw data was log base 2 transformed and normalized using positive controls and 5 housekeeping transcripts and then median centered along with 150 training data (from the 9830 nano110 project). All samples, including 150 training samples, were standardized to a 0 mean and unit variance. The PAM50 intrinsic subtype and claudin-low predictors were used to assign an intrinsic subtype (luminal A, luminal B, HER2-enriched, basal-like, and normal-like) to each tumor sample.5 Gene expression and clinical data were deposited in the Gene Expression Omnibus.
Clinical and Response Assessment
The baseline evaluations at enrollment included a history and physical examination, performance status determination, baseline laboratory measurements, a 12-lead electrocardiogram, an echocardiogram, and imaging studies to assess the tumor measurements. Patients were required to complete both the patient health questionnaire-9 (PHQ-9) and the generalized anxiety disorder 7-item (GAD-7) scale as a measure of mood and anxiety level. The medical history and focused physical examination were repeated on the first day of each cycle. Toxicity assessments were gathered weekly (days 1, 8, and 15) during cycle 1 and then on day 1 of the subsequent cycles. The tumor measurements were assessed using the Response Evaluation Criteria In Solid Tumors every 2 cycles.
Results
Patient Characteristics
A total of 25 breast cancer patients were enrolled at 2 institutions. The patient demographic data and baseline disease characteristics are summarized in Table 2. The median age of the women in the study was 50 years (range, 26 to 65 years). Most patients were white. A total of 10 patients had tumors that were ER+. Only 1 patient’s tumor was HER2 overexpressing. The remaining 15 patients had triple negative breast cancer (TNBC). The sites of metastatic disease included nodal/soft tissue in 11 patients, lung in 10 patients, bone in 8 patients, and liver in 7 patients. The median number of previous treatments was 2 (range, 0 to 6).
Table 2.
Patient Characteristics (n = 25)
| Characteristic | Value |
|---|---|
| Age (y) | |
| Median | 50 |
| Range | 26–65 |
| Race | |
| White | 22 |
| Black | 3 |
| Tumor subtype | |
| ER− PR− HER2− (TNBC) | 15 |
| ER+ | 10 |
| HER2+ | 1 |
| Metastatic site | |
| Nodal/soft tissue | 11 |
| Lung | 10 |
| Bone | 8 |
| Liver | 7 |
| Previous treatments | |
| Median | 2 |
| Range | 0–6 |
Abbreviations: ER = estrogen receptor; PR = progesterone receptor.
DLT and Determination of MTD
Of the 25 patients who received treatment in the study, 2 patients (1 in cohort 1 and 1 in cohort 3) were deemed not evaluable for DLT assessment because they did not receive an entire cycle of therapy (both because of rapid disease progression). The DLTs associated with buparlisib and capecitabine are summarized in Table 1. In all 4 cohorts, the first 3 evaluable patients enrolled in each treatment arm did not experience DLT. Therefore, a planned expansion of cohort 4 was initiated, with 1 patient of 5 experiencing a DLT of grade 3 confusion. Additionally, 4 of the 5 patients in this cohort experienced grade ≥ 3 toxicity during cycle 2 of treatment consisting of fatigue, photosensitivity/pruritus, rash, anxiety/hallucinations, mania, psychosis, depression, and concentration impairment. Given the cumulative toxicity in cohort 4, further enrollment to that cohort was halted, and an expansion of cohort 3 was conducted. Of the 13 total patients enrolled in cohort 3, 1 DLT was observed (grade 3 hyperglycemia); hence, the MTD was determined to be the dose given to cohort 3, consisting of buparlisib 100 mg once daily combined with capecitabine 1000 mg/m2 twice daily.
Adverse Events
All patients enrolled in the trial reported ≥ 1 treatment-related AE, with 13 patients (52%) experiencing grade ≥ 3 toxicity. The most common AEs of all grades associated with buparlisib and capecitabine were nausea (56%), hand-foot syndrome (HFS; 52%), mucositis (48%), diarrhea (40%), and rash (including photosensitivity; 36%; Table 3). The most common grade ≥ 3 toxicities were diarrhea and grade 3 elevation of AST and ALT (both presenting in 3 patients each). Other grade 3 toxicities included HFS and rash (occurring in 2 patients each), with the remaining 7 grade 3 and 4 AEs occurring in 1 patient each. A total of 10 patients (40%) experienced ≥ 1 AE related to psychiatric impairment, mood, memory, or cognition; 2 in cohort 1, 4 in cohort 3, and 4 in cohort 4. One patient experienced multiple grade 3 AEs (concentration impairment, confusion, and depression), and one reported grade 4 toxicities (anxiety, hallucinations, mania, and psychosis). Both patients had been treated in cohort 4, with the symptoms predominantly occurring in cycle 2 of treatment. The most common AEs resulting in dose delays included rash (including photosensitivity and pruritus), HFS, hyperglycemia, diarrhea, mood impairment, mucositis, and fatigue.
