Abstract
Background
Curative-intent treatment for localized hilar cholangiocarcinoma (HC) requires surgical resection. However, the effect of adjuvant therapy (AT) on survival is unclear. We analyzed the impact of AT on overall (OS) and recurrence free survival (RFS) in patients undergoing curative resection.
Methods
We reviewed patients with resected HC between 2000 and 2015 from the ten institutions participating in the U.S. Extrahepatic Biliary Malignancy Consortium. We analyzed the impact of AT on RFS and OS. The probability of RFS and OS were calculated in the method of Kaplan and Meier and analyzed using multivariate Cox regression analysis.
Results
A total of 249 patients underwent curative resection for HC. Patients who received AT and those who did not had similar demographic and preoperative features. In a multivariate Cox regression analysis, AT conferred a significant protective effect on OS (HR 0.58, p=0.013), and this was maintained in a propensity matched analysis (HR 0.66, p=0.033). The protective effect of AT remained significant when node negative patients were excluded (HR 0.28, p=0.001), while it disappeared (HR 0.76, p=0.260) when node positive patients were excluded.
Conclusions
Adjuvant therapy should be strongly considered after curative-intent resection for hilar cholangiocarcinoma, particularly in patients with node positive disease.
Keywords: adjuvant therapy, hilar cholangiocarcinoma, chemotherapy, biliary cancer, survival
Introduction
Cholangiocarcinoma accounts for 3% of gastrointestinal malignancies worldwide, with 3500 cases diagnosed per year in the United States, the majority of which (60-80%) arise in the perihilar region.1,2 Unresectable disease has a dismal prognosis, with a median survival of less than one year.1,3 Margin negative R0 resection provides the only chance for long-term cure.3,4 Despite this, resectable disease has a five-year survival between 20 and 50%.3,5–7 Adjuvant therapy (AT) has been advocated after resected hilar cholangiocarcinoma (HC) to improve outcomes.8–10
The role of AT for resected HC is a source of significant debate.11 Current National Comprehensive Cancer Network (NCCN) guidelines for AT in resected HC comment that more data is necessary in order to make firm conclusions.12 In two recent retrospective reviews of AT in biliary tract cancer (BTC), no survival benefit was found.7,13 Conversely, within the last ten years three single center retrospective studies have demonstrated a survival benefit with AT in resected extrahepatic cholangiocarcinoma.8–10 In the only published phase III randomized trial of AT that included patients with HC, and in the recently completed phase III trial of gemcitabine plus oxaliplatin in BTC, there was no significant survival benefit noted.14,15
Herein, we sought to determine the role of AT in resected HC by evaluating a large group of patients undergoing treatment with curative intent from the U.S. Extrahepatic Biliary Malignancy Consortium. We hypothesized that AT would improve both and recurrence free (RFS) overall survival (OS) in resected HC.
Methods
Patient Population
Our data source included all patients who underwent operative intervention with curative intent for extrahepatic biliary malignancy from January 2000 to April 2015 from ten institutions participating in the U.S. Extrahepatic Biliary Malignancy Consortium: Emory University, The Johns Hopkins University, Stanford University, Vanderbilt University, Washington University in St Louis, University of Wisconsin, University of Louisville, Wake Forest University, The Ohio State University, and New York University. Institutional Review Board approval for the study was obtained at all participating institutions.
All patient characteristics, operative/treatment data, and clincopathological data were gathered retrospectively via chart review (Table 1). Medical comorbidities, as coded via chart review, included: hypertension, diabetes, cardiac history, congestive heart failure, dyspnea, smoking history, severe chronic obstructive pulmonary disease (COPD), on a ventilator, acute renal failure, dialysis, chronic steroid use, disseminated cancer, and primary sclerosing cholangitis. We included all patients identified as undergoing a completed curative type resection for HC, excluding R2 resections. We analyzed the impact of AT on the primary outcome of OS and secondary outcome of RFS. OS was measured from the time of resection to death or last follow-up on chart review. Date of death was determined via chart review or the Social Security Death Index. RFS was defined as time from initial resection to recurrence, final documented follow up or death.
