Abstract
Stress granules are dynamic, conserved RNA-protein (RNP) assemblies that form when translation is limiting; and are related to pathological aggregates in degenerative disease. Mammalian stress granules are comprised of two structures – an unstable shell and more stable cores. Herein we describe methodology for isolation of stress granule cores from both yeast and mammalian cells. The protocol consists of first enriching for stress granule cores using centrifugation and then further purifying stress granule cores using immunoprecipitation. The stress granule core isolation protocol provides a starting point for assisting future endeavors aimed at discovering conserved RNA regulatory mechanisms and potential links between RNP aggregation and degenerative disease.
Keywords: Stress granule, RNP granule, Purification, Liquid-liquid phase separation
1. Description of theoretical basis and framework for the technique
Stress granules are conserved RNA-protein (RNP) assemblies that form when translation initiation is impaired [3]. Mammalian and yeast stress granules are comprised of both RNA and protein, with approximately half of proteins that localize to stress granules containing RNA-binding activity [6]. The presence of RNA is thought to be a critical stress granule scaffold as trapping mRNA in translation elongation impairs stress granule formation [2,3,7]. In addition, some proteins which localize to stress granules contain intrinsically disordered regions (IDR) which could promote physical protein-protein interactions and contribute to stress granule assembly [5,7,8,9,10,11,13]. Stress granules are comprised of a dense network of physical interactions and stress granule composition can change in response to different stressors [1,4,6]. Therefore, a broader understanding of stress granule composition is likely provide insights into RNP granule formation and RNA regulation.
Stress granules are dynamic structures which readily exchange components with the surrounding cytosol [3,8]. Mammalian stress granules are comprised of at least two phases: a dynamic phase separated shell that readily exchanges with the surrounding cytosol, and more stable RNP cores [6]. In contrast, yeast stress granules are largely comprised of a core RNP assembly, possibly with a proportionally smaller phase separated shell [6]. In both yeast and mammalian cells, stress granule cores form early during stress granule assembly suggesting these core complexes may provide the specific set of interactions necessary for seeding formation of a higher order liquid-like stress granule shell [12].
Purification of stress granules has been a major challenge in the field due to the dynamic and transient nature of stress granule shells. Recently, we established a protocol aimed at isolating the more stable stress granule core from both yeast and mammalian cells [6]. Consistent with these complexes being related to stress granules, we observe both yeast and mammalian stress granule cores are only observed under stress conditions and contain known stress granule components. Isolation of stable stress granule cores allowed for the identification of several novel members of the yeast and mammalian stress granule proteome.
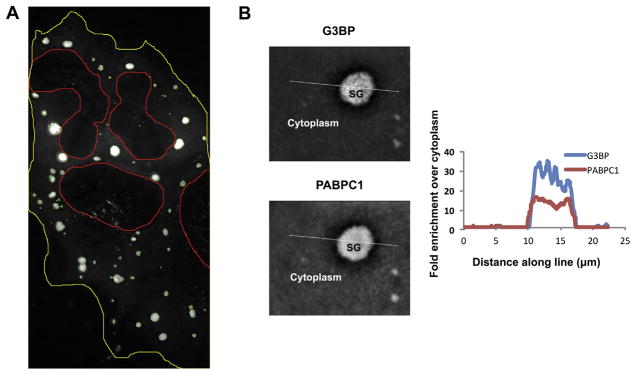
Here, we provide a detailed description of the stress granule core isolation protocol for both yeast and mammalian cells. A critical step in this protocol is to first enrich for large complexes prior to affinity purification. Although, components of stress granules enrich into stress granules, the majority of these proteins remain freely distributed throughout the cytosol during stress. For example, we estimate only 18% of G3BP1 is enriched into stress granules in U-2 OS cells during arsenite stress (Fig. 1A). Similarly, we estimate the partition coefficient of PABPC1 into stress granules is ~3X lower than that of G3BP (as assessed by SIM analysis (Fig. 1B). Since we have found free stress granule components are more efficiently selected in immunopurifications (data not shown), to avoid this free pool and purify stress granules, larger stress granule assemblies must first be enriched.
Fig. 1.
