Abstract
Typically, in studies designed to assess effects of irradiation on cognitive performance the animals are trained and tested for cognitive function following irradiation. Little is known about post-training effects of irradiation on cognitive performance. In the current study, 3-month-old male mice were irradiated with X-rays 24 hours following training in a fear conditioning paradigm and cognitively testing starting two weeks later. Average motion during the extinction trials, measures of anxiety in the elevated zero maze, and body weight changes over the course of the study were assessed as well. Exposure to whole body irradiation 24 hours following training in a fear conditioning resulted in greater freezing levels 2 weeks after training. In addition, motion during both contextual and cued extinction trials was lower in irradiated than sham-irradiated mice. In mice trained for cued fear conditioning, activity levels in the elevated zero maze 12 days after sham-irradiation or irradiation were also lower in irradiated than sham-irradiated mice. Finally, the trajectory of body weight changes was affected by irradiation, with lower body weights in irradiated than sham-irradiated mice, with the most profound effect 7 days after training. These effects were associated with reduced c-Myc protein levels in the amygdala of the irradiated mice. These data indicate that whole body X ray irradiation of mice at 3 months of age causes persistent alterations in the fear response and activity levels in a novel environment, while the effects on body weight seem more transient.
Keywords: cued fear, body weight, zero maze, irradiation, post-training
1. Introduction
Consequences of radiation exposure are perhaps most typically thought of in terms of cancer risk [1]. However, a growing body of evidence supports a pernicious influence of irradiation on neurobiological systems with profound behavioral and cognitive consequences [2]. As environmental whole body irradiation exposure might occur as part of a natural disaster, an accident at a nuclear facility, a military mission, or radiological terrorism, it is important to establish a body of knowledge from which to predict and anticipate important consequences and outcomes [3]. This is also important absent catastrophe when considering the incidence of radiological exposure of cancer patients. Radiological effects on behavioral and cognitive performance appear to be long lasting. Following total body irradiation (8 Gy) of 8-week-old mice, activity in the open field was reduced at 3 hours following irradiation, partially recovered within 24 hours, followed by a later decrease 4 days after irradiation with a slow recovery starting at 17 days [4]. Locomotion and body temperature, as assessed by telemetry, decreased rapidly following irradiation reaching a minimum between 13 and 17 days following irradiation and full recovery starting at 27 days [4]. Radiation effects on learning and memory have been reported 30 days or more after whole body irradiation of 3- or 8-week-old male mice (4 Gy) [5, 6]. Diffusion tension imaging (DTI) and in vivo proton nuclear magnetic resonance spectroscopy (MRS) performed 2 or more days following irradiation showed that the hippocampus and frontal cortex are especially sensitive to these early radiation effects [7] [8] thus implicating potentially retrograde mnemonic processes as being particularly sensitive. These effects might be age-dependent, as activity in the open field was not altered in 6-month-old mice 24 hours following receiving whole body irradiation at 2, 5, or 8 Gy [9].
In all these studies, the animals were trained and tested for cognitive function following irradiation. Thus, neurobehavioral processes reliant on previous experiences, conditioning, and memory were not assessed in isolation. Post-training irradiation is translational relevant. For example, soldiers, physicians and nurses, astronauts, emergency responders, and cancer patients can be exposed to irradiation exposure following learning experiences and formation of memories. Therefore, it would be important to assess whether memory alterations due to radiation exposure could be retrograde. Previously, we showed enhanced contextual and cued fear memory prior to and during extinction following post-training irradiation of one-month-old male mice [10]. Due to the established age-sensitivity of radiation effects in mice with increased sensitivity in younger mice, the prior observed enhanced contextual and cued fear memory prior to and during extinction following post-training irradiation following post-training irradiation of one-month-old male mice could be age-dependent. Thus, more adult mice might be conferred relative protection against effects of irradiation on contextual and cued fear memories. This could have significant consequences when anticipating potential human corrolaries, with possible focus on specific sequelae and subsequent intervention in younger or older populations. Therefore, in the current study 3-month-old male mice were irradiated with X-rays 24 hours following training in a fear conditioning paradigm and tested two weeks later for retention and extinction of hippocampus-dependent contextual fear memory or hippocampus-independent cued fear memory. Average motion during the extinction trials was analyzed as well, to determine whether these measures of the fear (escape) response were affected by post-training irradiation. As measures of anxiety might affect retention and extinction of fear, performance in the elevated zero maze was assessed as well. Finally, effects of irradiation on body weight changes over the course of the study were assessed.
Irradiation affects the levels of the v-myc avian myelocytomatotsis viral oncogene homolog (c-Myc) protein [11]. Recently, we reported that c-Myc plays an important role in the response of the brain in substance abuse [12]. Therefore, in this study we also assessed whether the behavioral changes were associated with alterations of c-Myc protein levels in pertinent brain regions.
