Abstract
We report a novel method for in situ imaging of microvascular permeability in inflamed gingival tissue, using state-of-the-art Cellvizio™ intravital endoscopic technology and a mouse model of ligature-induced periodontitis. The silk ligature was first placed at the upper left second molar. Seven days later, the ligature was removed, and the animals were intravenously injected with Evans blue. Evans blue dye, which selectively binds to blood albumin, was used to monitor the level of inflammation by monitoring vascular permeability in control non-diseased and ligature-induced experimental periodontitis tissue. More specifically, leakage of Evans blue-bound albumin from the micro-capillary to connective tissue indicates the state of inflammation occurring in the specific site. Evans blue leakage from blood vessels was imaged in situ by directly attaching the endoscope (mini Z tip) of the Cellvizio™ system to the gingival tissue without any surgical incision. Evans blue emission intensity was significantly elevated in gingiva of periodontitis lesions, but not control non-ligature placed gingiva, indicating that this technology can be used as a potential minimally invasive diagnostic tool to monitor the level of inflammation at the periodontal disease site.
1. Introduction
The advancement of micro-optical technology has led to the development of in situ confocal intravital imaging to monitor the real-time locomotion of immune cells and three-dimensional vasculature in live animals, as well as human patients (Pittet and Weissleder, 2011; Nacer et al., 2012). However, owing to its fixed optic, the application of intravital imaging by a conventional confocal microscope is limited to the preclinical research using small animals. However, the novel intravital endoscopic imaging technology of Cellvizio™(Mauna Kea Technologies) has addressed this limitation with a novel adaptive optics system employing micro-fiberscope technology. As shown in Figure 1, the optical device at the end of Cellvisio’s fiberscope can access the surface of mouse gingive. This technology has recently been approved by the U.S. FDA to visualize and detect abnormalities in the gastrointestinal and respiratory tracts. In addition, the Cellvizio™ system was successfully applied to image urothelial tumors in patients (Marien et al., 2017).
Figure 1.
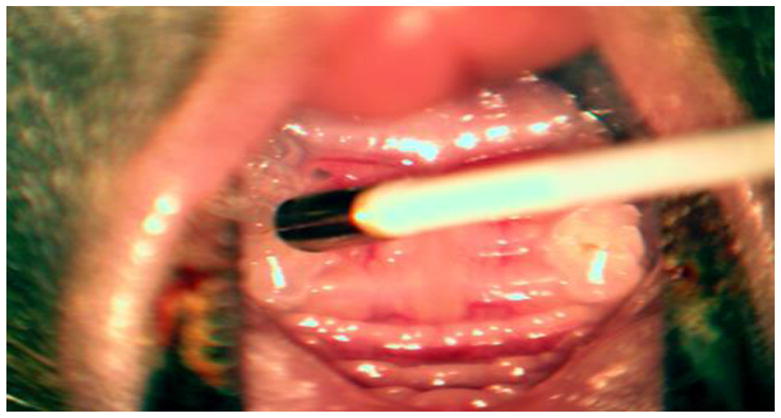
The size of mouse gingival crevice as compared to the size of Mini Z micro-fiberscope.
The Cellvizio™ Dual Band system is equipped with: 1) a laser scanning unit which allows the simultaneous recording of green and red fluorescence signal emissions (488 nm and 660 nm lasers, respectively) 2) a fibered microprobe that not only transports the laser beams to the site of observation, but also captures the fluorescent light emitted in the tissue, 3) a confocal processor with Cellvizio™ lab software, and 4) a pedal remote controller (Suppl. Fig. 1A–D). Apart from its approval for clinical applications, the robustness of the CellvizioTM system was recently demonstrated by successful detection of pathophysiological complications associated with cardiovascular diseases (Huang et al., 2016), oral cancer (Kossatz et al., 2016), as well as medication-related osteonecrosis of the jaw (MRONJ) (Movila et al., 2016) in various preclinical animal models.
It was reported that approximately 70% of adults in the United States have some form of periodontal disease (Eke et al., 2015), an oral mucosal inflammatory complication caused by polymicrobial infection of gingiva, leading to the destruction of both connective tissue and bone around a tooth. Currently, the estimated global economic impact of dental diseases is $442 billion per year (Listl et al., 2015). These alarming statistics call for the development of a novel approach to the early detection of periodontal disease and to monitor disease progression more precisely than the currently used method for clinical evaluation of this disease.
