Abstract
Sapium sebiferum, an ornamental and bio-energetic plant, is propagated by seed. Its seed coat contains germination inhibitors and takes a long time to stratify for germination. In this study, we discovered that the S. sebiferum seed coat (especially the tegmen) and endospermic cap (ESC) contained high levels of proanthocyanidins (PAs). Seed coat and ESC removal induced seed germination, whereas exogenous application with seed coat extract (SCE) or PAs significantly inhibited this process, suggesting that PAs in the seed coat played a major role in regulating seed germination in S. sebiferum. We further investigated how SCE affected the expression of the seed-germination-related genes. The results showed that treatment with SCE upregulated the transcription level of the dormancy-related gene, gibberellins (GAs) suppressing genes, abscisic acid (ABA) biosynthesis and signalling genes. SCE decreased the transcript levels of ABA catabolic genes, GAs biosynthesis genes, reactive oxygen species genes and nitrates-signalling genes. Exogenous application of nordihydroguaiaretic acid, gibberellic acid, hydrogen peroxide and potassium nitrate recovered seed germination in seed-coat-extract supplemented medium. In this study, we highlighted the role of PAs, and their interactions with the other germination regulators, in the regulation of seed dormancy in S. sebiferum.
Keywords: Sapium sebiferum, Seed dormancy, Tegmen, Endospermic cap, Proanthocyanidins, ABA, GA
Introduction
Seed germination is an important step in the plant life-cycle because it determines subsequent plant survival and reproductive success. The seed coat can play a role in regulating dormancy. Known mechanisms by which the seed coat regulates dormancy include the prevention of water uptake by an impermeable seed coat, or inhibiting gas exchange (McGill et al., 2017), or by mechanically constraining the embryo, or by containing germination inhibitors (Baskin & Baskin, 2014). Proanthocyanidins (PAs) are the chemical inhibitors of germination found in the seed coat of many plants. As early as 1914, Nilsson-Ehle showed that red seed coat colour in wheat is associated with extended dormancy period in comparison to that of white-grained wheat. In 1958, Miyamoto & Everson showed that the red pigment of the seed coat is a substance derived from catechins, which make up PAs. Red seeds of charlock (Sinapis arvensis L.) exhibit a reduced dormancy compared with black seeds (Durán & Retamal, 1989). In legumes, coloured seeds imbibe slower than white seeds and then germinate later (Kantar, Pilbeam & Hebblethwaite, 1996; Powell, 1989; Werker, Marbach & Mayer, 1979; Wyatt, 1977). In Rubus seed, PAs contribute to seed coat hardness and resulting seed dormancy (Wada, Kennedy & Reed, 2011). In Arabidopsis, the permeability and thickness of the testa are affected by the PAs and some structural elements altered in mutants, which may lead to effects on germination (Debeaujon, Leon-Kloosterziel & Koornneef, 2000). The seeds need gibberellins (GAs) to overcome the dormancy imposed by the seed coat (Debeaujon & Koornneef, 2000). PAs located in the seed coat can act as a doorkeeper to seed germination. Inhibitory effect of PAs on seed germination is due to de novo biogenesis of abscisic acid (ABA). Compared with wild-types, PA-deficient mutants contain a lower concentration of ABA during germination. PA regulation of seed germination is mediated by the ABA signalling pathway (Himi et al., 2002; Liguo et al., 2012). PAs modulate the activities of the Class III peroxidase that controlled the levels of reactive oxygen species (ROS) during seed germination (Jia et al., 2012, 2013).
Chinese tallow (Sapium sebiferum L.) belongs to the Euphorbiaceae family and is native to eastern Asia (Esser, 2002). It is popular because of its colourful autumn foliage (Zhao & Tao, 2015). The white waxy aril of the seeds contains highly saturated fatty acids and highly unsaturated oil is found in the seed (Boldor et al., 2010). Tallow has been used for manufacturing soap, candles, cloth and fuel, while the seed’s oil can be used for making native paints and varnishes (Brooks et al., 1987; Jeffrey & Padley, 1991). A single mature tree of S. sebiferum produces many seeds. The estimated yield of a S. sebiferum tree is 4,700 L of oil per hectare every year which far exceeds the average commercial yields of traditional oilseed crops (Boldor et al., 2010; Webster, Jenkins & Jose, 2006). That is why, S. sebiferum has recently become a species of interest as a source of biodiesel (Gao et al., 2016).
Sexual propagation is an easy method of commercial propagation, and it’s being used widely for the commercial propagation of a large number of plant species, including many bio-energetic plants like S. sebiferum. However, the poor rate of seed germination due to deep dormancy has seriously limited its use (Li et al., 2012).
Sapium sebiferum seeds have hard, dark brown to blackish seed outer testa and reddish brown inner tegmen. The tegmen encloses the endosperm, which in turn encloses the embryo. Tallow tree seeds readily imbibed water but the seed coat at the site of the radicle appeared to be a barrier to seed germination. Germination of cabbage seeds was inhibited when cabbage seeds were soaked in extracted solutions from S. sebiferum seed coat (Li et al., 2012). Moreover, it has also been discovered that endosperm extracts have a stronger inhibitory effect on cabbage seed germination than seed coat extracts (SCEs) of S. sebiferum (Qian et al., 2016). We hypothesize that germination inhibitors found in S. sebiferum could be the PAs. It is currently unknown which layer of the seed coat has the highest concentration of PAs. PAs inhibit seed germination by influencing ABA, GA and ROS regulatory genes (Debeaujon & Koornneef, 2000; Debeaujon, Leon-Kloosterziel & Koornneef, 2000; Jia et al., 2012, 2013; Liguo et al., 2012). Nitrates also play an important role during seed germination (Lara et al., 2014). It is unclear whether PAs respond to nitrate signalling. We tested whether exogenous application of an ABA biosynthesis inhibitor nordihydroguaiaretic acid (NDGA), gibberellic acid (GA3), hydrogen peroxide (H2O2) and potassium nitrate (KNO3) promote seed germination is the presence of SCE (which may contain PAs inside). We conducted several experiments to address these questions, to test our hypothesis and to demonstrate the mechanism involved in S. sebiferum seed dormancy.
Materials and Methods
Seed material collection and storage
S. sebiferum seeds were harvested from 6 plants grown in the experimental field of Hefei Institute of Physical Science, Chinese Academy of Sciences (31°52°02N, 117y17 07E), Anhui, China. Hefei has a humid subtropical climate with four distinct seasons. According to Anhui’s meteorological bureau, Hefei’s annual average temperature is 16.2 °C. Hefei’s annual average low temperature is 12.6 °C. Summers are hot and humid, with a July average of 28.3 °C. Its annual precipitation is over 1,000 mm. Seeds were collected in December 2016 from 6 trees, filled in nylon bags and stored at room temperature prior to use. The experiment was conducted from March to October 2017.
Chemicals and stock solutions
The GA3 (CAS# 77-06-5), NDGA (≥97%, CAS# 500-38-9) and Vanillin (CAS# 121-33-5) were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China. KNO3 (CAS# 7757-79-1), H2O2 (30%, CAS# 7722-84-1), sulphuric acid (H2SO4, CAS 7664-93-9) and hydrochloric acid (HCl, CAS# 7647-01-0) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Sodium hypochlorite (NaClO, CAS# 7681-52-9) was bought from Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China. A pure supply of PAs (UV ≥ 95%, CAS 4852-22-6) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Ethanol (99.7%, CAS# 64-17-5) and methanol (99.7%, CAS# 67-56-1) was purchased from Shanghai Titan Scientific Co., Ltd., Shanghai, China. Sodium hydroxide (NaOH, CAS# 1310-73-2) was purchased from Shanghai Chemical Reagent Co., Ltd., Shanghai, China. Murashige and Skoog (MS) medium was purchased from Qingdao Hope Bio-Technology Co., Ltd., Shandong, China. The GA3 and NDGA stock solutions (100 mM) were prepared by dissolving in 80% methanol. KNO3 stock solution (10%) was prepared by dissolving in distilled water. All stock solutions were diluted in distilled water to make working solutions.