2. Material and Methods
2.1. Animals
Three-month-old male C57Bl6/J wild-type mice (n = 70) purchased from the Jackson Laboratory (Bar Harbor, ME) were used for the experiments of this study, as described below in detail. The mice were housed under a constant 12 hr light: 12 hr dark cycle. Food (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO) and water were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University (OHSU), Portland, Oregon.
2.2. Experiment 1: Contextual fear conditioning
Forty mice were cognitively trained in a contextual fear conditioning paradigm, involving a ten-shock paradigm, consisting of 2-second 0.35 mA shocks, separated by 60-second inter-shock-intervals (ISI), with the first shock at 60 seconds from the beginning of the trial. The total length of the training session was 10 minutes. Twenty-four hours after training, all mice were brought to a room within the animal facility containing an X-ray irradiator (Rad Source RS2000 Biological Research Irradiator, Suwanee, GA). Half of the mice (randomly sorted into two groups until any differences in baseline measurements were non-significant) were placed in a new mouse cage fitting in the irradiator and received whole body irradiation at a dose of 4 Gy (dose rate: 1.25 Gy/min). This dose was selected as that was the dose we used previously to assess post-training irradiation effects on cognitive performance in one-month-old mice [10]. This dose could be relevant in the context of radiation therapy in cancer patients, nuclear contamination from power plants, military conflicts, and bioterrorism. The other half of the mice was placed in a new cage and received a sham-irradiation procedure by being placed into a new cage for the same duration of time. Fourteen days after training (or thirteen days after irradiation or sham-irradiation), the mice were tested for recall and extinction of conditioned fear, over a period of six days. The mice remained in the testing environment for an additional eight minutes to maintain the same 10-minute trial length in all trials. For assessment of contextual fear memory, the same environment was used as during training. For assessment of cued fear memory, a different environment was used compared to the one used during training. On day ten, recall of post-reinstatement hippocampus-dependent contextual fear memory was assessed by exposure to the training context. Mice were weighed the day after training (before irradiation), and every three days thereafter.
2.3. Experiment 2: Cued fear conditioning
In a separate experiment, to evaluate the contribution of non-hippocampus-dependent memory processes, thirty male mice were cognitively trained using a cued fear conditioning paradigm consisting of ten shocks. A 60-second habituation period was followed by 30-second tones (2800 Hz, 80 dB), co-terminating with 2-second 0.35 mA shocks, separated by 60-second inter-shock-intervals (ISI), and with a final 2-minute post-shock acquisition period. Twenty-four hours after training, the mice were irradiated with 4 Gy or sham-irradiated as described in Experiment 1. Two weeks (14 days) after training (13 days after irradiation), the mice were tested for recall and extinction of cued fear over eight days. Cued extinction trials consisted of the mouse being placed into an environment distinct from the one used during training (rounded walls, novel floor texture, cleaning with a 10% isopropanol solution). A 60-second baseline/habituation period was followed by five 60-second tone-presentations separated by 60-second inter-stimulus-intervals. Mice were weighed the day after training, and every three days thereafter.
2.4. Elevated Zero Maze
To determine whether potential differences in measures of anxiety might contribute to altered performance in fear conditioning tests, a subgroup of 20 mice from the cued fear experiment was tested for anxiety-like phenotype in the elevated zero maze. Because the potential anxiety phenotype in question required a temporal proximity to the fear conditioning extinction testing, mice were tested 12 days after irradiation or sham-irradiation, one day before the beginning of the extinction experiment.
The elevated zero-maze (Kinder Scientific, Poway, CA) consisted of four sections (6 cm wide), alternating between open and closed sections. Mice were placed into an open area of the maze and allowed to explore the maze for 10 minutes. An automated photo beam detection method (Kinder Motor Monitor software, Kinder Scientific, Poway, CA) was used to track mouse movements. Outcome measures were distance moved (cm) and percent time spent in the open areas.
2.5. Experiment 3: Western blot analysis
Amygdala and hypothalamus mouse tissues were homogenized in RIPA lysis buffer [0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl, 50 mM Tris pH 8.0] containing the phosphatase inhibitor sodium vanadate [NaV, 1mM]. Protein lysates were extracted by centrifugation and protein concentrations were calculated using the MicroBCA protein assay. Equal amounts of protein were separated on sodium dodecyl sulfate polyacrylamide (4–12% SDS-PAGE) gradient gels and transferred to a polyvinylidene difluoride (PVDF) membrane. Membrane blots were then blocked in tris-buffered saline and tween 20 (TBST) [1 × TBS, 0.1% Tween 20] containing 5% bovine serum albumin (BSA). The membranes were then incubated with c-Myc primary antibody diluted in TBST containing 1% bovine serum albumin (BSA) overnight at 4°C. Membranes were then washed in TBST [3×10min] before being incubated in secondary antibody [1:10,000 dilution] for one hour. Membranes were incubated in enhanced chemiluminescence (ECL) reagent before being exposed to CL-XPosure Film to detect protein changes. We used total protein as loading control, as described [13], as we noticed that c-Myc protein levels did not correlate well with the levels of the house keeping protein β-actin.
