Abstract
Rationale
Diurnal mechanisms are central to regulating host responses. Recent studies uncovered a novel family of mediators termed as specialized pro-resolving mediators (SPM) that terminate inflammation without interfering with the immune response. Little is known on their diurnal regulation.
Objective
Herein we investigated the diurnal regulation of SPM in humans and their role in controlling peripheral blood leukocyte and platelet activation.
Methods and Results
Using lipid mediator profiling and healthy volunteers we found that plasma concentrations of n-3 docosapentaenoic acid-derived D-series resolvins (RvDn-3 DPA) were regulated in a diurnal manner. The production and diurnal regulation of these mediators was markedly altered in patients at risk of myocardial infarct. These changes were associated with decreased 5-lipoxygenase expression and activity as well as increased systemic adenosine concentrations. We also found a significant negative correlation between plasma RvDn-3 DPA and markers of platelet, monocyte and neutrophil activation including CD63 and CD11b. Incubation of RvDn-3 DPA with peripheral blood from healthy volunteers and patients with cardiovascular disease significantly and dose-dependently decreased platelet and leukocyte activation. Furthermore, administration of RvD5n-3 DPA to apolipoprotein E deficient mice significantly reduced platelet-leukocyte aggregates, vascular thromboxane B2 concentrations and aortic lesions.
Conclusions
These results demonstrate that peripheral blood RvDn-3 DPA are diurnally regulated in humans and dysregulation in the production of these mediators may lead to cardiovascular disease.
Keywords: Vascular biology, omega-3, eicosanoids, monocyte, neutrophil, systemic inflammation, adenosine, polymorphonuclear neutrophils activation, pathogenesis, lipid mediators
Subject Terms: Biomarks, Inflammation, Mechanisms, Pathophysiology, Physiology
Introduction
Gaining an understanding of the basic mechanisms that regulate physiological responses to environmental changes is of interest since they may shed light into the ethiopathology of disease1. One basic environmental change that the body responds to is the light/dark cycle, which leads to a circadian regulation of a number of physiological functions, including leukocyte and platelet responses2,3. Disturbances to various aspects of these fundamental mechanisms are thought to be responsible for many of the diseases that afflict modern societies, including cardiovascular and metabolic disorders2–4. These conditions are characterized by a dysregulated inflammatory response, although the exact mechanisms that underlie this inflammatory state remain of interest.
Recent studies investigating the mechanisms engaged by the host to terminate ongoing inflammation uncovered a new genus of molecules, produced by leukocytes that reprogram both stromal and leukocyte responses5–10. These molecules, termed as specialized pro-resolving mediators (SPM), are produced via the enzymatic conversion of essential fatty acids, including n-3 docosapentaenoic acid (DPA), and are classified into four families: the lipoxins, resolvins, protectins and maresins8. SPM actively counter-regulate the production of pro-inflammatory mediators, including cytokines and eicosanoids, and regulate leukocyte trafficking and phenotype following both sterile and infectious challenge5–11. Furthermore, plasma SPM concentrations were recently found to reflect outcome in sepsis12 and increases plasma SPM concentrations in females are associated with improved endothelial function following challenge when compared with males13. Thus, peripheral blood SPM concentrations may provide insights into both physiological and pathological processes ongoing in the vasculature.
Given the potent vascular actions of SPM and the link between altered diurnal responses and a number of inflammatory conditions, including myocardial infarct14,15, we investigated whether systemic SPM levels were diurnally regulated. We also assessed whether the temporal regulation of SPM was associated with changes in leukocyte and platelet responses.
Methods
Detailed Methods are given in the Online Data Supplement. The authors declare that all supporting data are available within the article and its online supplementary files.