Currently, the level of periodontal inflammation is assessed by using the gingival index, a subjective method of grading the redness of gingiva. However this new in situ imaging technology can evaluate the level of inflammation by detecting the minute change of a pathophysiological event without subjective bias. Since this Cellvizio™ technology can achieve in situ imaging of microvascular permeability in a minimally invasive fashion, it potentially leads to a development of new point-of-care technology for the diagnosis/prognosis of periodontal disease, as well as other oral complications. To date, no study has ever employed fiber optic intravital endoscopic imaging for real-time monitoring of pathophysiological processes in the tissue of periodontal disease. Accordingly, using the Cellvizio™ device and a mouse model of ligature-induced periodontitis, we have developed a method to image and quantify the microvascular permeability in periodontal lesions. To visualize the micro-vasculature and detect it’s permeability in gingival tissues, Evans blue solution was used for its ability to specifically bind to serum albumin, but not other substances (Nacer et al., 2012; Movila et al., 2016). By visualizing the presence of Evans blue-bound albumin in the connective tissue around the blood vessel, we can detect the increased permeability of blood vessels, in turn reflecting the level of inflammation at the site.
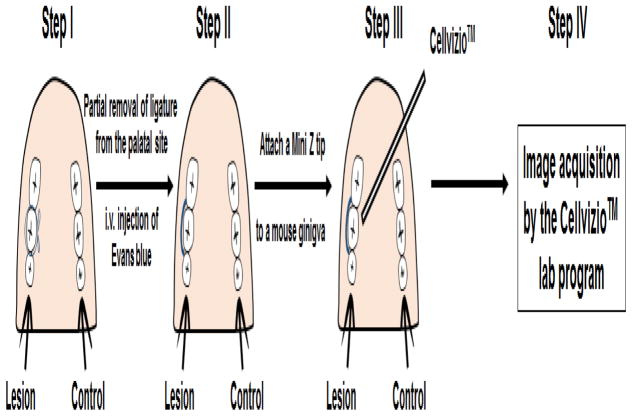
The basic protocol for this technique is summarized as follows (Fig. 2):
Figure 2.
Schematic illustration of developed intravital endoscopic imaging of experimental periodontal lesions in a mouse model of periodontitis using the Cellvizio™ device. Step I: Induce experimental periodontitis by placing a ligature at the second maxillary molar; Step II: Partially remove ligature at the palatal site and inject Evans blue solution i.v.; Step III: Attach a mini Z tip directly to the gingival tissue and image; Step IV: Collect data.
Induce experimental periodontitis by placing a silk ligature at the second maxillary molar, as we recently described (Matsuda et al., 2016).
Partially remove the silk ligature at the palatal site using scissors on day 7 after ligature placement.
Inject animals intravenously with 1% Evans blue solution in PBS.
Directly attach a mini Z tip fiber optic endoscope, which is connected to the Cellvizio™ Dual Band system, to gingival tissue.
Collect images using the Cellvizio™ lab program and analyze the captured digital images by Image J.
Material and Methods
2.1. Mice
Wild-type C57BL/6 mice (6–8 weeks old, males, 5 mice/group, Jackson Laboratories) were used in this study. This study was conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the experimental procedures were approved by the Forsyth Institutional Animal Care and Use Committee.
2.2. Histological analysis of Periodontal Tissue Sections
Mouse maxillae were dissected and immediately fixed in 10% formaldehyde overnight. Then, the tissues were decalcified in 10% EDTA solution for 2 weeks at 4°C. Decalcified samples were dehydrated in graded alcohol solutions, embedded in paraffin, and sectioned. Sections cut at 7-μm thickness in the mesial-distal plane were subjected to hematoxylin and eosin (H&E) staining. Next, images were captured at 10x and 20x maginifcation using a Leica DMLS light microscope. Finally, the number and diameter of blood vessels were quantified using Image J.
