Abstract
Parkinson disease (PD) related to homozygous mutations in the Pink1 gene is associated with nigrostriatal dopamine depletion and a wide range of sensorimotor deficits. In humans and animal models of PD, not all sensorimotor deficits are levodopa-responsive. We hypothesized that the underlying mechanisms of locomotion, limb control, and vocal communication behavior include other pathologies. Here, Pink1 −/− rats were treated with an oral dose of levodopa and limb motor and vocal communication behaviors were measured. Levodopa significantly improved some aspects of locomotion but did not improve ultrasonic vocalization intensity or frequency. Catecholamine concentrations in the striatum (SR), substantia nigra (SN), and locus coeruleus (LC) were analyzed to test the hypothesis that behavioral deficits would correlate to altered protein levels. There were no differences in dopamine concentrations in the SR and SN of Pink1 −/− animals compared to wildtype controls. There was a significant increase in norepinephrine concentration in the SN of Pink1 −/− animals. Moreover, an observed decrease in norepinephrine concentrations in the LC is consistent with the hypothesis that early-stage PD includes noradrenergic loss in the brainstem, and is congruent with a significant increase in catechol-O-methyltransferase expression in the LC of Pink1 −/− animals. Pearson correlations showed that increases in time to traverse a tapered balance beam are significantly associated with reductions in striatal dopamine. Ultrasonic vocalization complexity was positively correlated with LC norepinephrine concentrations. These data support the evolving hypothesis that differences in neural substrates and early-onset noradrenergic mechanisms in the brainstem may contribute to pathogenesis in the Pink1 −/− rat.
Keywords: Parkinson disease, rat, Pink1, catecholamine, locus coeruleus, basal ganglia, ultrasonic vocalization, levodopa
Introduction
Sensorimotor deficits in combination with selective degradation of dopaminergic circuitry are hallmark pathologies of Parkinson disease (PD), a progressive neurodegenerative disorder that affects 1-3% of the world’s population (de Lau et al., 2004). The cardinal signs of the disease-resting tremor, bradykinesia, and muscle rigidity-are all linked to significant reductions in nigrostriatal dopamine (Bernheimer et al., 1973, Stewart et al., 1995, Harel et al., 2004a, Harel et al., 2004b, Sung et al., 2010). However, early-onset signs such as cranial sensorimotor changes to voice as well as limb fine-sensorimotor skills, appear before these cardinal signs, and evidence suggests that other brainstem pathologies are present prior to nigral dopamine loss (Braak et al., 2003, Braak et al., 2004). To date, there is not a clear understanding of how this early brainstem pathology contributes to the overall manifestation of PD.
Here, we use a rat model of PD, that parallels human PD, to study the onset and progression of pathology (Cenci et al., 2002). The Pink1 −/− genetic rat model expresses a form of PD that is due to the complete knockout of the Pink1 gene; this loss of function mutation is related to the PARK6 phenotype of human familial PD (Dave et al., 2014). In humans, Pink1 monogenetic variants contribute to early-onset of PD with progressive deficits over time, nigrostriatal dopamine depletion, as well as other motor and non-motor deficits (Bonifati et al., 2002, Guo et al., 2011, Bonifati, 2012). At eight months of age, Pink1 −/− rats have cranial sensorimotor (vocalization and swallowing) and limb motor impairment (Dave et al., 2014, Grant et al., 2015), loss of dopaminergic cells within the substantia nigra (SN) (Dave et al., 2014), and reductions in noradrenergic cells in the locus coeruleus (Grant et al., 2015). However, at this early-stage time point there is not dopamine depletion in the striatum (SR) (Dave et al., 2014, Grant et al., 2015) suggesting that the 8 month time point is prior to significant nigrostriatal dopamine dysfunction. Consequently, other pathologies outside of the nigrostriatal circuitry, such as noradrenergic dysfunction, may be related to these early onset behavioral deficits.
Commonly, individuals with PD are prescribed dopamine precursors such as levodopa co-administered with carbidopa to improve many, but not all, of the signs and symptoms associated with dopamine loss. In humans, significantly improved cranial sensorimotor dysfunction, such as dysphonia, is non-responsive to levodopa while limb movement is (Dromey et al., 2000, Schulz and Grant, 2000, De Letter et al., 2006, D’Alatri et al., 2008, Plowman-Prine et al., 2009). The pharmacological recovery of behavioral deficits including cranial sensorimotor and limb motor function has not been documented in the Pink1 −/− rat model. To address this, we measured vocalization function and locomotive (limb) activity in the Pink1 −/− rat at baseline and after administration of a therapeutic dose of levodopa to test the hypothesis that levodopa would improve limb, but not vocal motor performance.
Data has shown that Pink1 −/− ultrasonic vocalization deficits correlate to noradrenergic cell loss in the locus coeruleus (LC) (Grant et al., 2015). Additionally, several studies have shown that degradation of catecholamines, norepinephrine in particular, occurs early in PD manifestation (Rommelfanger and Weinshenker, 2007). Consistent with a major pathologic landmark of PD, Pink1 −/− rats also exhibit significant aggregation of insoluble alpha-synuclein in nuclei that control and regulate motor behaviors (Grant et al., 2015, Kelm-Nelson et al., 2016b). Together, these data suggest that nervous system pathology contributes to early pathology in this rat model. In this study, we hypothesized that there would be reductions in dopamine within the SN and norepinephrine in the LC of Pink1−/− rats at 8 months of age. We also hypothesized that behavioral variables would correlate with catecholamine concentrations. Finally, by analyzing expression of key genes involved in catecholamine synthesis, transport, and metabolism such as tyrosine hydroxylase (Th), dopamine active transporter (Dat), and catechol-O-methyl transferase (Comt), Monoamine oxidase A (Mao-A), and norepinephrine transporter (solute carrier family 6 member 2, Net), we hypothesized that Pink1 −/− rats would show differential expression compared to age-matched WT in the SR, SN, and LC.
Experimental Procedures
A total of 34 animals were used. In Experiment 1, 8 male and 8 stimulus female rats were used. In Experiment 2, 18 male rats were used. Prior to study initiation, a power analysis was used to determine the number of animals for each experiment (0.80 power, alpha <0.05).
A. Experiment 1
Animals and habituation
Eight male Long-Evans rats, aged 8 mo, with a homozygous knockout of Pink1 (SAGE™ Research Labs, Boyertown, PA, USA (Baptista et al., 2013)), were housed in groups of two in standard polycarbonate cages (290 mm × 533 mm × 210 mm) with sawdust bedding on a reversed 12:12 hour light: dark cycle. A subset of females (n=8) from the animal colony were used to elicit vocalizations and not used as study subjects. All behavioral testing occurred during the dark period of the cycle under red light. Animals were handled each day for two weeks prior to the commencement of experiments and habituated to experimental procedures including gavage restraint. Food and water was available ad libitum. Food was restricted overnight before levodopa administration. All procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee (IACUC) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory animals.
Pharmacotherapy
Rats were orally gavaged one therapeutic dose (2mL) of 5/20 mg benserazide hydrochloride/ 3, 4-dihydroysyl-L-phenylalanine (Sigma Aldrich, USA). Benserazide inhibits peripheral metabolism of levodopa, which indirectly allows for penetration of levodopa across the blood brain barrier for a central nervous system effect (Kent et al., 1990). Behavioral testing occurred 60 minutes after dosing (Bredberg et al., 1994).