Results
Diurnal changes in peripheral blood n-3 DPA-derived SPM are regulated by acetylcholine in healthy volunteers
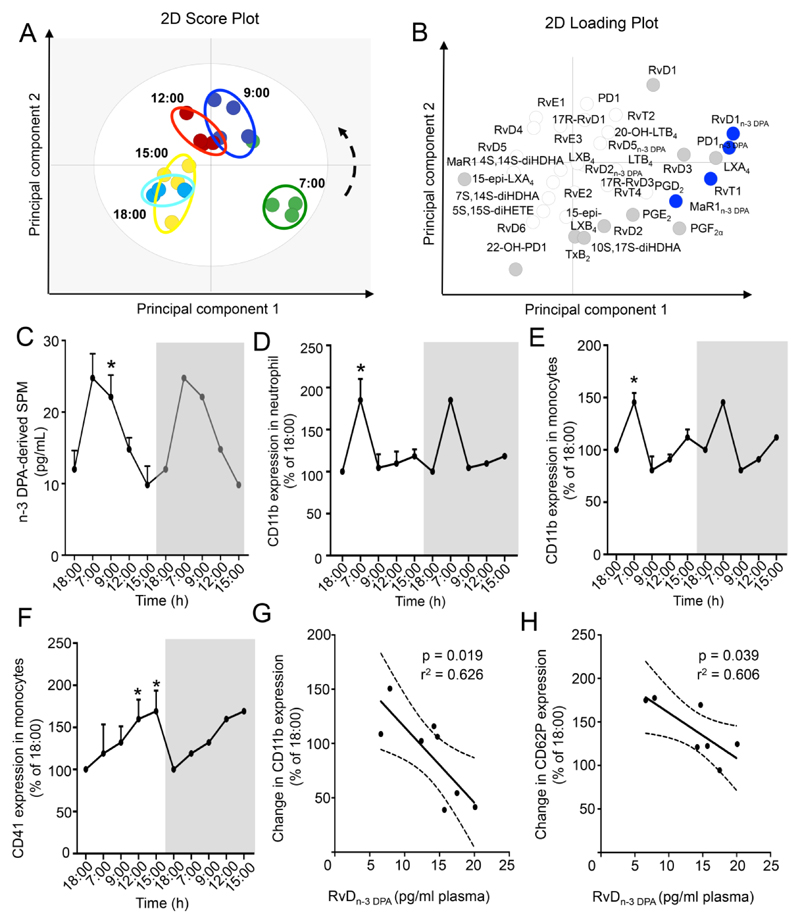
To investigate whether peripheral blood SPM concentrations are diurnally regulated we obtained plasma from healthy volunteers at distinct intervals during a 24h period (Online Table I). Here we identified mediators from all four major essential fatty acid metabolomes (Online Figure.I). Multivariate analysis of plasma lipid mediator (LM) profiles demonstrated a diurnal shift in plasma LM concentrations with a leftward shift in LM clusters from morning to evening profiles (Figure.1A,B). This shift was associated with an increase in the amounts of n-3 DPA derived mediators, including RvD1n-3 DPA and RvD5n-3 DPA from the evening (18:00h) to morning intervals (7:00 and 9:00h; 1C and Online Table II). Of note, diurnal changes in plasma RvDn-3 DPA concentrations were abrogated in mice lacking the main orchestrator of the molecular clock, BMAL1, in myeloid cells (Online Figure IG). These diurnal changes in RvDn-3 DPA were associated with a regulation of leukocyte and platelet activation that reached a maximum between 7:00 and 9:00h coincident with an increase in RvDn-3 DPA concentrations (Figure.1D-F, Online Figure.IIA). We also found a significant association between peripheral blood RvDn-3 DPA concentrations and morning leukocyte activation, where lower RvDn-3 DPA were associated with increased peripheral blood leukocyte and platelet activation (Figure.1G H and Online Figure.IIB,C).
Figure 1. Vascular n-3 DPA-derived SPM are diurnally regulated in human healthy volunteers.
Peripheral blood was collected from healthy volunteers at the indicated intervals and LM concentrations determined using LM profiling. (A) PLS-DA 2-dimensional score plot of the distinct LM-SPM profiles identified human plasma at the indicated intervals and (B) corresponding 2-dimensional loading plot. Grey ellipse in the score plots denotes 95% confidence regions. Grey and blue circles represent LM with a variable in importance score ≥ 1; n=4 healthy volunteers per interval. (C) n-3 DPA-derived SPM concentrations identified and quantified at each of the time intervals. Results are mean ± s.e.m, n = 7 per time point and expressed as pg/mL. * p ≤ 0.05 vs amounts at the 18h interval using Wilcoxon Signed Rank Test (D) Neutrophil CD11b expression. (E-F) Monocyte (E) CD11b and (F) CD41 expression. Results are mean±s.e.m, n = 7 volunteers per interval and expressed as percentage of 18:00h antigen expression. *p<0.05 vs 18:00h interval, determined using Wilcoxon Signed Rank Test. Results in the grey panel are re-plotted from the white portion to aid in visualization of rhythmicity. (G,H) Correlation between changes in monocyte (G) CD11b and (H) CD62P (9:00 to 18:00) expression and 9:00 RvDn-3 DPA concentrations. Results are representative of n=8 volunteers. Dashed line represents 95% confidence interval.