Seed coat extraction, application and PAs analysis
Seed coat extract was prepared as described by Li et al. (2012) with little modification. S. sebiferum tree seed coats were ground into powder. Ten grams of seed coat powder was dissolved in 200 mL of 80% (v/v) aqueous methanol and placed in a refrigerator at 4.0 °C for 24 h. After centrifugation (Allegra X-30R Centrifuge; Beckman Coulter, Inc. 250 S. Kraemer Boulevard Brea, CA, USA) at 4,500 rpm at 4.0 °C for 10 min, the supernatant was evaporated under vacuum at 40.0 °C.
Seed coat extract and PA medium for growing seeds were prepared by using 0.1%, 0.2% and 0.3% SCE and pure PAs in 0.5× Morashige and Skoog medium (MS) containing 15 g/L sucrose and 8 g/L agar before sterilization. All media were autoclaved at 121.0 °C for 22 min and poured into 9 cm diameter Petri dishes (20 mL each) under a laminar flow hood. Seed coat proanthocyanidin contents were analysed by conventional HCl–vanillin assay (Herald et al., 2014). Seed coat was ground under liquid nitrogen by a YLK Ball Mill Machine (YLT-04, Hunan, China), and 30 mg seed-coat powder was extracted for 20 min in 5 mL 1% HCl in methanol at 30.0 °C in a water bath. The extracts were centrifuged at 4,500 rpm for 5 min. One mL aliquots of the extract were dispensed into 2 culture tubes designated as sample and sample control. The tubes were incubated in a water bath at 30.0 °C for approximately 5 min. A working vanillin reagent was prepared daily by mixing equal amounts of 1% vanillin with 8% HCl solutions. Five mLs of the working vanillin reagent were added at 1 min intervals to the extract, and 5.0 mLs of 4% HCl were added to the sample control. The prepared tubes were incubated in a water bath at 30.0 °C for exactly 20 min, after which the absorbance was measured at 500 nm using nano-spectrophotometer of ScanDrop 100 (Analytik Jena AG, Überlingen, Germany). Final absorbance was calculated by subtracting the absorbance of the sample control from the corresponding vanillin-containing sample. Standard curves were developed using pure PAs (UV ≥ 95%) at concentrations that ranged from 0 to 100 μg/mL. ESC’s PAs were analysed by the previously used protocol as described by Xuan, Wang & Jiang (2014). Decoated seeds were dipped in 1% vanillin with 8% HCl solution and incubated in a water bath at 30.0 °C for 20 min. Photographs were taken by an Olympus SZX10 stereomicroscope having a TUCSEN 6.0 megapixel USB 2.0 colour camera.
Pre-germination treatments and germination conditions
Seeds were washed with 1% sodium hydroxide to remove white tallow. Sulphuric acid scarification was done by dipping seeds in 98.08% concentrated sulphuric acid at 4.0 °C for 10-, 20-, 30-, 40-, 50- and 60 min. After each time period of sulphuric acid treatment, seeds were washed in running tap water 5 times. For mechanical scarification, a cut was made by scissors in the seed coat on the opposite side of the radical. Intact seeds and scarified seeds were sown separately in 5 replicates (10 seeds in each pot) in 10 × 10 cm pots containing peat moss. We investigated the water uptake of intact seed and sulphuric acid scarified seed using the method of Li et al. (2012).
The seed coat was removed by dissection leaving the embryo (embryonic axis and endosperm) as a single unit, hereafter referred to as a ‘decoated seed.’ We used decoated seed to verify the effects of PAs and SCEs. Decoated seeds were sterilized by washing twice with 70% ethyl alcohol for 30 s and then incubated in 20% NaClO for 10 min. After rinsing off NaClO, the seeds were washed 3 times with autoclaved water and dried by blotting over sterilized filter papers. For the ESC experiments, ESC was removed with a sterilized blade in a laminar flow hood.
Sterilized decoated seeds were sown in SCE and PA mediums. To investigate the relationship of seed coat PAs with GA-, ABA-, ROS- and nitrates-signalling genes, we primed the decoated seeds in sterile water (for control), GA3 (50 μM), NDGA (50 μM), H2O2 (20 mM) and 0.4% KNO3 separately overnight (12 h) at room temperature (25.0 °C) and sowed the primed seed in 0.5× MS medium supplemented with 0.3% SCE. Germination conditions for all experiments were maintained as day/night temperatures of 25.0/20.0 °C, with 16 h light/8 h dark photoperiod, 150 μmol/m2/s photosynthetic photon flux density and 70% relative humidity. Protrusion of the radical from the micropyle was considered as the standard for seed germination. Germination data were recorded every day after germination started (5 days after imbibition). Shoot and root length were measured manually with a ruler. All seed germination pictures were taken by NIKON D90 containing NIKON DX AF-S NIKKOR 18-105 mm lens (NIKON Corporation, Tokyo, Japan).
Primer designing, RNA extraction, cDNA synthesis and RT–qPCR conditions
The full sequences of all genes were obtained by local blasting Arabidopsis amino-acids sequence in blast-2.2.31. A local blast library was built by flower-bud transcriptome (Accession: SRX656554, https://www.ncbi.nlm.nih.gov/sra/SRX656554) of S. sebiferum (Yang et al., 2015). The list of all genes’ full mRNA sequences is available in Data S1. Primers used for qPCR were designed by using primer premier 6. The Tm of the primers was between 59.0 and 61.0 °C and a list of all primers are given in Table S1. For gene expression analysis, seed samples were taken the third and sixth day after imbibition. Samples were frozen in liquid nitrogen and stored at −80.0 °C. RNA was extracted by using E.Z.N.A® plant RNA extraction kit (Omega Bio-tek, Inc., Norcross, GA) according to the given protocol. Five hundred nanograms RNA of each sample was reverse transcribed using cDNA synthesis SuperMix (TransGen Biotech., Shanghai, China) according to the given protocol. The cDNA samples were diluted 25× with sterile water. For each qPCR, 9 μL of the sample, 10 μL of the 2× QuantiNova SYBR Green PCR Master Mix (QIAGEN, Pudong, Shanghai, China) and 0.5 μL of each primer was added to make a final volume of 20 μL. The RT–qPCRs were run on a Light Cycler®96 (Roche Diagnostics, Indiana, USA). The qPCR program run consisted of the first step at 95.0 °C for 3 min and afterwards 45 cycles alternating between 15 s at 95.0 °C, 15 s at 60.0 °C and 15 s at 72.0 °C.
Statistical analysis
All data was arranged in Excel 2013 and statistical analyses were done in R Studio 1.1.383. All data were represented with mean ± standard deviation. Results from the different treatments were analysed separately. The significance of treatments was tested by one-way analysis of variance (ANOVA). Duncan’s multiple range test and the Tukey test were used to identify significant differences between pairs of means at P < 0.05.