Behavioral Testing
Ultrasonic vocalization testing
Ultrasonic vocalization testing occurred at baseline and 60 min after levodopa administration. An ultrasonic microphone with high directional properties, flat frequency response up to 150-kHz, and a working frequency response range of 10-180-kHz was used for recording 50-kHz ultrasonic vocalizations (CM16, Avisoft, Germany). Recording parameters were a 16-bit depth with a sampling rate of 250-kHz. The microphone was attached to a panel in the top center of a 10 cm × 10 cm × 12 cm sound-isolated Plexiglas chamber. To elicit vocalizations, the rat was placed in the home cage within this chamber. A sexually receptive female stimulus rat was placed in the cage and was removed when the male showed signs of socio-sexual interest. Male-only vocalizations from test subjects were recorded for 90 seconds (s).
Offline acoustic analysis was performed with a customized automated program using SASLab Pro (Avisoft, Germany). Spectrograms were built from each Avisoft-generated waveform with a Fast Fourier Transform (FFT) of 512 points, frame size of 100%, flat top window, and temporal resolution set to display 75% overlap frame set-up. A high pass filter was used to eliminate noise below 25-kHz. Calls were slowed down in order to listen and categorize the call type as reported by previous publications (Brudzynski and Pniak, 2002, McGinnis and Vakulenko, 2003, Ciucci et al., 2007). Two experienced raters, masked to experimental conditions, categorized each call into simple or frequency-modulated (Ciucci et al., 2007, Ciucci et al., 2009, Johnson et al., 2011). For each call category (simple, frequency modulated), acoustic analyses were performed using SASLab Pro (Avisoft, Germany) and include average: bandwidth (Hertz-Hz), peak frequency (kHz), intensity (decibel-dB), and duration (milliseconds-ms). In addition, we analyzed the call rate (calls per s) and the percent of complex calls produced.
Challenging beam: motor limb function
All rats were assayed for gross and fine motor limb function as each rat traversed a 165cm long ledged, tapered beam (Lafayette Instrument Company, Lafayette, IN) (Fleming et al., 2004b). The last 1/3 of the challenge beam has a tapered, reduced diameter that increases task complexity. Each rat was placed on a loading platform and allowed to traverse the beam toward his homecage for 5 trials. Masked raters then reviewed video footage and assessed the time to traverse the beam (s), time to traverse the final third of the beam (s), and the total number of foot faults, averaged across the 5 trials.
Cylinder test (spontaneous movement): forelimb and hindlimb use
All rats were tested for forelimb and hindlimb use. Rats were placed in an upright translucent acrylic cylinder, measuring 30 cm high and 20 cm in diameter, to encourage rearing and vertical exploration with the forepaws (similar to (Fleming et al., 2004b)). Two masked raters analyzed video recordings of the task, and the number of forelimb- and hindlimb-contacts was totaled over a 2 min period.
Gait
To assess locomotion (gait), rats were trained to walk across a paper-lined alley. During testing, each animal’s forelimbs (black) and hindlimbs (blue) were brushed with an organic, non-toxic paint. Animals were then placed at the beginning of the alley and as they walked they left their paw prints on the paper (similar to (Schallert et al., 1978, Fleming et al., 2004a)). Stride length for forelimbs and hindlimbs was determined by measuring the distance between paw prints. Only strides made while continuously walking (no stopping) were included in the analysis, this included stride 1 and 2, respectively. Stride lengths at the beginning and end were not counted; consequently, the distance in centimeters (cm) between step 1 and 2 (1-2) and step 2 and 3 (2-3) were measured.
B. Experiment 2
Animals
A separate cohort of 12 Pink1 −/− rats with a homozygous knockout of Pink1 and 6 aged-matched WT Long Evans rat brains were used ELISA and RT-qPCR experiments (Kelm-Nelson et al., 2015). The 8-month time point was selected for constitutive protein concentration and mRNA expression at a defined early to middle disease stage (Dave et al., 2014, Grant et al., 2015).
Tissue Preparation
At 8 months of age, animals were deeply anesthetized with isoflurane and rapidly decapitated. The brains were dissected out and immediately frozen and stored at −80°C. Brains were sliced in the coronal plane on a cryostat to a 250 μm thickness at −15°C and mounted on gelatin-coated glass slides. Specifically, 2 mm tissue bilateral samples within the rostral SR (Bregma 2.04 mm), SN (Bregma −4.92 mm), and 1mm samples from the LC (Bregma −9.72 mm) were micropunched using the Brain Punch Set (FST 18035-02, Foster City, CA, USA) transferred to microcentrifuge tubes and stored at - under a dissection microscope over dry ice (Figure 1). Tissue samples for protein and RNA experiments were 80 °C.
Figure 1. Rat brain schematic.
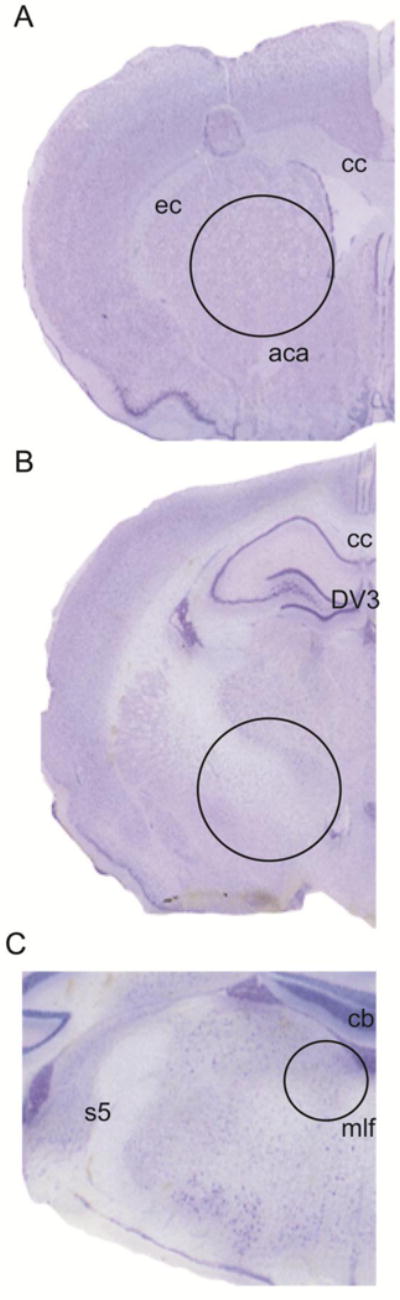
Location and sizes of (A) rostral striatum (SR); (B) substantia nigra (SN); and (C) locus coeruleus (LC) tissue samples collected for ELISA and real-time qPCR. Representative Nissl stained brain section, 50um thick sections. Photomicrograph at 10× magnification. Circular holes (represented by dashed circle) were centered within each region and illustrate the location rostral SR (Bregma 2.04 mm, 2mm punch), SN (Bregma −4.92 mm, 2mm punch), and LC (Bregma −9.72 mm, 1 mm punch). Color contrast has been optimized using Adobe Photoshop CC (Version 6) to clarify anatomical landmarks. Abbreviations: aca= anterior commissure, cb= cerebellum, cc= corpus callosum, DV3= dorsal 3rd ventricle, ec= external capsule, mlf=medial longitudinal fasciculus, s5=sensory root trigeminal nerve (Paxinos and Watson, 2005).
Protein Extraction
Brain tissue punches (left and right hemispheres for each animal) were homogenized using 300 μL of lysis buffer (N-PER Neuronal Protein Extraction Buffer (Thermo Scientific, Rockford, IL, USA) including a cocktail of protease (Sigma Aldrich, St. Louis, MO, USA), phosphatase inhibitors (Sigma Aldrich, St. Louis, MO, USA) and 200 mM PMSF (Sigma Aldrich, St. Louis, MO, USA)) and a hand Dremel tool for 30 s. Tissue homogenates were incubated on ice for 10 min, centrifuged at 12,000 rpm for 10 min at 4 °C to pellet the cell debris. The supernate was collected, aliquoted and stored at −80 °C for downstream analysis.
