Abstract
Abscisic acid-pretreated carrot (Daucus carota) somatic embryos survive dehydration upon slow drying, but fast drying leads to poor survival of the embryos. To determine whether the acquisition of desiccation tolerance is associated with changes in the physical stability of the cytoplasm, in situ Fourier transform infrared microspectroscopy was used. Although protein denaturation temperatures were similar in the embryos after slow or fast drying, the extent of the denaturation was greater after fast drying. Slowly dried embryos are in a glassy state at room temperature, and no clearly defined glassy matrix was observed in the rapidly dried embryos. At room temperature the average strength of hydrogen bonding was much weaker in the rapidly dried than in the slowly dried embryos. We interpreted the molecular packing to be “less tight” in the rapidly dried embryos. Whereas sucrose (Suc) is the major soluble carbohydrate after fast drying, upon slow drying the trisaccharide umbelliferose accumulates at the expense of Suc. The possibly protective role of umbelliferose was tested on protein and phospholipid model systems, using Suc as a reference. Both umbelliferose and Suc form a stable glass with drying: They depress the transition temperature of dry liposomal membranes equally well, they both prevent leakage from dry liposomes after rehydration, and they protect a polypeptide that is desiccation sensitive. The similar protection properties in model systems and the apparent interchangeability of both sugars in viable, dry somatic embryos suggest no special role of umbelliferose in the improved physical stability of the slowly dried embryos. Also, during slow drying LEA (late-embryogenesis abundant) transcripts are expressed. We suggest that LEA proteins embedded in the glassy matrix confer stability to these slowly dried embryos.
Desiccation tolerance is the capacity of an organism or tissue to regain vital metabolism after almost complete dehydration. Seeds, pollens, resurrection plants, mosses and ferns, nematodes, tardigrades, yeasts, fungi spores, and bacteria have this capacity (for review, see Crowe et al., 1992, 1997a; Vertucci and Farrant, 1995).
Carrot (Daucus carota) somatic embryos can be rendered tolerant to severe desiccation by a proper combination of treatments (Tetteroo et al., 1994, 1995, 1996, 1998). Addition of ABA at the proper stage of development, a sufficient slow-drying time (at least 4 d), and a subtle rehydration are the main requirements for the acquisition of desiccation tolerance. Fast drying within a few hours leads to an almost complete loss of viability. These rapidly dried somatic embryos have a considerably greater leakage of K+ and soluble carbohydrates than slowly dried embryos. The excessive leakage of cytoplasmic components upon rehydration is associated with irreversible changes in the plasma membranes. Formation of irreversible protein aggregates and an increased Tm have been detected in plasma membranes isolated after rehydration of the rapidly dried embryos, which did not occur in rehydrated plasma membranes of the slowly dried embryos (Tetteroo et al., 1996). This coincided with a decreased phospholipid content and an accumulation of free fatty acids in the rapidly dried embryos. Although no differences in surface morphology have been detected between the slowly dried, desiccation-tolerant and the rapidly dried, desiccation-sensitive embryos, freeze-fracture studies indicated clear morphological differences (Tetteroo et al., 1998). Two hours after rehydration, rapidly dried embryo cells had a disorganized appearance with few organelles visible, whereas slowly dried embryo cells had many organelles visible and appeared to be structurally intact. Because of these differences, the carrot somatic embryogenesis system is very suitable to study the mechanisms that are involved in the acquisition of desiccation tolerance.
Large amounts of soluble carbohydrates have been suggested to be involved in the acquisition of desiccation tolerance (Crowe et al., 1984). In fresh carrot somatic embryos, soluble sugars, mainly Suc, may make up more than 20% of the dry weight just before drying. With fast drying the sugar composition is more or less maintained. However, with slow drying the trisaccharide umbelliferose increases at the expense of Suc, and the monosaccharides disappear almost completely (Tetteroo et al., 1994). Umbelliferose is a characteristic sugar in the Apiaceae family (Hopf and Kandler, 1976). To our knowledge, the role of this trisaccharide in seeds has not been investigated, particularly with respect to desiccation tolerance.
Sugars may act as protectants of proteins and membranes in dehydrating seeds (Crowe et al., 1987, 1992). They may form a glassy state, which immobilizes cytoplasmic components and slows down all chemical reactions, including damaging free radical reactions (Leopold et al., 1994). In general, trisaccharides are better glass formers than disaccharides or monosaccharides (Slade and Levine, 1991; Roos, 1995). This might explain why umbelliferose is synthesized at the expense of Suc upon slow drying of the embryos. Besides sugars, proteins might influence the glassy properties of the cytoplasmic matrix. Model experiments have shown that proteins interact with sugars to form a glass of higher Tg than sugars alone (Kalichevsky et al., 1993; Bell and Hageman, 1996; Wolkers et al., 1998d). Slight drying usually is perceived by seeds as a signal to synthesize specific proteins, such as dehydrins (Blackman et al., 1991, 1992; Hsing et al., 1995), that are, like sugars, thought to play a role in the stabilization of dehydrating cells. It is possible, therefore, that during drying of the carrot somatic embryos a dense, solid-like glassy network is formed consisting of carbohydrates and proteins.
Recently, we applied in situ FTIR to assess the heat stability of proteins and the glassy cytoplasmic matrix in anhydrobiotic organisms such as pollen (Wolkers and Hoekstra, 1997), seeds (Golovina et al., 1997; Wolkers et al., 1998a), and dried leaves of the resurrection plant Craterostigma plantagineum (Wolkers et al., 1998c). This can provide information about the stability of the proteins and the properties of the matrix in which the proteins are embedded. Denaturation of endogenous proteins in dry tissues can be studied because of the changes in the amide-I band profile between 1700 and 1600 cm−1. Thus, we found that proteins in maturation-defective mutant seeds of Arabidopsis that are desiccation sensitive have much less heat stability than those in wild-type seeds (Wolkers et al., 1998a). The melting of cytoplasmic glasses can be derived from in situ IR spectra, using the shifts of the OH-stretch with temperature. Additional information regarding the intermolecular interactions through hydrogen bonding in the dry state can be derived from the rate of change of νOH with temperature, the WTC. This value is a measure of the average strength of hydrogen bonding and can be used to study the molecular packing density of cytoplasmic glasses in dry cells. Using this parameter, we found in model systems that proteins have a stabilizing effect on carbohydrate glasses by increasing the average strength of hydrogen bonding in the dry state (Wolkers et al., 1998d). In maturation-defective Arabidopsis seeds the reduced strength of hydrogen bonding as compared with wild-type seeds has been attributed to the reduced synthesis of maturation-specific proteins (Wolkers et al., 1998a).
We used in situ FTIR to study the heat stability of proteins and properties of the glassy matrix in slowly dried, desiccation-tolerant, and rapidly dried, desiccation-sensitive carrot somatic embryos. To explain the desiccation tolerance of the slowly dried somatic embryos by the considerably increased umbelliferose content, we analyzed the protecting properties of purified umbelliferose in model systems, which were compared with those of Suc. The roles of umbelliferose and de novo synthesized proteins in the acquisition of desiccation tolerance are discussed.