TABLE 1.
Association of Pretreatment, Surgery and Pathology Variables with Adjuvant Therapy
| Variable | No AT (n=95, 42%)
|
AT (n=129, 58%)
|
p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Pretreatment Data | |||||
| Age (mean and SD)* | 66 | 10 | 64 | 12 | 0.180 |
| Male | 53 | 56 | 82 | 64 | 0.240 |
| Race | 0.162 | ||||
| Caucasian | 73 | 77 | 105 | 82 | |
| Other | 22 | 23 | 24 | 19 | |
| ASA | 0.432 | ||||
| 1 | 1 | 1 | 1 | 1 | |
| 2 | 23 | 29 | 42 | 38 | |
| 3 | 50 | 64 | 66 | 59 | |
| 4 | 4 | 5 | 2 | 2 | |
| Comorbiditiesa | 0.141 | ||||
| Yes | 63 | 66 | 56 | 43 | |
| No | 32 | 34 | 73 | 57 | |
| Neo-adjuvant Therapy | 6 | 6 | 4 | 3 | 0.250 |
| Operative and Pathology Data | |||||
| Diagnostic Laparoscopy | 19 | 20 | 16 | `1 | 0.128 |
| Type of Operation | 0.017 | ||||
| BD Resection Only | 16 | 17 | 35 | 28 | |
| Right Hepatectomy and BD | 17 | 18 | 8 | 6 | |
| Left Hepatectomy and BD | 16 | 17 | 35 | 28 | |
| Extended Right Hepatectomy and BD | 20 | 21 | 16 | 13 | |
| Extended Left Hepatectomy and BD | 6 | 6 | 12 | 9 | |
| Right Trisectionectomy and BD | 10 | 10 | 12 | 9 | |
| Left Trisectionectomy and BD | 9 | 10 | 9 | 7 | |
| Method of Operationa | 0.177 | ||||
| Open | 93 | 98 | 128 | 99 | |
| Laparoscopic | 0 | 0 | 1 | 1 | |
| Converted to Open | 2 | 2 | 0 | 0 | |
| Frozen Section + | 24 | 29 | 49 | 46 | 0.015 |
| Intraoperative RBCs | 45 | 54 | 35 | 30 | 0.001 |
| R Status | 0.012 | ||||
| R0 | 71 | 75 | 77 | 60 | |
| R1 | 24 | 25 | 52 | 40 | |
| AJCC 7th. ed. T Stage18 | 0.803 | ||||
| T1 | 29 | 31 | 36 | 28 | |
| T2 | 47 | 49 | 72 | 56 | |
| T3 | 15 | 16 | 17 | 13 | |
| T4 | 4 | 4 | 4 | 3 | |
| Grade of Differentiation | 0.237 | ||||
| Well | 12 | 15 | 25 | 20 | |
| Moderate | 53 | 65 | 69 | 55 | |
| Poor | 15 | 19 | 32 | 25 | |
| Undifferentiated | 1 | 1 | 0 | 0 | |
| LVI | 27 | 34 | 49 | 45 | 0.124 |
| LN Positive | 17 | 20 | 55 | 49 | <0.001 |
| Postoperative Data | |||||
| Readmission ≤ 90 Days | 24 | 41 | 28 | 32 | 0.923 |
| Recurrence Locationb | 0.198 | ||||
| Local | 12 | 43 | 15 | 24 | |
| Distant | 11 | 39 | 31 | 50 | |
| Local + Distant | 5 | 18 | 16 | 26 | |
Abbreviations: AT, adjuvant therapy; SD, standard deviation; ASA, American Society of Anesthesiologists classification system; BD, bile duct; R status, resection status; AJCC, American Joint Committee on Cancer; LVI, lymphovascular invasion; LN, lymph node.