Quantification of percentage of G3BP and PABPC1 in granules. (A) Example of the quantification of percent of G3BP in granules taken from multiple U-2 OS cells expressing G3BP-GFP. Yellow line represents cytoplasm boundary. Red line represents boundary of the nucleus. Green lines represent stress granule boundary. Fraction of total intensity of GFP (G3BP) in stress granules was determined by comparing total intensity of all granules in image to total intensity within cell boundaries using ImageJ. (B) SIM image of the same granule imaged for G3BP (top) am PABPC1. Cytoplasm and stress granule (SG) are labeled. Graph shown alongside shows normalized quantification of intensity (left to right) along with white line shown in the image. Intensity is normalized to background subtracted average intensity in the cytoplasm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In brief, the approach used is that stress granules are first isolated from stressed cultures and enriched using centrifugation. Stress granules are further purified using immunoprecipitation with antibodies against known stress granule components. Together, this protocol provides a purified population of stress granule cores, which could be used for proteomic, transcriptomic, or biochemical experiments.
2. Yeast stress granule isolation protocol (cartooned in Fig. 2)
Fig. 2.
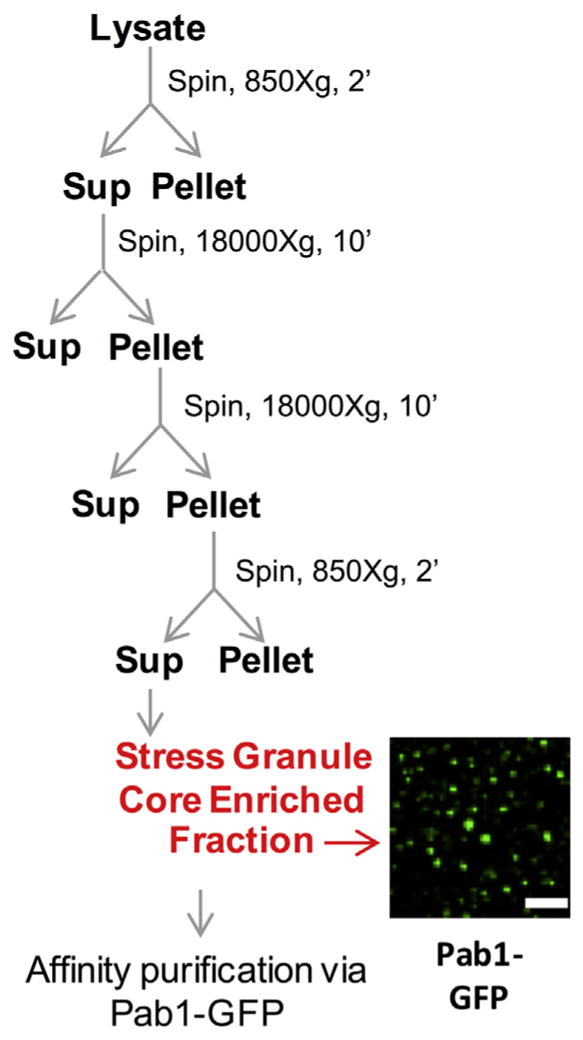
Isolation of yeast stress granule cores. Scheme for preparation of stress granule core enriched fraction from crude cell lysate from yeast cells. Image shows stress granule core enriched fraction from cells carrying Pab1-GFP.
-
-
1
Grow 1.2 L culture to log phase. Typically, we use a strain with a GFP labeled component of stress granules allowing the detection of stress granules at various steps along the protocol.
-
2
Apply stress. Stresses that induce stress granules in yeast include sodium azide, glucose deprivation, Vanillin, or heat shock [4,6].
-
3
Pellet cells 4000g, 1 min at room temperature in 50 mL falcons & freeze pellet in liquid N2.
-
1
-
a
Stopping point: Pellets can be stored at −80 °C. We routinely freeze down multiple cell pellets in advance of starting the stress granule core isolation protocol.
-
4
For each Falcon tube, re-suspend pellet in 500 μL lysis buffer on ice and transfer to 2 mL microcentrifuge tube and add glass beads (approx. 300–500 μL of glass beads). We recommend use of acid-washed glass beads (425–600 μm).
-
5
Lyse by vortexing tubes containing cell pellet mixed with glass beads for 2 min at 4 °C using a cell disruptor genie. Recover for 2 min on ice between vortexing cycles.