MATERIALS AND METHODS
Production, Drying, and Germination of Somatic Embryos
Seeds of carrot (Daucus carota L.) genotypes cv RS 1 and cv Trophy were provided by Royal Sluis (Enkhuizen, The Netherlands) and by Prof. Sacco de Vries (Wageningen Agricultural University), respectively.
Somatic embryos were produced, dried, and germinated as described previously (Tetteroo et al., 1995). Slow and fast drying were carried out as follows. Approximately 1 g of the freshly harvested somatic embryos was transferred to a sterile, plastic, 9-cm Petri dish by forceps, and the embryos were equally spread over the surface. The Petri dishes were closed and placed in hygrostats (Weges and Karssen, 1987). Slow drying was achieved by exposure for 3 d each to different RHs generated by different saturated salt solutions inside of the hygrostats at 25°C in the following order: NaCl (75% RH), Ca(NO3)2 (51% RH), and CaCl2 (30% RH). Fast drying within 4 h (final water content of approximately 0.05 g water/g dry weight) was obtained by placing the Petri dishes without covers in an air-flow cabinet. The RH of the air flow was approximately 30%, as monitored with a hygroscope (±2% RH, model Hygroskop DT, Rotronic, Zürich, Switzerland). When analyzed immediately after the 4 h of drying in the air-flow cabinet, the embryos had poor survival. The rapidly dried embryos were further equilibrated over saturated CaCl2 (30% RH) at 25°C with the slowly dried embryos until analysis. Water contents of the somatic embryos were analyzed by weighing samples before and after heating at 96°C for 36 h and calculating the water loss on a dry-weight basis.
Desiccation tolerance of the dry somatic embryos was evaluated by counting the number of germinated specimens. Approximately 100 dry embryos were placed on filter paper in a sterile, plastic, 6-cm Petri dish. Before imbibition the embryos inside of the closed Petri dish were prehumidified in moisture-saturated air for 4 h to prevent possible imbibitional damage (Hoekstra et al., 1989). After this treatment, 1 mL of B5 medium (Gamborg et al., 1968) was added to the embryos (Tetteroo et al., 1995). The Petri dish was sealed with laboratory film (Parafilm, American National Can, Greenwich, CT) and placed in an incubator with a 16 h d−1 photoperiod at 25°C. Somatic embryos were recorded as desiccation tolerant when they showed clear root growth within 10 d.
Extraction and Purification of Umbelliferose
Dry somatic embryos (10 g) were boiled in 200 mL of 80% methanol for 1 h. The embryos were then filtered and washed with 50 mL of 80% methanol, and the filtrates were combined. The methanol was removed from the filtrate by vacuum evaporation, and we defatted the remaining aqueous suspension by passing it through a C18 reversed-phase column (Waters). We then purified the suspension by passing it through a column of Polyclar AT (insoluble PVP, BDH Chemicals Ltd., Poole, UK). After the volume of the suspension was reduced, it was layered onto Sephadex QAE-A-25-formate and Sephadex SP-C-25-H+ columns (Pharmacia) and eluted with ultrapure water (Milli-Q system, Millipore) according to the method of Redgwell (1980). The eluate containing the sugar fraction was freeze-dried to reduce volume. If required, the Sephadex column-purification procedure was repeated. Umbelliferose was separated from the other sugars by preparative HPLC on a Shodex OH pak Q-2002 column (20 × 500 mm; Waters) by using ultrapure water at 55°C as the eluant at 3 mL min−1 and a refractive index detector (model SP 8430, Spectra Physics, San Jose, CA). Using HPLC (analytical PA-1 column, 9 × 250 mm, Dionex, Sunnyvale, CA; see also “Carbohydrate Analysis”) we further purified the umbelliferose. The NaOH in the eluant recovered from the column was removed using an anion self-generating suppressor (4 mm, Dionex) and 50 mm H2SO4 as the regenerant, with a flow rate of 5 mL min−1. The purified umbelliferose solution was then lyophilized to be used for the FTIR measurements and to determine the response factor for the quantitative analysis of umbelliferose by HPLC. The umbelliferose preparation purified according to the above procedure was characterized by one single peak in the HPLC chromatogram. Alternatively, extractions were made starting from 50 g of seed that was ground in 80% methanol in a mortar with a small amount of sand.
Carbohydrate Analysis
Per lot of lyophilized somatic embryos, approximately 10 mg was mixed with 1 mL of 80% methanol containing 1 mg of raffinose as the internal standard. The samples were kept at 76°C in a water bath for 15 min to extract the soluble carbohydrates and to inactivate enzymes. Subsequently, the methanol was evaporated in a Speedvac (Savant Instruments, Farmingdale, NY). The samples were then suspended in 1 mL of ultrapure water. After centrifugation in an Eppendorf centrifuge, the supernatants were diluted 50 times for HPLC analysis.
Carbohydrates were separated isocratically with an HPLC system equipped with a pulsed amperometric detector and a Carbopac PA-1 4- × 250-mm column with a guard column (Dionex). Carbohydrate peaks were identified by comparing retention times of standard solutions in two different elution programs. One-hundred millimolar NaOH was used as the eluant, and 1.1 m sodium acetate in 100 mm NaOH was used to clean the column after each run. The data were analyzed using an integrator (model SP 4400, Spectra Physics) and software (Labnet, Chromdat, Spectra Physics).
IR Spectroscopy
FTIR spectra were recorded on an IR spectrometer (model 1725, Perkin-Elmer) equipped with a liquid N2-cooled mercury/cadmium/telluride detector and a microscope (Perkin-Elmer) as described previously (Wolkers and Hoekstra, 1995).
The embryos were cross-sectioned in a glove box maintained at 30% RH using a stereomicroscope. The RH was maintained by a flow of air that was previously saturated with water vapor and then led through a cooling tower to remove a fraction of the water vapor. The RH was constantly monitored by the hygroscope. Before transfer to the IR spectrometer, slices of the embryo axes were pressed gently between two diamond windows that were hermetically sealed by a rubber ring in a temperature-controlled brass cell. In this way, rehydration of the samples during the transfer and during FTIR analysis was prevented. The microscopic image of the slice was segregated by a variable knife-edge aperture. IR spectra were recorded only when the slice was sufficiently translucent for IR in the OH-stretch and -amide regions. Thus, distortion of the shape of absorption bands was prevented. Temperature of the sample in the instrument was controlled with a computer-controlled device that activated a liquid N2 pump, in conjunction with a power supply for heating of the cell. The temperature of the sample was recorded using two PT-100 elements that were near the sample windows. The instrument was purged of water vapor with a dry-air generator (Balston, Maidstone, Kent, UK).