Results
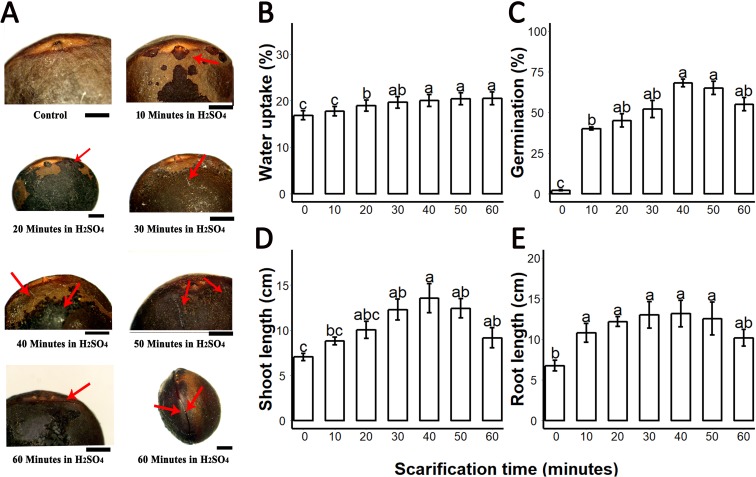
Sulphuric acid scarification promotes seed germination
We found that sulphuric acid digested the seed coat external surface and caused cracks. About 10 and 20 min incubation times digested the epidermal layer of the seed coat while the 30-, 40- and 50 min incubations caused mild cracks in the seed coat. But the 60 min incubation in sulphuric acid caused deep cracks in the seed coat (Fig. 1A). When we measured the PA contents of 0-, 10-, 20-, 30-, 40-, 50- and 60 min scarified seed, we found that sulphuric acid scarification degradation of the PA contents proportionally to the incubation time (Fig. 2).
Figure 1. Sulphuric acid (SA) scarification significantly promoted the seed germination of Sapium sebiferum.
(A), Effect of SA scarification time on seed coat; red arrows indicate the bruises, scars and cracks caused by SA. Bars 1 mm. (B), SA impacts on water uptake in the seed. (C), SA-induced seed germination of S. Sebiferum. (D and E), Impact of SA on shoot and root length of seedlings respectively. Shoot and root length were measured after 45 days of imbibition. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Duncan’s multiple range HSD post hoc test. The photographs were taken by Shah Faheem Afzal and Jun Ni.
Figure 2. Impact of sulphuric acid scarification on PA contents of S. Sebiferum seed coat.
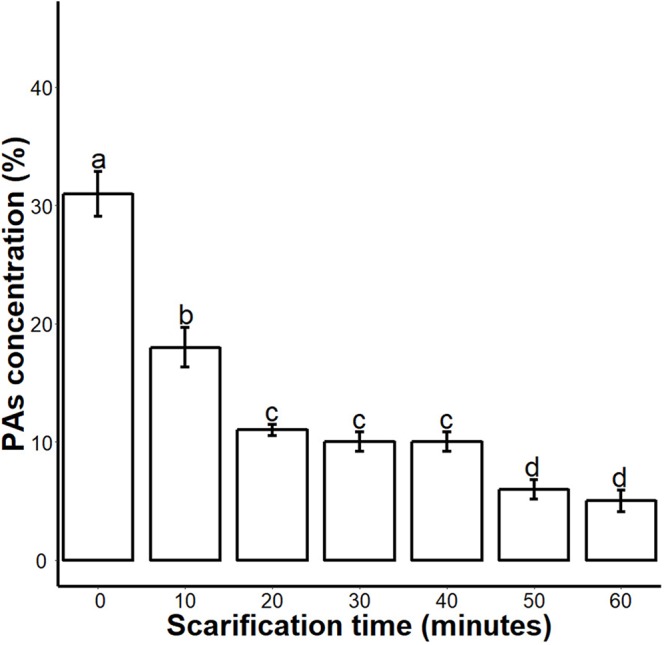
Seeds of S. Sebiferum were dipped in concentrated sulphuric acid for 10, 20, 30, 40, 50 and 60 min separately. PA contents of acid-scarified seeds were determined by vanillin assay. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
We investigated the water uptake of intact seeds and sulphuric-acid scarified seeds. Water uptake of untreated control seeds was 16.5–17.8% over 72 h; seeds treated with sulphuric acid for longer periods of time showed increasing water uptake to a maximum of 22.5% after 60 min of acid treatment. Our results showed that water uptake gradually increased from untreated (control) 0–10 min, 20-, 30-, 40-, 50- and 60 min. Water uptake percentages of 20-, 30-, 40-, 50- and 60 min scarified seed were significantly different from the control, but the water uptake percentage of 10 min scarified was not significantly different from control (Fig. 1B). We found that germination of intact seeds was 2%, and ranged from 40 to 65% in sulphuric-acid-scarified seeds while mechanically scarified showed 20% germination in 20 days. (Fig. 1C; Fig. S1). Intact and scarified seed’s germination were significantly different (P = 0.05).When we measured the root and shoot length of seedling of 45-day-old seedlings, we found that the seedlings whose seeds were chemically scarified by 30-, 40- and 50 min incubation in sulphuric acid, had more root and shoot length than the seedlings of 0-, 10-, 20- and 60 min incubated seeds in sulphuric acid (Figs. 1C and 1D).
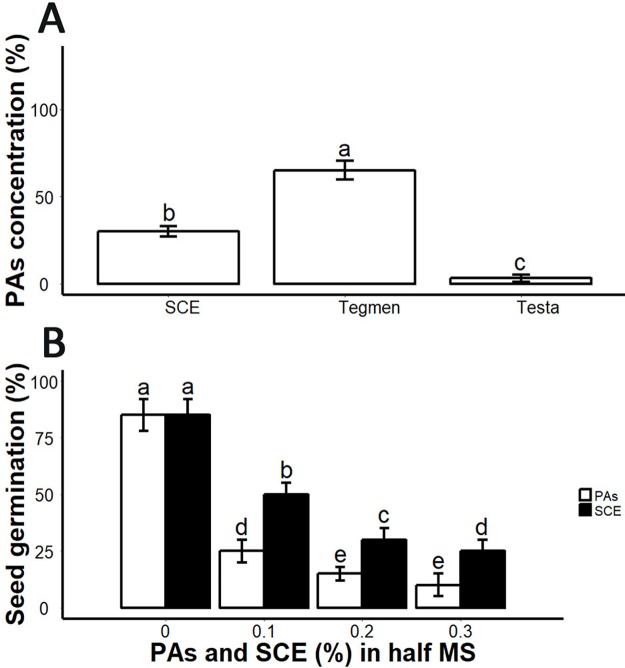
Tegmen and ESC contained PAs which inhibited seed germination
From germination analysis, we found that the decoated seeds showed 85 ± 5% seed germination within seven days (Fig. 3B; Fig. S2). SCE supplemented medium inhibited seed germination (Fig. 3B). We determined PA and SCE concentration in S. sebiferum seed coat testa and tegmen separately. We found that testa and tegmen contain 3 ± 2% and 65 ± 5% (mean ± SD) of PAs respectively, while SCE contained 30 ± 3% (mean ± SD) PAs (Fig. 3A). We also tested seed germination in proanthocyanidins-supplemented 0.5× MS, the results showed that PAs significantly inhibited the seed germination in S. sebiferum (Fig. 3B).
Figure 3. Impacts of exogenous application of SCE and PA on seed germination.