2.3. ELISA
Gingival tissues were collected from euthanized mice and then mechanically homogenized on ice with a mortar and pestle in PBS with 0.05% Tween 20 in the presence of 10 μg/ml of protease inhibitor cocktail (Thermo Scientific™). The collected suspension was centrifuged at 1500 rpm. Concentrations of TNF-α and IL-1β proteins were measured from the collected supernatant using commercial sandwich ELISA kits from BioLegend (San Diego, CA) according to the manufacturer’s recommendation.
2.4. Intravital confocal imaging of murine calvarial tissue
Mice were anesthetized by Ketamine (50 mg/kg)/Xylazine (10 mg/kg) (KX) cocktail, the heads were immobilized in a stereotactic holder, and the frontoparietal skull was exposed. Next, a glass cover slip (18 × 18 mm2 in size, 1.5 mm in thickness; Fisher Scientific) was fixed on the exposed bone tissue with Dermabond adhesive (Ethicon, Inc.), and the space between the cover slip and bone was filled with sterile PBS. The total time for preparation was less than 1 h. Then, mice were injected intravenously (i.v.) with 1% Evans blue (excitation: 470 and 520 nm, emission: 680 nm; Fisher Scientific) contrast solution in PBS to visualize the vascular lumen. The calvarial microvasculature was imaged with an inverted Zeiss 780 LSM confocal microscope 1 h after Evans blue injection. Confocal microscopy datasets were acquired with Zeiss Black Software. ImageJ was used for further image analysis. The total time required for animal preparation and imaging the object was ~ 4 h.
2.5. Intravital endoscopic imaging of murine calvarial tissue
To perform intravital endoscopic imaging by Cellvizio™ assay, mice were first anesthetized by intraperitoneal injection of KX cocktail to minimize discomfort and pain. Next, skin on the top of mice heads was shaved and sterilized. Then, a periosteum was exposed by making a 5 – 6-mm middle sagittal incision over the calvarium anterior to the line connecting both external ears using a scalpel. The required surgery time was about 30 – 45 min. Then, the mice were injected i.v. with 1% Evans blue solution in PBS and 1 h after injection, a mini Z fiber optic endoscope (diameter 0.94 mm; resolution 3.5 μm), as part of the Cellvizio™ device, was directly attached to the calvarial tissue through the incisor in the presence of a drop of PBS, and images were captured using the Cellvizio™ lab software. The Cellvizio™ lab software allowed us to record the fluorescence signal from Evans blue with the 660 nm laser. ImageJ was used for further image analysis. The total required time for animal preparation was ~ 2 h, invasive surgery was necessary.
2.6. Mouse model of ligature-induced periodontitis
Periodontitis was induced in the wild-type C57BL/6 mice (6–8 weeks old, males) following the method described in our previous publication (Matsuda et al., 2016). Briefly, animals were anesthetized by an intraperitoneal (i.p.) injection of KX cocktail to minimize discomfort and pain, and then a silk ligature (5.0 size; Johnson & Johnson, New Brunswick, NJ, USA) was placed and a knot made by tying the ligature ends at the palatal site of the upper left second molar.
2.7. Intravital endoscopic imaging of murine gingival tissue in an experimental periodontal lesion
Seven days later, the ligature knot was carefully cut from the palatal site, and the ligature-exposed palatal side was partially removed using Cohan-Vannas spring scissors (Fine Science Tools) under stereoscope. Five hours after removal of partial ligature, only the mice showing no visual bleeding were used for further studies. Next, animals were injected intravenously with 1% Evans blue solution in PBS. One hour after injection, the mini Z fiber optic endoscope was directly attached to gingival tissue from the palatal site of second molar. The total imaging time was less than 5 minutes. Cellvizio™ lab software was used to capture and save digital images.
To evaluate the level of inflammation induced in the ligatured site in comparison to the control non-ligature site, the fluorescent intensity of Evans blue dye in connective tissue was quantified in a 20 μm2 microscopic field using ImageJ. Altogether, 15 microscopic fields from each collected image were analyzed.
2.8. Measurement of the Evans blue amount in gingival tissue
To quantify the amount of Evans blue dye leakage from circulation into connective tissue, we used formamide to extract Evans blue from the collected gingival tissues as described in our earlier study (Nacer et al., 2012). Briefly, immediately after the imaging of gingiva using Cellvizio™, the mice were euthanized and gingival tissues were dissected from the jaw. Then, 300 μl of formamide were added to the collected gingiva to perform the dye extraction. After 3 days, 200 μl of solution were collected, followed by measuring the amount of Evans blue absorbed at 620 nm (OD620), using a plate reader.