2.6. Statistical analyses
Analyses were conducted using SPSS 22.0 software (Chicago, IL). Baseline measures between groups were analyzed by ANOVA, with treatment group as a between-subject variable. Comparisons of freezing, motion during shock, and body weight over time were performed as repeated measures ANOVA, with treatment as a between-subject variable and the time-unit as a within-subject variable. Cohort was added as a covariate to all analyses. Data were evaluated as to their satisfaction of assumptions for parametric statistics. For repeated measures analysis, if Mauchly’s test of sphericity was violated, Greenhouse-Geisser corrections were used and reported. For pairwise comparisons, Bonferroni’s posthoc tests were performed to compare selected values (between days and between groups). In case of a between-subject-variable interaction with a within-subject variable, the between-subject groups were separately analyzed to evaluate the potential difference in within-subject effects being mediated by the between-subject variable.
3. Results
3.1. Effects of irradiation on contextual fear
Baseline activity levels (prior to the first shock) were similar in both groups (Fig. 1A). To determine potential pre-existing group differences in sensitivity or perception to the aversive stimuli, average motion during the shocks was analyzed. The average motion during the shocks was also comparable and not significantly different in both groups (Fig. 1B). Acquisition of conditioned fear was assessed as immediate freezing during the inter-shock-interval (ISI) following a shock. Freezing levels during the ISIs on the training day increased with shock number in each group with no pre-existing group differences prior to radiation (Fig. 1C). As nearly all mice did not freeze immediately following the first shock, the first inter-shock interval was excluded from the analysis, as zero values can obscure parametric analysis of repeated measures.
Fig. 1.
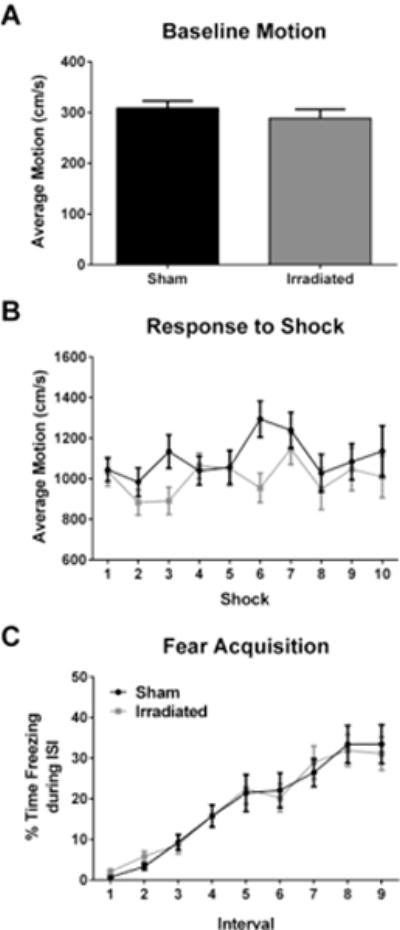
No effect of irradiation on acquisition of contextual fear. A. Activity during training at baseline, prior to the first shock. B. Average motion during the shocks during training. In panels B and C, the black line represents sham-irradiated mice and the grey line irradiated mice. C. Acquisition of contextual fear, analyzed as immediate freezing during the ISI following a shock. N = 20 mice/group.
Our study focused on between-trial extinction. Therefore, we analyzed the first five minutes of the extinction trials, to avoid the influence of habituation. Four animals (4 irradiated mice) were removed from the extinction analysis due to recording errors with the software. Two animals (1 sham-irradiated mouse and 1 irradiated mouse) were removed from the analysis based on outlier analysis (more than two standard deviations from the mean). Both groups showed extinction of contextual fear (effect of day: (F(2.118,65.651) = 3.743, p = 0.027, Fig. 2A). However, there was no radiation × day interaction, indicating that radiation did not affect the extinction of contextual fear per se. A multivariate analysis showed only a significant effect of irradiation on day 6 (F(1,31) = 4.545, p = 0.041).
Fig. 2.