For Age: columns n and % signify mean and standard deviation, respectively
Comorbidities determined via chart review, as discussed in methods section
% reflects percentage of the total number of patients that had a recurrence
Statistical Methods
The probability of OS and RFS were calculated in the method of Kaplan and Meier and analyzed using multivariate Cox regression analysis.16,17 Multivariate modeling was used to assess the impact of selected variables on both OS and RFS. Clinical judgment was used to select variables to evaluate from our dataset on univariate survival analysis, and these same variables were applied to our multivariate survival analysis. Patients who received AT were propensity score matched to patients who did not in a 1:1 ratio using variables which were found to be significantly associated with survival following resection (i.e. patient age, ASA, lymph node status, and grade) in the multivariable analysis. Propensity score matching was accomplished using a greedy matching algorithm with a caliper distance of 0.2 standard deviations of the logit of the propensity score.
Characteristics of the patient population receiving and not receiving AT were compared. Categorical variables were compared using the Fisher exact test or χ2 test as appropriate, and continuous variables were compared using the two-tailed t test. Statistical significance was set at p<0.05. STATA version 13.1 (StataCorp LP, College Station, TX, USA) was used for data analysis.
Results
Patient Population and Baseline Characteristics
249 patients underwent resection with curative intent for HC. In 224 patients (90%) AT status was known (129 receiving AT and 95 who did not). Baseline characteristics (age, sex, race, ASA classification, medical comorbidities, neo-adjuvant therapy status) were similar between groups (Table 1). A breakdown of AT status and type is depicted in Figure 1.
FIGURE 1.
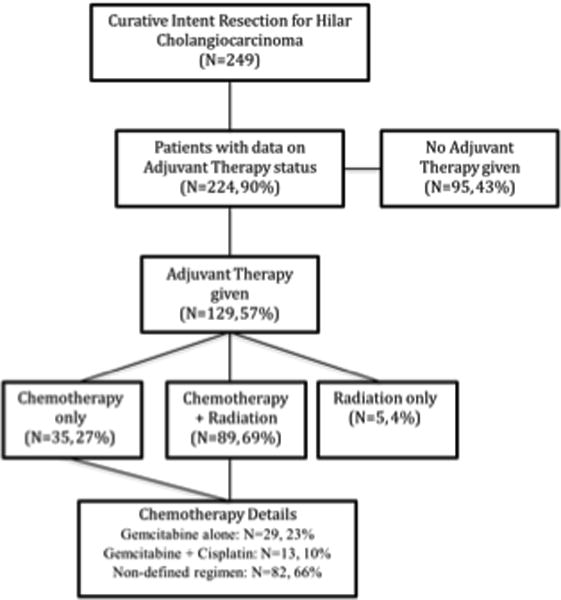
Breakdown of Patients by Adjuvant Therapy Type
Operative and Pathological Data
The operative and pathology data is presented in Table 1. There was no significant difference seen between groups for method of operation, AJCC T stage, presence of lymphovascular invasion, tumor grade and completion of preoperative diagnostic laparoscopy.18 As depicted in Table 1, those in the AT group tended to have more bile duct resections only or left hepatectomies (p=0.017), were more likely to have a positive frozen section (p=0.015), were less likely to receive an intraoperative blood transfusion (p=0.001), had a greater percentage of R1 resections (p=0.012), and had a greater rate of lymph node (LN) positivity (p<0.001). The mean number of LNs examined was similar between the no AT (4.47 ± 0.41) and AT (4.62 ± 0.46) groups (p=0.811).
Postoperative Data
Postoperatively, 90 day hospital readmission and location of recurrent disease were similar between groups. Length of stay was significantly greater in the no AT group at 19.5 ± 1.8 days, versus 11.2 ± 0.5 days in the AT group (p<0.001). See table 1 for more details.
Adjuvant Therapy Details
In the AT group (N=129), 89 patients (69%) received combined chemoradiotherapy, 35 patients (27%) received only chemotherapy, and 5 patients (4%) received only radiation therapy (Figure 1). See Figure 1 for further information regarding chemotherapy regimen.