Table 3.
Most Frequent Adverse Events
| Adverse Event | Total Patients | Grade | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Nausea | 14 (56) | 10 | 3 | 1 | 0 |
| HFS | 13 (52) | 1 | 10 | 2 | 0 |
| Oral mucositis | 12 (48) | 10 | 2 | 0 | 0 |
| Diarrhea | 10 (40) | 7 | 0 | 3 | 0 |
| Rash (including photosensitivity) | 9 (26) | 3 | 4 | 2 | 0 |
| Fatigue | 8 (32) | 5 | 2 | 1 | 0 |
| Vomiting | 8 (32) | 4 | 4 | 0 | 0 |
| Depression | 6 (24) | 2 | 3 | 1 | 0 |
| Dyspepsia | 6 (24) | 6 | 0 | 0 | 0 |
| Memory/cognitive impairment | 6 (24) | 4 | 1 | 1 | 0 |
| Other mood disturbancea | 5(20) | 2 | 1 | 1 | 1 |
| Dysgeusia | 5(20) | 1 | 4 | 0 | 0 |
| Anxiety | 5(20) | 1 | 3 | 0 | 1 |
| Peripheral neuropathy | 5(20) | 2 | 3 | 0 | 0 |
| Hyperglycemia | 4 (16) | 3 | 1 | 0 | |
| Pruritus | 4 (16) | 3 | 1 | 0 | |
| Constipation | 4 (16) | 3 | 1 | 0 | 0 |
| Elevated AST/ALT | 4 (16) | 1 | 0 | 3 | 0 |
| Dizziness | 4 (16) | 3 | 1 | 0 | 0 |
| Nail discoloration/ridging | 3(12) | 3 | 0 | 0 | 0 |
Data presented as n (%).
Abbreviations: ALT = alanine transaminase; AST = aspartate aminotransferase; HFS = hand-foot syndrome.
Other mood disturbances included irritability (2 patients), mania (1 patient), vivid dreams (1 patient), psychosis (2 patients), hallucinations (1 patient), confusion (1 patient), and euphoria (1 patient).
At baseline and throughout the study, the patients completed both the PHQ-9 and the GAD-7 surveys. The mean highest PHQ-9 values for each dose level were 4, 6, 4, and 9. The mean highest GAD-7 values for each dose level were 3, 3, 4, and 7. Of these, only 3 patients had a score clinically suggestive of depression (> 10 on the PHQ-9) and 4 patients had a score suggestive of clinically relevant anxiety (> 10 on the GAD-7). All of these occurred in cohorts 3 and 4. The 2 patients in cohort 4 with the highest scores for both surveys also experienced grade 3 toxicity that included anxiety, hallucinations, depression, and impaired concentration.
Tumor Response and Clinical Benefit
Of the 25 patients who received treatment in the trial, 17 were evaluable for response. The median number of cycles received for all patients was 4 (range, 2 to 32). One patient in cohort 3 with heavily pretreated TNBC and lung metastases exhibited a complete response after receiving 11 cycles of treatment. She received a total of 32 cycles and discontinued because of mood changes that resolved with withdrawal of therapy. Four patients (2 ER+, 1 HER2+, and 1 TNBC) experienced a partial response to treatment, with a maximum percentage of change from baseline ranging from 34% to 67%. The remaining evaluable patients (4 ER+ and 8 TNBC) demonstrated stable disease, 3 of whom continued with treatment for ≥ 6 months. Of all 25 patients enrolled in the trial, 7 patients had died at study completion, none within 28 days of receiving treatment and all attributed to disease progression.