2.9. Statistical analysis
All data are displayed as the mean value and the error bars indicate standard error. Statistical significance was evaluated using Student’s t-test. A p value of <0.05 was considered statistically significant. Data were analyzed using PAST 2.1 statistical software.
2. Results and Discussion
3.1. Induction of periodontitis changed the level of pro-inflammatory cytokines and the structure of blood vessels in the connective tissue
In response to the ligature attachment, the production of TNF-α and IL-1β pro-inflammatory cytokines in the gingival tissue homogenate was significantly elevated in comparison to the control non-ligature attached site (Suppl. Fig. 2).
Furthermore, the number and diameter of blood vessels were significantly elevated in the connective tissue at the site that received ligature compared to the control non-ligature site (Suppl. Fig. 3A–D), indicating that inflammation induced by ligature attachment changed the structure of blood vessels in the periodontal connective tissue. These data correlate well with an earlier report which demonstrated that the number of blood vessels and their size were larger in human ginigiva affected by periodontitis (Yoshida et al., 2011). This group has also reported that the permeability of blood vessels of human gingiva is increased during periodontitis. Thus, vascular permeability could be used as a diagnostic tool of the level of periodontal inflammation.
3.2. Comparison of image quality between intravital confocal imaging and intravital endoscopic imaging
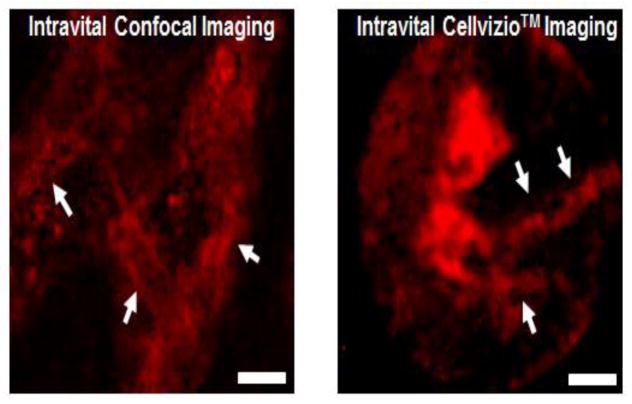
In order to monitor real-time blood vessel integrity as a diagnostic tool to determine the level of inflammation in live animals, in situ confocal intravital imaging technology was previously developed (Pittet and Weissleder, 2011). However, this conventional confocal microscopy assay has low potential for clinical applications owing to the structural limitation of confocal microscopy. Because of the fixed optic on the body of the confocal microscope, accessing tissues and organs inside the body is difficult. For this reason, we surgically exposed the bone of calvaria and demonstrated that the Cellvizio™ technology could capture intravital images of periosteum in murine calvaria at resolution equal to that of traditional confocal microscopy (Fig. 3).
Figure 3.
Comparison of intravital image quality between of mouse calvaria using traditional confocal microscopy (Zeiss LSM 760) and Cellvizio™ confocal endoscopy. Animals (n=5/experiment) were injected i.v. with 1% Evans Blue solution in PBS. Using both confocal systems we were able to detect blood circulation contrasted with Evans blue dye (shown in red) in murine calvaria. Arrowheads show blood vessels. Scale bar = 20 μm.