Univariate Analysis of RFS/OS
Median follow up after resection for the group was 19.8 months. RFS for the group as a whole was 13.5% at 5 years, with a 16.3 month median RFS. OS for the group was 17.0% at 5 years, with a median OS of 21.7 months. For the no AT group RFS at 5 years was 14.3%, with a median RFS of 13.3 months, while for the AT group 5 year RFS was 11.0%, with a 17.4 month median RFS (p=0.25). For the no AT group OS at 5 years was 21.3%, with a median 20.0 month OS, while for the AT group 5 year OS was 14.2%, with a 21.9 month median OS (p=0.25).
LN positivity, age ≥60 years old, and worse tumor grade were all associated with decreased RFS, Table 2. Factors associated with decreased OS on univariate analysis include: LN positivity, and age ≥60 years old. Tumor size greater than 2cm, non-R0 resection status, increased ASA class, comorbidities, and concomitant liver resection had no significant association with RFS or OS. Tumor grade had no significant relationship with OS.
TABLE 2.
Significant Relationships on Univariate Analysis and Multivariate Cox Regression Analysis for Recurrence Free and Overall Survival
| Univariate Analysis
| |||||
|---|---|---|---|---|---|
| RFS
|
OS
|
||||
| N | Median Survival (mo) | p value | Median Survival (mo) | p value | |
| LN Status | 0.004 | 0.011 | |||
| Negative | 136 | 17.3 | 24.3 | ||
| Positive | 81 | 13.7 | 17.6 | ||
| Age | 0.044 | 0.008 | |||
| <60 yrs | 72 | 20.1 | 27.8 | ||
| ≥60 yrs | 176 | 15.0 | 18.2 | ||
| Grade | 0.022 | 0.081 | |||
| Well Diff. | 43 | 20.0 | 27.8 | ||
| Mod Diff. | 132 | 16.0 | 19.3 | ||
| Poorly Diff. | 53 | 12.5 | 22.2 | ||
| Undifferentiated | 1 | – | 4.8 | ||
| Multivariate Analysis
| ||||
|---|---|---|---|---|
| RFS
|
RFS
|
OS
|
OS
|
|
| p | aHR (95% CI) | p | aHR (95% CI) | |
| Adjuvant Therapy | 0.005 | 0.55 (0.37-0.84) | 0.013 | 0.58 (0.38-0.89) |
| LN Positive | 0.001 | 2.06 (1.39-3.04) | 0.002 | 1.95 (1.28-2.95) |
| Age (Increasing) | 0.005 | 1.02 (1.01-1.04) | 0.011 | 1.02 (1.01-1.04) |
| ASA Status (Higher*) | 0.003 | 0.54 (0.37-0.81) | 0.073 | 0.69 (0.46-1.04) |
| Grade (vs. Well Diff.) | ||||
| Mod Diff. | 0.597 | 1.13 (0.72-1.76) | 0.573 | 1.14 (0.72-1.80) |
| Poorly Diff. | 0.015 | 1.89 (1.13-3.16) | 0.166 | 1.44 (0.86-2.43) |
| Undifferentiated | 0.025 | 10.53 (1.34-82.74) | 0.029 | 9.97 (1.26-78.94) |
Abbreviations: RFS, recurrence free survival; OS, overall survival; N, number at risk; mo, months; LN, lymph node; R status, resection status; yrs, years; Mod, moderately; Diff., differentiated; aHR, adjusted hazard ratio; ASA, American Society of Anesthesiologists classification system.
