Abstract
OBJECTIVES
To report the results of and to identify problems with implementing a screening program to detect critical congenital heart defects (CCHDs) in newborns by using differential pulse oximetry (POx).
METHODS
Charts of all live-born infants from 4 Yale–New Haven health system hospitals in Connecticut between January 1 and December 31, 2014, were reviewed.
RESULTS
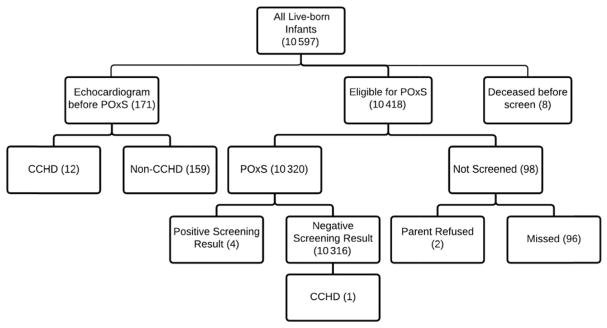
Of 10 589 newborns, 171 (1.6%) underwent an echocardiogram before screening, 10 320 (97.5%) were screened by POx, and 98 (0.9%) were not screened. Thirteen newborns (0.1%) were diagnosed with a CCHD. No infants with CCHDs were identified through POx screening (POxS) alone. Eleven (85%) were already suspected of having a CCHD lesion on the basis of prenatal ultrasound, 1 (8%) was diagnosed because of clinical concern before undergoing screening, and 1 (8%) had a false-negative screening result, but a CCHD was identified after an echocardiogram was performed because a murmur was heard. Four infants with a positive POx screen showed noncritical cardiac lesions by echocardiogram. The majority of infants were screened within the recommended 24 to 72 hours of age interval and had POx screens that were interpreted and documented correctly. Of 10 316 infants with negative POx screens, 52.1% were still in the Yale–New Haven Hospital health system at 1 year of age and no CCHD lesions were listed in their charts.
CONCLUSIONS
Although a CCHD screening program was effectively implemented, perhaps because most children with a CCHD (85%) were detected antenatally by ultrasound, in our hospital system POxS did not lead to a substantial increase in the early identification of CCHDs.
Congenital heart disease (CHD) is the most common type of birth defect, with an estimated incidence of 8 to 9 cases per 1000 births.1 Approximately 25% of children with CHD have critical CHD (CCHD), a structural defect associated with hypoxemia in the newborn period that requires surgical intervention before 1 year and, without intervention, can lead to significant morbidity and mortality.1,2 Pulse oximetry (POx) is a noninvasive and painless test that estimates the percentage of oxygenated hemoglobin in the blood and is used as a screening test for CCHD1,3 An echocardiogram is performed as a confirmatory test.1 POx screening (POxS) is expected to primarily detect lesions such as hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, dextro-transposition of the great arteries, tricuspid atresia, and truncus arteriosus.1,4 Other CCHD lesions that may be detected by POxS, but not as reliably, include coarctation of the aorta, double outlet right ventricle, Ebstein anomaly, interrupted aortic arch, severe pulmonary or tricuspid valve stenosis, and single ventricle complex.1,4
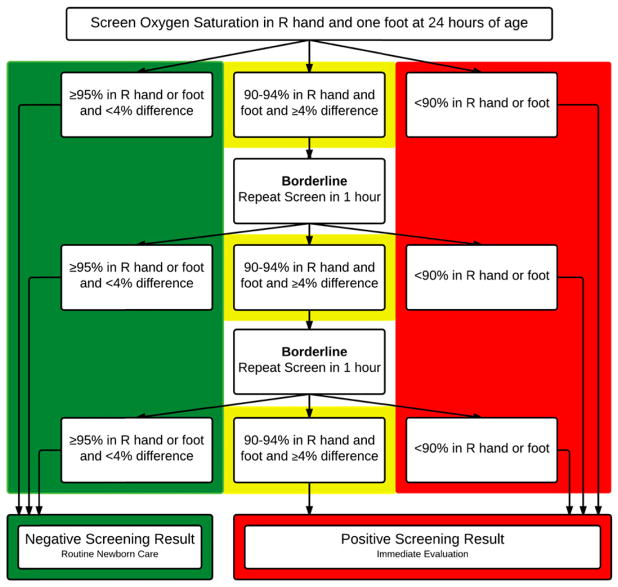
Since January of 2013, hospitals in Connecticut are required by law to screen all infants for CCHD.5 The legislation allows hospitals discretion over the type of testing to be used. CCHD screening results are not reportable to the Connecticut Department of Health, but hospitals may be audited in the event of a concern or during a routine onsite visit.5 We developed a screening program for CCHD for all newborns in 4 hospitals in the Yale–New Haven Hospital (YNHH) system. The screening protocol was adapted from an evidence-based algorithm proposed by the American Academy of Pediatrics, which is the basis for most screening protocols in newborns across the country (Fig 1).3 In the YNHH CCHD screening protocol, CCHD screening is considered complete if the infant has either POxS or an echocardiogram before hospital discharge. Infants are eligible for POxS at ≥24 hours of age and on room air. The recommended timing of POxS for CCHD is after 24 hours of age to limit false positives associated with