Pharmacokinetics
The mean buparlisib plasma concentrations in each cohort on days 1, 8, and 22 of treatment are summarized in Table 4. With no PK data collected for buparlisib alone for each patient, the potential PK interactions between buparlisib and capecitabine were evaluated by (1) comparing differences in the PK of buparlisib at increasing doses (50, 80, and 100 mg) combined with a constant dose of capecitabine (1000 mg/m2); and (2) comparing differences in the PK of buparlisib at the same dose (100 mg) combined with varying doses of capecitabine (1000 and 1250 mg/m2). In cohorts 1, 2, and 3, all of whom had received the same dose of capecitabine (1000 mg/m2), an inconsistent increase in buparlisib concentrations at 2 hours after buparlisib on days 8 and 22. These results raise the possibility that a PK interaction might exist between buparlisib and capecitabine, because the exposure of buparlisib increased at a much greater extent at 100 mg compared with buparlisib at 80 mg with a constant dose of capecitabine.
Table 4.
Mean Buparlisib Concentrations
| Cohort | Buparlisib Dose (mg/d) |
Capecitabine Dose (mg/m2) Twice Daily |
Buparlisib Concentration (ng/mL) | ||||
|---|---|---|---|---|---|---|---|
| Day 1, 2-h After Buparlisib |
Day 8, Before Buparlisib |
Day 8, 2-h After Buparlisib |
Day 22, Before Buparlisib |
Day 22, 2-h After Buparlisib |
|||
| 1 | 50 | 1000 | 151 ± 89 | 386 ± 69 | 407 ± 86 | 583 ± 176 | 466 ± 184 |
| 2 | 80 | 1000 | 252 ± 76 | 495 ± 41 | 561 ± 11 | 522 ± 406 | 661 ± 165 |
| 3 | 100 | 1000 | 511 ± 210 | 673 ± 226 | 728 ± 249a | 912 ± 454 | 1004 ± 317b |
| 4 | 100 | 1250 | 635 ± 90 | 786 ± 64 | 1390 ± 300a | 1228 ± 298 | 1386 ± 135b |
Statistically significant difference in buparlisib concentrations at day 8, 2 hours after buparlisib administration between cohorts 3 and 4 (P = .049).
Statistically significant difference in buparlisib concentrations at day 22, 2 hours after buparlisib administration between cohorts 3 and 4 (P = .014).
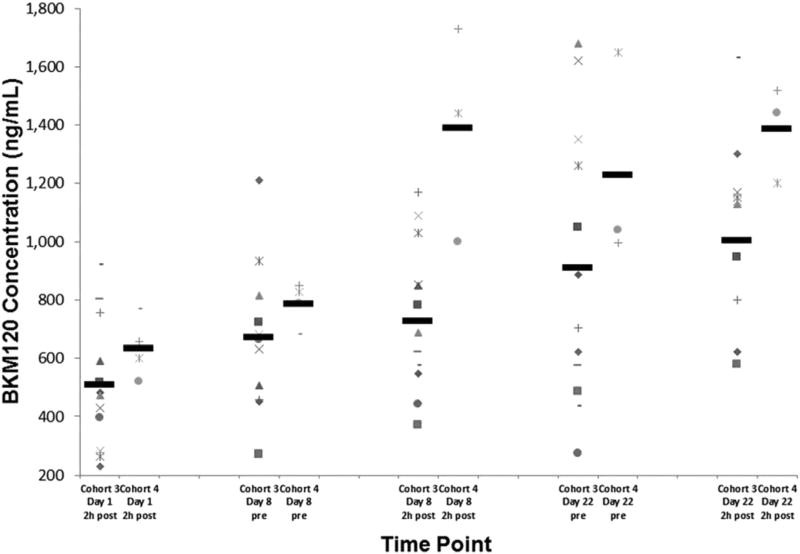
The buparlisib plasma concentrations on days 1, 8, and 22 collected before and 2 hours after buparlisib administration in cohorts 3 and 4 are presented in Figure 1. These patients all received a buparlisib dose of 100 mg daily. The higher dose of capecitabine in cohort 4 (1250 mg/m2) was associated with greater exposure of buparlisib on all days compared with the dose of capecitabine in cohort 3 (1000 mg/m2). On day 8, a statistically significant increase was found in the buparlisib concentrations collected 2 hours after administration in cohort 4 (1390 ± 300 ng/mL) compared with cohort 3 (728 ± 249 ng/mL; P = .049; Table 4). Similarly, a statistically significant increase in buparlisib concentrations was detected on day 22 (2 hours after administration) in cohort 4 (1386 ± 135 ng/mL) compared with cohort 3 (1004 ± 317 ng/mL; P = .014; Table 4). These results suggest that the higher dose of capecitabine in cohort 4 (1250 mg/m2) compared with that in cohort 3 (1000 mg/m2) increased the exposure of buparlisib, despite a constant dose of buparlisib (100 mg).