ASA Higher signifies ASA class of 3 or greater
Multivariate Cox Regression Modeling of RFS
Utilizing multivariate cox regression analysis we created a model to evaluate RFS and OS (Table 2). AT, tumor size >20mm, LN positivity, resection status (R1 versus R0), increased age, higher ASA status (III/IV), comorbidities, concomitant liver resection, and tumor grade (moderate, poor or undifferentiated) were included in the model. Of these variables, AT (p=0.005, HR 0.55) and higher ASA status (p=0.003, HR 0.54) were found to be significantly predictive of improved RFS. LN positive final pathology (p=0.001, HR 2.06), increased age (p=0.005, HR 1.02), poorly differentiated pathology (p=0.015, HR 1.89), and undifferentiated pathology (p=0.025, HR 10.53) were all found to be significantly predictive of worse RFS. Tumor size >20mm (p=0.140, HR 1.36), R1 versus R0 resection status (p=0.720, HR 1.08), presence of comorbidities (p=0.420, HR 1.161), concomitant liver resection (p=0.70, HR 1.09), and moderately differentiated pathology (p=0.597, HR 1.13) were found to be non-significant predictors of RFS on multivariate analysis.
Multivariate Cox Regression Modeling of OS
AT (p=0.014, HR 0.58) was found to significantly predict improved OS. The benefit seen with AT on OS is further displayed in Table 2. LN positive final pathology (p=0.002, HR 1.95), increased age (p=0.011, HR 1.02), and undifferentiated pathology (p=0.029, HR 9.97) were all found to significantly predict decreased OS. Tumor size >20mm (p=0.587, HR 1.12), R1 versus R0 resection status (p=0.372, HR 1.21), higher ASA classification (p=0.073, HR 0.69), concomitant liver resection (p=0.727, HR1.08), moderately differentiated pathology (p=0.573, HR 1.14), and poorly differentiated pathology (p=0.166, HR1.44) were found to be non-significant predictors of OS on multivariate analysis.
Propensity Matching of AT and no AT Groups
We next matched patients who received AT to those who did not based on age, LN status, ASA, and tumor grade using propensity scores (Supplemental Table). We matched based on these variables because they were the only independent predictors of survival in our multivariate analysis (Table 2) other than AT. As displayed in Figure 2, matched patients who received AT had significantly improved RFS (17.7 months vs 10.9 months, p=0.015, HR 0.61), as well as OS (21.5 months vs 13.5 months, p=0.033, HR 0.66).
FIGURE 2.
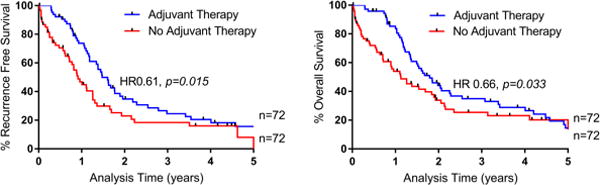
Overall and Recurrence Free Survival Based on Adjuvant Therapy Status for Propensity Matched Patients
Multivariate Cox Regression Modeling Subgroup Analysis of OS
The multivariate analysis was redone separating chemotherapy and chemoradiotherapy, with both groups demonstrating a similar OS benefit (both HR 0.6) to the combined analysis shown in table 2 (HR 0.58). When only LN positive patients were included in the multivariate model, AT continued to be associated with improved OS (HR 0.28, p=0.001). When only node negative patients were included in the model, AT was no longer significantly associated with improved OS (HR 0.76, p=0.26).
Discussion
To our knowledge, our current analysis represents the largest in a Western cohort that explores the role of AT on outcomes in resected HC. The majority of the patients in our study who underwent AT received both chemotherapy and radiation therapy (69%), with 27% only receiving chemotherapy and the rest only radiation therapy. Although AT had no survival benefit on univariate analysis, likely reflecting the increased rate of nodal positivity and R1 resection margin in this group (Table 1), on multivariate analysis as well as on a propensity matched analysis (Figure 2), we demonstrated an association between having received AT, and improved OS and RFS. This relationship disappeared when removing the node positive patients from the multivariate analysis. Therefore, we are able to draw the conclusion that adjuvant chemo/chemoradiotherapy is significantly associated with improved survival in patients undergoing curative resection for HC who harbor positive LNs on final pathology.