normal variation during the newborn transition period1,6,7 but within 72 hours because screening after this time may be too late.6 For example, if there is a ductal-dependent CCHD and the duct closes, the infant may become symptomatic and decompensate before screening is performed. Minimum documentation in the electronic medical record required for each infant who undergoes CCHD screening by POx at the YNHH includes age at time of screening (hours), pre- and postductal oxygen saturation (%), interpretation of the result (positive; negative; borderline, repeat in 1 hour), and follow-up of any positive or borderline result.
FIGURE 1.
YNHH CCHD screening algorithm adapted from the American Academy of Pediatrics.10 R, right.
Screening for CCHD with POx in asymptomatic newborns has been found to increase the detection of CCHD with good sensitivity and excellent specificity in settings in which the proportion of infants in whom CCHD is detected prenatally by ultrasound is relatively low.8,9 However, the incremental benefit of screening for CCHD by POx may be lower in settings in which CCHD is frequently detected antenatally.2 The purpose of our study was to report the results of our screening program to detect CCHD in newborns and to evaluate problems with the implementation of the screening program, such as number of infants not screened, lack of follow-up of positive POx screens, screening outside of the recommended 24 to 72 hours of life, incorrect interpretation of POx results, inadequate documentation of results, and false-negative POx screens.
METHODS
We reviewed the charts of infants delivered at all 4 YNHH system hospitals between January 1 and December 31, 2014. We defined an early and late screening as those performed at <24 hours of age or at >72 hours of age, respectively.10 Live-born infants who died before CCHD screening were excluded. We reviewed the charts of infants whose CCHD screening result by POx was negative for any CCHD lesion diagnosed after hospital discharge and before 1 year of age. We included infants who remained in our electronic medical record system as evidenced by primary care, subspecialty, or emergency department visits in the first 12 months of life. The study was approved by the Yale Human Investigation Committee.
RESULTS
The characteristics of our sample are shown in Table 1. Eight live-born infants who died before CCHD screening were excluded. Of 10 589 newborns, 171 (1.6%) had an echocardiogram before screening because of either an abnormality detected on prenatal ultrasound (n = 40) or an abnormal postnatal finding (n = 131), 10 320 (97.5%) were screened by POx, and 98 (0.9%) did not undergo screening (96 were missed in error and parents refused in 2 instances). Thirteen newborns (0.1%) were diagnosed with CCHD, all of whom had an echocardiogram because of either an abnormal prenatal ultrasound (11; 85%) or a heart murmur (2; 15%). Of the 2 evaluated because of a heart murmur, 1 infant in whom the murmur was heard at <24 hours of age was found to have total anomalous pulmonary venous return and the other infant, who had had a POxS result in the NICU that was negative at day of life 10 and in whom the murmur was heard at day of life 13, was found to have coarctation of the aorta. For this newborn, although there was not an oxygen gradient (99% preductal and 100% postductal), there were decreased femoral pulses on physical examination and a ≥30 mm Hg gradient in blood pressures (right upper extremity: 99/49; left upper extremity: 85/54; left lower extremity: 67/44; and right lower extremity: 63/42). Pediatric Cardiology thought that this was a “critical” cardiac lesion because the newborn required prostaglandin and, in the absence of early detection, the newborn could have presented in cardiogenic shock. Surgical repair was performed at age 1 month.