(A), PA contents in SCE, tegmen and testa of S. Sebiferum seed coat. (B), Impact of different concentrations of SCE and PAs on seed germination. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
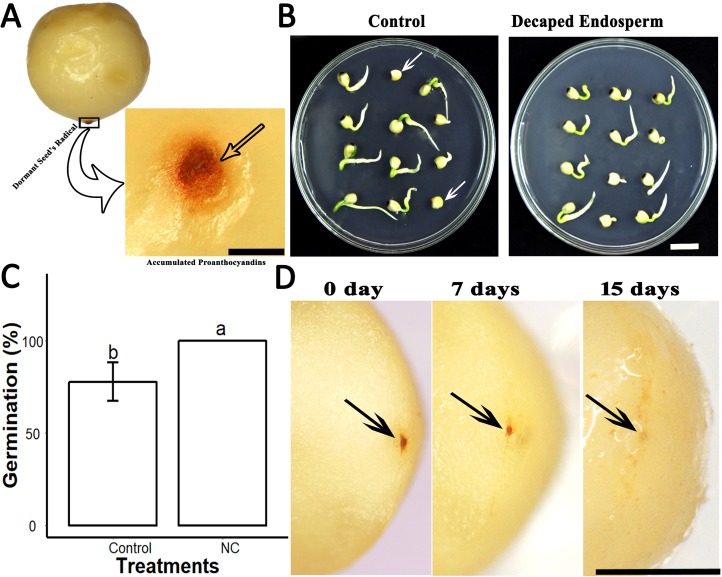
Further, we found that the seeds of S. sebiferum contain a dark brownish colour ESC. When cultivated on 0.5× MS, we found that dark brownish ESC became darker in some dormant seeds (Fig. 4A). When we removed that ESC and cultivated those ESC removed seed on 0.5× MS, we found the seed without ESC showed 100% seed germination within 5 days which was significantly different than the seed with ESC (Figs. 4B and 4C). We hypothesized that the dark brownish ESC might accumulate PAs, which inhibited seed germination. Then we determined PAs by vanillin assay and interestingly we found that ESC gives red colour which is an indication of PAs. We also tested the dynamic changes in PAs in ESC of intact seeds cultivated in peat moss media. We found that the intensity of PAs gradually decreased with imbibition time, and, after complete diminishing of PAs in ESC, the seed showed signs of germination (Fig. 4D).
Figure 4. PAs in the endospermic cap affected the seed germination.
(A), Accumulation of PAs in endospermic cap of dormant seed. (B and C), Decaping of endospermic cap significantly promoted seed germination as compared to control (with endospermic cap). (D), Dynamic changes of PAs in the endospermic cap of non-dormant seed. Bars in (A) and (D) 2 mm, (B) 1 cm. The photographs were taken by Shah Faheem Afzal.
Effect of SCE on the expression level of dormancy-related genes
We examined the relationship between the seed coat dormancy and the expression levels of GA-, ABA-, ROS- and nitrates-related genes. To investigate whether this effect was because of PAs, we compared the relative expression of GA-, ABA-, ROS-, nitrates- and dormancy-related genes between the control, SCE and PA treatments. It is very important to check the expression of dormancy-specific genes while studying seed dormancy. Delay of Germination 1 (DOG1) is a dormancy-specific gene which positively regulates seed dormancy (Dekkers et al., 2016; Footitt et al., 2017). We found that the expression level of SsDOG1 was significantly higher in SCE and PAs as compared to control on the third and sixth days of imbibition. But the expression level of SsDOG1 at both time points was not significantly different between SCE and PAs (Fig. 5A).
Figure 5. Effect of SCE on the expression of seed dormancy-related gene (SsDOG1) and ABA-related genes.
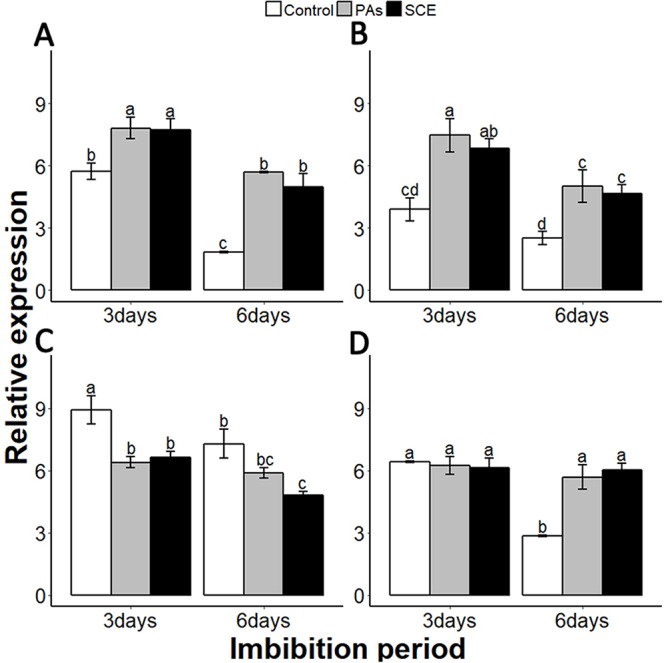
(A), SsDOG1 (B), SsNCED6 (C), SsCYP707A2 and (D), SsABI3. The expression of SsDOG1, SsNCED6, SsCYP707A2 and SsABI3 were determined by qRT-PCR on third and sixth day after treatment. SsACTIN was used as the reference gene. Control, seed grown in half MS medium. PAs, proanthocyanidins-supplemented half MS medium. SCE, half MS medium supplemented with seed-coat extract. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
It has been reported that dormant seeds have high levels of ABA (Millar et al., 2010). To find the transcriptional changes of ABA-related genes during different imbibition periods of different treatments, we selected 9-cis-epoxycarotenoid dioxygenases 6 (NCED6), INSENSITIVE3 (ABI3) and CYP707A2 as ABA biosynthesis, signalling and catalyzing genes, respectively (Dekkers et al., 2016; Footitt et al., 2011). We also found that PAs and SCE both promoted the expression level of SsNCED6 on both the third and sixth days of imbibition (Fig. 5B). In SCE and PAs, the SsCYP 707A2 expression decreases gradually with time, while in the control SsCYP 707A2 expression is higher during both third and sixth days of imbibition (Fig. 5C). Expression levels of SsABI3 were not significantly different between control, PAs and SCE on the third day of imbibition. Interestingly, on the sixth day of imbibition, the SsABI3 expression level remained the same in SCE and PAs but dropped in the control (Fig. 5D).
Gibberellins are plant hormones that play an important role in seed germination. we selected a GA-biosynthesis gene (GA3OX1), a GA-inactivating gene (GA2OX), GA negative regulator genes GAI (GIBBERELLIC ACID INSENSITIVE) and RGL2 (REPRESSOR-OF-GA1 2) (Lee et al., 2002; Matsushita et al., 2007; Ravindran et al., 2017; Rieu et al., 2008; Shen et al., 2016). PAs and SCE significantly repressed the SsGA3OX1 expression level as compared to the control on the third day, while SsGA3OX1 transcript remained unchanged on the sixth day in both SCE and PAs. Expression levels of SsABI3 were not significantly different between control, PAs and SCE on the third day of imbibition. Interestingly, on the sixth day of imbibition, the SsABI3 expression level remained the same in SCE and PAs but dropped in the control (Fig. 5D). In PAs and SCE, the expression level of SsGA2OX increased on the third day and then decreased insignificantly on the sixth day of imbibition (Fig. 6A). In the control treatment, SsGA2OX transcription decreased gradually with time (Fig. 6C). SsRGL2 and SsGAI expression levels were higher in SCE and PAs as compared to the control during both the third and the sixth days of imbibition (Figs. 6B and 6D, respectively).
Figure 6. Effect of SCE on the expression of GA-related genes.