Total protein concentrations were first verified using a Nanodrop system (Thermo Scientific, Wilmington, DE, USA) and subsequently quantitatively determined using a bicinchoninic acid protein assay (BCA Protein Assay Kit; Thermo Scientific Pierce, Rockford, IL, USA) adopted for microtiter plates for all samples (1:2 dilution in nuclease-free water, in triplicate) following the manufacturer’s protocol. Bovine serum albumin (BSA; Thermo Scientific Pierce, Rockford, IL, USA) was used as the standard. Following a 30 min incubation, the plate was read at 562 nm using an BioTek® Eon® spectrophotometer (BioTek, Winooski, VT, USA) and analyzed with BioTek’s Gen5™ v 2.0 data analysis software.
Dopamine and Norepinephrine ELISA
To quantitatively determine the levels of dopamine and norepinephrine, normalized total protein concentrations (100 μg/mL per sample, as determined in pilot studies as the optimal concentration) were plated on a 96-well ELISA plate (BA E-5500; 2-CAT (N-D) Research ELISA™, Rocky Mountain Diagnostics, Colorado Springs, CO, USA) according to manufacturer’s instructions. Briefly, standards, control solutions and sample aliquots, diluted with distilled water, were pipetted into individual extraction plate wells. Plates were incubated by shaking at 600 rpm with TE buffer, acylation buffer plus acylation reagent, and hydrochloric acid. Extracted samples, standards, and controls were transferred to individual microtiter plate wells, mixed with enzyme solution, and incubated for 37 °C for 2 h. Sample, standard, and control well aliquots were transferred to pre-coated dopamine and noradrenaline microtiter strip wells, mixed with 50 μL of respective primary antiserum for 15–20 h at 2–8 °C. Wells were washed and incubated with enzyme conjugate for 30 min, then incubated for 20–30 min with substrate. After termination of reactions with stop solution, absorbance for each well was read at 450 nm on an Eon Plate Reader (BioTek, Winooski, VT, USA). All samples were run in duplicate within a single assay. Data inclusion criteria consisted of protein concentrations above the manufacturer’s specified limit of detection, intra- and inter-assay coefficients of variance below 20%, as well as a standard curve R2 of at least 0.990. Sensitivities of the commercial EIA according to manufacturer’s specification indicate a limit of detection: dopamine 0.25 ng/mL × correction factor and noradrenaline 0.1 ng/mL × correction factor. This assay is specific (cross reactivity to: derivatized adrenaline (noradrenaline, 0.14%; dopamine 0.03%), derivatized dopamine (noradrenaline, 0.2%; dopamine 100%), and derivatized noradrenaline (noradrenaline, 100%; dopamine 0.87%). The total protein concentration in each sample duplicate was averaged for each respective assay.
RNA Extraction
Sample order was randomized during processing. Tissue was homogenized with an electric Dremel tool, and total RNA was extracted with the Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (Catalog No. 732-6830; Bio-Rd, Hercules, CA, USA) according to manufacturer’s instructions. Total RNA was measured using a Nanodrop system (Thermo Scientific, Wilmington, DE, USA). DNase treated RNA (100 ng/μL per cDNA reaction) was converted into single-stranded cDNA using the Invitrogen SuperScript III First-Strand Synthesis System (Catalog No. 18080-05; Invitrogen, Carlsbad, CA, USA).
Primer design and verification
NCBI Primer Blast was used to design primers for control reference genes (Bactin and Gapdh) and genes of interest (Table A1) using the rat (Rattus norvegius) genome and based on hypothesis-driven selection. Reference genes were based on previous work in Pink1 −/− models (Kelm-Nelson et al., 2016c). Additionally, Netprimer (PREMIER Biosoft, Palo Alto, CA, USA) was used to examine secondary structure of all primers to dimers and non-specific amplification products. Specificity for each primer pair was confirmed using melt curve analysis; all primer runs yielded single peak melt curves indicating amplification of single gene products. Furthermore, the qPCR reaction product for each gene was sequenced using Sanger sequencing with both forward and reverse primers at the University of Wisconsin Biotechnology Center to confirm that sequences match intended targets. Using NCBI BLAST all sequences were confirmed to match the intended targets.
Table A1.
RT qPCR information.
| Gene [Rattus norvegicus (Norway rat)] |
Gene Abbreviation |
Accession Number |
Net Primer Score |
Direction | Primer Sequences |
T (°C) |
Product (bp) |
FASTA (Aligned) |
|---|---|---|---|---|---|---|---|---|
| Actb actin, beta | Bactin | NM_031144.3 | 92 | Forward | TGTGGATTGGT GGCTCTATC |
58 | 149 | AGATGTGGATC GTCCGGCCCCT TTCTAGGCGGA |
| Backward | AGAAAGGGT GT AAAACGCAG |
|||||||
| Gapdh glyceraldehyde-3-phosphate dehydrogenase | Gapdh | NM_017008.4 | 100 | Forward | GGATACTGAGA GCAAGAGAGA |
59 | 106 | ATCCCAACTCG CCCTCACAATT |
| Backward | TTATGGGGTCT GGGATGGAA |
|||||||
| Th tyrosine hydroxylase | Th | NM_012740.3 | 100 | Forward | CTTTGACCCAG ACACAGCA |
59 | 123 | CCAGCCTGTGT ATGACGCCAAC |
| Backward | TGGATACGAGA GGCATAGTTC |
|||||||
| Slc6a3 solute carrier family 6 (neurotransmitter transporter) | Dat | NM_012694.2 | 100 | Forward | CAACTCCACCC TCATCAACC |
60 | 70 | CTCAGGGAAGC ATGCCCTATGT TGCGTGGAGTT TGGCATCAGAG |
| Backward | TTTCTTGCTCC AGGTCTCCC |
|||||||
| Comt catechol-O-methyltransferase | Comt | NM_012531.2 | 100 | Forward | AAGACCGCTAC CTTCCAGAC |
60 | 132 | TGGCCTGCTGC GCTGACAACGT ACTTCCTGGCG |
| Backward | TGCTGCTCCCT CTCACATAC |
|||||||
| Maoa monoamine oxidase A | Mao-a | NM 033653.1 | 100 | Forward | TTTGTGTAGCG TCCAAGGTG |
60 | 103 | GAAGTGTTCCC TCCATGTGATA |
| Backward | AAAGTGCCAAGGGTAGTGTG | |||||||
| Slc6a2 solute carrier family 6 member 2 | Net | NM_031343.1 | 100 | Forward | GCATTGAGAAG GCAGAGAGG |
59 | 135 | GGGACCCCACA ATGCCTGGCCC TGGGGGGTGC |
| Backward | CAGT AGAGCAA GGAAGGCAC |
Gene of interest (abbreviation; http://www.ncbi.nlm.nih.gov/gene), NCB accession number, Net Primer Score (Premier Biosoft), forward and backward primer sequences, melting temperature, product length and FASTA (Aligned Sequence) for each of the genes tested.
Quantitative real-time PCR (RT qPCR)
Relative gene expression was determined using real-time qPCR analysis following the MIQE guidelines for quantitative real-time PCR experiments [42]. All samples were prepared in reaction tubes containing the respective sample cDNA, nuclease-free water, characterized forward and reverse primers (5μM concentration, University of Wisconsin Biotechnology Center) and SsoFast EvaGreen Supermix (Catalog No. 172-5201). On each qPCR plate, five standards were run (1:10 serial dilutions, starting at 500 ng/μL) with a Non Template negative control. Samples and standards were run in triplicate on each plate. Plates were read with the BioRad CFX96 Touch Real-Time PCR Detection System (Catalog No. 185-5195, Bio-Rad, Hercules, CA, USA). Briefly, each qPCR run entailed of an initiation step at 95 °C for 30 s, 40 cycles of 95 °C for 5 c, a 30 s annealing phase, a 20 s elongation phase at 72 °C, and a melt curve from 60-88 °C, 0.5 degrees for each 5 s step. All plates were read following each elongation and melt curve stage.