TABLE 1.
Subject Characteristics
| Value | |
|---|---|
| Total number | 10 589 |
| Sex, n (%) | |
| Male | 5394 (51.0) |
| Female | 5195 (49.0) |
| Gestational age, n (%) | |
| Term (>37 weeks) | 9584 (90.5) |
| Late preterm (35–37 weeks) | 559 (5.3) |
| Preterm (<35 weeks) | 446 (4.2) |
| Birth weight, n (%) | |
| AGA (2500–4000 g) | 8802 (83.0) |
| LGA (>4000 g) | 980 (9.3) |
| SGA (<2500 g) | 807 (7.6) |
| Screening location, n (%) | |
| Well nursery | 9484 (89.6) |
| NICU | 1040 (9.8) |
| Inpatient pediatric unit | 65 (0.6) |
| Infant race,a n (%) | |
| White | 5556 (53.5) |
| Otherb | 2165 (20.4) |
| Black or African American | 1642 (15.5) |
| Asian | 479 (4.5) |
| Unknown | 747 (7.1) |
| Infant ethnicity,a n (%) | |
| Non-Hispanic | 7844 (74.1) |
| Hispanic or Latino | 2211 (20.9) |
| Unknown | 534 (5.0) |
AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
Infant race and ethnicity were obtained from the medical record and are as self-reported by the mother at the time of admission to the hospital.
“Other” includes those reported by the mother as “Other” as well as Native Hawaiian or Other Pacific Islander, American Indian, or Alaska Native.
No infants with CCHD were identified through POxS alone. The CCHD lesions in the other 11 infants included hypoplastic left heart syndrome (n = 3), tetralogy of Fallot (n = 2), dextro-transposition of the great arteries (n = 2), tricuspid atresia (n = 1), double outlet right ventricle (n = 2), and single ventricle complex (n = 1). Of 10 320 infants screened by POx alone, only 4 infants (0.04%) had positive POxS results, all of whom were found to have non-CCHD lesions on echocardiogram (Table 2, Fig 2).
TABLE 2.
Infants with Positive CCHD Screening by POxS
| Infant
|
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Gestational age, wk | 39 2/7 | 40 4/7 | 41 2/7 | 38 |
| Birth weight, kg | 4.14 | 4.43 | 3.32 | 3.33 |
| Age at screening, h | 25 | 27 | 31 | 24 |
| Preductal oxygen saturation, % | 93 | 84 | 99 | 99 |
| Postductal oxygen saturation, % | 100 | 95 | 89 | 86 |
| Main echocardiogram 3ndings | Small atrial septal defect, patent ductus arteriosus | Moderate–large atrial septal defect | Patent foramen ovale, patent ductus arteriosus, right ventricle dilation | Moderate tricuspid regurgitation, severely elevated right ventricle pressure |
N = 4.
FIGURE 2.
Results of the CCHD screening program.
Only 98 infants (0.9%) did not have CCHD screening documented before hospital discharge (96 were erroneously not screened and the parents of 2 infants refused screening). Four infants with positive POxS results were found to have non-CCHD lesions on echocardiogram. All 4 infants subsequently had outpatient follow-up with pediatric cardiology.