Investigations into mechanism(s) regulating peripheral blood n-3 DPA derived SPM demonstrated that plasma ACh concentrations mirrored those of RvDn-3 DPA reaching a maximum at 7:00h (Online Figure IIIA), suggesting that ACh may regulate the RvDn-3 DPA in peripheral blood given its role in SPM biosynthesis16. Incubation of whole blood with ACh increased RvDn-3 DPA concentrations, including RvD2n-3 DPA, under both static and flow conditions (Online Figure IIIB-E and; Table III); an increase that was not linked with a selective mobilization of n-3 DPA by ACh (Online Table IV). Of note, incubation of peripheral blood with norepinephrine17 (n=6 donors; 0.1-10µM) or cortisol18 (Online Table V, 1-10µM), which are both diurnally regulated in the circulation, did not significantly augment the production of n-3 DPA derived mediators in peripheral blood from healthy volunteers. In line with these findings, administration of a glucocorticoid receptor antagonist (mifepristone) or an antagonist to β1-, β2- and α1-adrenoceptors (carvedilol) to mice reduced diurnal increases in platelet-leukocyte interactions without significantly altering plasma RvDn-3 DPA (Online Figure IVA, B). Intriguingly, incubation of human peripheral blood with anti-PSGL1 antibody prior to the addition of either PAF or FMLP decreased both platelet-leukocyte aggregates and RvDn-3 DPA concentrations (Online Figure IVC). These findings suggest that RvDn-3 DPA production is regulated by ACh in peripheral blood and that platelet-leukocyte heterotypic aggregates contribute to RvDn-3 DPA production in stimulus dependent.
RvDn-3 DPA reduce leukocyte and platelet activation in peripheral blood
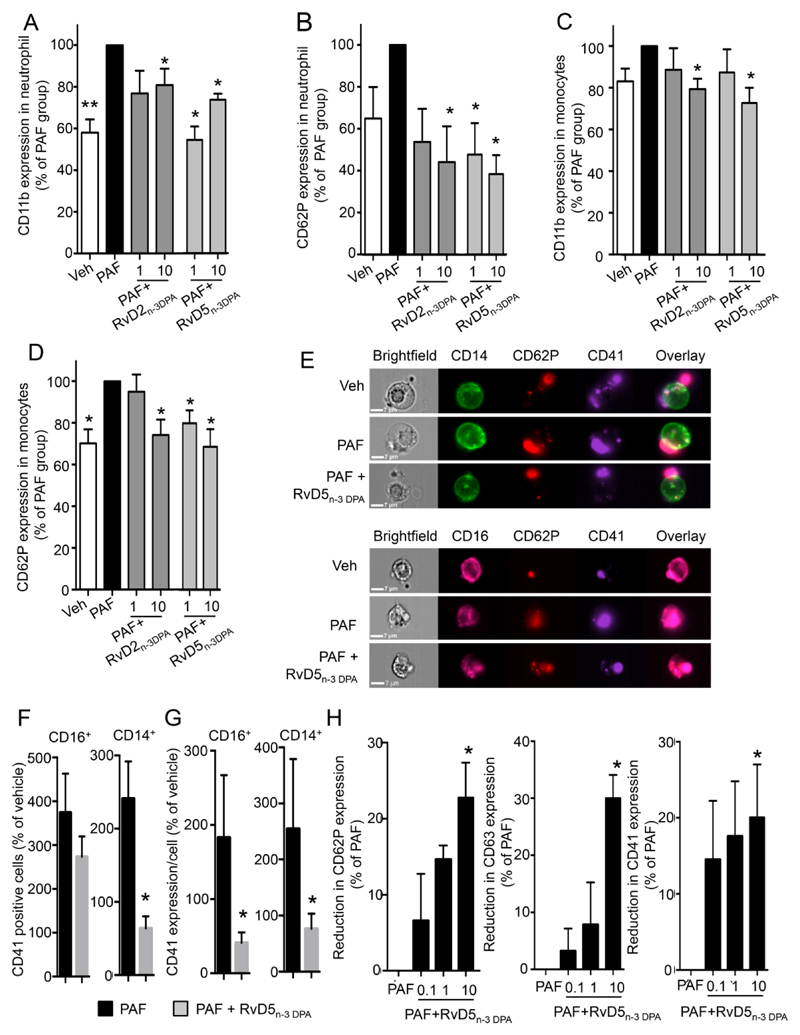
We next investigated the actions of RvDn-3 DPA in regulating monocyte, neutrophil and platelet activation as well as platelet-leukocyte aggregates. Incubation of human peripheral blood with RvD2n-3 DPA led to dose dependent decreases in neutrophil CD11b expression and in neutrophil-platelet aggregates measured as decreases in neutrophil CD62P (Figure 2A,B) and CD41 expression (n=5 donors; ~20% decrease at 10nM) when compared with cells incubated with platelet activating factor (PAF) alone. In these incubations we also found a significant reduction in monocyte activation where RvD2n-3 DPA, gave dose dependent decreases in monocyte expression of CD11b, and in the platelet markers CD62P (Figure 2C,D) and CD41 (n=5 donors; ~29% decreased at 10nM). Similar findings were also made when healthy volunteer whole blood was incubated with RvD5n-3 DPA that resulted in dose-dependent decreases in neutrophil and monocyte CD11b expression as well as in leukocyte-platelet aggregates (Figure.2). To obtain insights into the regulation of platelet-leukocyte interactions by RvDn-3 DPA we used ImageStream and found that RvD5n-3 DPA reduced both the number of leukocytes that carried platelets (i.e. CD41 positive cells) and the number of platelets tethered to each leukocyte, measured as function of the number of pixels positive for CD41 staining per cell (Figure 2E-G).