For qPCR, data inclusion criteria consisted of run efficiencies between 90% and 110% as well as an R2 of at least 0.990. The mean Ct value for each sample was defined as the average cycle number at which each sample triplicate crossed the amplification threshold, which was set a priori to 200 RFU. The mean Ct values for each sample were transformed via the Pfaffl Method to yield individual relative expression level values for each gene of interest for each rat [43].
Statistics
All statistical analyses were conducted with SigmaPlot® 12.5 (Systat Software, Inc., San Jose, CA). Data are presented as means +/− standard error of the mean (SEM). Data variables were transformed if data failed to conform to assumptions for statistical models: normality (using the Kolmogorov-Smirnov test), and equal variance (Levene’s test for homogeneity of variance). In cases where data failed assumptions, data was either rank transformed and transformed data was used for subsequent statistical analysis, or a Wilcoxon Signed Rank Test (Z score) was used. For gene analysis data that violated assumptions, a Mann-Whitney Rank Sum tests was used (U statistic).
For Experiment 1 data analysis, paired student’s t-tests were used to evaluate differences between baseline and levodopa conditions for each assay as detailed above. Additionally, in Experiment 2, independent student’s t-tests were used to evaluate differences between genotype for each brain region for both protein concentrations and relative mRNA expression. A one-way analysis of variance (ANOVA) with a Fisher’s Least Squared Differences post-hoc method was used to compare protein concentrations across brain regions. Pearson’s correlation analysis was performed for behavioral variables (challenge beam, cylinder, ultrasonic vocalization) previously collected on this dataset (Kelm-Nelson et al., 2015) and catecholamine concentrations. Due to the exploratory nature of this work and the associated statistical type II error, no corrections were made for multiple comparisons. The critical level for significance was set, a priori, at p<0.05 for all testing. In some instances, sample sizes were reduced. Data trends for 0.10>p>0.05 are indicated as appropriate and effect sizes (Cohen’s d or Pearsons r value) are reported (Cohen, 1992, Rosenthal, 1994).
Results
Baseline and levodopa data (mean +/− SEM) for behavioral assays are presented in Table 1; WT data taken from Grant et al., 2015 are also presented as a comparison. There were no significant differences for any of the acoustic measures for simple calls between baseline and treatment (p>0.10); therefore, results presented below are for FM ultrasonic vocalizations only.
Table 1.
Motor behavioral assays.
| Behavior at 8 months of age | Mean (SEM) Wildtype$ | Mean (SEM) Pinkl −/− Baseline | Mean (SEM) Pinkl −/− Levodopa |
|---|---|---|---|
| Challenge Beam (Whole) | 2.12 (0.14) | 3.666 (0.611) | 3.217 (0.423) |
| Challenge Beam (Last 1/3) | 1.491 (0.059) | 2.373 (0.685) | 1.688 (0.262) |
| # Foot faults | 0.46 (0.12) | 0.667 (0.349) | 0.429 (0.14) |
| Hindlimb Steps | 18.92 (2.41) | 29.143 (5.045) | 22.857 (3.384) |
| Forelimb Steps | 21.5 (1.73) | 53 (9.859) | 31 (5.282) |
| Gait (steps 1-2 forelimb R) | Not tested | 13.133 (0.986) | 14.533 (1.041) |
| Gait (steps 2-3 forelimb R) | Not tested | 10.917 (1.058) | 15.825 (1.023) |
| Gait (steps 1-2 hindlimb R) | Not tested | 13.55 (1.056) | 14.386 (0.559) |
| Gait (steps 2-3 hindlimb R) | Not tested | 10.8 (2.064) | 16.043 (0.775) |
| # Calls | Not tested | 120 (15.74) | 126.143 (19.202) |
| Call Rate | Not tested | 1.33 (0.175) | 1.402 (0.123) |
| Complex Calls (%) | 62.23 (3.65) | 58 (5.29) | 4 5.7 (5.13) |
| Average FM Duration | 0.043 (0.0021) | 0.0484 (0.00413) | 0.0596 (0.00766) |
| Average FM Bandwidth | 21496 (1185.6) | 17377.46 (875.938) | 18823.92 (454.33) |
| Average FM Intensity | −44.675 (0.5025) | −54.101 (0.566) | −56.179 (0.626) |
| Average Peak FM Frequency | 55494 (911.83) | 54832.25 (597.588) | 52007.7 (351.216) |
Means (SEM) for limb motor and vocal motor variables for Pink1 −/− animals at baseline and after administration of levodopa. Wildtype data is provided as a comparison. *Non-transformed data,
Data extracted from Grant et al., 2015.
Average frequency-modulated ultrasonic vocalizations
Representative spectrograms are illustrated in Figure 2 A and B. Compared to baseline values, there were no significant differences in the call rate (t(6)= −0.22, p=0.42; Figure 2 C). There was a trend between treatment groups for the percent complex calls (t(6)=1.75, p=0.07; d=0.96); specifically, when administered levodopa the % complex calls decreased (Figure 2 D).
Figure 2. Average frequency modulated ultrasonic vocalizations.
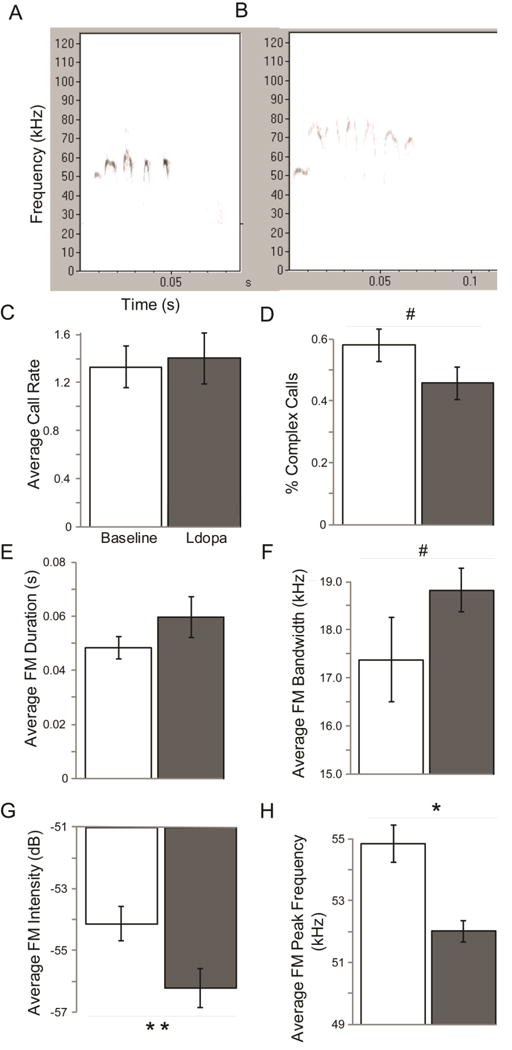
Representative spectrograms (x axis: time (s), y-axis: frequency in (kHz)) of frequency modulated ultrasonic vocalizations in Pink1 −/− rats (A) baseline (at 8 months of age) and (B) 60 minutes post-administration of levodopa (ldopa). Means (+/− SEM) of acoustic variables at baseline (white bar) and after levodopa administration (gray bar) for (C) Average call rate (calls/2 min); (D) % complex calls; (E) average frequency modulated duration (s); (F) average frequency modulated bandwidth (Hz); (G) average frequency modulated intensity (dB), (H) average frequency modulated peak frequency (Hz). Statistical significance between baseline and levodopa conditions indicated by bar and ** p<0.01, * p<0.05, #, p<0.10 (trend). Abbreviations: SEM=standard error of the mean, s=second, Hz=hertz, dB=decibels.