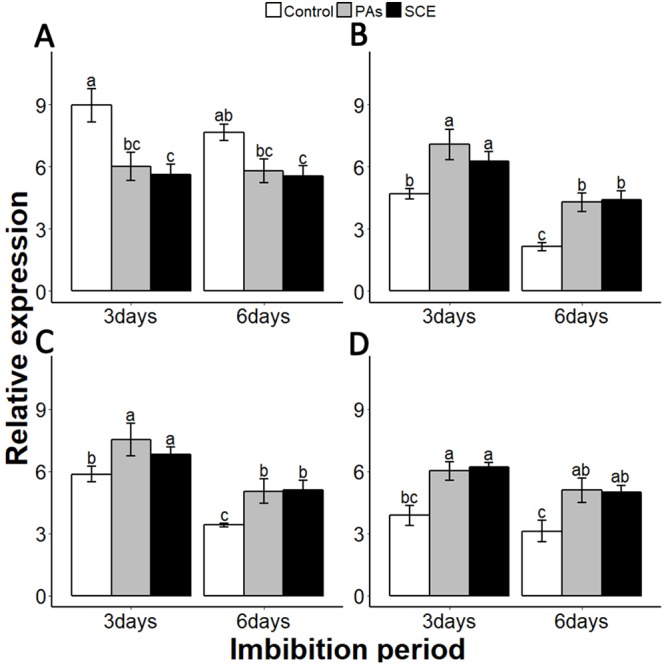
(A), SsGA3OX1 (B), SsGAOX (C), SsRGL2 and (D), SsGAI. The expression of SsGA3OX1, SsGAOX, SsRGL2 and SsGAI was determined by qRT-PCR on third and sixth days after treatment. SsACTIN was used as the reference gene. Control, seed grown in half MS medium. PAs, proanthocyanidins-supplemented (0.1%) half MS medium. SCE, half MS medium supplemented with seed-coat extract (0.3%). Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
Reactive oxygen species are highly active during seed germination. MITOGEN-ACTIVATED PROTEIN KINASE (MPKs) protein regulates the ROS signalling. Among the MPKs genes, MPK6 is highly active during seed germination (Oracz et al., 2009; Oracz & Karpinski, 2016). In our experiments, the effects of PAs and SCE were negative on SsMPK6 transcription. Relative expression of the SsMPK6 decreased over time in the seed growing on SCE and PAs. On the other hand, in the control treatment, the transcription levels of the SsMPK6 increased from the third to the sixth days of imbibition (Fig. 7A).
Figure 7. Impact of SCE on expression levels of ROS- and nitrates-signalling genes.
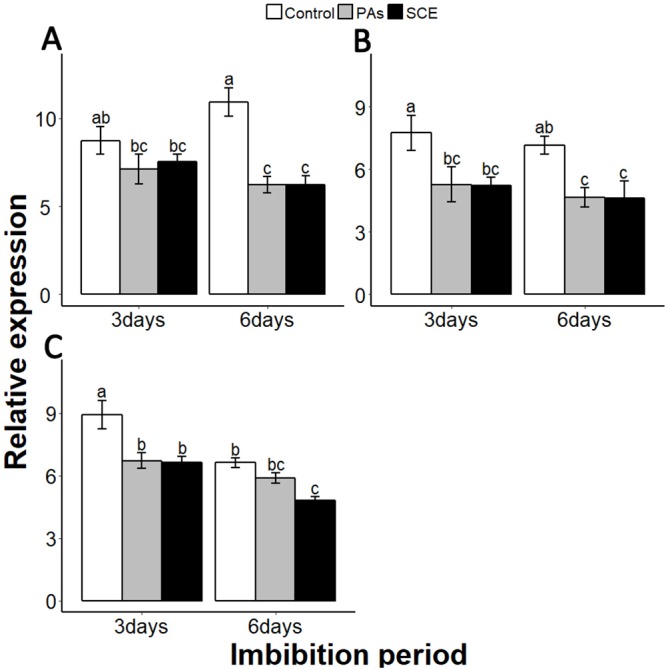
(A), SsMPK6 (B), SsNLP8 and (C), SsCIPK23. The expression of SsMPK6, SsNLP8 and SsCIPK23 were determined by qRT-PCR on the third and sixth days after treatment. SsACTIN was used as the reference gene. Control, seeds grown in half MS medium. PAs, proanthocyanidins-supplemented half MS medium. SCE, seeds cultivated on half MS medium supplemented with seed-coat extract. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
Among the soil nutrients, nitrates play an important role in seed germination with a specific molecular mechanism (Lara et al., 2014). To investigate the impact of seed coat on nitrates signalling, we selected nitrates-signalling genes NIN-LIKE-PROTEIN 8 (NLP8) and CBL-INTERACTING PROTEIN KINASE 23 (CIPK23) (Footitt et al., 2017; Yan et al., 2016). The transcript level of SsNLP8 was higher in the control as compared to PAs and SCE treatments in both third and the sixth days of imbibition (Fig. 7B). PAs and SCE treatments decreased the expression level of SsCIPK23 gradually as compared to the control on both the third and sixth imbibition days (Fig. 7C).
We found interactions of seed coat PAs with the germination regulators. After seven days imbibition, the control, GA3, NDGA, H2O2 and KNO3 priming showed 36 ± 4.8, 97.22 ± 4.8, 91.9 ± 1, 93.44 ± 3 and 97.22 ± 4.8 (mean ± SD) percent germination respectively on 0.5× MS medium supplemented with 0.3% SCE. We found that all of our treatments significantly (P = 0.05) promoted germination as compared to the control (Fig. 8; Fig. S3).
Figure 8. Inhibitory effects of SCE on seed germination was alleviated by GA3, NDGA, H2O2 and KNO3.
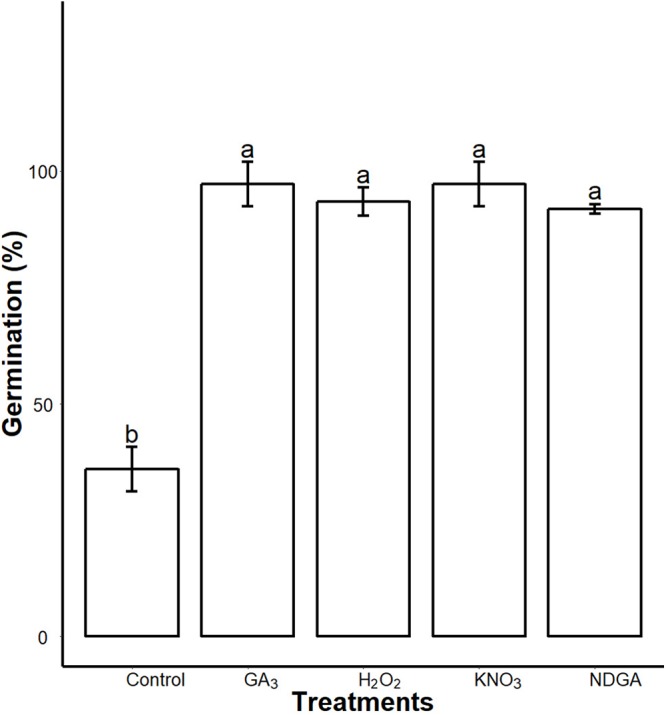
Seeds were primed in double distilled water (Control), 50 μΜ GA3, 50 μM NDGA, 20 mM H2O2 and 0.4% KNO3 and were sowed in 0.3% SCE-supplemented half MS medium. The seed germination rate was recorded seven days after imbibition. Data shown are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05 using Tukey’s HSD post hoc test.
Discussion
The data presented here show the causes of dormancy in S. sebiferum seed. PAs found in the seed coat and the ESC were the major controllers of dormancy in S. sebiferum seed. Our results show that S. sebiferum intact seeds are dormant, imbibe water and increase in germination if the covering structures such as the seed coat and endosperm cap are removed. Seeds showing this combination of features fit into the non-deep physiological dormancy category of Baskin & Baskin (2014). The causes of dormancy in these seeds can lie in the covering structures like seed coat (Baskin & Baskin, 2014) and ESC. If the covering structures control dormancy, there are a number of known mechanisms by which they do so; they can be impermeable to water, they can mechanically constrain the embryo from germinating, they can contain chemical inhibitors of germination and they can prevent the exit of chemical inhibitors of germination present in the embryo.
S. sebiferum intact seeds take up water, and so the seed coat is permeable to water. These results are in agreement with the results of Li et al. (2012). Recently, McGill et al. (2017) also reported that the rapid uptake of water within the first hour of imbibition indicates that the Myosotidium hortensia seed coat is not acting as a water impermeable barrier preventing germination, which suggests that water impermeability is not the reason for dormancy in S. sebiferum seed.