Figure 2. RvD2 n-3 DPA and RvD5n-3 DPA reduce monocyte, neutrophil and platelet activation in healthy volunteer peripheral blood.
Blood was collected from healthy volunteers and incubated with RvD2n-3 DPA, RvD5n-3 DPA (0.1nM, 1nM or 10nM) or vehicle (PBS) for 15min (37°C) then with PAF (100ng/ml; 30min; 37°C). Cell activation and leukocyte-platelet aggregates were assessed using flow cytometry. (A,B) Cumulative neutrophil (A) CD11b and (B) CD62P expression. (C,D) Monocyte (C) CD11b and (D) CD62P expression. Results are mean of n=5 per condition and expressed as percentage change from PAF incubated cells. *p<0.05, **p<0.01 compared to PAF using Wilcoxon Signed Rank Test. (E) Images depicting platelet monocyte (top panel) and (bottom panel) platelet-neutrophil aggregates. (F) Percent CD41 positive neutrophils (CD16+ cells; left panel) and monocytes (CD14+ cells; right panel). (G) Expression of CD41 on neutrophils (CD16+ cells; left panel) and monocytes (CD14+ cells; right panel). Results are representative of n=4-5 donors per group. *p<0.05 vs PAF group using Mann–Whitney test. (H) Platelet rich plasma was incubated with vehicle (PBS) or RvD5n-3 DPA 0.1-10nM (15min; 37°C) and PAF (100nM; 30min; 37°C). Adhesion molecule expression evaluated using flow cytometry. Results are mean±s.e.m. n=5 healthy volunteers. *p<0.05 compared to Vehicle group using Friedman’s test followed by Dunn’s multiple comparisons test.
We next investigated whether RvDn-3 DPA displayed direct anti-platelet actions. Incubation of RvD5n-3 DPA with platelet rich plasma (PRP) led to a dose dependent reduction in PAF mediated upregulation of CD62P, CD63 and CD41 expression (Figure 2H).
Reduced RvDn-3 DPA concentrations correlate with increased peripheral blood cell activation in CVD patients
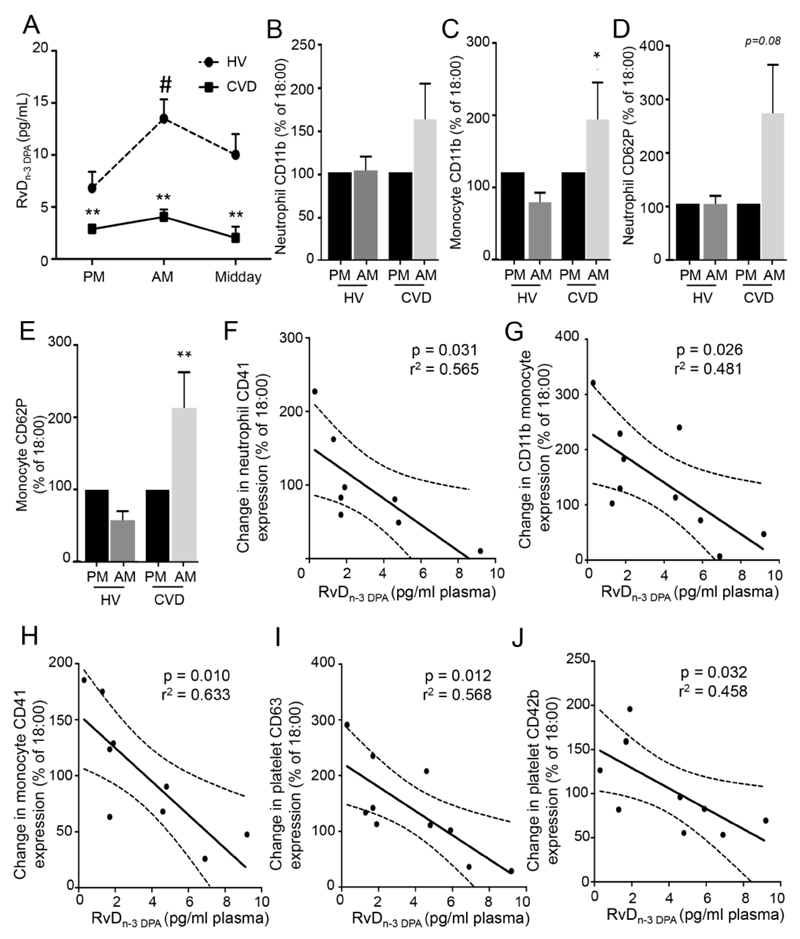
Given the role that systemic platelet and leukocyte activation plays in CVD we next assessed RvDn-3 DPA concentrations in CVD patients. Using lipid mediator profiling we found significant decreases in plasma RvDn-3 DPA concentrations and a marked impairment in their diurnal regulation in CVD patients when compared to healthy volunteers (Figure 3A, Online Table VII).