There was no difference between baseline and levodopa treatments for the average frequency modulated duration (Z=1.18, df=6, p=0.30; Figure 2 E). There was a trend for an increase in bandwidth (t(6)= −1.60, p=0.080; d=0.85) when animals were given levodopa compared to baseline (Figure 2 F). There was a significant decrease in the average frequency modulated peak frequency (t(6)=4.62, p=0.0018) when Pink1 −/− rats were given levodopa compared to baseline (Figure 2 G). There was also a significant decrease in the average frequency modulated intensity (loudness) on levodopa compared to baseline measures (t(6)=2.20, p=0.035; Figure 2 H).
Challenge beam
There were no significant differences in the time to traverse the challenge beam (t(6) =1.05), p=0.17), the time to traverse the last 1/3 of the challenge beam (t(6)=1.02, p=0.17), or the number of foot faults (Z=−0.73, p=0.63) between baseline and levodopa conditions (Figure 3 A-C).
Figure 3. Average behavioral data.
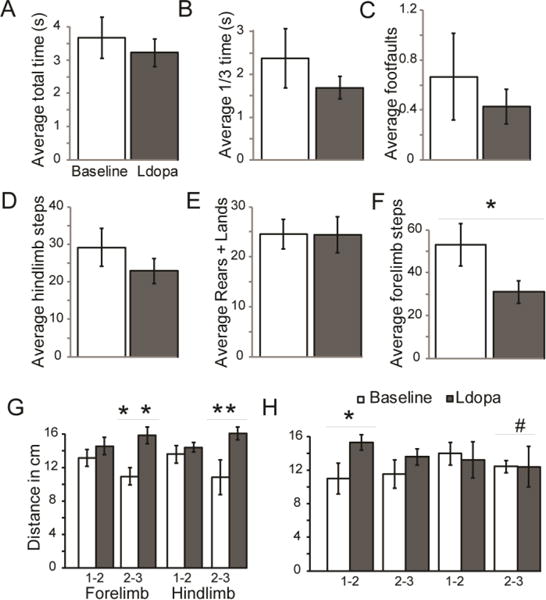
Means (+/− SEM) in Pink1 −/− rats at baseline (white bar) and levodopa (ldopa, gray bar). Challenge beam variables included (A) average total time to traverse the beam (s); (B) average time to traverse the last 1/3 of the tapered beam (s); (C) total number of foot faults. Cylinder test (spontaneous movement) variables included the (D) average number of hindlimb steps; (E) total number of forelimb steps; (F) the average number of rears + lands during at 2-minute testing period. Gait analysis for (G) right side and (H) left side included distance (cm) between steps 1-2 and 2-3 for both forelimbs and hindlimb. p<0.10 Statistical significance between baseline and levodopa conditions indicated by bar and ** p<0.01, * p<0.05, #, (trend).
Cylinder test (spontaneous activity)
Compared to baseline measures, the Pink1 −/− animals had no significant change in the number of hindlimb steps (t(6)=1.31, p=0.12; Figure 3 D), or rears and lands (t(6)=0.045, p=0.48; Figure 3 E) when levodopa was administered. However, there was a significant difference between baseline and levodopa for the number of forelimb touches (t(6)=2.67, p=0.019); specifically, on levodopa animals had fewer forelimb movements (Figure 3 F). During the cylinder test, there was no significant difference in the amount of time animals spent grooming at baseline compared to levodopa (t(6)=1.27, p=0.12; data not shown).
Gait
There was no significant difference between right forelimb step 1-2 (t(10)= −0.98, p=1.18) or right hindlimb step 1-2 (t(11)= −0.73, p=0.24). However, there was a significant difference between right step 2-3 for both forelimb (t(10)= −3.34, p=0.0038) and hindlimb (t(9)= −2.87, p=0.0092); specifically, when administered levodopa Pink1 −/− animals took a longer stride (Figure 3 G). Moreover, there was a significant difference between left forelimb step 1-2 (t(11)= −2.19, p=0.025), but not left forelimb step 2-3 (t(7)= −1.11, p=0.15), left hindlimb 1-2 (t(11)=0.28, p=0.39) or left hindlimb 2-3 (t(5)=0.0074, p=0.50) (Figure 3 H).
Total protein concentrations
There were no significant differences between genotypes for the total protein (normalized to initial tissue amount) in SR (t(14)= −0.63, p=0.27), SN (t(12)= −0.92, p=0.19), or LC (t(16)=0.67, p=0.26) (Table 2).
Table 2. Protein concentrations.
| Brain Region | WT Mean Total Protein (μg/mL) (SEM) | WT Dopamine (pg/mL) | WT Norepinephrine (pg/mL) | Pinkl −/− Mean Total Protein (μg/mL) (SEM) Pinkl −/− | Pinkl −/− Dopamine (pg/mL) | Pinkl −/− Norepinephrine (pg/mL) |
|---|---|---|---|---|---|---|
| Striatum (SR) | 169.431 (30.273) | 13136.26 (2606.49) | N/A | 145.431 (21.841) | 9864.92 (1040.17) | N/A |
| Substantia Nigra (SN) | 139.197 (38.537) | 814.97 (386.15) | 1211.18 (360.41) | 115.91 (6.852) | 1162.21 (220.8 | 2016.38 (222.87) |
| Locus Coeruleus (LC) | 60.99 (11.011) | N/A | 1674.20 (492.25) | 74 (12.566) | N/A | 882.53 (125.04) |
Protein concentrations. Means (SEM) for total protein (μg/ml_), dopamine and norepinephrine concentrations (pg/mL) in wildtype (WT) and Pinkl −/− rats (non-transformed data).
Dopamine and norepinephrine protein concentrations
Several samples were below the limit of detection as specified from the manufacturer and this is reflected in the sample sizes; they were not included in the analysis. Dopamine concentrations in the LC and norepinephrine in the SR were not analyzed. Average concentrations (SEM) are provided in Table 2.
Within the SR, there was a trend for a difference in dopamine concentration between genotypes (t(11)=1.43, p=0.09; d=0.91); specifically there was a decrease in the protein concentrations of Pink1 −/− animals compared to WT controls (Figure 4 A). There was no difference in dopamine concentrations between genotypes in the SN (t(12)= −0.74, p=0.24; Figure 4 A).
Figure 4. Average protein data.
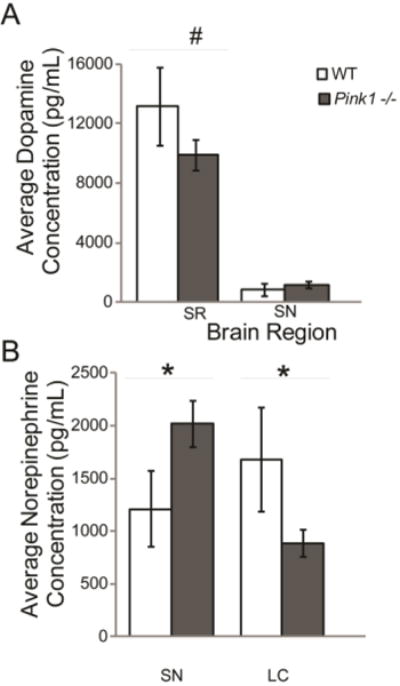
Means (+/− SEM) in wildtype (white bar) compared to Pink1 −/− (gray bar). (A) Average ELISA dopamine (pg/mL) concentration in WT and Pink1 −/− rats in SR and SN; (B) Average ELISA norepinephrine (pg/mL) concentration in WT and Pink1 −/− rats in SN, and LC. Statistical significance between genotypes indicated by bar and ** p<0.01, * p<0.05, #, p<0.10 (trend).