-
6
Repeat step 5 two additional times.
-
7
Poke hole in bottom of 2 mL microcentrifuge tube using a 18G ½ needle, heated to white hot over a flame. Place the microcentrifuge tube in a 15 mL Falcon tube and spin at 2000g, 2 min to collect lysate.
-
4
-
b
We recommend that the lysate be microscopically inspected for the presence of GFP-tagged stress granule cores to assess lysis efficiency (see note in Section 4).
-
8
Transfer supernatant from 15 mL tube to a new 1.5 mL microcentrifuge tube.
-
9
Spin 1.5 mL microcentrifuge tube at 18,000g, 10 min at 4 °C. Following spin, discard supernatant.
-
10
Re-suspend pellet in 1 mL of stress granule lysis buffer.
-
11
Spin 1.5 mL microcentrifuge tube at 14,000g, 10 min at 4 °C. Following spin, discard supernatant.
-
12
Re-suspend pellet in 50 μL stress granule lysis buffer per 1.5 mL microcentrifuge tube. To increase stress granule core yield, multiple preparations can be combined together into a new 1.5 mL microcentrifuge tube (see Section 5).
-
13
Spin 850g, 2 min at 4 °C. Transfer supernatant to new 1.5 mL microcentrifuge tube. The supernatant represents the yeast stress granule core enriched fraction.
-
8
-
c
We recommend to microscopically inspect GFP-tagged stress granule core enriched fraction. We recommend following microscopic inspection to increase the volume of the stress granule enriched faction to 470 μL stress granule lysis buffer per 1.5 mL microcentrifuge tube.
-
14
Add RNaseIN at 1:100 final dilution.
-
15
Equilibrate Dynabeads (procedure outlined in Section 4).
-
16
Mix 30 μL Dynabeads and solution from Step 14 (stress granule core enriched fraction) and rotate on nutator at room temperature for 15 min to pre-clear the stress granule enriched fraction prior to immunoprecipitation.
-
17
Remove Dynabeads using magnetic and transfer supernatant to a new 1.5 mL microcentrifuge tube.
-
18
Repeat step 17 to ensure all Dynabeads are removed prior to addition of antibody.
-
19
Add 20 μL of anti-GFP antibody and rotate on nutator at room temperature for 60 min.
-
14
-
d
Stopping point: antibody incubation can be performed overnight with nutating at 4 °C.
-
20
Spin at 14,000g for 15 min at 4 °C. This step is to remove unbound, excess antibody. Prior to spinning, more stress granule lysis buffer can be added if the volume being spun is too little. This will ensure that all antibody can be effectively removed without loosing stress granules.
-
21
Discard supernatant, re-suspend pellet in 500 μL lysis buffer and add 5 μL of RNaseIN (1:100 final dilution).
-
22
Add equilibrated 60 μL Dynabeads and mix with solution in step 21.
-
23
Bind by rotating on a nutator at room temperature for 15 min. Wash Dynabeads, 5 min per wash in lysis buffer at 4 °C. Repeat three times.
-
20
-
e
We recommend to microscopically inspect binding efficiency of GFP-tagged stress granules on Dynabeads following wash steps.
-
24
Wash 5 min in Wash Buffer 1 at 4 °C.
-
25
Wash 2 min in Wash Buffer 2 at 4 °C.
-
26
Wash 5 min in Lysis buffer at 4 °C. Repeat twice and transfer to new tube
-
24
-
f
We recommend to microscopically inspect immunoprecipitation efficiency of GFP-tagged stress granules on Dynabeads.
3. Mammalian stress granule isolation protocol (cartooned in Fig. 3)
Fig. 3.