Figure 3. Systemic n-3 DPA-derived SPM are reduced and leukocyte activation is increased in patients with CVD.
Peripheral blood from patients diagnosed with cardiovascular disease (CVD) was collected at 16:00-18:00h (PM), 9:00h (AM) and 12:00 (midday) LM identified and quantified using LM-profiling (see methods for details). (A) Plasma RvDn-3 DPA concentrations. Results are mean±s.e.m. and expressed as pg/mL. n=14 CVD patients for AM, PM; n=5 for midday group and n=7 healthy volunteers (HV). *p≤0.05 and **p≤0.01 compared to respective HV group using Mann-Whitney test; # p≤0.05 vs PM values using Friedman’s test followed by Dunn’s multiple comparisons test. (B-E). Whole blood was incubated with fluorescently labeled antibodies and cell activation as well as leukocyte-platelet aggregates were assessed using flow cytometry. (B,C) CD11b expression on (B) neutrophils and (C) monocytes. (D,E) CD62P expression on (D) neutrophils and (E) monocytes. *p≤0.05, **p≤0.01 compared to HV determined using using Wilcoxon Signed Rank Test. n=5 HV and, 9 CVD patients. (F-J) Correlation between percent changes (AM to PM) in (F) neutrophil CD41 (G-H) monocyte (G) CD11b and (H) CD41 and (I-J) platelet (I) CD63 and (J) CD42b expression and 9:00h RvDn-3 DPA concentrations. Results are representative of n=8-10 patients. Dashed line represents 95% confidence interval.
Flow cytometric analysis of peripheral blood leukocyte from CVD patients demonstrated increases in the expression of CD11b on both neutrophils and monocytes from CVD patients when compared with healthy volunteers (Figure.3B-E). This was coupled with increases in platelet-neutrophil and platelet-monocyte aggregates in peripheral blood from CVD patients (Figure.3B-E). In addition, we found a significant correlation between peripheral blood RvDn-3 DPA concentration and leukocyte and platelet activation as demonstrated by a negative correlation between RvDn-3 DPA and neutrophil CD41, monocyte CD41, and platelet CD63 and CD42b expression (Figure 3F-J).
In order to establish the mechanisms leading to the downregulation of RvDn-3 DPA biosynthesis we next assessed the expression of the RvDn-3 DPA biosynthetic enzymes in peripheral blood leukocytes. Flow cytometric assessment of peripheral blood myeloid cells from healthy volunteers and CVD patients demonstrated a dysregulation in diurnal expression of SPM biosynthetic enzymes in CVD leukocytes (Figure VA-C). These findings suggest that an impaired expression of these enzymes may contribute to altered RvDn-3 DPA biosynthesis.