For protein studies, the spectral region between 1800 and 1500 cm−1 was selected. This region contains the amide-I and -II absorption bands of the protein backbones. Deconvolved and second-derivative spectra were calculated using the interactive Perkin-Elmer routine for Fourier self-deconvolution. The parameters for the Fourier self-deconvolution were a smoothing factor of 15.0 and a width factor of 30.0 cm−1. Second-derivative spectra were smoothed over 19 data points. For glass studies, the broad band between 3500 and 3000 cm−1, arising from OH-stretching vibrations, was selected. A wave number-versus-temperature plot of this band was used to calculate the Tg of cytoplasmic glasses or sugars (Wolkers et al., 1998c). We have defined the point of intersection of the two lines regressed to the linear parts of the plot as Tg. The position of the symmetric CH2 stretching vibration band (lipids) around 2853 cm−1 and the C=O stretching vibration band (proteins) around 1635 cm−1 was determined from second-derivative spectra (19 data points smoothing factor). The bands were selected and normalized to unity, and the band position was calculated as the average of the spectral positions at 80% of the total peak height. This procedure allowed a precise determination of the peak position, irrespective of noise.
Preparation of Liposomes for FTIR Analysis and Leakage Studies
Egg PC in CHCl3 (Fluka) was used without further purification. After the CHCl3 was removed in a vacuum overnight, the dry egg PC was rehydrated in water at a concentration of 10 mg mL−1. When required, carbohydrates were added externally to the egg PC suspension to give a mass ratio of 5:1 (sugar:egg PC). Subsequently, we produced unilamellar vesicles by passing the suspension 35 times through one 100-nm-pore size polycarbonate filter (Nuclepore, Pleasanton, CA) as described previously (Van Bilsen et al., 1994). For IR spectroscopy, 5-μL samples were dried directly on circular CaF2 windows (13 × 2 mm) for at least 3 h in a stream of dry air at 23°C (<3% RH). Before the samples were removed from the dry-air box, another window was placed on top of the sample window, with a rubber ring in between. Hydrated liposome samples were concentrated by ultracentrifugation, and the pellet was used for FTIR analysis.
For leakage studies, the vesicles were produced at 10 mg mL−1 in 1 mm Tes, pH 7.5, containing 0.25 m Suc and 100 mm CF (Serva, Heidelberg, Germany; purified according to the method of Klausner et al. [1981]). After being passed through one 100-nm-pore size polycarbonate filter 35 times, the external Suc and CF were removed from the liposomes by gel filtration (Sephadex G-50). A typical concentration of egg PC after filtration was 3 mg mL−1. Samples of approximately 30 μg of egg PC containing different concentrations of the various sugars in a total volume of 30 μL were dried in the caps of Eppendorf tubes for 3 h in dry air (3% RH; dried to a final water content of approximately 0.02 g water/g dry weight). After rehydration of the sample in 1 mL of 1 mm Tes buffer, pH 7.5, in the closed Eppendorf tubes, the fluorescence of CF was measured, and the percentage of CF retention was calculated according to the method of Crowe and Crowe (1988). The excitation wavelength was 490 nm and the emission wavelength was 515 nm.
RNA Extraction and Northern Hybridization
For the isolation of RNA, 30 to 50 mg of embryo material was taken at 24-h intervals during slow drying, starting at 0 h, and then rapidly dried for 4 h in a flow cabinet. Subsequently, the embryos were ground with a mortar and pestle in the presence of liquid N2. Lysis of the material was done in 50 mm Tris-HCl buffer, containing 0.5 m NaCl, 50 mm EDTA, and 10 mm β-mercaptoethanol, pH 8.5. Subsequently, the mixture was homogenized in a mixture of 6% 2-butanol, 1% triisopropylnaphtalene disulfonate, 2% para-aminosalicylate, and 4% SDS and extracted with phenol:chloroform (1:1, v/v). RNA was precipitated overnight with 2 m LiCl, and poly(A+) RNA was obtained by affinity chromatography on oligo(dT)-cellulose. Poly(A+) RNA was electrophoresed on a glyoxal/DMSO gel and blotted onto a nylon membrane (Gene Screen Plus, DuPont) as described by Sambrook et al. (1989). Hybridization was done using a random-primed DNA-labeling kit (Boehringer Mannheim). The dehydrin clone B18 (Close et al., 1989) was used as the probe.
RESULTS
In Situ Protein Secondary Structures as Influenced by Drying Rate
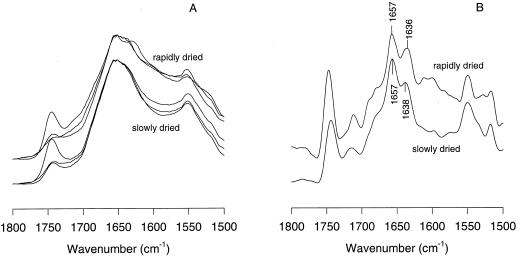
Somatic embryos grown in a Suc- and ABA-containing medium were subjected to slow and fast drying. Slowly dried embryos usually germinated for more than 90%, whereas the germination of the rapidly dried embryos varied between 0% and 30%. Figure 1A depicts the in situ IR spectra of slowly and rapidly dried somatic embryos in the 1800 to 1500 cm−1 region. The band around 1740 cm−1 in this region is attributable to ester bonds arising from lipids. The differences in peak height around 1740 cm−1 were the result of uncontrolled losses of neutral lipid during the sandwiching of the sample between the diamond windows and were not taken into consideration any further. The amide-I band around 1650 cm−1 and the amide-II band around 1550 cm−1 arise from the protein backbone (Wolkers and Hoekstra, 1995). For protein structural studies we have focussed on the amide-I band. Slight but consistent differences can be observed between the amide-I band profiles of slowly and rapidly dried somatic embryos. The slowly dried embryos had a relatively stronger absorption around 1654 cm−1 than the rapidly dried embryos. Sometimes, a clear band around 1632 cm−1 was observed in spectra of rapidly dried somatic embryos, which may be indicative of intermolecular protein clusters (denaturation) or protein breakdown.
Figure 1.
A, IR absorption spectra in the 1800 to 1500 cm−1 region of slowly and rapidly dried carrot somatic embryos. B, The spectra after deconvolution.
Because the original absorption spectra yielded rather broad bands in the amide-I region, second-derivative and deconvolution analyses were used to resolve details. Deconvolution analysis shows that the amide-I band of the slowly and rapidly dried somatic embryos is composed of two major bands located at approximately 1657 and 1637 cm−1 (Fig. 1B). The band around 1657 cm−1 can be assigned, at least partly, to α-helical structures, and the band around 1637 cm−1 can be assigned to turn and β-sheet structures (Wolkers et al., 1998b, and refs. therein). After deconvolution (Fig. 1B), the slight differences in relative band heights between spectra of the slowly and rapidly dried embryos, which were already visible in Figure 1A, were more pronounced.