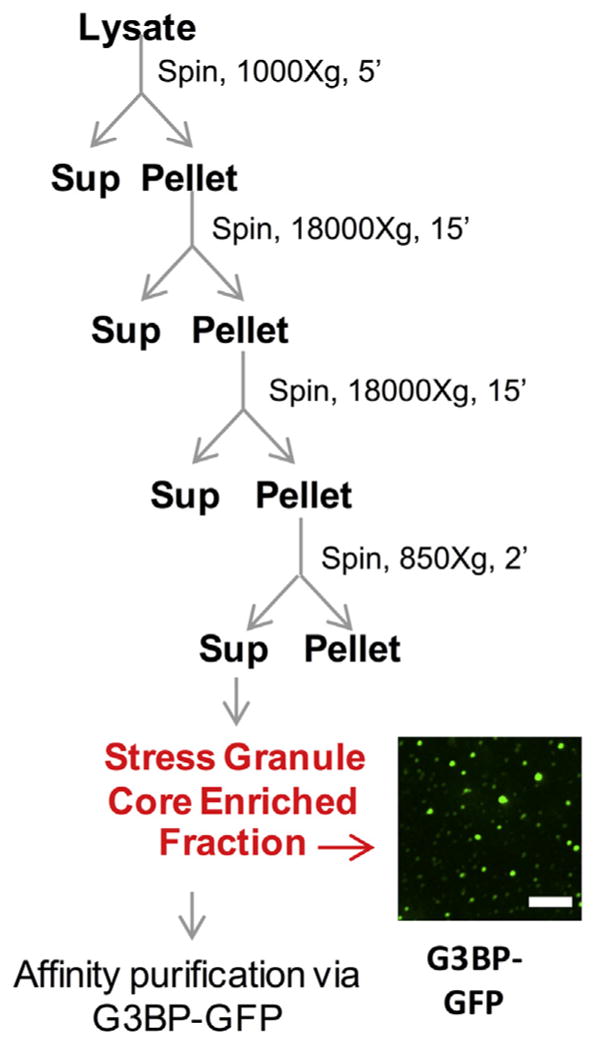
Isolation of mammalian stress granule cores. Scheme for preparation of stress granule core enriched fraction from crude cell lysate from U-2 OS cells expressing G3BP-GFP. Image shows stress granule core enriched fraction from cells carrying G3BP-GFP.
-
-
1
Plate U-2 OS expressing G3BP1-GFP at desired volume/density and grow overnight. Traditionally, we recommend isolating stress granule cores from cells that are 80–90% confluent on the day of harvest. Further, culturing cells in 15 cm tissue-culture dishes is recommended as this makes harvesting of cells more efficient (see note in Section 5).
-
2
Exchange media 1 h before stress with fresh media. We have found this step improves reproducibility in stress induction.
-
3
Apply stress. Stresses that induce stress granules in mammalian cells include but are not limited to sodium arsenite, glucose deprivation, ER-stress, osmotic stress, proteasome inhibition, heat shock [3].
-
4
Aspirate media, wash with fresh media, and add 5 mL of complete media. We recommend avoiding washing in PBS as PBS can induce stress granule formation if left on harvested cells for prolonged periods (see note in Section 5).
-
5
Scrape cells and collect into a 50 mL Falcon tube.
-
6
Pellet cells at 1500g, 3 min at room temperature. Note, all washing and pelleting steps should be performed in less than 10 min to avoid formation of visible stress granule cores.
-
7
Aspirate media and flash freeze pellet with liquid N2. Pellets can be stored at −80 °C. Of note, for mammalian cells, we routinely combine and snap freeze 5 × 15 cm plates for a single pellet.
-
1
-
a
Stopping point: We recommend growing and harvesting cells in advance of starting the stress granule core isolation protocol.
-
8
Thaw pellet on ice for 5 min.
-
9
Re-suspend in 1 mL of stress granule lysis buffer.
-
10
Syringe lysis with 25G 5/8 needle on ice. 5 passages through needle are normally sufficient to lyse cells.
-
8
-
b
We recommend to microscopically inspect for presence of GFP-tagged stress granule cores to assess lysis efficiency (see note in Section 4).
-
11
After lysis, spin at 1000g, 5 min at 4 °C to pellet cell debris. Discard pellet and transfer supernatant to 1.5 mL microcentrifuge tube.
-
12
Spin 18,000g, 20 min at 4 °C. Discard supernatant.
-
13
Re-suspend pellet in 1 mL of stress granule lysis buffer, spin 18,000g, 20 min at 4 °C. Discard supernatant.
-
14
Re-suspend pellet in 300 μL stress granule lysis buffer.
-
15
Spin 850g, 2 min at 4 °C. Transfer supernatant to new 1.5 mL microcentrifuge tube. The supernatant represents the yeast stress granule core enriched fraction.