Of 10 316 infants with negative POxS at the time of birth, we were able to review postdischarge records of 52.1% (n = 5367) of them and none had evidence of CCHD at that time. Six infants had died and diagnosis at time of death was sudden unexpected infant death (n = 4), complications from Otohara syndrome (n = 1), and complications from spinal muscular atrophy type 1 (n = 1). The infant with spinal muscular atrophy type 1 had a normal echocardiogram.
Of the 10 320 infants who underwent screening, 9799 (94.9%) were screened between 24 and 72 hours. Of the other 521 infants (5.1%), 389 represented delayed screens (screened at >72 hours of age) and 132 were early screens (screened at < 24 hours of age). Most infants with POxS completed after 72 hours of age (94.1%) had been admitted to the NICU. The median age at screening for infants with delayed screens was 7 days, with a maximum of 104 days.
Incorrect interpretation of results (eg, screening results classified as normal when abnormal or vice versa) was uncommon (0.1%). Of 10 320 infants screened by POx, 6.5% (n = 635) did not have all components of minimum documentation required in the YNHH protocol.
DISCUSSION
More than 99% of newborns at our hospitals were successfully screened for CCHD. Most CCHD lesions in our study population were detected prenatally and no CCHD lesions were detected by POxS alone. Only 4 infants had positive POxS results. All 4 were found to have noncritical CHD lesions. The rate of false-positive results (0.04%) was low compared with rates (0.3–0.5%) reported in some other studies,8,9 but is similar to the false-positive rate of 0.05% reported in newborns screened after 24 hours of life,9 which is when screening was performed in our program. The infant who was diagnosed with coarctation of the aorta after a negative POxS result is an important reminder of the limitations of POxS for CCHD. Clinicians need to have a high index of suspicion for lesions such as coarctation of the aorta that may not reliably be detected by POxS.
Although none of the infants with a positive POxS had a CCHD lesion, all 4 did have non-CCHDs (Table 2). There are potential benefits of POxS in identifying non-CCHDs but yet clinically important conditions such as septal defects. Surveillance and reporting of non-CCHD lesions detected by POx will provide additional information that may expand the list of conditions targeted by POxS.11
For the majority of infants who underwent POxS, the screening was completed as recommended with few missed screens and appropriate follow-up of positive screens. Infants should be placed on room air before POxS for this screening to perform optimally. The majority of screens performed at >72 hours of life occurred in infants who had been admitted to the NICU and were receiving supplemental oxygen. Goetz et al12 found similar results in their NICU population, with 67.4% of infants screened at >48 hours due to oxygen requirements. Other studies have highlighted problems with POxS in the NICU,12–14 and this is an area that requires further study to develop an optimal screening protocol.
All components of POxS results (age at time of screening [hours], pre- and postductal oxygen saturation [%], interpretation of the result [positive; negative; borderline, repeat in 1 hour], and follow-up of any positive or borderline result) were documented in most instances. Recording the results of POxS so that it is useful to the clinician is more complex than those of hearing or metabolic screening. For example, it is important that the specific oxygen saturations are recorded and interpreted correctly because failure to do so can affect clinical decision-making.
There are some limitations to our study. We were able to conduct longer-term follow-up to see if CCHD was detected in any of the infants in only approximately half of the newborns, because many did not have records of subsequent visits in the YNHH health system through 1 year of age. However, it is likely that most (many of whom would be seeing their own pediatricians in the immediate area) would have returned to our hospital system if CCHD had been diagnosed. Of 6 infants who died, 4 deaths were due to sudden unexpected infant death. In discussions with physicians directly involved with these 4 cases, the deaths were attributed to unsafe sleep conditions.
In summary, we successfully implemented CCHD screening in our large tertiary care hospital system. Due to a high percentage of antenatal detection in our center (85%), POxS may not lead to a substantial increase in the early identification of CCHD in our population, although hospitals with lower rates of antenatal detection of CCHD may find POxS to be an important method to increase early detection of CCHD. Data on the cost-effectiveness of CCHD screening in the United States are becoming available.16 It will be important to assess the cost-effectiveness of POxS in hospitals with different rates of antenatal detection of CCHD.