There was a significant difference in the concentration of norepinephrine (t(13)=1.88, p=0.042); there was a significant increase in the norepinephrine concentrations of Pink1 −/− animals in the SN compared to WT controls (Figure 4 B). Additionally, there was a significant decrease in the norepinephrine concentrations in the LC of Pink1 −/− animals compared to WT (t(14)=1.67, p=0.05; Figure 4 B).
Pearson correlations
To compare to ELISA protein values in each brain region within the Pink1 genotype, previously collected behavioral data for catalepsy, cylinder, and challenge beam was used for correlation analysis with brain protein concentrations (Figure 5 A-F) (Kelm-Nelson et al., 2015). All correlations are listed in Tables 3 and 4.
Figure 5. Limb motor degeneration over time of Pink1 −/− rats.
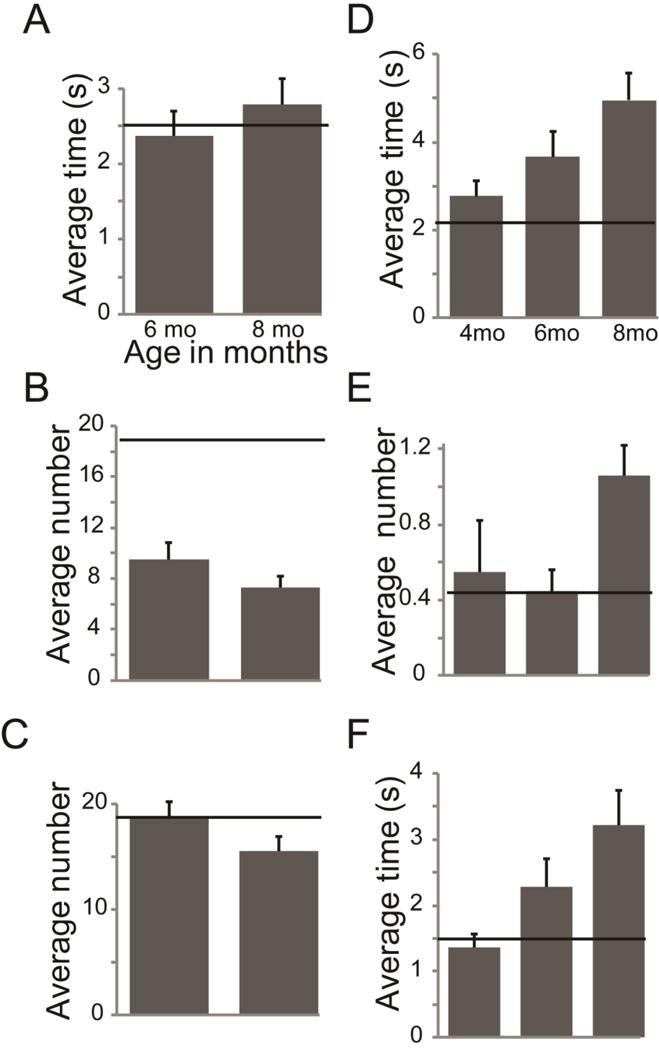
(A) Catalepsy time to disengage (s); (B) cylinder-hindlimb number of steps; (C) cylinder-forelimb number of steps; (D) time to traverse challenge beam (s); (E) number of foot faults on the challenge beam; (F) time to traverse the last 1/3 of the challenge beam (s). X-axis is age of animals during testing and Y-axis is behavior variable + SEM. Horizontal black line represents average wildtype value for each behavioral assay as assessed in previous reports (Ringel et al., 2013, Grant et al., 2015).
Table 3.
Motor and Protein: Pearson Correlations
| ELISA | |||
|---|---|---|---|
| Behavior | Dopamine | Norepinephrine | Brain Region |
| Catalepsy | −0.552 (0.124) | 0.220 (0.635) | SR |
| 0.475 (0.140) | 0.179 (0.599) | SN | |
| Not tested | −0.204 (0.524) | LC | |
| Challenge Beam (Whole) | −0.920 (0.00328)* | 0.445 (0.376) | SR |
| −0.310 (0.455) | −0.502 (0.205) | SN | |
| Not tested | −0.377 (0.317) | LC | |
| Challenge Beam (Last 1/3) | −0.567 (0.184) | 0.607 (0.201) | SR |
| −0.0230 (0.957) | −0.168 (0.691) | SN | |
| Not tested | −0.150 (0.699) | LC | |
| # Foot faults | −0.564 (0.187) | −0.277 (0.547) | SR |
| −0.207 (0.593) | 0.0588 (0.881) | SN | |
| Not tested | 0.659 (0.0383)* | LC | |
| Hindlimb Steps | −0.0414 (0.916) | 0.705 (0.0771)# | SR |
| −0.0347 (0.919) | −0.186 (0.583) | SN | |
| Not tested | −0.327 (0.299) | LC | |
| Forelimb Steps | −0.265 (0.491) | −0.120 (0.798) | SR |
| 0.560 (0.0732)# | 0.269 (0.425) | SN | |
| Not tested | −0.494 (0.103) | LC | |
Gross motor behavioral variables correlated to ELISA dopamine concentrations and norepinephrine concentrations, respectively. Data presented are r (p value) for each brain region. SR=striatum, SN=substantia nigra, LC=locus coeruleus. Non-transformed data. Significant finding
(p<0.05);
trend (0.10<p<0.05).
Table 4.
Vocal and Protein: Pearson Correlations
| ELISA | |||
|---|---|---|---|
| Behavior | Dopamine | Norepinephrine | Brain Region |
| # Calls | −0.0847 (0.872) | −0.183 (0.817) | SR |
| −0.264 (0.567) | −0.471 (0.286) | SN | |
| Not tested | −0.325 (0.433) | LC | |
| Call Rate | −0.0847 (0.873) | −0.183 (0.817) | SR |
| −0.264 (0.567) | −0.471 (0.286) | SN | |
| Not tested | −0.325 (0.433) | LC | |
| Complex Calls (%) | 0.804 (0.05)* | −0.597 (0.403) | SR |
| 0.0899 (0.848) | 0.415 (0.355) | SN | |
| Not tested | 0.646 (0.0837)# | LC | |
| Average FM Duration | −0.122 (0.818) | −0.382 (0.618) | SR |
| 0.279 (0.544) | 0.109 (0.817) | SN | |
| Not tested | 0.455 (0.258) | LC | |
| Average FM Bandwidth | 0.355 (0.489) | −0.369 (0.613) | SR |
| −0.00398 (0.993) | 0.0968 (0.836) | SN | |
| Not tested | −0.0676 (0.874) | LC | |
| Average FM Intensity | −0.468 (0.349) | −0.266 (0.734) | SR |
| 0.402 (0.371) | 0.193 (0.679) | SN | |
| Not tested | −0.535 (0.172) | LC | |
| Average Peak FM Frequency | −0.00434 (0.993) | −0.172 (0.828) | SR |
| −0.379 (0.402) | −0.271 (0.556) | SN | |
| Not tested | −0.492 (0.215) | LC | |
Vocal motor behavioral variables correlated to ELISA dopamine concentrations and norepinephrine concentrations, respectively. Data presented are r (p value) for each brain region. SR=striatum, SN=substantia nigra, LC=locus coeruleus. Non-transformed data. Significant finding
(p<0.05);
trend (0.10<p<0.05).
Pearson correlation analysis determined that there was an association between motor behavior and catecholamine concentrations. Specifically, within the Pink1 genotype, SR dopamine levels were significantly negatively correlated with challenge beam time (r=−0.92, p= 0.0033; Figure 6 A). Specifically, lower concentrations of dopamine in the SR are associated with long times to traverse the beam. Within the SN, there was a trend for dopamine concentrations to be positively correlated with the number of forelimb steps in the cylinder test (r=0.56 (large effect), p=0.073; Figure 6 B); increased SN dopamine concentrations are associated with increased forelimb movements. There were significant associations between SR dopamine (r=0.80, p=0.05; Figure 6 C); increased SR dopamine is associated with an increase in the number of complex ultrasonic vocalizations produced. Additionally, there was a trend for LC norepinephrine to be correlated to percent complex ultrasonic vocalizations (r=0.65 (large effect), p=0.08; Figure 6 D).