Figure 1.
Buparlisib Plasma Concentrations Collected on Days 1, 8, and 22 (Before and 2 Hours After Buparlisib Administration) Displayed From Patients in Cohorts 3 and 4. These Patients (Each Represented With a Different Symbol) Received the Same Dose of Buparlisib (100 mg Daily) but With Increasing Doses of Capecitabine (1000 mg/m2 and 1250 mg/m2, Respectively). Mean Values for Each Time Point Are Represented by the Black Horizontal Line. Despite the Same Administered Dose of Buparlisib, the Higher Dose of Capecitabine in Cohort 4 Was Associated With Higher Buparlisib Exposure. This Was Statistically Significant 2 Hours After Buparlisib on Both Days 8 and 22
PIK3CA Mutational Analysis
A total of 21 patients had archived tissue available for PIK3CA mutational analysis. Of those, 4 had insufficient DNA quantity for testing. Of the remaining 17 samples, 3 contained an activating mutation in PIK3CA (2 in exon 20 and 1 in exon 9). The presence of an activating mutation did not appear to correlate with a greater likelihood of response to buparlisib. Of the 3 mutated tumors, 1 patient demonstrated progressive disease during cycle 2 and died approximately 1 month after discontinuing treatment. The remaining 2 patients had stable disease (nonmeasurable but evaluable) but discontinued treatment during the trial at the onset of cycle 3 because of toxicity.
Gene Expression Profiling
FFPE tumor samples were available from 14 patients, of which 7 had sufficient RNA for Nanostring analysis. Intrinsic molecular subtyping revealed 4 basal-like (57%), 1 luminal A (14%), 1 luminal B (14%), and 1 normal-like tumor (14%). The patient who had experienced a complete response to treatment, continuing therapy for 32 cycles, had a tumor with a basal-like subtype. Two patients with a partial response (continuing therapy for 5 and 15 cycles) had tumors with luminal B and luminal A subtypes, respectively. The small sample size and the scant number of tumors with analyzable RNA made this analysis exploratory, because we lacked power for any significant correlations with response or survival.
Discussion
The present study is a phase I trial of the oral PI3K inhibitor buparlisib combined with capecitabine in patients with metastatic breast cancer. The MTD of the combination was determined to be buparlisib 100 mg daily combined with capecitabine 1000 mg/m2 twice daily. This dose of buparlisib is consistent with the MTD reported in the first-in-human phase I trial of single-agent buparlisib.8 The DLT associated with buparlisib and capecitabine in our study included grade 3 hyperglycemia and confusion. Approximately one half of patients in the study experienced grade ≥ 3 toxicity. The most common AEs were diarrhea, AST and ALT elevation, HFS, and rash. The median treatment time was 12 weeks, with 1 patient demonstrating a complete response.
We evaluated for potential PK interactions between buparlisib and capecitabine by comparing both the differences in PK of buparlisib at increasing doses (50, 80, and 100 mg) combined with a constant dose of capecitabine (1000 mg/m2) and the differences in the PK of buparlisib at the same dose (100 mg) combined with increasing doses of capecitabine (1000 and 1250 mg/m2). We observed an inconsistent increase in buparlisib concentrations on days 8 and 22 collected 2 hours after buparlisib administration (Table 4). These results suggest that a PK interaction between buparlisib and capecitabine might exist, because the exposure of buparlisib did not demonstrate a linear increase, despite a constant dose of capecitabine.
Additionally, at a constant 100-mg dose of buparlisib, increased exposure of buparlisib was documented, with the higher dose of capecitabine administered in cohort 4 (1250 mg/m2) compared with the lower dose of capecitabine in cohort 3 (1000 mg/m2; Figure 1). This was a statistically significant difference on days 8 and 22 between cohorts 3 and 4 (P = .049; Table 4). These results suggest that the higher dose of capecitabine in cohort 4 (1250 mg/m2) compared with the dose in cohort 3 (1000 mg/m2) increased the exposure of buparlisib, perhaps by altering the absorption and/or the metabolism of buparlisib. However, the metabolism pathways of the 2 drugs are not especially overlapping to explain the potential drug–drug interactions. Preclinical data showed buparlisib to be a sensitive CYP3A4 substrate, an enzyme that has insignificant bearing on capecitabine metabolism. This finding has not been reported in studies of capecitabine combined with other oral targeted agents, including everolimus, sunitinib, and sorafenib, all of which have demonstrated no PK interactions.18–20 A phase I study reported by our group of buparlisib combined with mFOLFOX6 (modified leucovorin calcium, 5-fluorouracil, oxaliplatin) reported an increased incidence of grade 3 and 4 toxicity with the combination than would be expected from either the PI3K inhibitor or the chemotherapy agent alone.21 In that study, the MTD of buparlisib combined with mFOLFOX6 was only 40 mg, which, we believe, suggests a potential interaction between 5-fluorouracil derivatives and PI3K inhibitors.