The only published phase III randomized trial that evaluated AT in HC was from 2002 by Takada et. al.14 This study of 508 patients with pancreaticobiliary malignancies, enrolled from 1986-1992, included 139 patients with cholangiocarcinoma, and randomly assigned patients to surgery alone or surgery with adjuvant mitomycin C plus 5-fluoruracil. They demonstrated no significant survival benefit in the cholangiocarcinoma cohort (OS AT 26.7% vs. 24.1% no AT at 5 years). In contrast to this study, our present analysis evaluates a more modern cohort of patients representing contemporary surgical outcomes (see Table 3 for further comparison details).
TABLE 3.
Studies Evaluating Adjuvant Chemotherapy ± Radiation for Resected Hilar Cholangiocarcinoma
| Investigator/Year | Country | Type Study | Years Data | # EHBTC/Total # Study | % Total Hilar | % AT | 5 year % OS AT | OS AT (months) | AT Regimen | Improved Survival with AT? | Improved Survival AT-Node Positive Subset? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Takada14 2002 |
JAPAN | Phase III RCT, multicenter | 1986-1992a | 139b/508 | – | 50 | 26.7c | – | MMC + 5FU | No | – |
|
|
|
||||||||||
| Borghero22 2008 |
USA | Retro, single centerd | 1984-2005 | 65/65 | 55 | 65e | 36 | 31 | ChemoXRT (5FU based) | – | – |
|
|
|
||||||||||
| Lim8 2009 |
KOREA | Retro, single center | 2000-2006 | 120/120 | 49 | 75 | (3yr) 62.6 | – | CCRT vs. CCRT+ 5FU based chemo | Yes | No |
|
|
|
||||||||||
| Kim9 2011 |
KOREA | Retro, single center | 2001-2009 | 168/168 | 53.9 | 68 | 36.5 | – | 5FU/LCV + XRT | Yes | Yes |
|
|
|
||||||||||
| Murakami10 2011 |
JAPAN | Retro, single center | 1990-2009 | 106/127 | 39 | 39 | 47 | – | GEM based | Yes | Yes |
|
|
|
||||||||||
| Park20 2011 |
KOREA | Retro, single center | 1998-2007 | 101/101 | 35 | 84 | 34 | 24 | All XRT, 84% chemo (most CAP based) | – | – |
|
|
|
||||||||||
| Glazer13 2012 |
USA | Retro, single center | 1978-2009 | 40/197 | – | 49.1 | – | 46/53f | CAP/GEM based, 15.3% ChemoXRT | No | – |
|
|
|
||||||||||
| Horgan19 2012 |
N/A | Meta-analysis | 1960-2010 | −/6712 | – | – | – | – | Multiple regimens | No | Yes |
|
|
|
||||||||||
| Ben- Josef21 2015 |
USA | Phase II Trial, multicenter | 2008-2012a | 35/79 | 48 | 86g | (2yr) 65 | 35 | GEM + CAP, 87% also XRT | – | – |
|
|
|
||||||||||
| Kang7 2016 |
KOREA | Retro, single center | 1991-2010 | 260/260 | 100 | 48.8 | 21.2 | 24.8 | ChemoXRT (5FU based), some just XRT/chemo | No | Yes |
|
|
|
||||||||||
| Edeline15 2017 |
EUROPE | Phase III RCT, multicenter | 2009-2014 | 69/193 | 8 | – | – | – | GEM + OX | No | No |
Abbreviations: EHBTC, extra hepatic biliary tract cancer; AT, adjuvant therapy; OS, overall survival; RCT, randomized controlled trial; MMC, mitomycin C; 5FU, 5-fluorouracil; retro, retrospective; chemoXRT, chemotherapy and radiation therapy; CCRT, concurrent chemoradiotherapy; LCV, leucovirin; XRT, radiation therapy; GEM, gemcitabine; CAP, capecitabine; OX, oxaliplatin.