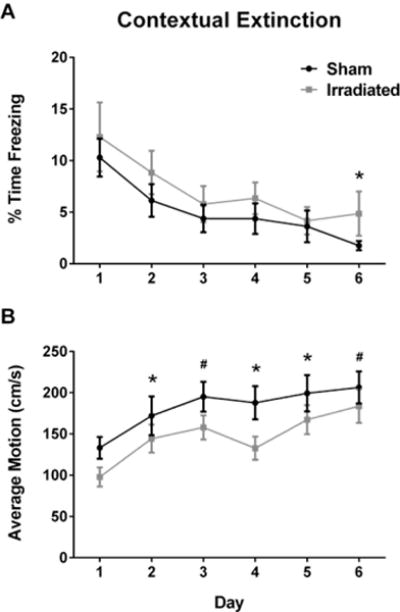
A. Effect of irradiation on extinction of contextual fear memory. B. Effect of irradiation on average motion during the contextual extinction trials. In panels A and B, the black line represents sham-irradiated mice and the grey line irradiated mice. The X-axis in both panels indicate the day of measurement. *p < 0.05; #p = 0.05. N = 16–19 mice/group.
We analyzed motion during the extinction trials as well (Fig. 2B). There was an effect of irradiation on average motion during the extinction trials (F(1,31) = 6.570, p = 0.015), with lower motion levels in irradiated than sham-irradiated mice. The multivariate analysis revealed effects of irradiation on days 2 (F(1,31) = 4.469, p = 0.043), 4 (F(1,31) = 6.777, p = 0.014), and 5 (F(1,31) = 4.933, p = 0.034), with a trend towards an effect of irradiation on day 3 (F(1,31) = 4.172, p =0.05), and 6 (F(1,31) = 3.803, p = 0.060). On day ten, recall of post-reinstatement contextual fear memory was assessed. Sham-irradiated and irradiated mice showed comparable recall of hippocampus-dependent contextual fear.
3.2. Effects of irradiation on cued fear
Baseline activity levels (prior to the first shock) were similar in both groups (Fig. 3A). There were no group differences in motion during the shock, and the average motion during the shocks was comparable in both groups (Fig. 3B). Acquisition of cued fear was assessed as freezing during the inter-shock-interval (ISI) following a tone-shock pairing. Freezing levels during the ISIs during training increased with shock number (F(7,196) = 16.767, p < 0.0001) and was comparable in both groups (Fig. 3C).
Fig. 3.
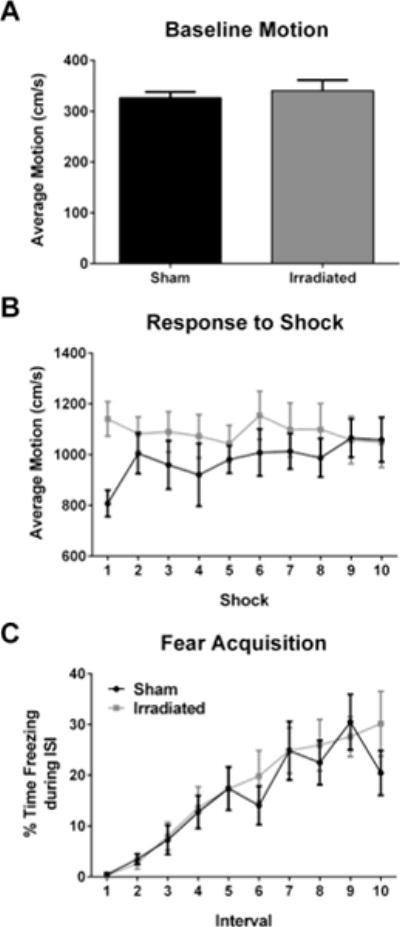
No Effect of irradiation on acquisition of cued fear. A. Activity during training at baseline, prior to the first shock. B. Average motion during the shocks on the training day. C. Acquisition of cued fear, analyzed as freezing during the ISI following tone-shock pairings. In panels B and C, the black line represents sham-irradiated mice and the grey line irradiated mice. N = 15 mice/group.
During extinction, there was no difference in motion prior to the first tone during the extinction trials (not shown), which suggests that the mice did not experience any generalized anxiety differences. As our study focused on between-trial extinction, we analyzed freezing during the first three tones of the extinction trials. There was an effect of radiation (F(1,27) = 8.146, p = 0.008), with higher freezing levels in irradiated than sham-irradiated mice (Fig. 4A). Multivariate analysis revealed effects of irradiation on days 2 (F(1,27) = 5.928, p = 0.022) and 3 (F(1,27) =7.32, p = 0.012). Sham-irradiated and irradiated mice showed similar freezing levels the day following re-instatement (not shown).
Fig. 4.