Elevated plasma adenosine reduces RvDn-3 DPA biosynthesis in CVD patients
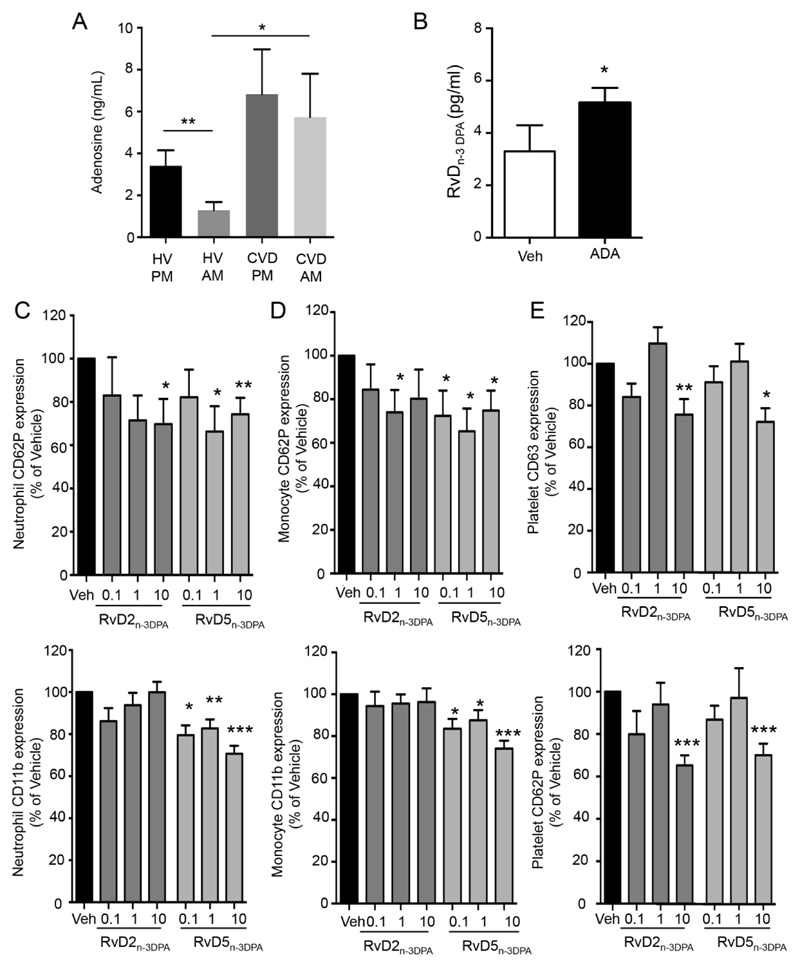
Given that we found a significant reduction in 7-HDPA concentrations in peripheral blood from CVD patients (Online Figure VIA) we investigated whether adenosine, which is known to regulate 5-LOX activity19 was elevated in plasma from these patients. LC/MS-MS analysis demonstrated an increase in the peripheral blood adenosine levels at all intervals tested when compared with healthy volunteers (Figure.4A). Incubation of peripheral blood from CVD patients with adenosine deaminase (ADA), the enzyme that degrades adenosine20, led to an increase in plasma RvDn-3 DPA concentrations (Figure.4B). We also found significant increases in plasma ACh levels and decreased 15-LOX expression in peripheral blood leukocytes in CVD patients (Online Figure V, VI). Of note, 17-HDPA concentrations, a marker of 15-LOX activity5, remained unchanged (Online Figure VIB), suggesting that increases in ACh compensated for the decreased enzyme expression.
Figure 4. Increased adenosine downregulates the concentrations of the protective RvDn-3 DPA in CVD patients.
(A) Plasma adenosine concentrations were determined using LC/MS-MS. Results are mean±s.e.m. n=7 HV and, 9 CVD patients. *p<0.05, **p<0.01 using Mann-Whitney Test. (B) Peripheral blood from CVD patients was incubated with ADA (1U; 37°C; 20min) then perfused (0.3Pa, 20min, 37°C). Plasma was collected and RvDn-3 DPA concentrations determined using LC/MS-MS based lipid mediator profiling. Results are mean ± s.e.m. n=6 donors. *p<0.05, using paired Mann-Whitney test. (C-E) Whole blood was incubated with RvD2n-3 DPA or RvD5n-3 DPA (0.1nM, 1nM or 10nM) or vehicle (PBS containing 0.01% EtOH) for 45min (37°C). Expression of (C) neutrophil CD62P and CD11b (D) monocyte CD62P and CD11b and (E) platelet CD63 and CD42P was investigated using flow cytometry and fluorescently labeled antibodies. Results are mean±s.e.m and expressed as percentage of Vehicle (Veh) incubated cells. n=8 patients per interval. * p<0.05 vs Veh group using Wilcoxon Signed Rank Test.
Reduced leukocyte activation by RvD2n-3 DPA and RvD5n-3 DPA in patient peripheral blood/
In order to test whether there was a relationship between the increased systemic inflammation and reduced n-3 DPA derived SPM we next investigated whether RvDn-3 DPA regulated patient peripheral blood leukocyte responses. RvD2n-3 DPA and RvD5n-3 DPA dose-dependently decreased platelet-neutrophil and platelet-monocyte aggregates as well as leukocyte and platelet activation (Figure.4C-E). These leukocyte and platelet directed actions were also retained when peripheral blood was incubated with PAF (Online Figure VII). Thus, these findings suggest that reductions in circulating RvDn-3 DPA lead to increased peripheral blood leukocyte and platelet activation in CVD patients.