Heat Stability of Endogenous Proteins
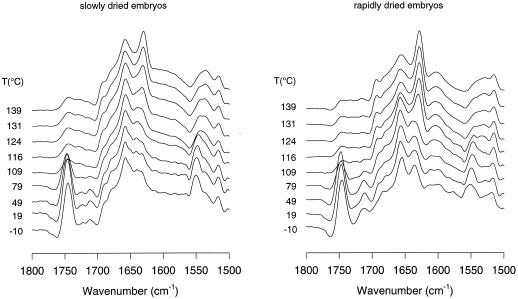
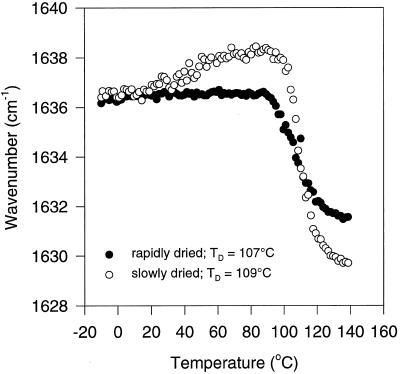
When dry, intact tissues are heated and monitored with respect to protein secondary structure, information can be obtained concerning the intrinsic heat stability of the proteins in their native environment (Wolkers and Hoekstra, 1997; Wolkers et al., 1998a). In the slowly and rapidly dried somatic embryos, the band at approximately 1637 cm−1 shifted with temperature to approximately 1630 cm−1, which is characteristic of intermolecular extended β-sheet structures (Fig. 2). This can be interpreted as protein denaturation and is irreversible, i.e. after cooling the bands did not return to their original positions. Figure 2 also shows that the peak height at 1630 cm−1 in spectra of the heat-denatured, rapidly dried embryos was more pronounced than in those of the slowly dried embryos. This indicates that the extent of protein denaturation is greater in the rapidly dried somatic embryos. The α-helical band at approximately 1657 cm−1 hardly shifted in position with temperature for both drying treatments. The heat-induced protein Td was derived from a plot of the position of the turn/β-sheet band (1637 cm−1) versus the temperature (Fig. 3). In the slowly and rapidly dried embryos, the band position sharply fell to lower wave numbers above 90°C, indicating that denaturation had begun in both types of embryos. Td values, which were derived from Figure 3 with the help of first-derivative analysis of the curve fitted through the data, were 109°C and 107°C for slowly and rapidly dried embryos, respectively.
Figure 2.
Deconvolved IR absorption spectra in the 1800 to 1500 cm−1 region of slowly and rapidly dried carrot somatic embryos as a function of temperature.
Figure 3.
Wave number-versus-temperature plots (FTIR) of the amide-I band denoting turn and β-sheet protein structures of slowly and rapidly dried carrot somatic embryos (both 0.055 g water/g dry weight). Data of four individual embryos were averaged. Td values were calculated with the help of first-derivative analysis of the curve fitted through the data.
Properties of the Dry Cytoplasmic Glassy Matrix
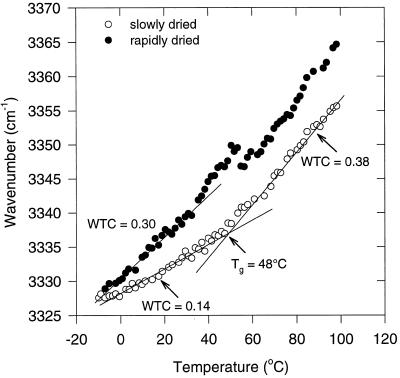
FTIR was used to study the glassy matrix in the slowly and rapidly dried somatic embryos. For this purpose, the νOH (at approximately 3330 cm−1), arising mainly from the sugar OH groups, was monitored as a function of temperature (Wolkers et al., 1998c). Two linear regression lines could be drawn in the νOH-versus-temperature plot of the slowly dried embryos with an intersection point at 48°C (Fig. 4). The temperature at the intersection point can be considered as Tg. This indicates that the slowly dried embryos are in a glassy state at room temperature. The slopes of the regression lines, the WTC values, were 0.14 and 0.38 cm−1/°C below and above Tg, respectively, and are indicative of the average strength of the hydrogen-bonding interactions. In the rapidly dried embryos no clear break in the νOH-versus-temperature plot could be observed, which indicates that there is not one defined Tg. Moreover, the WTC of 0.30 cm−1/°C in the rapidly dried embryos below 40°C was much higher than that of the slowly dried ones. The higher WTC of the rapidly dried embryos suggests a less tight hydrogen-bonding network, which is associated with a more loosely packed glassy structure in these embryos.
Figure 4.
Wave number-versus-temperature plot (FTIR) of the OH-stretching vibration band of slowly and rapidly dried carrot somatic embryos (both 0.055 g water/g dry weight). Data of four individual embryos were averaged.
Effect of Drying Rate on the Composition of Soluble Carbohydrates
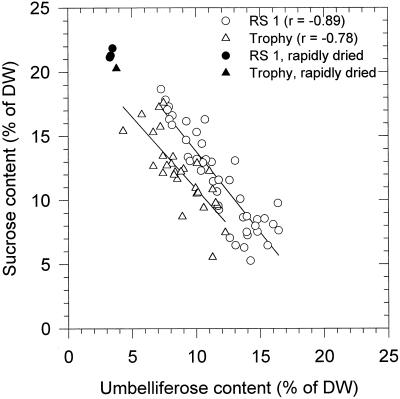
It was reported previously that slow drying leads to the accumulation of the trisaccharide umbelliferose in carrot somatic embryos (Tetteroo et al., 1994, 1995). This accumulation does not occur when the embryos are subjected to fast drying. Because umbelliferose, apart from Suc, may make up a considerable portion of the total dry weight, an important role was attributed to this trisaccharide in the stabilization of the dry cytoplasm. During protocol development for optimizing desiccation tolerance of the somatic embryos, a large number of different treatments were given. The variables in these experiments were the genotypes (cvs RS 1 and Trophy) and the concentration of added ABA and Suc in the maturation medium. Whereas omission of ABA from the maturation medium and fast drying led to reduced viability, the ABA and Suc concentrations used resulted in optimal survival of the somatic embryos. Figure 5 shows a plot of the umbelliferose against the Suc contents in each individual lot of slowly dried embryos that had germination percentages of 95% or more. The contents of Suc and umbelliferose in a few rapidly dried embryo lots of poor viability (10%–28%) are also shown. It is clear that slow drying leads to increased contents of umbelliferose. However, the amounts observed were variable, without apparent effect on desiccation tolerance. The linear regression coefficients of the lines that can be drawn through the data points for both cultivars (r = −0.89 and −0.78 for cvs RS 1 and Trophy, respectively) suggest that umbelliferose is produced at the expense of Suc. The apparent interchangeability between these sugars and the generally excellent survival after drying suggest further that Suc and umbelliferose may be equally important in relation to desiccation tolerance.
Figure 5.
Correlation between the Suc and umbelliferose contents in each lot of viable (germination percentage ≥95%), slowly dried carrot somatic embryos of the cvs RS 1 and Trophy. The treatment variables were Suc and ABA concentrations in the maturation medium. The filled symbols represent the Suc and umbelliferose content after fast drying. DW, Dry weight.