In our study, if the seed coat is removed, germination does increase substantially, as would be predicted from the model that the seed coat could have mechanical constraints. Our results show that mechanical scarification can slightly break the seed dormancy but scarification with sulphuric acid which degrades the germination inhibitors greatly improves germination. These results suggest that any mechanical constraint by the seed coat on germination is minor, compared to the major role played by germination inhibitors in the seed coat. Our results are in agreement with the results of Wada, Kennedy & Reed (2011), which showed that the effectiveness of sulphuric acid for Rubus seed scarification was likely due to degradation of PAs in the testa. The acid both weakened the seed coat and degraded the inhibitors (PAs) which the seed coat contains, and these inhibitors restrict germination of decoated seeds. Our results suggest that covering structures like tegmen contain germination inhibitors. These inhibitors restrict germination of decoated seeds, so removal of the seed coat results in 85% germination of the decoated seeds. The ESC also contains inhibitors, and the removal of the ESC results in 100% germination. It is also possible that the ESC is mechanically restraining the extra 15% of seeds from germinating, but the evidence we present here (vanillin assay) about the changes in the colour of the ESC support the chemical inhibition model.
Proanthocyanidins found in the tegmen and the ESC are the inhibitors of seed germination in S. sebiferum and have a molecular mechanism to inhibit seed germination. In our study, SCE (containing PAs) and pure PA treatments impacted the expression level of genes involved in GA/ABA homeostasis, which suggests that the seed-coat induced the seed dormancy by influencing transcription levels of ABA and GA biosynthesis or degradation genes and unbalance between GA/ABA homeostasis (Debeaujon & Koornneef, 2000; Debeaujon, Leon-Kloosterziel & Koornneef, 2000; Liguo et al., 2012). Seeds primed in GA3 and NDGA recovered seed germination on a SCE-supplemented medium, which confirms the above-suggested model. ROS and nitrates are also important factors in seed germination due to their role in the maintenance of ABA/GA homeostasis (Debeaujon & Koornneef, 2000; Jia et al., 2012; Lara et al., 2014; Liu et al., 2010; Yan et al., 2016; Zhou et al., 2015). Exogenous application of PAs and SCE in growing medium significantly reduced the transcription level of ROS- and nitrates-signalling genes. H2O2− and KNO3− primed seed recovered the germination on SCE-containing medium. These results are in agreement with previously reported results that a mutation of Arabidopsis with transparent testa 8 (TT8) lacked PA accumulation in its testa, produced a high level of H2O2 after imbibition, and had higher germination rate (Jia et al., 2013; Liu et al., 2010). H2O2 is the main kind of ROS in plants which regulates seed germination through GA/ABA metabolism and signalling, (Jia et al., 2012, 2013; Liu et al., 2010). KNO3 is a source of nitrates which activates the nitrate reductase enzyme and regulates the expression of nitrates-signalling genes NLP8 and CIPK23 which induce seed germination. NLP8 binds directly to the promoter of CYP707A2 and reduces ABA levels in a nitrate-dependent manner (Footitt et al., 2017; Liu et al., 2010; Yan et al., 2016).
Conclusion
Our research offers a quick and easy method for germinating S. sebiferum seeds for nursery growers. Moreover, this experiment will provide a basis for researchers to understand the mechanisms involved in S. sebiferum seed dormancy. S. sebiferum seeds contain PAs in the seed coat (tegmen layer) and ESC. PAs impact the transcription of ABA-, GA-, ROS- and nitrates-related genes, and cause dormancy in S. sebiferum seed. In our experiments, we found dynamic changes in PA levels in the ESC. To generalize this result, more investigations of seeds of other species are required.
Supplemental Information
A, B, C, D, E, F and G are 0-(control), 10-, 20-, 30-, 40-, 50- and 60 minutes incubation with concentrated sulfuric acid respectively. H, mechanical scarification effect on seed germination. Seeds of every treatment were sown in each 10×10 cm pot with 3 replicates. Photographs were taken after one month of seed sowing. I, graphical comparisons of seed germination of intact seed, mechanical and sulfuric acid scarified seeds. Data were collected 30 days after seed sowing. Data shown are means±sd (n=3). Means with different letters are significantly different at P < 0.05 using Tuckey’s HSD post hoc test. The photographs were taken by Shah Faheem Afzal and Jun Ni.
A, 0.5×MS (control). B, 0.3% SCE+0.5×MS. C, 0.1% PAs+0.5×MS. For each treatment, twelve seeds were sown in a 9 diameter cm Petri plate separately. All treatments were replicated 5 times. The photographs were taken on the 7th day of imbibition by Shah Faheem Afzal. Bar = 2cm.
A, control (sterile water). B, 50 μΜ GA3. C, 50 μΜ NDGA. D, 20 mM H2O2 and E, 0.4% KNO3 priming overnight at room temperature and the primed seed of all treatments were grown separately on 0.3% SCE+0.5×MS in 9 cm Petri plates (12 seeds per plate) for 7 days. These photographs were taken on the 7th day of imbibition. Bars = 1 cm. The photographs were taken by Shah Faheem Afzal.
The full sequences of all genes were obtained by local blasting Arabidopsis amino-acids sequence in blast-2.2.31. A local-blast library was built by S. sebiferum flower-bud transcriptome (Accession: SRX656554, https://www.ncbi.nlm.nih.gov/sra/SRX656554). The CDS regions of genes were translated by https://web.expasy.org/translate/.
Fifty grams of S. sebiferum seeds were taken for this experiment. The water uptake was calculated by weighing seeds before and after imbibition. Data were collected was taken 72 hours after imbibition.
Incubation time (minute), H2SO4 scarification time. Data were collected one month after seed sowing.
Incubation time (minute), H2SO4 scarification time. Data were collected 45 days after seed sowing.
PAS (%), PAs (%) content in seed coat after H2SO4 scarification. PAs (%) content was calculated by comparing with the absorbance of pure proanthocyanidins.
The CDS of each gene was used to design primers. All the primers for qPCR were designed by using Primer Premier 6.0.
Seed coat layers were separated into testa and tegmen, and were ground by a mortar and a pestle in the flow of liquid nitrogen. SCE was prepared as described in the main manuscript. Thirty milligrams of each sample was taken for vanillin assay. Data were collected was taken by comparing the absorbance of samples with a standard curve of pure proanthocyanidins.
SCE and PAs were added in half MS. Twelve seeds were sown in each 10 cm2 Patri plate. Data were collected after 7 days of imbibition.
IDS, imbibition days. Data shows the Δ Ct value f each gene. The Δ Ct value was calculated by subtracting Ct value of the reference gene SsACTIN from the Ct value of each gene.
Germination, germination (%), Seeds were primed in double distilled water (Control), 50 μΜ GA3, 50 μM NDGA, 20 mM H2O2 and 0.4% KNO3 and were sowed in 0.3% SCE-supplemented half MS medium. Twelve seeds were sown in each 9 cm diameter Petri dishes. Data were collected after 7 days of imbibition.
MS, mechanical scarification. CS, sulfuric acid scarification. IT, intact seed. Twently seeds of every treatment were sown in each 10×10 cm pot with 3 replicates. Data were collected after one month of seed sowing.
Acknowledgments
The authors are thankful to Ghulam Ali Bugti, school of plant protection, Anhui Agriculture University, for his wise suggestions for improving the quality of manuscript.
Funding Statement
This work was funded by Anhui Natural Science Foundation (1708085QC70), the National Natural Science Foundation of China (11375232&31500531), the Science and Technology Service program of Chinese Academy of Sciences (KFJ-STS-ZDTP-002&KFJ-SW-STS-143-4), the Grant of the President Foundation of Hefei Institutes of Physical Science of Chinese Academy of Sciences (YZJJ201502&YZJJ201619), the major special project of Anhui Province (16030701103), and the research and technology project of Anhui province (1501031079). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Songling Fu, Email: fusongl001@outlook.com.