RvD5n-3 DPA reduces systemic leukocyte and platelet activation and protects against vascular disease in ApoE-/- mice
We next investigated whether the leukocyte-directed actions of RvD5n-3 DPA were also retained in vivo. In ApoE-/- mice fed western diet for 6 weeks, RvD5n-3 DPA administration reduced circulating platelet leukocyte-aggregates and activation (Figure VIIIA,B). LC/MS-MS analysis of aortic sections from mice given RvD5n-3 DPA demonstrated distinct lipid mediator profiles when compared with mice given vehicle. Here we found increases in a number of SPM including MaR1 and 15-epi-LXA4 (Figure VIIIC and Online Table VIII). In addition, Oil red-O staining demonstrated that RvD5n-3 DPA administration reduced aortic lesions (Figure VIIID). Together these findings demonstrate that the protective actions of RvD5n-3 DPA are also retained in vivo leading to reduced vascular disease.
Discussion
In the present studies we uncovered a novel host protective mechanism centered on the diurnal regulation of systemic RvDn-3 DPA. Recent findings implicate the neuronal system in regulating tissue pro-resolving mediator biosynthesis via release of the neurotransmitter ACh, a mechanism that is central in maintaining tissue resolution tone16. Results from the present studies demonstrate that the vascular levels of this neurotransmitter in healthy volunteers are diurnally regulated and increase during the early morning hours (Online Figure III). Incubation of peripheral blood from healthy volunteers with ACh increased RvDn-3 DPA, indicating that this neurotransmitter was also involved in controlling the production of these molecules in peripheral blood. We also found that the contribution of platelet leukocyte aggregates in RvDn-3 DPA biosynthesis is stimulus dependent whereby alteration of platelet leukocyte aggregates using different strategies (Online Figure IV) did not always significantly regulate RvDn-3 DPA biosynthesis. These findings suggest that additional mechanisms are involved in regulating RvDn-3 DPA production during these cellular interactions. This is also in line with the distinct biological actions associated with these heterotypic cell aggregates 21–25 in the onset and propagation of systemic inflammation, actions that are linked with the production of a distinct mediator repertoire21–25.
Given the protective actions that RvDn-3 DPA exert in regulating peripheral blood cell responses (Figures.1-4) and tissue protection6 the marked reductions in plasma RvDn-3 DPA (~3 fold lower) during the early morning hours indicate that alterations in RvDn-3 DPA production may contribute to cardiovascular disease onset and propagation. While we cannot completely rule out an influence of the medications taken by these patients in regulating RvDn-3 DPA the following lines of evidence support a role for increased adenosine in the downregulation of this pathway in CVD patients: 1) the medications taken by these patients are not known to directly regulate either 15-LOX or 5-LOX; 2) 7-HDPA concentrations, the marker of 5-LOX activity, were significantly downregulated in peripheral blood from CVD patients; 3) 17-HDPA and 13-HDPA concentrations, markers of 15-LOX and COX activity, were not different in the morning hours, highlighting a specific loss in 5-LOX activity in CVD patients; 4) 5-LOX was significantly downregulated in human peripheral blood leukocytes; 5) adenosine, known regulator of 5-LOX activity19, was significantly increased in plasma from CVD patients; 6) incubation of patient peripheral blood with adenosine deaminase significantly upregulated (~2 fold) plasma RvDn-3 DPA concentrations. Thus, these results suggest that increases in circulating adenosine are responsible for altered peripheral blood levels of these SPM in CVD patients. These findings are also in line with findings made with peripheral blood from healthy volunteers demonstrating that reducing adenosine concentration during coagulation upregulates SPM biosynthesis20.
In summation, the present findings uncover a novel protective pathway centered on the diurnal regulation of vascular n-3 DPA-derived resolvins that is lost in patients with CVD. Thereby, strategies to restore peripheral blood RvDn-3 DPA, including n-3 DPA supplementation that was recently shown to increase plasma RvD5n-3 DPA in healthy volunteers26, may be a useful therapeutic option. In addition, RvDn-3 DPA-based therapeutics may also provide new opportunities for fine-tuning the increased inflammatory status present in these patients, dampening systemic inflammation and reducing vascular disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Disruptions in diurnal rhythms are linked with exacerbated inflammatory responses.
Specialized pro-resolving mediators (SPM) promote resolution ofinflammation and facilitate tissue repair/regeneration.
What New Information Does This Article Contribute?
Circulating concentrations of RvDn-3 DPA are regulated in a diurnal manner in healthy humans.
RvDn-3 DPA regulate platelet leukocyte activation, indicating that their increases in healthy volunteers is part of a physiological, protective, counterregulatory mechanism.
In patients with cardiovascular disease (CVD), a decrease in RvDn-3 DPA was associated with an increased plasma adenosine concentrations and platelet leukocyte activation.