Comparison of Stabilizing Properties of Umbelliferose and Suc
In an attempt to ascribe the changes in physical stability as observed by in situ FTIR to the elevated amounts of umbelliferose in the slowly dried embryos, several properties of umbelliferose were tested in model systems and compared with those of Suc.
Glass-Forming Properties
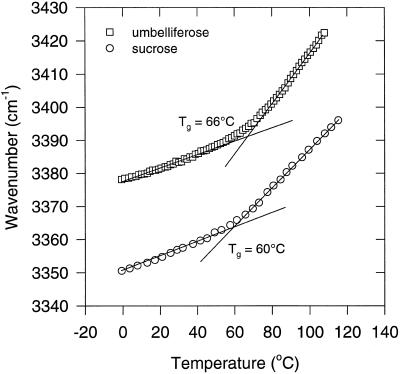
The glass-forming properties of pure umbelliferose and Suc were monitored using FTIR, as was done for the intact somatic embryos. Figure 6 shows the νOH-versus-temperature plots of umbelliferose and Suc, from which the Tg values for dry umbelliferose and Suc glasses were determined at 66°C and 60°C, respectively. Before the measurements, the samples were heated to 80°C for several minutes, in which all water detectable in the IR spectra was removed. The WTC values in the glassy state were 0.20 cm−1/°C for umbelliferose and Suc.
Figure 6.
Wave number-versus-temperature plots (FTIR) of the OH-stretching vibration band of dry umbelliferose and Suc glasses. The Tgs were determined from the intersection between the regression lines in the liquid and glassy states.
Protein Protection
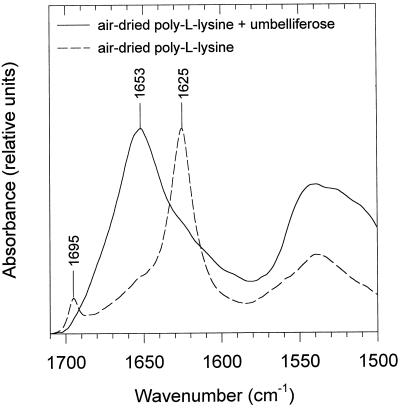
One hypothesized role of sugars in desiccation tolerance is interaction during drying with proteins, which would prevent dehydration-induced conformational changes (Carpenter and Crowe, 1989; Wolkers et al., 1998d). To study whether umbelliferose is effective in this respect, we selected poly-l-Lys, a synthetic polypeptide that undergoes structural changes with freeze-drying (Prestrelski et al., 1993) and air-drying (Wolkers et al., 1998d). As in Figure 6, the samples were heated to 80°C for several minutes before the measurements. When poly-l-Lys was air-dried in the presence of umbelliferose, a broad band at 1653 cm−1 dominated, representing a random coil structure (Fig. 7). A similar spectrum can be observed when poly-l-Lys is dried in the presence of Suc (Wolkers et al., 1998d). Without carbohydrate, drying led to absorption bands at 1625 and 1695 cm−1, which are indicative of extended β-sheet structure. In the hydrated state poly-l-Lys exists entirely in the random coil conformation (Tiffany and Krimm, 1969; Jackson et al., 1989; Wolkers et al., 1998d). The above results show that umbelliferose, similar to Suc, can prevent dehydration-induced conformational transitions of poly-l-Lys.
Figure 7.
Representative IR absorption spectra of the amide I region of poly-l-Lys (57.4 kD) air-dried (30% RH) in the presence or absence of umbelliferose.
Interactive Effect with Protein on Glassy Behavior
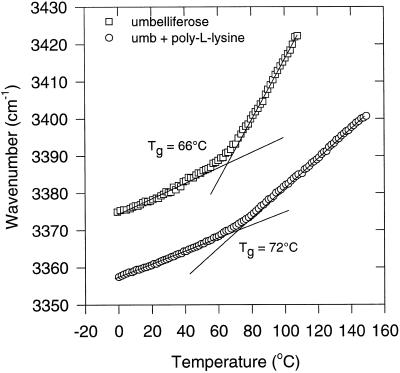
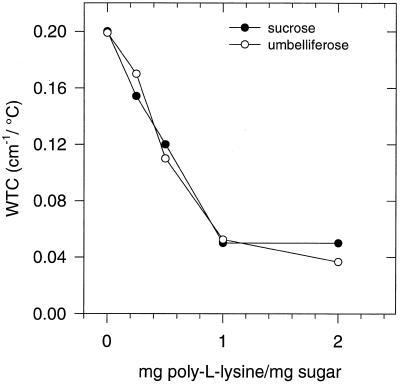
Sugars and proteins may form a tight hydrogen-bonding network when dried together, which leads to increased molecular stability and elevated Tgs. We studied the glassy behavior of dried umbelliferose/poly-l-Lys mixtures (preheated) on the basis of the temperature-dependent shifts in the positions of the OH-stretching band (Fig. 8). Poly-l-Lys is particularly suitable in this respect, because it lacks OH groups, thus, only sugar OH groups were studied. With increasing amounts of the polypeptide in the sugar polypeptide mixture, Tg increased. The WTCg decreased from 0.20 to 0.05 cm−1/°C in the range from 0 to 1 mg poly-l-Lys/mg umbelliferose, respectively (Fig. 9). The decrease in WTCg on addition of the polypeptide is interpreted to mean that poly-l-Lys directly interacts through hydrogen bonding with umbelliferose to form a tightly packed molecular network. Suc acts similarly in this respect.
Figure 8.
Wave number-versus-temperature plot (FTIR) of the OH-stretching vibration band of dry umbelliferose and of an umbelliferose/poly-l-Lys glass (0.25 mg poly-l-Lys/mg umbelliferose [umb]). Tg values were determined from the intersection points of the regression lines in the liquid and glassy states.
Figure 9.
Effect of increasing amounts of poly-l-Lys on the WTCg values in dry umbelliferose and Suc glasses. The values were calculated from wave number-versus-temperature plots as shown in Figure 8. The lsd at P = 0.05 was 0.03 cm−1/°C.
Protection of Liposomes
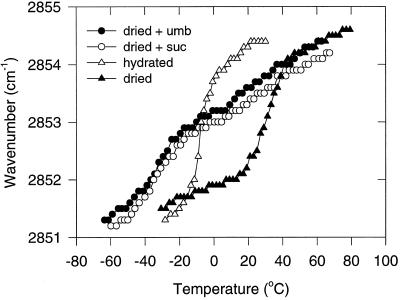
Sugars in anhydrobiotic organisms may also play a role in the protection of membranes. By interacting with the polar headgroups of phospholipids, sugars were found to depress Tm of model membranes (Crowe et al., 1992, 1996). Using FTIR we measured the position of the absorption band attributed to the CH2 symmetric stretching vibration of the acyl chains in egg PC liposomes. During the transition from the gel to the liquid crystalline phase, there is a wave number shift from 2851 to 2854 cm−1 (Hoekstra et al., 1989). Figure 10 shows that umbelliferose can prevent the dehydration-induced increase of Tm in egg PC liposomes. Whereas hydrated liposomes have a Tm at approximately −8°C and the air-dried liposomes at 32°C, the Tm of liposomes dried in the presence of umbelliferose was −35°C, far below that of the hydrated control. Suc had an identical effect on the dry liposomes. Thus, the lipid bilayer remains in the liquid crystalline phase during dehydration at 20°C, which is one of the prerequisites for the protection of liposomes in the dry state (Crowe et al., 1994, 1996, 1997b). Also, a slight shift between 10°C and 50°C could be observed for both sugars, which points to a slightly inhomogeneous interaction with the headgroups. The samples were not preheated, because this would lead to fusion of the liposomes (Crowe et al., 1997b).