Years patients enrolled
Intrahepatic cholangiocarcinoma included in group with no exact numbers given
For extrahepatic cholangiocarcinoma group
Most at a single center
Only R1 and/or N1 underwent chemoXRT
46/53 month med survival chemo/chemoXRT groups
% completed
A 2016 Korean retrospective study, that included 260 patients with resected HC, demonstrated no survival benefit with a predominantly fluorouracil based AT regimen, with an OS of 21.2% at 5 years for those receiving AT, versus 16.7% for those not receiving AT.7 A survival benefit was noted in the node positive cohort. A 2011 retrospective analysis that included 50 patients with HC demonstrated benefit with adjuvant chemotherapy (OS 47% AT vs. 36% no AT at 5 years), and similar to our study this benefit was limited to the node positive group.10 A meta-analysis from Horgan et. al. included 6,712 patients with BTC, and although there was no improvement in survival for patients receiving AT after surgical resection, again, in line with our data, a survival benefit was seen in patients with node positive disease.19 The recently released results from the phase III PRODIGE 12-ACCORD 18 European randomized controlled trial of adjuvant gemcitabine plus oxaliplatin in resected BTC demonstrated no impact of AT on survival in HC, even in those with node positive disease.15 This trial was limited by the small number of patients with HC accrued (N=15).
Of note, the 5-year median survival of 21.7 months in our patient cohort is lower than prior studies. This is likely reflective of different patient populations with variable tumor biology. For instance, our mean age was 65±11 years. Takada et. al. excluded patients 75 years or older and had a mean population age of 61, similar to other studies.7,14,20,21 In addition, each study represented a different population with specific exclusion criteria.9,14,20,21 Furthermore, tumor characteristics differ widely between studies. For example, the SWOG 0809 phase II trial had an R1 resection margin in 32% of patients and Kim et. al. had 0 patients with a positive resection margin.9,21 For our study, 40% of patients in the AT group had an R1 resection. Finally, prior studies have included a heterogeneous population of pancreaticobiliary malignancies, while ours is one of the only to focus solely on HC.8–10,13,14,19–22
Our data is in line with a study from Matsuo et. al., who examined 157 patients with resected HC, and showed that LN positivity and worse tumor grade imparted significantly decreased survival.4 Our demonstration of decreased OS and RFS for patients with positive LNs is consistent with many prior studies.4,7,9,10 Matsuo et. al. also linked concomitant hepatectomy with improved survival for HC, although this was not supported by our data.4
Of note, R0 resection was not an independent predictor of improved survival on multivariate analysis. This contrasts with prior reports.3,4,10 However, it is consistent with other data that demonstrated no appreciable independent benefit with R0 resection.7,20,21 Our lack of benefit for R0 resection may be due to the relatively high use of AT, which was more likely to be used after an R1 resection. In addition, our rate of node positive resection for the cohort was 37%, which is comparable to several prior studies, but is significantly greater than others, such as the retrospective study by Kang et. al. that reported a 25% LN positivity rate.7,8,10
Our study is limited by its retrospective nature. Although we strived to created concise definitions across the member institutions that would easily allow translation from each hospital system, we acknowledge that there are cases where the coded data would likely differ depending upon the reviewer. In addition, we recognize that the 15 year collection period over multiple centers leads to considerable patient and treatment heterogeneity. Although we acknowledge these drawbacks to our dataset, the relatively large number of patients included in our study, in combination with the fact that 2000-2015 represents a more modern cohort than most prior Western studies, we do feel that our analysis fills a significant gap in the literature.13,22 Our data also lacks significant detail regarding chemotherapy regimens (see Figure 1), with only 34% of the patients receiving chemotherapy in our database having a defined regimen. This is a function of the limited granularity afforded by our retrospective database. Although we feel that these missing 66% of regimens from 10 academic centers from 2000 to the present likely represent variations of fluorouracil/gemcitabine based therapy, we are unable to make strong specific recommendations. Our conclusions are strengthened by our propensity matched analysis, and although the number of patients was insufficient to match for all preoperative variables, we were able to match for all variables that were significant in our multivariate model.