-
11
-
c
We recommend to microscopically inspect GFP-tagged stress granule core enriched fraction.
-
16
Pre-clear stress granule enriched fraction by adding equilibrated 30 μL Dynabeads and rotating on nutator at 4 °C for 30 min. Bring volume up to 500 μL total in stress granule lysis buffer. Dynabead equilibration procedure is outlined in Section 4.
-
17
Remove Dynabeads using a magnetic and transfer supernatant to a new 1.5 mL microcentrifuge tube
-
18
Repeat step 17 to ensure all Dynabeads are removed prior to addition of antibody.
-
19
Add 20 μL of anti-GFP antibody and rotate on nutator at 4 °C for 1hr.
-
16
-
d
Stopping point: antibody incubation can be performed overnight with nutating at 4 °C.
-
20
Spin 14,000 RPM, 20 min at 4 °C. Discard supernatant to remove any unbound antibody. Prior to spinning, more stress granule lysis buffer can be added if the volume being spun is too little to ensure all antibody can be effectively removed without loosing stress granules.
-
21
Re-suspend pellet in 500 μL of stress granule lysis buffer and add RNaseIN at final dilution of 1:100.
-
22
Add 60 μL equilibrated Dynabeads to solution in step 21.
-
23
Bind for 3 h at 4 °C by rotating on nutator. Wash Dynabeads 5 min in stress granule lysis buffer. Repeat 3 times.
-
20
-
e
We recommend to microscopically inspect binding efficiency of GFP-tagged stress granules on Dynabeads following initial set of washes.
-
24
Wash 2 min in wash buffer 1 at 4 °C.
-
25
Wash 5 min in wash buffer 2 at 4 °C.
-
26
Wash 5 min in stress granule lysis buffer at 4 °C. Repeat wash twice and then transfer to new tube.
-
24
-
f
We recommend to microscopically inspect immunoprecipitation efficiency of GFP-tagged stress granules on Dynabeads.
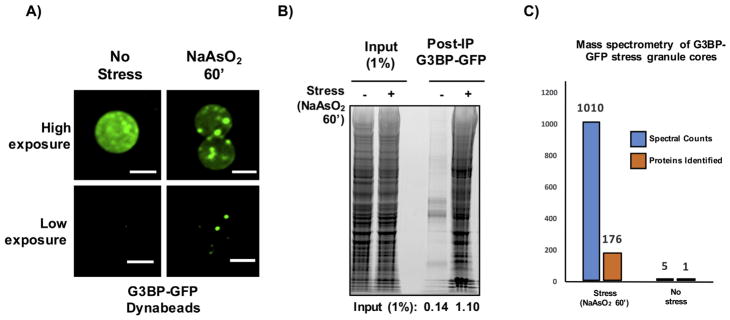
For both yeast and mammalian preparations, we recommend examining isolation efficiency and levels of background using a combination of methods including microscopic visualization, detection of proteins on SDS-PAGE gel using Sypro Ruby, and mass spectrometry (Fig. 4).
Fig. 4.
Expected results for mammalian stress granule core isolation. A) Representative images of Dynabeads following immunoprecipitation of G3BP-GFP using anti-GFP antibody. B) SYPRO Ruby staining following affinity purification of G3BP-GFP stress granule cores. Quantification is normalized to input and corrected for background using ImageJ. C) Mass spectrometry results of G3BP-GFP stress granule cores. Scale bars represent 2 μm.
4. Discussion of equipment
Table 1 provides details for recommended select reagents and equipment required for stress granule core isolation.
Table 1.