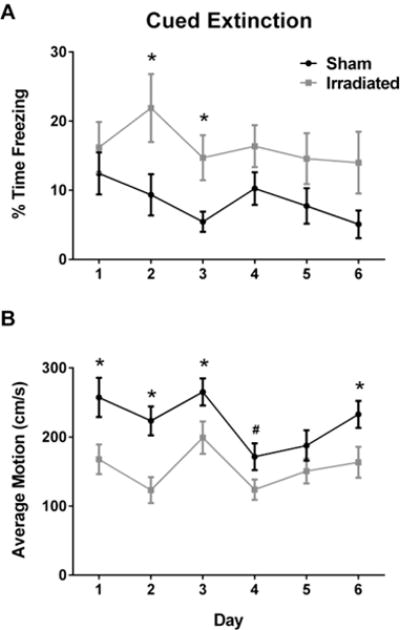
A. Effect of irradiation on extinction of cued fear memory. B. Effect of irradiation on average motion during the cued extinction trials. In panels A and B, the black line represents sham-irradiated mice and the grey line irradiated mice. The X-axis in both panels indicate the day of measurement. *p < 0.05; #p = 0.05. N = 15 mice/group.
We next analyzed motion during the extinction trials (Fig. 4B). There was an effect of irradiation (F(1,27) = 15.069, p = 0.001) with lower motion levels in irradiated than sham-irradiated mice. The multivariate analysis revealed effects of irradiation on days 1 (F(1,27) = 7.483, p = 0.011), 2 (F(1,27) = 13.614, p =0.001), 3 (F(1,27) = 4.566, p = 0.042), and 6 (F(1,27) = 5.689, p = 0.0024), with a trend towards an effect of irradiation on day 4 (F(1,27) = 4.119, p = 0.052).
3.3. Effects of irradiation on body weights and on performance in the elevated zero maze
Mice were weighed the day after training (before irradiation), and every three days thereafter. The trajectory of body weight change was significantly altered in irradiated animals, as shown by a day × irradiation interaction (F(3.393,213.743) = 8.556, p = 0.000). Multivariate analysis showed lower body weights in irradiated than sham-irradiated mice on day 7 (p = 0.002) and day 10 (p = 0.036), with a trend towards lower body weights on day 13 (p = 0.063, Fig. 5). Comparing the body weights on the last day with those on day 1, there was a significant group × time interaction (F(1,38) = 10.51, p = 0.0025). The effect of time was more pronounced in the irradiated (p = 0.0065) than the sham-irradiated (p = 0.03) mice. At both time point, there was no difference between the two groups (beginning weights: sham-irradiated: 25.46 g, irradiated: 25.89 g; t(76) = 0.8031, p = 0.8489; end weights: sham-irradiated: 25.62 g; irradiated: 24.97 g; t(76) = 1.223, p = 0.4500).
Fig. 5.
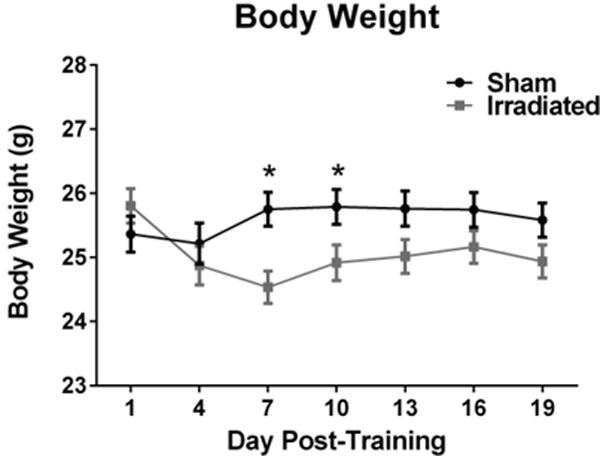
Effects of irradiation on body weights of mice trained and tested for contextual and cued fear conditioning. There was a day × irradiation interaction with significantly lower body weights in irradiated mice than sham-irradiated mice on days 7 and 10. *p < 0.05 versus irradiated mice on the same day. N = 31–34 mice/group.
Anxiety and activity levels were assessed in the elevated zero maze. The percent time spent in the more anxiety-provoking open areas of the maze was not significantly different between sham-irradiated and irradiated mice (t(14) = 1.166, p = 0.2632), indicating similar anxiety levels in the groups (Fig. 6A). However, irradiated mice showed lower overall activity levels than sham-irradiated mice (t(14) = 3.763, p = 0.0021, Fig. 6B).
Fig. 6.
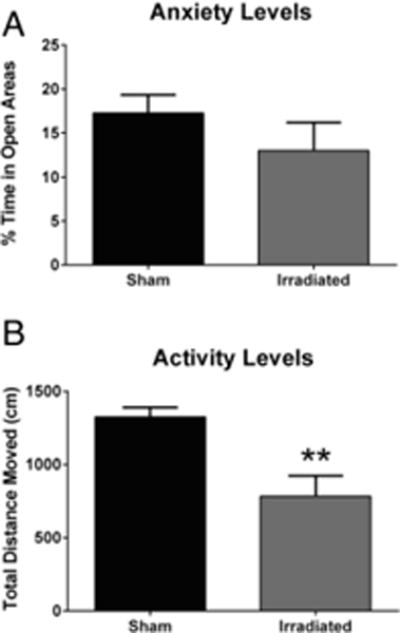
A. No effects of irradiation on measures of anxiety in the elevated zero maze. N = 10 mice/group. B. Effect of irradiation on activity levels in the elevated zero maze. **p < 0.01 versus sham-irradiated mice. N = 10 mice/group.