Figure 10.
Representative wave number-versus-temperature plots (FTIR) of the CH2 symmetric stretch of egg PC liposomes. The different conditions were: hydrated, air-dried (3% RH), and air-dried (3% RH) in the presence of either Suc or umbelliferose (umb).
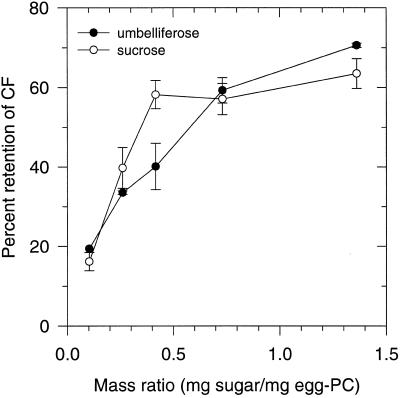
Figure 11 shows the effect of air-drying on the retention of the fluorescent label CF in egg PC liposomes that were mixed with increasing amounts of umbelliferose in a total volume of 30 μL. Umbelliferose provided retention of entrapped CF to a similar extent as Suc.
Figure 11.
Retention of trapped CF in rehydrated egg PC liposomes that were air-dried (3% RH) at room temperature for 3 h in the presence of varying amounts of umbelliferose or Suc. Data are means of triplicate leakage experiments. Error bars (±sd) are indicated when they exceed the symbol size.
Expression of a Dehydrin Transcript (B18) during Drying
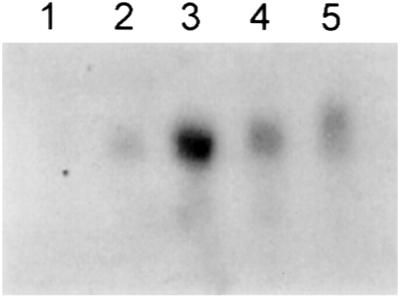
Whereas the type of sugar used has an effect on the glassy properties of the cytoplasm, proteins also can have an effect (Wolkers et al., 1998d, and refs. therein). Therefore, we extracted mRNAs regularly during slow drying to study whether there is transcription of mRNA coding for dehydration-specific proteins. The slow-drying treatments were always followed by 4 h of fast drying so that the mRNA extraction was performed on completely dehydrated embryos at all sample points. Figure 12 shows the mRNA expression of the dehydrin transcript B18 during slow drying of the somatic embryos. From this blot it can be seen that at zero time, which represents rapidly dried embryos, there is no expression of dehydrins. The expression increases during the slow drying, reaching a maximum level of expression after 48 h.
Figure 12.
Northern hybridization of RNA isolated from carrot somatic embryos with the dehydrin probe B18 (Close et al., 1989). Time course of changes in mRNA levels of B18 mRNA during slow drying of the embryos (lane 1, 0 h; lane 2, 24 h; lane 3, 48 h; lane 4, 72 h; lane 5, 96 h).
DISCUSSION
Carrot somatic embryos acquire the capacity to become desiccation tolerant by the addition of ABA to the maturation medium, but the actual tolerance requires slow drying for several days (Tetteroo et al., 1995). Fast drying within a few hours results in poor survival. Therefore, we used the carrot somatic embryo system to study the mechanisms of desiccation tolerance. In particular, we compared the macromolecular stability of slowly and rapidly dried embryos in relation to the differences in molecular composition between them.
Protein Secondary Structure
Rapidly dried somatic embryos have leaky plasma membranes, decreased phospholipid contents, elevated free fatty acid contents, and irreversible protein aggregates in their plasma membranes (Tetteroo et al., 1996). However, all of these signs of cellular breakdown were observed after rehydration of the dried embryos. Thus, postmortem phenomena might have been observed rather than primary damage due to drying. To study the primary effects of drying, it is necessary to assess the embryos in the dry state, which the development of FTIR has made possible (Crowe et al., 1984; Wolkers and Hoekstra, 1995, 1997; Wolkers et al., 1998a, 1998c).
Our first objective was to study changes in overall protein secondary structure associated with the acquisition of desiccation tolerance. We found that slow drying led to a slightly greater relative proportion of α-helical structures in the dry state. Despite this slight difference, to a large extent, the overall protein secondary structures of the slowly and rapidly dried somatic embryos resembled one another. However, in some of the rapidly dried embryos signs of protein breakdown were observed (Fig. 1). A more severe breakdown was observed after fast drying of immature maize zygotic embryos (Wolkers et al., 1998b).
The slightly greater relative proportion of α-helical structure to the overall protein secondary structure also could indicate that additional proteins are synthesized during the slow-drying treatment. In general, drying can induce the synthesis of LEA (late-embryogenesis abundant) or LEA-like proteins, for which a role in cellular protection is assumed. We show that transcripts of a gene coding for a LEA-like protein are expressed during slow drying of the somatic embryos (Fig. 12). During the fast drying, time is simply lacking for the synthesis of new proteins. Previously, we reported on an increased proportion of α-helical structures in maize embryos that acquire desiccation tolerance (Wolkers et al., 1998b). A possible explanation for such an increased α-helical content could be that newly synthesized proteins adopt an α-helical conformation in the dry state. Strikingly, a purified group III LEA-like protein from pollen adopts an α-helical structure in the dried state, although it has an unordered structure in solution (W.F. Wolkers, unpublished data). Because of the expression of LEA-like transcripts during slow drying (Fig. 12), we suggest that the observed increase in the proportion of α-helical structures in desiccation-tolerant carrot somatic and maize zygotic embryos can be attributed, at least in part, to newly synthesized LEA proteins.
Heat Stability of Endogenous Proteins
During drying, the cytoplasm of the embryos transforms into a glassy matrix, which is thought to immobilize macromolecular and cellular structures, thus providing stability (Williams and Leopold, 1989; Leopold et al., 1994). Using FTIR we were able to assess in situ the stability of endogenous proteins embedded in this dry glassy matrix. No differences in protein denaturation temperatures between rapidly and slowly dried embryos were found. However, the extent of protein denaturation was greater in the rapidly dried embryos, as was deduced from the greater relative proportion of irreversible protein aggregates (Fig. 2). Apparently, proteins are immobilized to a lesser extent in the rapidly dried embryos, which permits more heat-induced protein-protein interactions (denaturation) in the dry cytoplasmic matrix of these embryos.