NCCN guidelines make no definitive AT recommendations for resected HC, but rather comments on observation versus fluoropyrimidine based chemoradiation versus gemcitabine or fluoropyrimidine based chemotherapy.12 For advanced or metastatic disease, recent data demonstrated a survival benefit with gemcitabine plus oxaliplatin as compared to fluorouracil (4.6 vs. 9.5 month, for fluorouracil and gemcitabine plus oxaliplatin groups, respectively), and a pooled analysis of clinical trials from 2007 also supported the combination of gemcitabine plus platinum based chemotherapy.23,24 Gemcitabine plus cisplatin has a category 1 recommendation per NCCN guidelines for advanced/metastatic disease.12 Our data supports the use of AT after resection of node positive HC, and with the adoption of updated chemotherapy regimens we are hopeful for future improvement in survival.
Our multi-institutional study of 249 patients who underwent resection for HC, shows an association of AT and improved survival both on multivariate and propensity matched analysis, with the effect limited to those with node positive disease. It is likely that with the discovery of more active regimens in HC, AT will be more effective and incorporated into future treatment protocols.25–27
Supplementary Material
Synopsis.
Adjuvant therapy is an independent predictor of improved survival in resected hilar cholangiocarcinoma. Adjuvant therapy should be strongly considered after curative-intent resection for hilar cholangiocarcinoma, particularly in patients with node positive disease.
Acknowledgments
Funding: No funding sources to report
Footnotes
Invitation to Publish: Not invited
Conflict of Interest No conflicts of interest to report
References
- 1.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 2.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Annals of Surgery. 1996;224(4):463–73. doi: 10.1097/00000658-199610000-00005. discussion473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of Surgery. 2001;234(4):507–17. doi: 10.1097/00000658-200110000-00010. discussion517–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215(3):343–355. doi: 10.1016/j.jamcollsurg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey J-N. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17(8):691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 7.Kang MJ, Jang J-Y, Chang J, et al. Actual Long-Term Survival Outcome of 403 Consecutive Patients with Hilar Cholangiocarcinoma. World J Surg. 2016 May;:1–9. doi: 10.1007/s00268-016-3551-9. [DOI] [PubMed] [Google Scholar]
- 8.Lim K-H, Oh D-Y, Chie EK, et al. Adjuvant concurrent chemoradiation therapy (CCRT) alone versus CCRT followed by adjuvant chemotherapy: which is better in patients with radically resected extrahepatic biliary tract cancer?: a non-randomized, single center study. BMC Cancer. 2009;9(1):345. doi: 10.1186/1471-2407-9-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Han S-S, Park S-J, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853–e859. doi: 10.1016/j.ijrobp.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18(3):651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 11.Cereda S, Belli C, Reni M. Adjuvant treatment in biliary tract cancer: to treat or not to treat? World J Gastroenterol. 2012;18(21):2591–2596. doi: 10.3748/wjg.v18.i21.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson Al B. NCCN Clinical Practice Guidelines in Oncology, Hepatobiliary Cancers. 2016 Jun;:1–121. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazer ES, Liu P, Abdalla EK, Vauthey J-N, Curley SA. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg. 2012;16(9):1666–1671. doi: 10.1007/s11605-012-1935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 15.Edeline J, Bonnetain F, Phelip JM, Watelet J. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. San Francisco: http://meetinglibrary.asco.org/print/2547036. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 2012;53(282):457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 17.Cox DR. Breakthroughs in Statistics. New York, NY: Springer New York; 1992. Regression Models and Life-Tables; pp. 527–541. Springer Series in Statistics. [DOI] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL. AJCC Cancer Staging Manual. 7th. New York: Springer Inc; 2010. [Google Scholar]
- 19.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 20.Park J-H, Choi EK, Ahn SD, et al. Postoperative chemoradiotherapy for extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2011;79(3):696–704. doi: 10.1016/j.ijrobp.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33(24):2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15(11):3147–3156. doi: 10.1245/s10434-008-9998-7. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28(30):4581–4586. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
- 24.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Löffler MW, Anoop Chandran P, Laske K, et al. Personalized peptide vaccine induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016 Jul; doi: 10.1016/j.jhep.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohal DPS, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol. 2013;24(12):3061–3065. doi: 10.1093/annonc/mdt416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