The Tumor Genome Atlas (TCGA) Network reported that PIK3CA mutations are present in as many as 45% of breast cancer cases across all subtypes; hence, PI3K inhibitors represent an attractive treatment strategy for metastatic patients.1 An observational study of 90 patients with a variety of advanced tumor types reported that PIK3CA-mutated cancers (specifically in exon 20) demonstrated greater response rates to PI3K/AKT/mTOR inhibitors compared with wild-type tumors treated with their best phase I option.22 Only 3 patients in our study had tumors with activating mutations in PIK3CA (2 in exon 20), which precluded a meaningful association with response to buparlisib. Other potential biomarkers of response have been reported, including aberrations in PTEN, AKT, and INPP4B, which were not evaluated in our study. Additionally, the coexistence of other pathway mutations with PIK3CA mutations might increase the risk of resistance to PI3K inhibitors. For example, in a review of 66 PIK3CA-mutant tumors treated with PI3K/AKT/mTOR pathway inhibitors, none of the 16 patients with concomitant KRAS mutations demonstrated a response to therapy.23 Therefore, understanding in more depth how the tumor kinome adjusts to PI3K pathway inhibition might provide additional preclinical data for how to best combine buparlisib with other targeted agents in future clinical trials.
A total of 7 patients had tumors with sufficient RNA for Nanostring analysis to determine the intrinsic molecular subtype. Of these, basal-like tumors were found in 4 patients, luminal subtypes in 2 patients (1 A and 1 B), and normal breast tissue-like in 1 patient. The 2 luminal tumors did not harbor PIK3CA mutations in our study despite the greater reported incidence in this subtype. However, of the basal-like tumors, 1 patient achieved a complete response to treatment (with no evidence of an activating PIK3CA mutation). Although basal-like tumors typically have lower rates of PIK3CA mutations (7%), TCGA breast cancer data reported a greater incidence of PTEN mutations or loss (35%) in basal-like tumors than in other subtypes.1 Basal-like tumors also demonstrated widespread genomic instability with increased copy number/ amplification of many downstream components of the PI3K and RAS-RAF-MEK pathway, including PIK3CA (49%), BRAF (30%), and EGFR (23%). These features might have contributed to the robust response of the basal-like tumor to buparlisib in our study. However, the small sample size of our study and the inability to determine whether patients’ clinical benefit was driven by the capecitabine itself limited any meaningful evaluation of the molecular subsets.
In addition to our study, buparlisib has been studied in combination with other agents, mostly hormonal therapies in breast cancer patients with ER+ tumors. The BELLE-2 trial, a large randomized phase III trial of 1147 patients with advanced hormone-positive breast cancer, randomized patients to fulvestrant either alone or combined with buparlisib.24 All patients in that trial had previously been treated with aromatase inhibitors and had developed progression. The investigators reported a statistically significant improvement in PFS with fulvestrant plus buparlisib of 6.9 months compared with 5.0 months with fulvestrant alone. A planned exploratory analysis of clinical outcomes in those patients with PIK3CA mutations detected in circulating tumor DNA revealed a significant difference in PFS in the buparlisib-treated patients versus placebo but not in those lacking a mutation. The same randomization of fulvestrant as a single agent or combined with buparlisib was applied to the BELLE-3 trial with 432 postmenopausal breast cancer patients enrolled with previous disease progression with both an aromatase inhibitor and an mTOR inhibitor.9 An improvement in PFS from 1.8 to 3.9 months was reported, suggesting that resistance to the mTOR pathway could be overcome by targeting PI3K. Again, the improvement in PFS was more pronounced for patients with mutant PIK3CA genes. Although these results provide further rationale for targeting the PI3K pathway in breast cancer, many concerns were raised regarding the toxicity of this combination, most notably the mood disturbances and suicidal ideation. Biologically, this can be attributed to the ability of small molecules to cross the blood–brain barrier, with future emphasis on developing isoform-specific PI3K inhibitors that target mutant variations of the protein only.