Properties of the Viscous-Solid Matrix
To link the differences in heat denaturation behavior between the slowly and rapidly dried embryos to possible differences in glassy behavior, heat-induced shifts in the OH stretch were investigated. We found that slowly dried somatic embryos were in a glassy state at room temperature (Tg = 48°C) and that no clearly defined Tg could be observed for the rapidly dried embryos. Furthermore, the WTC values below 40°C were considerably higher for the rapidly dried specimens than for the slowly dried ones. This means that the average strength of intermolecular hydrogen bonding, or the molecular packing density, is greater in the slowly dried embryos than in the rapidly dried embryos. The reduced molecular packing density may account for the reduced protein stability in the rapidly dried somatic embryos. High WTC values and less protein stability were also found in desiccation-sensitive, maturation-defective mutant seeds of Arabidopsis (Wolkers et al., 1998a).
Role of Umbelliferose in Slowly Dried Carrot Somatic Embryos
Whereas Suc is the major soluble carbohydrate after fast drying (up to 20% of the dry weight), after slow drying, the trisaccharide umbelliferose accumulates at the expense of Suc up to 15% of the dry weight. During fast drying, time is lacking for the synthesis of umbelliferose. For seeds in general, the accumulation of oligosaccharides has been linked with a better long-term survival in the dry state (Horbowicz and Obendorf, 1994; Horbowicz et al., 1995). Also, in the present work elevated umbelliferose contents evoked by slow drying correlated well with improved desiccation tolerance. Particularly, the ratio of Suc to oligosaccharides is considered important in this respect rather than Suc and the oligosaccharides in their absolute amounts (Bernal Lugo and Leopold, 1995; Horbowicz et al., 1995). It was suggested that even small amounts of oligosaccharides prevent the crystallization of Suc (Caffrey et al., 1988), thus improving survival in the dry state. However, in our work the rapidly dried, desiccation-sensitive embryos still contained umbelliferose for approximately 3% of the dry weight, which may have prevented Suc crystallization.
Because of the quantitative importance of the shift in sugar composition with the acquisition of desiccation tolerance in carrot somatic embryos, we compared some protective properties of umbelliferose with those of Suc to explain the better physical stability of the slowly dried embryos.
Stabilizing Properties of Umbelliferose Compared with Suc
Both umbelliferose and Suc form a glassy state upon air-drying. The Tg of umbelliferose was slightly higher than that of Suc, 66°C compared with 60°C, respectively. Both sugars are equally effective in depressing the Tm of dry egg PC liposomes. Thus, the liposomes remain in the liquid crystalline phase during dehydration at ambient temperatures; for protection of liposomes in the dry state, this is one of the prerequisites (Crowe et al., 1994, 1996). Another prerequisite is prevention of liposome fusion, which depends on the presence of good glass-forming compounds (Crowe et al., 1997b). As discussed above, umbelliferose and Suc are examples of good glass formers. It is therefore no surprise that both sugars are able to retain CF inside liposomes after dehydration in their presence. Likewise, raffinose and stachyose have this ability but monosaccharides do not (Crowe et al., 1997b).
It has been suggested that some disaccharides have a role in the protection of proteins during drying (Carpenter et al., 1987). We tested the effect of umbelliferose and Suc on the retention of the aqueous structure of poly-l-Lys and found that both sugars are equally effective in preventing dehydration-induced conformational transitions of this polypeptide. Umbelliferose also interacts with poly-l-Lys to form a more stable glassy structure than does umbelliferose alone. In this respect, umbelliferose is equally effective as Suc (Fig. 9).
Taken together, umbelliferose does not have superior stabilizing properties when compared with Suc. This might explain the apparent interchangeability between these sugars in slowly dried embryos having greater survival of desiccation (Fig. 5). Apparently, umbelliferose and Suc are equally important in relation to desiccation tolerance. However, this does not rule out the possibility that umbelliferose adds to stability that becomes manifest during dry storage.
Possible Role of LEA Proteins versus Umbelliferose
The rapidly dried somatic embryos are different from the slowly dried embryos in that they lack dehydration-induced protein synthesis and further accumulation of umbelliferose. Such proteins may have an important impact on the glassy matrix of the dry embryos. We previously showed that LEA proteins purified from pollen increased the Tg and the molecular packing density of a Suc glass (W.F. Wolkers, unpublished data). Based on the similar physical properties of Suc and umbelliferose, we suggest that the lesser physical stability of the rapidly dried embryos is most likely due to a lack of dehydration-induced proteins rather than to a lack of accumulated umbelliferose.
ACKNOWLEDGMENTS
We thank Dr. Renske van der Veen and Sander van Hal for their help with the northern hybridization experiments. We thank Dr. Henk A. Schols (Food Science Department, Wageningen Agricultural University, The Netherlands) and Dr. Steef M. De Bruijn (Laboratory of Plant Physiology, Wageningen Agricultural University, The Netherlands) for their help with the Dionex experiments.
Footnotes
This project was financially supported by the Life Sciences Foundation, which is subsidized by the Netherlands Organization for Scientific Research.
Corresponding author; e-mail folkert.hoekstra@algem.pf.wau.nl; fax 31–317–484740.
Abbrevations: CF, 5(6) carboxyfluorescein; FTIR, Fourier transform IR spectroscopy; νOH, band position of the OH stretch; PC, phosphatidylcholine; Td, denaturation temperature (midpoint); Tg, glass transition temperature; Tm, gel-to-liquid crystalline phase transition temperature; WTC, wave number-temperature coefficient; WTCg, wave number-temperature coefficient in the glassy state.