Acknowledgments
FUNDING: This work was supported in part by a National Heart, Lung, and Blood Institute Medical Student Research Fellowship, National Institutes of Health (award T35HL007649; to Ms Klausner), and by grant UL1 TR001863 from the National Center for Advancing Translational Science at the National Institutes of Health and the NIH Roadmap for Medical Research. Funded by the National Institutes of Health (NIH).
Footnotes
Ms Klausner and Dr Loyal conceptualized and designed the study, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Shapiro, Elder, and Colson reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Mahle WT, Newburger JW, Matherne GP, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young; ; Council on Cardiovascular Nursing; and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery; Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124(2):823–836. doi: 10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LC, Lieberman E, O’Leary E, Geggel RL. Prenatal and newborn screening for critical congenital heart disease: findings from a nursery. Pediatrics. 2014;134(5):916–922. doi: 10.1542/peds.2014-1461. [DOI] [PubMed] [Google Scholar]
- 3.Mahle WT, Martin GR, Beekman RH, III, Morrow WR Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 4.Garg LF, Van Naarden Braun K, Knapp MM, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2) doi: 10.1542/peds.2013-0269. Available at: www.pediatrics.org/cgi/content/full/132/2/e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.State of Connecticut Department of Public Health. Senate Bill no. 56. Public Act no. 12–13. [Accessed May 23, 2016];An act concerning critical congenital heart disease screening for newborns. Available at: www.ct.gov/dph/cwp/view.asp?a=3138&q=531512.
- 6.Peterson C, Ailes E, Riehle-Colarusso T, et al. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manja V, Mathew B, Carrion V, Lakshminrusimha S. Critical congenital heart disease screening by pulse oximetry in a neonatal intensive care unit. J Perinatol. 2015;35(1):67–71. doi: 10.1038/jp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao QM, Ma XJ, Ge XL, et al. Neonatal Congenital Heart Disease Screening Group. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384(9945):747–754. doi: 10.1016/S0140-6736(14)60198-7. [DOI] [PubMed] [Google Scholar]
- 9.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 10.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5) doi: 10.1542/peds.2011-1317. Available at: www.pediatrics.org/cgi/content/full/128/5/e1259. [DOI] [PubMed] [Google Scholar]
- 11.Oster ME, Aucott SW, Glidewell J, et al. Lessons learned from newborn screening for critical congenital heart defects. Pediatrics. 2016;137(5):e20154573. doi: 10.1542/peds.2015-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz EM, Magnuson KM, Eickhoff JC, Porte MA, Hokanson JS. Pulse oximetry screening for critical congenital heart disease in the neonatal intensive care unit. J Perinatol. 2016;36(1):52–56. doi: 10.1038/jp.2015.150. [DOI] [PubMed] [Google Scholar]
- 13.Iyengar H, Kumar P, Kumar P. Pulse-oximetry screening to detect critical congenital heart disease in the neonatal intensive care unit. Pediatr Cardiol. 2014;35(3):406–410. doi: 10.1007/s00246-013-0793-2. [DOI] [PubMed] [Google Scholar]
- 14.Suresh GK. Pulse oximetry screening for critical congenital heart disease in neonatal intensive care units. J Perinatol. 2013;33(8):586–588. doi: 10.1038/jp.2012.161. [DOI] [PubMed] [Google Scholar]
- 15.Prudhoe S, Abu-Harb M, Richmond S, Wren C. Neonatal screening for critical cardiovascular anomalies using pulse oximetry. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F346–F350. doi: 10.1136/archdischild-2012-302045. [DOI] [PubMed] [Google Scholar]
- 16.Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132(3) doi: 10.1542/peds.2013-0332. Available at: www.pediatrics.org/cgi/content/full/132/3/e595. [DOI] [PMC free article] [PubMed] [Google Scholar]