3.4. Effect of irradiation on c-Myc protein in the brain
Western blot analysis was used to detect changes in c-Myc protein in irradiated animals. In the amygdala, we observed a significant decrease in c-Myc protein levels in mice that were irradiated when compared to amygdala of mice that were sham treated (Fig. 7). This decrease in c-Myc protein levels was not detected in the hypothalamus of mice that were irradiated (Fig. 7).
Fig. 7.
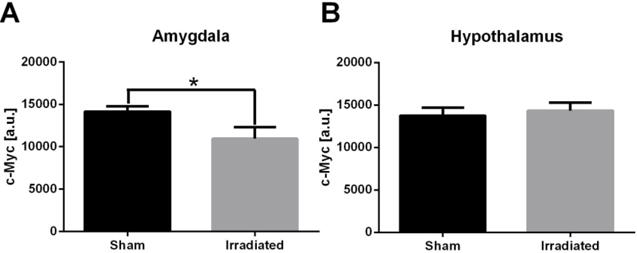
C-Myc protein levels in the amygdala (A) and hypothalamus (B) of sham-irradiated and irradiated mice. N = 8 mice/brain region/treatment.
4. Discussion
The data of the current study shows that exposure to whole body irradiation 24 hours following cued fear conditioning training results in greater freezing levels 2 weeks after training. In addition, motion during both contextual and cued extinction trials was lower in irradiated than sham-irradiated mice. In mice trained for cued fear conditioning prior to sham-irradiation or irradiation, activity levels in the elevated zero maze 12 days after irradiation were also lower in irradiated than sham-irradiated mice. Finally, the trajectory of body weight changes was affected by irradiation with lower body weights in irradiated than sham-irradiated mice, with the most profound effect 7 days after training. These data indicate that whole body X ray irradiation causes persistent alterations in the fear response and activity levels in a novel environment, while the effects on body weight seem more transient. These data are pertinent to human radiation exposure related to nuclear accidents or subsequent cleanup efforts at nuclear power plants [14–17], military missions, and dirty bomb scenarios [3, 18, 19].
Irradiation of 3-month-old mice in the current study did not affect anxiety-like behavior two weeks after training. Therefore, it is unlikely that measures of anxiety modulate the observed effects on the fear response during the extinction trials. In contrast, enhanced measures of anxiety were observed following post-training irradiation of 1-month-old mice [10]. The enhancement in freezing levels during extinction trials of cued fear but not contextual fear suggests that limbic areas like the amygdala might be more important for this effect than the hippocampus. However, the effect of irradiation on average motion during the contextual extinction trials suggests that the hippocampus might be involved as well. Interestingly, the reverse pattern was seen in 1-month-old mice with more profound effects of post-training irradiation on freezing levels during contextual than cued extinction trials [10]. The effect of post-training irradiation on body weights was more pronounced in 1-month-old [10] than 3-month-old mice. While the effect of irradiation on body weights was more transient at 3 months of age, this effect was persistent at 1 month of age. These data indicate that the age at irradiation critically modulates the effects of post-training irradiation.
Effects of post-training irradiation on memory might not be limited to X ray or 137Cs irradiation and also occur following space irradiation. Recently, detrimental effects of irradiation with 56Fe (25 cGy, 600 MeV/n) or 16O (5 cGy, 600 MeV/n) 2–4 hours after the training session in an object recognition test was shown to impair novelty preference in rats 18 hours after irradiation [20]. Besides the age at radiation exposure, the effects of post-training irradiation on memory might be dependent on the cognitive task, radiation type, dose, and energy, and the interval between irradiation and memory assessment. Genetic factors might be important modulators as well. For example, training and memory testing three months following 28Si irradiation showed impaired contextual fear memory in B6D2F1 mice [21] but enhanced contextual fear memory in C57BL/6J mice [22].
Our data show reduced c-Myc protein levels in the amygdala of irradiated mice that show enhanced cued fear memory and reduced activity levels in the elevated zero maze. These reduced c-Myc protein levels in the amygdala might relate to the behavioral and cognitive alterations seen in these post-training irradiated mice. Cued fear memory involves the amygdala [23–28] and the amygdala plays an important role in regulating anxiety levels [28, 29] as well. Future efforts are warranted to determine the mechanism underlying the role of c-Myc in amygdala-related behavioral and cognitive performance.