LITERATURE CITED
- Bell LN, Hageman MJ. Glass transition explanation for the effect of polyhydroxy compounds on protein denaturation in dehydrated solids. J Food Sci. 1996;61:372–378. [Google Scholar]
- Bernal-Lugo I, Leopold AC. Seed stability during storage: raffinose content and seed glassy state. Seed Sci Res. 1995;5:75–80. [Google Scholar]
- Blackman SA, Obendorf RL, Leopold AC. Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol. 1992;100:225–230. doi: 10.1104/pp.100.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol. 1991;96:868–874. doi: 10.1104/pp.96.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M, Fonseca V, Leopold C. Lipid-sugar interactions. Plant Physiol. 1988;86:754–758. doi: 10.1104/pp.86.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JF, Crowe JH. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry. 1989;28:3916–3922. doi: 10.1021/bi00435a044. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Martin B, Crowe LM, Crowe JH. Stabilization of phosphofructokinase during air drying with sugars and sugar/transition metal mixtures. Cryobiology. 1987;24:455–464. doi: 10.1016/0011-2240(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Close TJ, Kortt AA, Chandler PM. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989;13:95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM. Factors affecting the stability of dry liposomes. Biochim Biophys Acta. 1988;939:327–334. doi: 10.1016/0005-2736(88)90077-6. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Carpenter JF, Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Carpenter JF, Prestrelski SJ, Hoekstra FA, De Araujo P, Panek AD. Anhydrobiosis: cellular adaptations to extreme dehydration. In: Dantzler WH, editor. Handbook of Physiology: Section 13, Comparative Physiology, Vol 2. Oxford, UK: Oxford University Press; 1997a. pp. 1445–1477. [Google Scholar]
- Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:570–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Nguyen KHN, Crowe LM. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim Biophys Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Leslie SB, Crowe LM. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 1994;31:355–366. doi: 10.1006/cryo.1994.1043. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Oliver AE, Hoekstra FA, Crowe LM. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology. 1997b;35:20–30. doi: 10.1006/cryo.1997.2020. [DOI] [PubMed] [Google Scholar]
- Gamborg O, Miller R, Ojima K. Nutrient requirements of suspension cultures of soybean roots cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Golovina EA, Wolkers WF, Hoekstra FA. Long term stability of protein secondary structure in dry seeds. Comp Biochem Physiol. 1997;117A:343–348. [Google Scholar]
- Hoekstra FA, Crowe JH, Crowe LM (1989) Membrane behavior in drought and its physiological significance. In RB Taylorson, ed, Recent Advances in the Development and Germination of Seeds. Plenum Press, New York, pp 71–88
- Hopf H, Kandler O. Physiologie der umbelliferose. Biochem Physiol Pflanz. 1976;169:5–36. [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols [review and survey] Seed Sci Res. 1994;4:385–405. [Google Scholar]
- Horbowicz M, Obendorf RL, McKersie BD, Viands DR. Soluble saccharides and cyclitols in alfalfa (Medicago sativa L.) somatic embryos, leaflets, and mature seeds. Plant Sci. 1995;109:191–198. [Google Scholar]
- Hsing YIC, Chen ZY, Shih MD, Hsieh JS, Chow TY. Unusual sequence of group 3 LEA mRNA inducible by maturation or drying soybean seeds. Plant Mol Biol. 1995;29:863–868. doi: 10.1007/BF00041175. [DOI] [PubMed] [Google Scholar]
- Jackson M, Haris PI, Chapman D. Conformational transitions in poly-l-lysine: studies using Fourier transform infrared spectroscopy. Biochim Biophys Acta. 1989;998:75–79. [Google Scholar]
- Kalichevsky MT, Blanshard JMV, Tokarczuk PF. Effect of water content and sugars on the glass transition of casein and sodium caseinate. Int J Food Sci Technol. 1993;28:139–151. [Google Scholar]
- Klausner RD, Kumar JN, Blumenthal R, Flavin M. Interaction of tubulin with phospholipid vesicles. I. Association with vesicles at the phase transition. J Biol Chem. 1981;256:5879–5885. [PubMed] [Google Scholar]
- Leopold AC, Sun WQ, Bernal-Lugo I. The glassy state in seeds: analysis and function. Seed Sci Res. 1994;4:267–274. [Google Scholar]
- Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF. Dehydration-induced conformational transitions in protein and their inhibition by stabilizers. Biophys J. 1993;65:661–671. doi: 10.1016/S0006-3495(93)81120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgwell RJ. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980;107:44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Roos YH. Phase Transitions in Foods. London: Academic Press; 1995. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Slade L, Levine H (1991) A food polymer science approach to structure-property relationships in aqueous food systems: non-equilibrium behavior of carbohydrate-water systems. In H Levine, L Slade, eds, Water Relationships in Food. Plenum Press, New York, pp 29–101 [DOI] [PubMed]
- Tetteroo FAA, Bomal C, Hoekstra FA, Karssen CM. Effect of abscisic acid and slow drying on soluble carbohydrate content in developing embryoids of carrot (Daucus carota L.) and alfalfa (Medicago sativa L.) Seed Sci Res. 1994;4:203–210. [Google Scholar]
- Tetteroo FAA, de Bruijn AY, Henselmans RNM, Wolkers WF, van Aelst AC, Hoekstra FA. Characterization of membrane properties in desiccation-tolerant and -intolerant carrot somatic embryos. Plant Physiol. 1996;111:403–412. doi: 10.1104/pp.111.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteroo FAA, Hoekstra FA, Karssen CM. Induction of complete desiccation tolerance in carrot (Daucus carota) embryoids. J Plant Physiol. 1995;145:349–356. [Google Scholar]
- Tetteroo FAA, van Aelst AC, Von Recklinghausen IR, Golovina EA, Hoekstra FA. Membrane permeability, morphology and desiccation tolerance of Daucus carota somatic embryos as influenced by drying rate. Protoplasma. 1998;202:202–212. [Google Scholar]
- Tiffany ML, Krimm S. Circular dichroism of the “random” polypeptide chain. Biopolymers. 1969;8:347–359. [Google Scholar]
- Van Bilsen DGJL, Hoekstra FA, Crowe LM, Crowe JH. Altered phase behavior in membranes of aging dry pollen may cause imbibitional leakage. Plant Physiol. 1994;104:1193–1199. doi: 10.1104/pp.104.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Farrant JM (1995) Acquisition and loss of desiccation tolerance. In J Kigel, G Galili, eds, Seed Development and Germination. Marcel Dekker, New York, pp 237–271
- Weges R, Karssen CM. The influence of desiccation following pretreatment on germination of lettuce seeds. Acta Hortic. 1987;215:173–178. [Google Scholar]
- Williams RJ, Leopold AC. The glassy state in corn embryos. Plant Physiol. 1989;89:977–981. doi: 10.1104/pp.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers WF, Alberda M, Koornneef M, Léon-Kloosterziel KM, Hoekstra FA. Properties of proteins and the glassy matrix in maturation-defective mutant seeds of Arabidopsis. Plant J. 1998a;16:133–143. doi: 10.1046/j.1365-313x.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Bochicchio A, Selvaggi G, Hoekstra FA. Fourier transform infrared microspectroscopy detects changes in protein secondary structure associated with desiccation tolerance in developing maize embryos. Plant Physiol. 1998b;116:1169–1177. doi: 10.1104/pp.116.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers WF, Hoekstra FA. Aging of dry desiccation-tolerant pollen does not affect protein secondary structure. Plant Physiol. 1995;109:907–915. doi: 10.1104/pp.109.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers WF, Hoekstra FA. Heat stability of proteins in desiccation tolerant cattail pollen (Typha latifolia): a Fourier transform infrared spectroscopic study. Comp Biochem Physiol. 1997;117A:349–355. [Google Scholar]
- Wolkers WF, Oldenhof H, Alberda M, Hoekstra FA. A Fourier transform infrared microspectroscopy study of sugar glasses: application to anhydrobiotic higher plant cells. Biochim Biophys Acta. 1998c;1379:83–96. doi: 10.1016/s0304-4165(97)00085-8. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Van Kilsdonk M, Hoekstra FA. Dehydration-induced conformational changes of poly-l-lysine as influenced by drying rate and carbohydrates. Biochim Biophys Acta. 1998d;1425:127–136. doi: 10.1016/s0304-4165(98)00059-2. [DOI] [PubMed] [Google Scholar]