Many biological processes, including peripheral blood platelet and leukocyte activation, are regulated in a diurnal manner in healthy people. In patients with CVD, this diurnal activation of peripheral blood platelets and leukocytes is associated with an increased incidence of thrombus formation and infarct in the early morning hours. The mechanisms that counterregulate this diurnal peripheral blood cell activation remain of interest. Herein, we found that RvDn-3 DPA, a novel family of protective mediators produced by white blood cells, are upregulated during peak peripheral blood cell activation in healthy volunteers and temper platelet and leukocyte responses. Of note, in patients with CVD, the production and diurnal regulation of these molecules was lost; an observation that was associated with increased platelet and leukocyte activation. These findings indicate that diurnal increases peripheral RvDn-3 DPA counterregulate physiological activation of peripheral blood cells, and disruptions in these pathways may lead to vascular disease.
Acknowledgements
The authors also thank Dr. David Collier (QMUL) for insightful discussions.
Sources of Funding
This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant number: 107613/Z/15/Z), funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant number: 677542), and the Barts Charity (Grant number: MGU0343). This work was also partly funded by MRC Advance Course Masters (Grant number: MR/J015741/1), a Wellcome Trust Infrastructure Grant (Ref 101604/Z/13/Z) and Science Foundation Ireland (SFI) Starting Investigator Research Grant 13/SIRG/2130.This work forms part of the research areas contributing to the translational research portfolio of the Cardiovascular Biomedical Research Unit at Barts, which is supported and funded by the National Institute for Health Research.
Nonstandard Abbreviations and Acronyms
- Ach
acetylcholine
- ADA
adenosine deaminase
- CVD
cardiovascular disease
- DPA
docosapentaenoic acid
- LM
lipid mediator
- Rv
resolvins
- SPM
specialized pro-resolving mediators
Footnotes
Disclosures
The authors declare no competing financial interests
References
- 1.Majno G, Joris I. Cells, tissues, and disease : principles of general pathology. ed 2nd. New York: Oxford University Press; 2004. [Google Scholar]
- 2.Ingle KA, Kain V, Goel M, Prabhu SD, Young ME, Halade GV. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol. 2015;309:H1827–1836. doi: 10.1152/ajpheart.00608.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlpine CS, Swirski FK. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ Res. 2016;119:131–141. doi: 10.1161/CIRCRESAHA.116.308034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puttonen S, Oksanen T, Vahtera J, et al. Is shift work a risk factor for rheumatoid arthritis? The Finnish Public Sector study. Ann Rheum Dis. 2010;69:779–780. doi: 10.1136/ard.2008.099184. [DOI] [PubMed] [Google Scholar]
- 5.Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21:1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017 doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang MJ, Sansbury BE, Hellmann J, et al. Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation. Circulation. 2016;134:666–680. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dona M, Fredman G, Schwab JM, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalli J, Colas RA, Quintana C, et al. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations With Survival and Clinical Outcomes. Crit Care Med. 2017;45:58–68. doi: 10.1097/CCM.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathod KS, Kapil V, Velmurugan S, et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 2017;127:169–182. doi: 10.1172/JCI89429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert K, Bernier J, Bourque-Riel V, Malick M, Rousseau G. Resolvin D1 Reduces Infarct Size Through a Phosphoinositide 3-Kinase/Protein Kinase B Mechanism. J Cardiovasc Pharmacol. 2015;66:72–79. doi: 10.1097/FJC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 15.Kain V, Ingle KA, Colas RA, et al. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity. 2017;46:92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura S, Fujitaka M, Sakura N, Ueda K. Circadian rhythms in plasma cortisone and cortisol and the cortisone/cortisol ratio. Clin Chim Acta. 1997;266:83–91. doi: 10.1016/s0009-8981(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 19.Krump E, Picard S, Mancini J, Borgeat P. Suppression of leukotriene B4 biosynthesis by endogenous adenosine in ligand-activated human neutrophils. J Exp Med. 1997;186:1401–1406. doi: 10.1084/jem.186.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris PC, Libreros S, Chiang N, Serhan CN. A cluster of immunoresolvents links coagulation to innate host defense in human blood. Sci Signal. 2017;10 doi: 10.1126/scisignal.aan1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furman MI, Barnard MR, Krueger LA, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 22.Pfluecke C, Tarnowski D, Plichta L, et al. Monocyte-platelet aggregates and CD11b expression as markers for thrombogenicity in atrial fibrillation. Clin Res Cardiol. 2016;105:314–322. doi: 10.1007/s00392-015-0922-4. [DOI] [PubMed] [Google Scholar]
- 23.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 24.Abdulnour RE, Sham HP, Douda DN, et al. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2016;9:1278–1287. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brancaleone V, Gobbetti T, Cenac N, et al. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markworth JF, Kaur G, Miller EG, et al. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 2016;30:3714–3725. doi: 10.1096/fj.201600360R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.