3.3. Intravital endoscopic imaging of murine gingiva
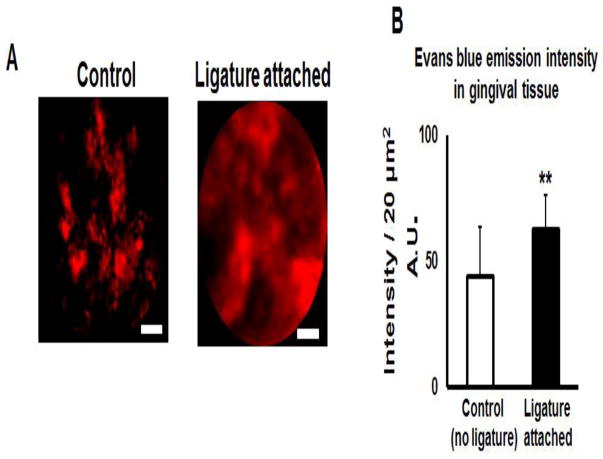
Next, we tested the ability of Cellvizio™ to capture the images of microvasculature in murine gingiva. The total imaging time using Cellvizio™ was less than 5 minutes in the anesthetized mice, and mice showed no complications or signs of discomfort after the imaging procedure. Cellvizio™-based imaging of the mouse gingival tissue identified a massive leakage of Evans blue from circulation into the connective tissue in mice induced of periodontitis, compared to control group (Fig. 4A). Quantification of the Evans blue emission intensity from the collected images has demonstrated a significant elevation in the ligature-induced periodontal lesion compared to control animals (Fig. 4B). To quantify the amount of Evans blue leakage, we have extracted the dye from the collected gingival tissues using formamide and then measured the absorption at 620 nm. As expected, the gingival tissues collected from ligature-induced periodontal lesions exhibited a significant elevation of Evans blue (OD 620) compared to the control non-ligature placed gingival tissues (Suppl. Fig. 4) indicating that the amount of Evans blue leaking into connective tissue correlated with the level of inflammation in periodontal lesions. These data corresponds to our earlier study (Nacer et al., 2012), reporting that massive leakage of Evans blue occurs only from the pathologically damaged blood vessels.
Figure 4.
Intravital imaging of gingival tissue in a mouse model of ligature-induced periodontitis by the Cellvizio™ system. A: Mouse gingivae exhibit elevated fluorescence intensity of Evans blue dye in connective tissue of periodontal lesions. B: Quantification of Evans blue fluorescence intensity of Evans blue. A.U. – arbitrary units. n=5 animals/condition. Student’s t-test was used to evaluate the statistical significance. Scale bar = 20 μm. **p<0.01.
Taken together, these results suggested that Cellvizio™ can detect the level of inflammation in periodontal disease by intravital imaging and the vascular permeability at the microscopic level. Currently, all available diagnostic imaging assays such as positron emission tomography, dynamic contrast-enhanced magnetic resonance imaging, perfusion computed tomography, and ultrasound are used to evaluate microcirculation. However, these technologies are unable to capture the image of microcirculation, owing to technical resolution limitations (Charnley et al., 2009). On the other hand, our results indicate that Cellvizio™ technology can be used to image in a minimally invasive manner, using Evans blue, a contrast-enhanced fluorescence imaging agent which makes a stable complex with albumin.
4. Conclusions
The Cellvizio™ technology was originally developed for real-time micro-imaging of the gastrointestinal, pulmonary and cardiovascular systems. In this study, we showed its robust capacity to image blood leakage from circulation and to monitor vascular permeability in mouse gingival tissue induced of experimental periodontitis. Therefore, Cellvizio™ imaging technology can be used as a real-time diagnostic device in a minimally invasive manner to detect the level of inflammation of periodontitis.
Supplementary Material
Supplemental Figure 1. Components of the Cellvizio™ Dual Band system. 1: a laser scanning unit that generates the scanned laser excitation at 488 nm and 660 nm; 2: confocal processor with Cellvizio™ lab software; 3: a fibered microprobe which transports the scanned laser beam to the site of observation and captures the fluorescent light emitted back from the tissue; 4: a pedal-operated remote controller.
Supplemental Figure 2. The levels of TNF-α and IL-1β were significantly elevated at the site that received ligature compared with the healthy non-ligature site. N=5 mice/group. **p<0.01, ***p<0.001.
Supplemental Figure 3. Histological images of hematoxylin and eosin (H&E) staining of experimental periodontitis tissues at lower x10 (A) and higher x20 (B) magnification. The tissues were collected 7 days after placement of 5.0 silk suture. C. Quantification of the number of blood vessels per 50 μm2 in control tissues and diseased periodontal lesions. D. Diameter of blood vessels in in control tissues and diseased periodontal lesions. N = 5 animals/condition. **p<0.01.
Supplemental Figure 4. Evaluation of emission intensity (OD 620) of Evans blue dye extracted by formamide from gingival tissues of control and ligature-attached animals. n= 5 animals/condition. Student’s t-test was applied to evaluate statistical significance. ***p<0.001.