Lifang Wu, Email: lfwu@ipp.ac.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Faheem Afzal Shah conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, seed collection, Taking photographs.
Jun Ni conceived and designed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, taking photographs.
Jing Chen performed the experiments.
Qiaojian Wang performed the experiments, contributed reagents/materials/analysis tools, seed collection.
Wenbo Liu performed the experiments.
Xue Chen analysed the data, contributed reagents/materials/analysis tools.
Caiguo Tang analysed the data, contributed reagents/materials/analysis tools.
Songling Fu conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Lifang Wu conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
Gene sequences are provided in Data S1.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Dataset Files.
References
- Baskin & Baskin (2014).Baskin C, Baskin JM. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. San Diego: Academic Press; 2014. [Google Scholar]
- Boldor et al. (2010).Boldor D, Kanitkar A, Terigar BG, Leonardi C, Lima M, Breitenbeck GA. Microwave assisted extraction of biodiesel feedstock from the seeds of invasive Chinese tallow tree. Environmental Science & Technology. 2010;44(10):4019–4025. doi: 10.1021/es100143z. [DOI] [PubMed] [Google Scholar]
- Brooks et al. (1987).Brooks G, Morrice NA, Ellis C, Aitken A, Evans AT, Evans FJ. Toxic phorbol esters from Chinese tallow stimulate protein kinase C. Toxicon: Official Journal of the International Society on Toxinology. 1987;25(11):1229–1233. doi: 10.1016/0041-0101(87)90141-3. [DOI] [PubMed] [Google Scholar]
- Debeaujon & Koornneef (2000).Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiology. 2000;122(2):415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon, Leon-Kloosterziel & Koornneef (2000).Debeaujon I, Leon-Kloosterziel KM, Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiology. 2000;122(2):403–414. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers et al. (2016).Dekkers BJ, He H, Hanson J, Willems LA, Jamar DC, Cueff G, Rajjou L, Hilhorst HW, Bentsink L. The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development. Plant Journal. 2016;85(4):451–465. doi: 10.1111/tpj.13118. [DOI] [PubMed] [Google Scholar]
- Durán & Retamal (1989).Durán JM, Retamal N. Coat structure and regulation of dormancy in Sinapis arvensis L. Seeds. Journal of Plant Physiology. 1989;135(2):218–222. doi: 10.1016/s0176-1617(89)80180-4. [DOI] [Google Scholar]
- Esser (2002).Esser H-J. A revision of Triadica Lour. (euphorbiaceae) Harvard Papers in Botany. 2002;7:17–21. [Google Scholar]
- Footitt et al. (2011).Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20236–20241. doi: 10.1073/pnas.1116325108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt et al. (2017).Footitt S, Olcer-Footitt H, Hambidge AJ, Finch-Savage WE. A laboratory simulation of Arabidopsis seed dormancy cycling provides new insight into its regulation by clock genes and the dormancy-related genes DOG1, MFT, CIPK23 and PHYA. Plant Cell & Environment. 2017;40(8):1474–1486. doi: 10.1111/pce.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2016).Gao RX, Su ZS, Yin YB, Sun LN, Li SY. Germplasm, chemical constituents, biological activities, utilization, and control of Chinese tallow (Triadica sebifera (L.) Small) Biological Invasions. 2016;18(3):809–829. doi: 10.1007/s10530-016-1052-2. [DOI] [Google Scholar]
- Herald et al. (2014).Herald TJ, Gadgil P, Perumal R, Bean SR, Wilson JD. High-throughput micro-plate HCl-vanillin assay for screening tannin content in sorghum grain. Journal of the Science of Food and Agriculture. 2014;94(10):2133–2136. doi: 10.1002/jsfa.6538. [DOI] [PubMed] [Google Scholar]
- Himi et al. (2002).Himi E, Mares DJ, Yanagisawa A, Noda K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. Journal of Experimental Botany. 2002;53(374):1569–1574. doi: 10.1093/jxb/erf005. [DOI] [PubMed] [Google Scholar]
- Jeffrey & Padley (1991).Jeffrey BSJ, Padley FBJ. Chinese vegetable tallow-characterization and contamination by stillingia oil. Journal of the American Oil Chemists Society. 1991;68(2):123–127. doi: 10.1007/bf02662332. [DOI] [Google Scholar]
- Jia et al. (2012).Jia LG, Sheng ZW, Xu WF, Li YX, Liu YG, Xia YJ, Zhang JH. Modulation of anti-oxidation ability by proanthocyanidins during germination of Arabidopsis thaliana seeds. Molecular Plant. 2012;5(2):472–481. doi: 10.1093/mp/ssr089. [DOI] [PubMed] [Google Scholar]
- Jia et al. (2013).Jia L, Xu W, Li W, Ye N, Liu R, Shi L, Bin Rahman AN, Fan M, Zhang J. Class III peroxidases are activated in proanthocyanidin-deficient Arabidopsis thaliana seeds. Annals of Botany. 2013;111(5):839–847. doi: 10.1093/aob/mct045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantar, Pilbeam & Hebblethwaite (1996).Kantar F, Pilbeam CJ, Hebblethwaite PD. Effect of tannin content of faba bean (Vicia faba) seed on seed vigour, germination and field emergence. Annals of Applied Biology. 1996;128(1):85–93. doi: 10.1111/j.1744-7348.1996.tb07092.x. [DOI] [Google Scholar]
- Lara et al. (2014).Lara TS, Lira JMS, Rodrigues AC, Rakocevic M, Alvarenga AA. Potassium nitrate priming affects the activity of nitrate reductase and antioxidant enzymes in tomato germination. Journal of Agricultural Science. 2014;6(2):72–80. doi: 10.5539/jas.v6n2p72. [DOI] [Google Scholar]
- Lee et al. (2002).Lee SC, Cheng H, King KE, Wang WF, He YW, Hussain A, Lo J, Harberd NP, Peng JR. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes & Development. 2002;16(5):646–658. doi: 10.1101/gad.969002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2012).Li SX, Gu HB, Mao Y, Yin TM, Gao HD. Effects of tallow tree seed coat on seed germination. Journal of Forestry Research. 2012;23(2):229–233. doi: 10.1007/s11676-011-0217-1. [DOI] [Google Scholar]
- Liguo et al. (2012).Liguo J, Qiuyu W, Nenghui Y, Liu R, Weifeng X, Zhi H, Rubaiyath Bin Rahman ANM, Xia Y, Zhang J. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana. Journal of Integrative Plant Biology. 2012;54(9):663–673. doi: 10.1111/j.1744-7909.2012.01142.x. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2010).Liu Y, Ye N, Liu R, Chen M, Zhang J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany. 2010;61(11):2979–2990. doi: 10.1093/jxb/erq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita et al. (2007).Matsushita A, Furumoto T, Ishida S, Takahashi Y. AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiology. 2007;143(3):1152–1162. doi: 10.1104/pp.106.093542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill et al. (2017).McGill CR, Park MJ, Williams WM, Outred HA, Nadarajan J. The mechanism of seed coat-imposed dormancy revealed by oxygen uptake in Chatham Island forget-me-not Myosotidium hortensia (Decne.) Baill. New Zealand Journal of Botany. 2017;56(1):38–50. doi: 10.1080/0028825x.2017.1383922. [DOI] [Google Scholar]
- Millar et al. (2010).Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant Journal. 2010;45(6):942–954. doi: 10.1111/j.1365-313x.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto & Everson (1958).Miyamoto T, Everson EH. Biochemical and physiological studies of wheat seed pigmentation. Agronomy Journal. 1958;50(12):733–734. doi: 10.2134/agronj1958.00021962005000120005x. [DOI] [Google Scholar]
- Nilsson-Ehle (1914).Nilsson-Ehle H. Zur kenntnis der mit der keimungsphysiologie des weizens in zusammenhang stehenden inneren faktoren. Z Pflanzenzücht. 1914;2:153–187. [Google Scholar]