Two studies have combined buparlisib with trastuzumab (1 of which also contained paclitaxel) for patients with HER2+ breast cancer with progression during trastuzumab-based therapies.25,26 The combination was well tolerated, and in both studies, the MTD of buparlisib was 100 mg daily. Although both were small studies, the promising disease control rates seen in this refractory population did lend credence to the hypothesis that inhibiting the PI3K pathway might reverse resistance to HER2 targeted therapies. Lapatinib, a dual anti-HER2/EGFR tyrosine kinase inhibitor, is approved in combination with capecitabine in patients with HER2+ breast cancer and has also been studied in combination with buparlisib. However, a phase I study of buparlisib and lapatinib that enrolled 24 patients with refractory HER2+ breast cancer reported increased toxicity with 5 patients experiencing DLTs in the first cycle of treatment.27 No evidence was found of a PK drug–drug interaction. The recommended phase 2 dose of buparlisib was therefore reported as 80 mg daily with 1000 mg of lapatinib. In an unpublished cohort of our study, HER2+ patients received buparlisib combined with capecitabine and lapatinib. In the first cohort of 4 patients, buparlisib was administered at 60 mg daily for 21 days with lapatinib 750 mg daily and 1000 mg/m2 of capecitabine twice daily for 14 days. Within 1 cycle, one half of the patients experienced DLT; therefore, treatment was discontinued. This occurred although all 3 drugs were administered at doses less than the recommended phase 2 dose, which begs the question regarding whether dual inhibition of HER2 and EGFR in conjunction with PI3K inhibition leads to increased toxicity.
Conclusion
The present phase I trial of the oral PI3K inhibitor buparlisib plus capecitabine in patients with metastatic breast cancer found the combination to be generally well tolerated, with several patients demonstrating prolonged responses. Similar to other larger studies of buparlisib in breast cancer patients, psychiatric effects of buparlisib were observed in our study, with 20% of patients reporting mood disturbances but with limited grade 3/4 toxicity. The MTD of the combination was 100 mg of buparlisib daily combined with capecitabine 1000 mg/m2 twice daily. The PK analysis suggested the possibility of a drug–drug interaction with a statistically significant increase in buparlisib concentrations at higher capecitabine doses. Unexpectedly low rates of PIK3CA mutations (3 of 21) were observed, and only 2 of 7 tumors with subtyping available were luminal, resulting in a limited exploration of these predictive markers. One patient with a basal-like tumor experienced a complete response during the study. The basal-like tumor is a subtype reported to have increased amplification of many components of the PI3KandRAS-RAF-MEKpathways. Further study of the combination of buparlisib with chemotherapeutic agents, although not unreasonable, should be pursued with caution to minimize toxicity and, ideally, will involve more definitive correlative studies to better predict those patients who would be more likely to respond to PI3K inhibitors to avoid undue toxicity.
Clinical Practice Points
PIK3CA mutations are the most common genetic abnormality in breast cancer; hence, PI3K inhibitors represent an attractive treatment strategy for advanced breast cancer patients.
Buparlisib is an oral pan-class I PI3K inhibitor that has been studied in combination with both hormonal therapies and chemotherapeutic agents, showing safety and some efficacy.
Capecitabine is an oral fluoropyrimidine and is among the preferred single agents for treatment of metastatic breast cancer.
In our phase I study of buparlisib plus capecitabine in patients with metastatic breast cancer, the MTD of the combination was buparlisib 100 mg daily and capecitabine 1000 mg/m2 twice daily.
The DLTs associated with buparlisib and capecitabine in our study included grade 3 hyperglycemia and confusion; the most common AEs were diarrhea, AST and ALT elevation, HFS, and rash.
The PK analysis showed a statistically significant increase in buparlisib concentrations at higher capecitabine doses, suggesting a potential drug–drug interaction.
The only complete response during the study was in 1 patient with a basal-like tumor; no association was seen between the presence of PIK3CA mutations and the response to buparlisib.
Acknowledgments
The authors thank the patients who participated in the present study and their families, the referring physicians, the clinical research associates, and trial coordinators, all of whom contributed to the completion of the study. This work was supported by funds from the National Cancer Institute Breast SPORE program (grant P50-CA58223-09A1), the Susan G. Komen Foundation (grant SAC 110044 ECD), and Novartis Pharmaceuticals, Inc.