Highlights.
Cellvizio™ is an intravital endoscopic technology.
An assay to image periodontal lesions using Cellvizio™ was developed.
Evans blue dye is a fluorescent marker of vasculitis in periodontal lesions.
The assay could detect Evans blue leakage into surrounding tissue in a minimally invasive manner.
Acknowledgments
This study was supported by The Forsyth Pilot grant and NIH grants AG-053615 (A.M.), DE-03420, DE-18499, DE-19917, and DE- 007327 (T.K.) and by Massachusetts Life Science Center Competitive Capital Program (P.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Charnley N, Donaldson S, Price P. Imaging angiogenesis. Methods in molecular biology. 2009;467:25–51. doi: 10.1007/978-1-59745-241-0_2. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of periodontology. 2015;86:611–22. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Sachse FB, Hitchcock RW, Kaza AK. Sensitivity and Specificity of Cardiac Tissue Discrimination Using Fiber-Optics Confocal Microscopy. PloS one. 2016;11:e0147667. doi: 10.1371/journal.pone.0147667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossatz S, Brand C, Gutiontov S, Liu JT, Lee NY, Gonen M, Weber WA, Reiner T. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Scientific reports. 2016;6:21371. doi: 10.1038/srep21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listl S, Galloway J, Mossey PA, Marcenes W. Global Economic Impact of Dental Diseases. Journal of dental research. 2015;94:1355–61. doi: 10.1177/0022034515602879. [DOI] [PubMed] [Google Scholar]
- Marien A, Rock A, Maadarani KE, Francois C, Gosset P, Mauroy B, Bonnal JL. Urothelial Tumors and Dual-Band Imaging: A New Concept in Confocal Laser Endomicroscopy. Journal of endourology. 2017;31:538–544. doi: 10.1089/end.2016.0892. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Movila A, Suzuki M, Kajiya M, Wisitrasameewong W, Kayal R, Hirshfeld J, Al-Dharrab A, Savitri IJ, Mira A, Kurihara H, Taubman MA, Kawai T. A novel method of sampling gingival crevicular fluid from a mouse model of periodontitis. Journal of immunological methods. 2016;438:21–25. doi: 10.1016/j.jim.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movila A, Mawardi H, Nishimura K, Kiyama T, Egashira K, Kim JY, Villa A, Sasaki H, Woo SB, Kawai T. Possible pathogenic engagement of soluble Semaphorin 4D produced by gammadeltaT cells in medication-related osteonecrosis of the jaw (MRONJ) Biochemical and biophysical research communications. 2016;480:42–47. doi: 10.1016/j.bbrc.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS pathogens. 2012;8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–91. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Noguchi K, Imura K, Miwa Y, Sunohara M, Sato I. A morphological study of the blood vessels associated with periodontal probing depth in human gingival tissue. Okajimas folia anatomica Japonica. 2011;88:103–9. doi: 10.2535/ofaj.88.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Components of the Cellvizio™ Dual Band system. 1: a laser scanning unit that generates the scanned laser excitation at 488 nm and 660 nm; 2: confocal processor with Cellvizio™ lab software; 3: a fibered microprobe which transports the scanned laser beam to the site of observation and captures the fluorescent light emitted back from the tissue; 4: a pedal-operated remote controller.
Supplemental Figure 2. The levels of TNF-α and IL-1β were significantly elevated at the site that received ligature compared with the healthy non-ligature site. N=5 mice/group. **p<0.01, ***p<0.001.
Supplemental Figure 3. Histological images of hematoxylin and eosin (H&E) staining of experimental periodontitis tissues at lower x10 (A) and higher x20 (B) magnification. The tissues were collected 7 days after placement of 5.0 silk suture. C. Quantification of the number of blood vessels per 50 μm2 in control tissues and diseased periodontal lesions. D. Diameter of blood vessels in in control tissues and diseased periodontal lesions. N = 5 animals/condition. **p<0.01.
Supplemental Figure 4. Evaluation of emission intensity (OD 620) of Evans blue dye extracted by formamide from gingival tissues of control and ligature-attached animals. n= 5 animals/condition. Student’s t-test was applied to evaluate statistical significance. ***p<0.001.