Figure 6. Significant Pink1 −/− protein concentrations correlated to behavioral variables.
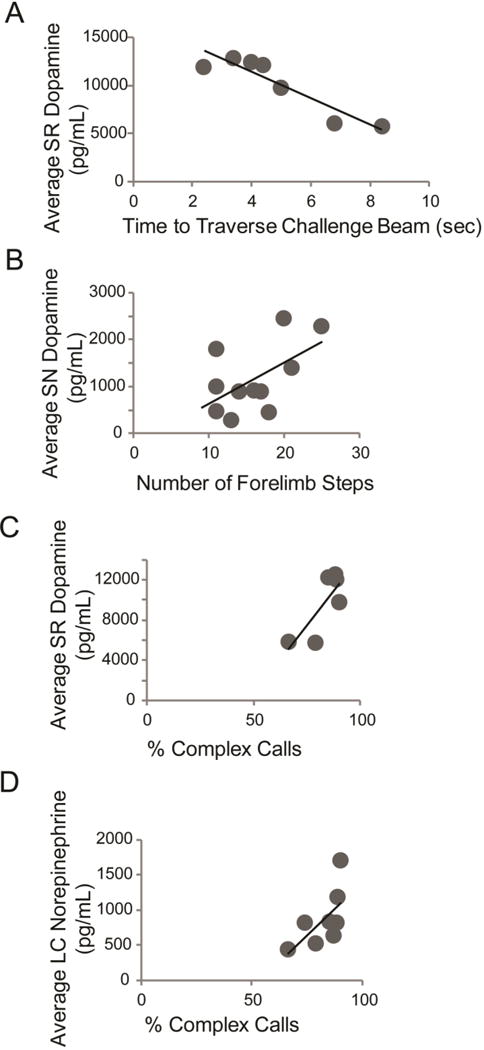
(A) Average Pink1 −/− striatum (SR) dopamine concentration (pg/mL) and time to traverse the challenge beam in s; (B) Average SR norepinephrine concentration (pg/mL) and number of hindlimb steps in the cylinder test; (C) Average substantia nigra (SN) dopamine concentration and number of forelimb steps in the cylinder test; (D) Average SR dopamine and % complex calls; (E) Average locus coeruleus norepinephrine concentration and % complex calls. Each dark circle represents one individual Pink1 −/− rat.
RT-qPCR
There was no significant difference in the normalized, total RNA concentrations (Table 5) between genotypes within the SR (t(16)=0.98, p=0.17), SN (U=25, p=0.33) or LC (U=22, p=0.29).
Table 5.
Average total RNA Concentrations.
| Genotype | Striatum | Substantia Nigra | Locus Coeruleus |
|---|---|---|---|
| Wildtype | 7.75 (2.549) | 5.331 (0.461) | 6.712 (1.888) |
| Pink1 −/− | 10.375 (1.408) | 5.418 (1.231) | 3.916 (0.675) |
Means (SEM) for wildtype and Pink1 −/− animals in each brain region.
Mean (SEM) for WT and Pink1 −/− genotypes are displayed in Table 6. There were no statistically significant findings in the relative expression of Th in the SR (t(16)=0.55, p=0.30), SN (t(15)=0.31, p=0.76), or LC (t(15)=0.20, p=0.42) (Figure 7 A). There was a trend for a difference in dopamine active transporter between genotypes in the SR (t(16)=1.45, p=0.083, d=0.77) with Pink1 −/− animals exhibiting an increase in Dat relative expression compared to WT, but not the SN (t(15)=0.001, p=1.0) (Figure 7 B). There were significant differences in expression of Comt within in the LC (t(15)=1.97, p=0.034); the Pink1 −/− animals exhibited an increase in Comt expression compared to WT. There were no significant differences in Comt expression in the SN (t(16)=0.37, p=0.36) (Figure 7 C). There were no significant difference in Mao-a expression in the SR (t(16)=0.64, p=0.26) or SN (t(16)=0.64, p=0.26; Figure 7 D). There were no significant differences in Net expression within the LC (t(15)=0.20, p=0.42; Figure 7 E).
Table 6.
RT qPCR data
| qPCR data SR. | ||
|---|---|---|
| Gene | Mean (SEM) Wildtype | Mean (SEM) Pink1 −/− |
| Th | 1.401 (0.353) | 1.009 (0.148) |
| Dat# | 0.939 (0.127) | 1.101 (0.0846) |
| Mao-a | 0.998 (0.146) | 1.099 (0.121) |
| Comt | 1.034 (0.201) | 1.090 (0.105) |
| qPCR data SN. | ||
|---|---|---|
| Gene | Mean (SEM) Wildtype | Mean (SEM) Pink1 −/− |
| Th | 2.505 (0.992) | 3.374 (2.297) |
| Dat | 0.788 (0.337) | 1.998 (1.410) |
| Mao-a | 1.415 (0.387) | 1.146 (0.331) |
| Comt | 1.789 (0.677) | 1.157 (0.363) |
| qPCR data LC. | ||
|---|---|---|
| Gene | Mean (SEM) Wildtype | Mean (SEM) Pink1 −/− |
| Th | 2.041 (1.051) | 2.222 (1.052) |
| Dat | 1.431 (0.309) | 1.695 (0.374) |
| Comt* | 0.789 (0.221) | 1.459 (0.274) |
| Mao-a | 1.538 (0.703) | 1.569 (0.562) |
| Net | 3.363 (2.016) | 2.528 (1.292) |
Means (SEM) for wildtype and Pinkl −/− animals in each brain region. Non-transformed data. Significant finding
(p<0.05);
trend (0.10<p<0.05).
Figure 7. RT qPCR data.
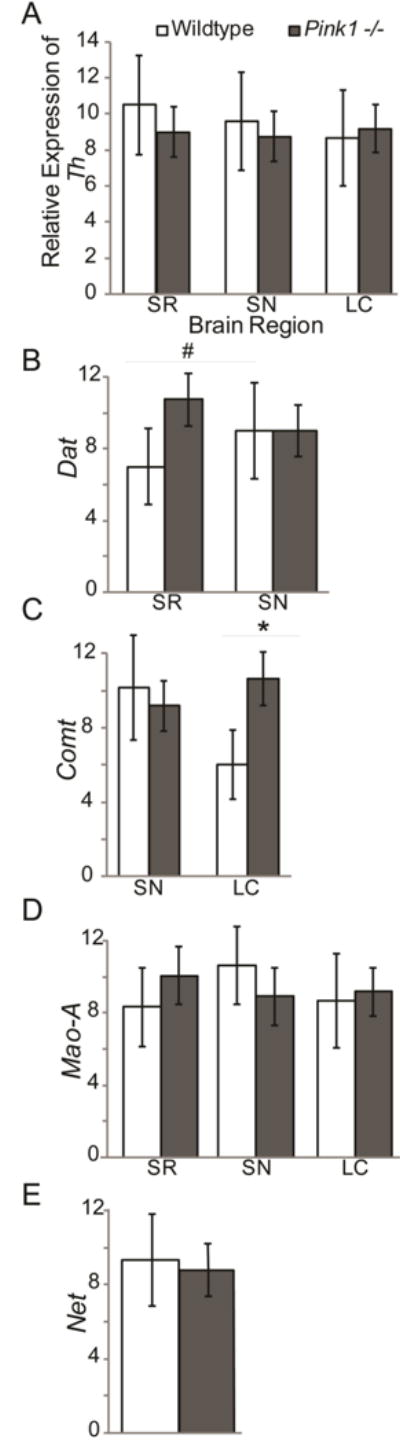
Means (+/− SEM) in wildtype (white bars) and Pink1 −/− (gray bars) rats in striatum (SR), substantia nigra (SN), and locus coeruleus (LC). Relative mRNA expression of (A) tyrosine hydroxylase (Th); (B) dopamine active transporter (Dat) in SR and SN; (C) catechol-O-methyltransferase (Comt) in SN and LC; (F) monoamine oxidase A (Mao-A); (F) solute carrier family 6 member 2 (Net) in LC. All data was analyzed with the Pfaffl Method, normalized to reference genes (βactin, Gapdh) expression. Statistical significance between genotype conditions indicated by bar and ** p<0.01, * p<0.05, #, p<0.10 (trend).