The reduced c-Myc protein levels in the amygdala of irradiated mice might be relevant to cancer as well. Pathways downstream of receptor tyrosine kinases such MAPK/ERK and PI3K/Akt/mTOR that indirectly control c-Myc protein stability are often overexpressed or mutated in brain tumors. MAPK/ERK kinase inhibitors have been shown to dephosphorylate c-Myc and reduce cell proliferation and anchorage-independent growth of rhabdomyosarcoma [30], a soft tissue sarcoma in children. mTOR occurs in a complex, mTORC1, that directly phosphorylates and inhibits PP2A which can lead to sustained c-Myc protein activity by dephosphorylating serine-62 within c-Myc’s transactivation domain. Consequently, clinical inhibitors of PI3K/mTOR are used to degrade c-Myc in neuroblastoma. In gliomas where suppressor genes like p53 and Pten are inactivated, c-Myc is critical for tumor maintenance. c-Myc inhibition will suppress tumors by promoting differentiation of the glioma cells [31] and inhibiting glioma cancer stem cells [32]. While the c-Myc protein is a validated target for cancer therapies, targeting a transcription factor that lack clear binding domains is challenging. Based on our data, irradiation could play a key role in combating brain tumors by reducing c-Myc protein levels.
In summary, the data of the current study show enhanced freezing during cued fear extinction and reduced motion during contextual and cued fear extinction in mice that received whole body irradiation following acquisition of fear conditioning two weeks earlier. Activity levels in the elevated zero maze were reduced as well following post-training irradiation. More transient effects on body weight changes following post-training irradiation were observed as well. These behavioral and cognitive changes are pertinent to radiation exposure as part of a nuclear accident, military mission, or dirty bomb scenario. The mechanism underlying these post-training radiation effects might involve c-Myc protein levels in the amygdala. Future efforts are warranted to determine the detailed mechanisms underlying these post-training radiation effects.
Highlights.
Post-training irradiation increases freezing levels
Post-training irradiation reduces motion in extinction trials
Post-training irradiation reduces activity levels in the zero maze
Post-training irradiation transiently reduces body weights
Acknowledgments
This work was supported by NASA Grants # NNX10AD59G and # NNJ12ZSA001N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has competing financial interests or other conflicts of interest.
References
- 1.Cucinotta FA, Kim M-HY, Chappell LJ. Space Radiation Cancer Risk Projections and Uncertainties. NASA Center for AeroSpace Information. 2013 [Google Scholar]
- 2.Cucinotta F, Wang H, Huff J. Risk of acute or late central nervous system effects from radiation exposure. 2011 http://humanresearchroadmapnasagov/evidence/reports/CNSpdf.
- 3.Obenaus A, Mickley A, Bogo V, West B, Raber J. Chapter 7 in Military Consequences of Nuclear Warfare’. Henry M Jackson Foundation for the advancement of military medicine; 2012. Behavioral and neurophysiological changes of radiation exposure. [Google Scholar]
- 4.Van der Meeren A, Lebanon-Jacobs L. Behavioural consequences of an 8 Gy total body irradiation in mice: regulation by interleukin-4. Can J Physiol Pharmacol. 2001;79:140–3. [PubMed] [Google Scholar]
- 5.Rosi S, Ferguson R, Fishman K, Allen A, Raber J, Fike JR. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PlOS One. 2012;7:e31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen A, Chakraborti A, Eilertson K, Sharma S, Baure J, Habdank-Kolaczkowski J, et al. Radiation exposure to juvenile mice induces a heightened sensitivity to traumatic brain injury in adulthood. Int J Radiat Oncol Biol Phys. 2013;90:214–223. doi: 10.3109/09553002.2014.859761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi R, Khan A, Rana P, Haridas S, Kumar H, Manda K, et al. Radiation-induced early changes in the brain and behavior: serial diffusion tensor imaging and behavioral evaluation after graded doses of irradiation. J Neurosci Res. 2012;90:2009–19. doi: 10.1002/jnr.23073. [DOI] [PubMed] [Google Scholar]
- 8.Rana P, Khan A, Modi S, Hermanth Kumar B, Javed S, Tripathi R, et al. Altered brain metabolism after whole body irradiation in mice: a preliminary in vivo 1H MRS study. Int J Radiat Biol. 2013;89:212–8. doi: 10.3109/09553002.2013.734944. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Haridas S, Trived R, Khushu S, Manda K. Early cognitive changes due to whole body gamma-irradiation: A behavioral and diffusion tensor imaging study in mice. Exp Neuro. 2013 doi: 10.1016/j.expneurol.2013.06.005. Epub 2013, June 11. [DOI] [PubMed] [Google Scholar]