Equipment and catalog numbers used during stress granule core isolation.
| Recommended lab equipment | |||||
|---|---|---|---|---|---|
| Yeast and mammalian culture equipment | |||||
| Cell disruptor genie | |||||
| Table top centrifuge (4 °C, max speed ≥18,000g) | |||||
| Widefield or confocal microscope | |||||
| 18 × 18-1.5 microscopy coverslips | |||||
| Reagent | Company | Catalog number | |||
|
| |||||
| Glass beads acid-washed 425–600 μM | Sigma-Aldrich | G8772-1KG | |||
| Dynabeads protein A | Thermo Fisher | 10002D | |||
| anti-GFP rabbit IgG fraction | Life Technologies | A11122 | |||
| RNasin plus RNase inhibitor | Promega | N2615 | |||
| Antifoam B emulsion | Sigma-Aldrich | A5757 | |||
| Complete™, mini, EDTA-free Protease inhibitor cocktail | Sigma-Aldrich | 11836170001 | |||
| Reagent | Company | Catalog number | Concentration (Initial) | IP’s for downstream mass spec analysis | IP’s for non-mass spec downstream applications |
|
| |||||
| anti-GFP rabbit IgG fraction | Life Technologies | A11122 | 2 μg/μL | 0.5 μg total antibody added | 20–40 μg total antibody added |
4.1. Buffers
4.1.1. Stress granule lysis buffer
50 mM Tris HCl pH 7.4, 100 mM Potassium acetate, 2 mM Magnesium acetate, 0.5 mM DTT, 50 μg/mL Heparin, 0.5% NP40, 1:5000 Antifoam B, 1 complete mini EDTA free protease inhibitor tablet 50/mL of lysis buffer. *Add RnaseIN 1 U/μL right before lysis.
4.1.2. Wash buffer 1
Stress granule lysis buffer + 2 M Urea.
4.1.3. Wash buffer 2
Stress granule lysis buffer + 300 mM Potassium acetate.
4.2. Microscopic visualization of yeast and mammalian stress granule cores
We recommend microscopic inspection at steps indicated above during stress granule core isolation to assure the preparation is proceeding well. Microscopic inspection of stress granule cores is performed at room temperature. Representative images for yeast and mammalian stress granule core enriched fractions are provided in Figs. 2 and 3 respectively. Of note, when visualizing GFP-positive stress granule cores on Dynabeads, Dynabeads can be weakly autofluorescent in the GFP channel (Fig. 4A).
4.2.1. Protocol
Remove 4 μL stress granule prep at steps outlined above.
Spot 4 μL onto a glass slide and apply a microscope cover slip.
Invert microscope slide and allow stress granule cores to settle onto cover slip (2–3 min).
Using an oil-objective lens (we recommend use of 100×), visualize GFP-positive stress granule cores using cover slip to establish focal plane.
4.3. Equilibrating and DEPC treating Dynabeads
We recommend DEPC treating Protein A Dynabeads as we have empirically determined Protein A Dyanbeads can harbor RNases.
4.3.1. Protocol
Transfer required volume of Dynabeads for stress granule isolation to 1.5 mL microcentrifuge tube.
Aspirate storage solution from Dynabeads using magnet.
Re-suspend Dynabeads in 1 mL PBS containing 1 μL DEPC.
Mix for 1 h at room temperature with nutator.
Separate beads from solution using magnets again
Wash with 1 mL PBS, 0.05% NP40 for 5 min at 4 °C. We have found addition of 0.05% NP40 helps prevent Dynabeads sticking to the sides of microcentrifuge tubes. Sticking of Dynabeads to the side of microcentrifuge tubes can result in diminished Dynabead yield and insufficient washing.
Equilibrate Dynabeads to stress granule lysis buffer by washing with 1 mL stress granule lysis buffer. Repeat 3 times.
Re-suspend with stress granule lysis buffer + 4 μL RnaseIN
5. Troubleshooting hints
5.1. Insufficient yield of stress granule cores following isolation
5.1.1. Possible solution: Increasing starting materials
Increasing the starting amount of cells can improve yield downstream of stress granule core isolation. To increase starting material, we recommend preparing and freezing down multiple cell pellets for either yeast or mammalian cells. We recommend performing cell lysis independently and combining at stress granule core enriched fraction step before immunoprecipitation steps.
5.1.2. Possible solution: Inefficient antibody binding
We have also observed some antibodies to stress granule components are not efficient at immunoprecipitation (data not shown). A simple way to test if the epitope for a given antibody is accessible on stress granule cores is to image cores incubated with antibodies and a fluorescent secondary antibody. We recommend performing this step on stress granule enriched fractions. Including a secondary only control is useful for determining specificity of primary antibody binding.