C.K.A. reports research funding provided by Novartis, Sanofi, toBBB, Geron, Angiochem, Merrimack, Puma Biotechnology, Lilly, Merck, and Oncothyreon. L.A.C. reports research funding from Genentech. C.M.P. is an equity stockholder and board of director member of BioClassifer LLC and is listed as inventor on patent applications for the Breast PAM50 assay. E.C.D. reports research funding from Novartis, Genentech, Cerulean, Bayer, Pfizer, and Merck and a family member who receives consulting income from Novartis.
Footnotes
Disclosure
The remaining authors declare that they have no competing interests.
References
- 1.Cancer Genoma Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–96. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Bild AH, Parker JS, Gustafson AM, et al. An integration of complementary strategies for gene-expression analysis to reveal novel therapeutic opportunities for breast cancer. Breast Cancer Res. 2009;11:R55. doi: 10.1186/bcr2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creighton CJ, Fu X, Hennessy BT, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer IA. Clinical implications of mutations in the PI3K pathway in HER2+ breast cancer: prognostic or predictive? Curr Breast Cancer Rep. 2015;7:210–4. doi: 10.1007/s12609-015-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 9.Di Leo A, Seok Lee K, Ciruelos E, et al. BELLE-3: a phase III study of buparlisib + fulvestrant in postmenopausal women with HR+, HER2−, aromatase inhibitor-treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor-based treatment, Presented at the San Antonio Breast Cancer Symposium 2016, abstract s4-07. Cancer Res. 2017;77:S4–07. [Google Scholar]
- 10.Baselga J, Im SA, Iwata H, et al. MPIK3CA status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR+/HER2− advanced breast cancer (BC): First results from the randomized, phase III BELLE-2 trial; Presented at the San Antonio Breast Cancer Symposium; 2015. abstract s6-01. [Google Scholar]
- 11.Martin M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4) Ann Oncol. 2017;28:313–20. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 12.Smyth LM, Monson KR, Jhaveri K, et al. A phase 1b dose expansion study of the pan-class I PI3K inhibitor buparlisib (BKM120) plus carboplatin and paclitaxel in PTEN deficient tumors and with dose intensified carboplatin and paclitaxel. Invest New Drugs. 2017 doi: 10.1007/s10637-017-0445-0. [DOI] [PMC free article] [PubMed]
- 13.Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25:3853–8. doi: 10.1200/JCO.2007.11.9776. [DOI] [PubMed] [Google Scholar]
- 14.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 15.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 16.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–42. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–54. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 18.Awada A, Gil T, Whenham N, et al. Safety and pharmacokinetics of sorafenib combined with capecitabine in patients with advanced solid tumors: results of a phase 1 trial. J Clin Pharmacol. 2011;51:1674–84. doi: 10.1177/0091270010386226. [DOI] [PubMed] [Google Scholar]
- 19.Deenen MJ, Klumpen HJ, Richel DJ, et al. Phase I and pharmacokinetic study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced solid malignancies. Invest New Drugs. 2012;30:1557–65. doi: 10.1007/s10637-011-9723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney CJ, Chiorean EG, Verschraegen CF, et al. A phase I study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol. 2010;28:4513–20. doi: 10.1200/JCO.2009.26.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McRee AJ, Sanoff HK, Carlson C, Ivanova A, O’Neil BH. A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest New Drugs. 2015;33:1225–31. doi: 10.1007/s10637-015-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutations in advanced cancers: characteristics and outcomes. Oncotarget. 2012;3:1566–75. doi: 10.18632/oncotarget.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013;73:276–84. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–16. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saura C, Bendell J, Jerusalem G, et al. Phase Ib study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res. 2014;20:1935–45. doi: 10.1158/1078-0432.CCR-13-1070. [DOI] [PubMed] [Google Scholar]
- 26.Zambrano CC, Schuler MH, Machiels JP, et al. Phase lb study of buparlisib (BKM120) plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC) J Clin Oncol. 2014;32(suppl):627. [Google Scholar]
- 27.Gonçalves A, Guerin M, Isambert N, et al. PIKHER2: a phase Ib study evaluating oral BKM120 in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Mol Cancer Ther. 2015;14(suppl 2):A118. doi: 10.1016/j.ejca.2017.08.025. [DOI] [PubMed] [Google Scholar]