- 10.Olsen R, Marzulla T, Raber J. Impairment in extinction of contextual and cued fear following post-training whole body irradiation Frontiers. 2014;8:231. doi: 10.3389/fnbeh.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuka M, Hatakenaka M, Ishigami K, Masuda K. Expression of the c-myc and c-fos genes as a potential indicator of late radiation damage to the kidney. Int J Radiat Oncol Biol Phys. 2001;49:169–73. doi: 10.1016/s0360-3016(00)01371-7. [DOI] [PubMed] [Google Scholar]
- 12.Akyniyeke T, Weber SJ, Davenport ATD, Baker EJ, Daunais JB, Raber J. Effects of alcohol on c-Myc protein in the brain. Beh Brain Res. 2016 doi: 10.1016/j.bbr.2016.11.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton SL, Roche SL, Hurtado ML, Oldknow KJ, Farquharson C, Gillingwater TH, et al. Total Protein Analysis as a Reliable Loading Control for Quantitative Fluorescent Western Blotting. PLOSOne. 2013;8:e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.House R, Sax S, Rumack E, Holness D. Medical management of three workers following a radiation exposure incident. Am J Ind Med. 1992;22:249–57. doi: 10.1002/ajim.4700220210. [DOI] [PubMed] [Google Scholar]
- 15.Shigemura J, Tanigawa T, Saito I, Nomura S. Psychological distress in workers at the Fukushima nuclear lower plants. J Am Med Assoc. 2012;308:667–9. doi: 10.1001/jama.2012.9699. [DOI] [PubMed] [Google Scholar]
- 16.Havenaar J, Rumyantezeva G, van den Brink W, Poelijoe N, van den Bout J, van Engeland H, et al. Long-term mental health effects of the Chernobyl disaster: an epidemiologic survey in two former Soviet regions. Am J Psychiatr. 1997;154:1605–7. doi: 10.1176/ajp.154.11.1605. [DOI] [PubMed] [Google Scholar]
- 17.Rahu K, Rahu M, Tekkel M, Bromet E. Suicide risk among Chernobyl cleanup workers in Estonia stil increased: an updated cohort study. Ann Epidemiol. 2006;16:917–9. doi: 10.1016/j.annepidem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Giesecke J, Burns J, Barrett E, Bayrak E, Rose A, Slovic P, et al. Assessment of the regional economic impacts of catastrophic events: CGE analysis of resource loss and behavioral effects of an RDD attack scenario. Risk Analysis. 2012;32:583. doi: 10.1111/j.1539-6924.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 19.Chin F. Scenario of a dirty bomb in an urban environment and acute management of radiation poisoning and injuries. Singapore Med J. 2007;48:950–7. [PubMed] [Google Scholar]
- 20.Rabin BM, Poulose S, Carrihill-Knoll K, Ramirez F, Bielinski D, Heroux N, Shukitt-Hale B. Acute effects of exposure to (56)Fe and (16)O particles on learning and memory. Radiat Res. 2015;184:143–50. doi: 10.1667/rr13935.1. [DOI] [PubMed] [Google Scholar]
- 21.Raber J, Marzulla T, Stewart BA, Kronenberg A, Turker M. 28Si irradiation impairs contextual fear memory in BD2F1 mice. Radiat Res. 2015;183:708–712. doi: 10.1667/RR13951.1. [DOI] [PubMed] [Google Scholar]
- 22.Raber J, Rudobeck E, Allen A, Allen B, Rosi S, Nelson G, et al. 28Silicon radiation-induced enhancement of synaptic plasticity in the hippocampus of naive and cognitively tested mice. Radiat Res. 2014;181:362–8. doi: 10.1667/RR13347.1. [DOI] [PubMed] [Google Scholar]
- 23.Stork O, Stork S, Pape HC, Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learn Mem. 2001;8:209–19. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tye K, Prakash R, Kim S-J, Fenno L, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bideirectional control of anxiety. Nature. 2011 doi: 10.1038/nature09820. epub ahead of print March 9 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Meer P, Pfankuch T, Raber J. Reduced histamine levels and H3 receptor antagonist-induced histamine release in the amygdala of Apoe−/− mice. J Neurochem. 2007;103:124–30. doi: 10.1111/j.1471-4159.2007.04705.x. [DOI] [PubMed] [Google Scholar]
- 26.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker D, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 28.Yilmazer-Hanke D, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res. 2003;145:145–59. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 29.Young BJ, Leaton RN. Amygdala central nucleus lesions attenuate acoustic startle stimulus-evoked heart rate changes in rats. Behavioral Neuroscience. 1996;110:228–37. doi: 10.1037//0735-7044.110.2.228. [DOI] [PubMed] [Google Scholar]
- 30.Marampon F, Ciccarelli C, Zani B. Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol Cancer. 2006;5:31. doi: 10.1186/1476-4598-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng H, Ying H, Yan H, Kimmelman A, Hiller D, Chen A, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, WAng H, Li Z, Wu Q, Lathia JD, McLendon RE, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLOSOne. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]