5.2. Absence of detectable stress granule cores
5.2.1. Potential problem: Incomplete lysis
We have observed incomplete cell lysis can negatively impact stress granule core yields by “trapping” stress granules cores in poorly lysed cell debris. This is particularly true for mammalian stress granule core isolation. We have observed improved lysis without impairing stress granule core integrity using the following approaches:
Repeated freeze thaw: mammalian cell pellets are repeatedly snap frozen in liquid N2 and thawed at room temperature followed by pelleting of cell debris at 1000g, 5 min. Additional syringe lysis can improve lysis under these conditions.
Serial sonication: mammalian cell pellets are re-suspended in 5 mL stress granule lysis buffer. Pellets are sonicated on ice using 10-s pulses followed by 20 s of recovery. This is repeated twice. Following cells are recovered for 10 min on ice. The disadvantage of this approach is the user now has to use a larger volume when concentrating heavy complexes by ultracentrifugation.
Of note, for yeast, cells grown to high OD cells may require increasing buffer to cell ratio at the stage of the lysis to ensure complete lysis and removal of large cell debris.
5.2.2. Potential problem: Loss of GFP signal from cell line
As we use GFP-tagged versions of stress granule components (e.g. GFP-G3BP) to track and immunoprecipitated stress granule cores during the purification procedure, low expression of these constructs can negatively impact stress granule core isolation. We recommend checking GFP-expression of your respective cell line prior to starting the stress granule core isolation protocol. If low expression is observed (defined as less than 60% of the cells expressing your respective construct), we recommend reselecting or sorting your cell population.
5.2.3. Potential problem: Inadequate detection by microscopy
Inadequately detecting stress granule cores by microscopy can erroneously give the impression of a poor stress granule core preparation. We recommend the use of oil-objectives on either confocal or widefield microscopes and following the procedure outlined in Section 4. Also, the addition of inert beads can assist in finding the appropriate focal plane on coverslips.
5.3. Presence of stress granule cores in unstressed preparations
5.3.1. Potential problem: PBS-induced stress granule induction
In some experiments we observe prolonged incubation in PBS following cell scrapping is sufficient to induce stress granule formation. To avoid the induction of stress granules in control samples, we recommend washing cells in complete media instead of PBS and keeping washing/pelleting steps to less than 10 total minutes. Similarly, we observe fixation using 1% formaldehyde induces small stress granule induction. As most fixation protocols recommend fixing for greater than 10 min at room temperature, fixation protocols may need to be adjusted or the inclusion of additional controls or more stringent analysis may be necessitated. For example, for proteomics of granules isolated from fixed cells, we rely on increased detection (defined as greater than twofold spectral counts) of stress granule proteins to define a protein as being ‘enriched’ into a stress granule.
It is important to note, similar to mammalian cells, unstressed yeast can also be stressed during harvesting and pelleting. Prolonged sample preparation time can induce granules in unstressed cells. Therefore, we recommend rapid isolation of unstressed cells during the harvesting procedure to avoid stress granule induction.
Acknowledgments
Funding
This work was funded by NIH-F30N2093682 (J.R.W.), NIH-GM045443 (R.P.), and the Howard Hughes Medical Institute (R.P.).
We thank Sarah Mitchell and members of the Parker Lab for helpful discussions and feedback on the manuscript.
References
- 1.Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, Ivanov P. Stress-specific differences in assembly and composition of stress granules and related foci. J Cell Sci. 2017 doi: 10.1242/jcs.199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchan JR. MRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014;11:1019–1030. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchan JR, Yoon J-H, Parker R. Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae. J Cell Sci. 2011;124:228–239. doi: 10.1242/jcs.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol. 2007;179:437–449. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of Tia-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollet S, Cougot N, Wilczynska A, Dautry F, Kress M, Bertrand E, Weil D. Translationally repressed mRNA transiently cycles through stress granules during stress. Mol Biol Cell. 2008;19:4469–4479. doi: 10.1091/mbc.E08-05-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Toretsky JA, Wright PE. Assemblages: functional units formed by cellular phase separation. J Cell Biol. 2014;206:579–588. doi: 10.1083/jcb.201404124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149:1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R. Distinct stages in stress granule assembly and disassembly. eLife. 2016;5:e18413. doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, Gladfelter AS. RNA controls PolyQ protein phase transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]