- Oracz et al. (2009).Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiology. 2009;150(1):494–505. doi: 10.1104/pp.109.138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oracz & Karpinski (2016).Oracz K, Karpinski S. Phytohormones signaling pathways and ROS involvement in seed germination. Frontiers in Plant Science. 2016;7:864–869. doi: 10.3389/fpls.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell (1989).Powell AA. The importance of genetically determined seed coat characteristics to seed quality in grain legumes. Annals of Botany. 1989;63(1):169–175. doi: 10.1093/oxfordjournals.aob.a087720. [DOI] [Google Scholar]
- Qian et al. (2016).Qian CM, Zhou J, Chen L, Su YY, Dai S, Li SX. Bioassay of germination inhibitors in extracts of Sapium sebiferum seeds of different provenance. Journal of Horticultural Science & Biotechnology. 2016;91(4):341–346. doi: 10.1080/14620316.2016.1160543. [DOI] [Google Scholar]
- Ravindran et al. (2017).Ravindran P, Verma V, Stamm P, Kumar PP. A novel RGL2–DOF6 complex contributes to primary seed dormancy in Arabidopsis thaliana by regulating a GATA transcription factor. Molecular Plant. 2017;10(10):1307–1320. doi: 10.1016/j.molp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Rieu et al. (2008).Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell. 2008;20(9):2420–2436. doi: 10.1105/tpc.108.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen et al. (2016).Shen C, Wang X, Zhang L, Lin S, Liu D, Wang Q, Cai S, Eltanbouly R, Gan L, Han W. Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development. Horticulture Research. 2016;3(1):16059–16068. doi: 10.1038/hortres.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, Kennedy & Reed (2011).Wada S, Kennedy JA, Reed BM. Seed-coat anatomy and proanthocyanidins contribute to the dormancy of Rubus seed. Scientia Horticulturae. 2011;130(4):762–768. doi: 10.1016/j.scienta.2011.08.034. [DOI] [Google Scholar]
- Webster, Jenkins & Jose (2006).Webster CR, Jenkins MA, Jose S. Woody invaders and the challenges they pose to forest ecosystems in the eastern United States. Journal of Forestry. 2006;104:366–374. [Google Scholar]
- Werker, Marbach & Mayer (1979).Werker E, Marbach I, Mayer AM. Relation between the anatomy of the testa, water permeability and the presence of phenolics in the genus Pisum. Annals of Botany. 1979;43(6):765–771. doi: 10.1093/oxfordjournals.aob.a085691. [DOI] [Google Scholar]
- Wyatt (1977).Wyatt JE. Seed coat and water absorption properties of seed of near-isogenic snap bean lines differing in seed coat color. Journal American Society for Horticultural Science. 1977;102:478–480. [Google Scholar]
- Xuan, Wang & Jiang (2014).Xuan L, Wang Z, Jiang L. Vanillin assay of Arabidopsis seeds for proanthocyanidins. Bio-protocol. 2014;4:e1309 [Google Scholar]
- Yan et al. (2016).Yan D, Vanathy E, Vivian C, Masanori O, Matthew I, Mitsuhiro K, Akira E, Ryoichi Y, Asher P, Gong Y. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nature Communications. 2016;7:13179–13190. doi: 10.1038/ncomms13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang M, Wu Y, Jin S, Hou J, Mao Y, Liu W, Shen Y, Wu L. Flower bud transcriptome analysis of Sapium sebiferum (Linn.) Roxb. and primary investigation of drought induced flowering: pathway construction and G-Quadruplex prediction based on transcriptome. PLOS ONE. 2015;10(3):e0118479. doi: 10.1371/journal.pone.0118479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao & Tao (2015).Zhao D, Tao J. Recent advances on the development and regulation of flower color in ornamental plants. Frontiers in Plant Science. 2015;6:261–274. doi: 10.3389/fpls.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2015).Zhou J, Yin YT, Qian CM, Liao ZY, Shu Y, Li SX. Seed coat morphology in Sapium sebiferum in relation to its mechanism of water uptake. Journal of Horticultural Science & Biotechnology. 2015;90(6):613–618. doi: 10.1080/14620316.2015.11668723. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, B, C, D, E, F and G are 0-(control), 10-, 20-, 30-, 40-, 50- and 60 minutes incubation with concentrated sulfuric acid respectively. H, mechanical scarification effect on seed germination. Seeds of every treatment were sown in each 10×10 cm pot with 3 replicates. Photographs were taken after one month of seed sowing. I, graphical comparisons of seed germination of intact seed, mechanical and sulfuric acid scarified seeds. Data were collected 30 days after seed sowing. Data shown are means±sd (n=3). Means with different letters are significantly different at P < 0.05 using Tuckey’s HSD post hoc test. The photographs were taken by Shah Faheem Afzal and Jun Ni.
A, 0.5×MS (control). B, 0.3% SCE+0.5×MS. C, 0.1% PAs+0.5×MS. For each treatment, twelve seeds were sown in a 9 diameter cm Petri plate separately. All treatments were replicated 5 times. The photographs were taken on the 7th day of imbibition by Shah Faheem Afzal. Bar = 2cm.
A, control (sterile water). B, 50 μΜ GA3. C, 50 μΜ NDGA. D, 20 mM H2O2 and E, 0.4% KNO3 priming overnight at room temperature and the primed seed of all treatments were grown separately on 0.3% SCE+0.5×MS in 9 cm Petri plates (12 seeds per plate) for 7 days. These photographs were taken on the 7th day of imbibition. Bars = 1 cm. The photographs were taken by Shah Faheem Afzal.
The full sequences of all genes were obtained by local blasting Arabidopsis amino-acids sequence in blast-2.2.31. A local-blast library was built by S. sebiferum flower-bud transcriptome (Accession: SRX656554, https://www.ncbi.nlm.nih.gov/sra/SRX656554). The CDS regions of genes were translated by https://web.expasy.org/translate/.
Fifty grams of S. sebiferum seeds were taken for this experiment. The water uptake was calculated by weighing seeds before and after imbibition. Data were collected was taken 72 hours after imbibition.
Incubation time (minute), H2SO4 scarification time. Data were collected one month after seed sowing.
Incubation time (minute), H2SO4 scarification time. Data were collected 45 days after seed sowing.
PAS (%), PAs (%) content in seed coat after H2SO4 scarification. PAs (%) content was calculated by comparing with the absorbance of pure proanthocyanidins.
The CDS of each gene was used to design primers. All the primers for qPCR were designed by using Primer Premier 6.0.
Seed coat layers were separated into testa and tegmen, and were ground by a mortar and a pestle in the flow of liquid nitrogen. SCE was prepared as described in the main manuscript. Thirty milligrams of each sample was taken for vanillin assay. Data were collected was taken by comparing the absorbance of samples with a standard curve of pure proanthocyanidins.
SCE and PAs were added in half MS. Twelve seeds were sown in each 10 cm2 Patri plate. Data were collected after 7 days of imbibition.
IDS, imbibition days. Data shows the Δ Ct value f each gene. The Δ Ct value was calculated by subtracting Ct value of the reference gene SsACTIN from the Ct value of each gene.
Germination, germination (%), Seeds were primed in double distilled water (Control), 50 μΜ GA3, 50 μM NDGA, 20 mM H2O2 and 0.4% KNO3 and were sowed in 0.3% SCE-supplemented half MS medium. Twelve seeds were sown in each 9 cm diameter Petri dishes. Data were collected after 7 days of imbibition.
MS, mechanical scarification. CS, sulfuric acid scarification. IT, intact seed. Twently seeds of every treatment were sown in each 10×10 cm pot with 3 replicates. Data were collected after one month of seed sowing.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Dataset Files.