Discussion
The purpose of these experiments was to study the primary effects of levodopa on motor behavior and to characterize catecholamine content in the SR, SN, and LC in the Pink1 −/− rat model of PD. In the first experiment, we hypothesized that peripheral administration of levodopa would improve limb, but not cranial sensorimotor (vocalization) functions. We showed that levodopa improves some aspects of locomotion, specifically gait stride and forelimb movements. However, there were no improvements in challenge beam performance or hindlimb cylinder measures. Pink1 −/− vocalizations did not significantly improve with levodopa to average WT conspecific levels; moreover, intensity and peak frequency were reduced further compared to baseline measures. Additionally, there were no differences in other vocalization parameters including call rate and complexity as well as duration and bandwidth of the call. Subsequently, we confirmed our second hypothesis that Pink1 −/− rats have a reduction of norepinephrine in the LC. This is the first study to report that SN norepinephrine is increased in this rodent model. Additionally, the present study confirms that nigrostriatal dopamine concentrations in Pink1−/− rats are not changed compared to WT, consistent with previous reports (Grant et al., 2015). Within the Pink1 −/− genotype, individual differences in striatal catecholamine concentrations are correlated with limb motor function. Therefore, it is likely that catecholamine loss may contribute to individual behavioral deficits in this early-stage model. The qPCR results show that, compatible with norepinephrine protein results, there is a significant increase in Comt, the gene coding for the enzyme that is involved in the catabolism of norepinephrine. Other catecholamine-related genes, including Th, Dat, Mao-A, and Net, were largely unchanged. Together, these data support that the Pink1 −/− rat model is a valid model to study early-onset Parkinsonism.
Ultrasonic vocalization intensity and peak frequency do not improve with levodopa
Rats in this study were given an acute oral dose of levodopa and within-subject changes to behaviors were analyzed. We found that ultrasonic vocalization intensity and peak frequency are significantly reduced with acute drug administration compared to baseline. The lack of improvement in Pink1 −/− vocalizations with levodopa is consistent with levodopa administration in the 6-hydroxydopamine (6-OHDA) infusion model of PD where a single dose of levodopa did not improve duration, bandwidth, or intensity (Kelm-Nelson et al., 2016a). Likewise, this study provides significant evidence that the Pink1 −/− rat model is similar to human PD in that human vocalization quality is also largely unaffected by levodopa administration (Schulz and Grant, 2000, De Letter et al., 2006, Plowman-Prine et al., 2009). In addition to the reductions in ultrasonic vocalization complexity, intensity, and peak frequency, we observed pilo-erection and hunched posture after drug administration indicative of commonly seen side effects. Exercise-based speech and voice training improves vocal quality in humans (Fox et al., 2006, Sapir et al., 2007), the 6-OHDA model (Kelm-Nelson et al., 2016a), as well as the Pink1 −/− model (Kelm-Nelson et al., 2015), which implicates the involvement of motor and motivational brain circuitry beyond the nigrostriatal dopamine pathway in vocalization behavior. However, rats were tested after a single oral dose of levodopa, which is not reflective of patient treatment regimens. Therefore, it is not clear whether long-term administration would have a net benefit on vocalizations in this model.
Levodopa improves some limb motor function
In general, Pink1 −/− rats demonstrate difficulty traversing a challenge beam (increased foot faults and reductions in speed in the challenging portions), fewer forelimb and hindlimb movements in a cylinder, and shorter gait stride length (Dave et al., 2014, Grant et al., 2015). Here, we show that levodopa improves gait stride, but no other aspects of limb motor function. The brain metabolism of levodopa includes the catabolism into dopamine and subsequently norepinephrine. Thus, it is feasible that the improvement (increases in length) in gait stride is due to increase in LC norepinephrine, as previously observed in humans (Tohgi et al., 1993); however, we did not find that these measures correlated to each other. Significant differences for gait stride were side-dependent (Yoshioka, 1930, GÜVen et al., 2003). Asymmetries in brain regions were not assessed because brain regions were combined for each individual rat. Future studies of chronic levodopa administration in this rat model are necessary to show long-term rescue of gait deficits. Within the Pink1 −/− rat model, we show that an individual’s striatal dopamine concentration is related to challenge beam performance. Specifically, reductions in striatal dopamine correlated to longer transit times. Likewise, lower concentrations of substantia nigra dopamine correspond to reduced forelimb steps in the cylinder. Motor findings in the rat parallel human PD, in which reductions in nigrostriatal dopamine is correlated to motor performance. Levodopa has additional effects on brainstem regions regulating gait and limb motor movements (Ryczko and Dubuc, 2017{Jordan, 2008 #1824)}; thus, it is possible that the acute levodopa administration influences these structure and contribute to motor improvements. Additionally, administration of levodopa in humans improves gait disturbances including slow, short-stepped shuffling gait patterns, stride length, and balance (i.e. falls) (Burleigh-Jacobs et al., 1997, O’Sullivan et al., 1998, Schaafsma et al., 2003a, Schaafsma et al., 2003b).
Pink1 −/− rats have disrupted catecholamine concentrations
Congruent with previous TH immunohistochemistry analysis by Grant et al. (2015), socially-housed Pink1 −/− rats show no significant difference in nigrostriatal TH compared to WT (Grant et al., 2015). This finding is in contrast to Dave et al. (2014) that showed a TH cell loss (50%) in the SN, but no difference in striatal TH density (Dave et al., 2014). Here, the experimental methodology was similar to Grant et al. (2015); all rats in this study received daily experimenter handling and daily social interactions with a female which has been shown to impact brain morphology (Bezard et al., 2003). Alternatively, discrepancies in SN cell numbers between Dave et al. 2014 and Grant et al. 2015 may be related to other behavioral modifications including diet or light cycle, and tissue processing and cell counting methodologies. Previous studies have shown that lesions to the substantia nigra can lead to the increase in extracellular striatal dopamine concentrations, as a compensatory change resulting from insult (Robinson and Whishaw, 1988). Additional changes or adaptations to dopamine terminals result in the compensation or normalization of extracellular concentrations of dopamine, which may account for the non-significant differences in striatal dopamine concentrations in this early-stage model. This mechanism likely plays a role in the effectiveness of levodopa as a treatment (see above), as well as recovery of behavioral functions (Kelm-Nelson et al., 2015). Consistent with these data, Pink1 −/− mice do not show differences in the number of dopamine neurons in the SN or striatal density measures compared to WT(Kelm-Nelson et al., 2017); however, the researchers do observe decreases in evoked DA release in striatal slices using amperometric recordings (Kitada et al., 2007). As we studied an early-stage timepoint, it is possible that we have not observed significant pathological differences because of the striatal compensatory changes. Additionally, because we evaluated general catecholamine protein concentrations it is possible that we did not identify localized differences including neurotransmitter release or reuptake.
Lower brainstem nuclei appear to be particularly susceptible to early damage, prior to the degeneration of nigrostriatal neuron pathways (Braak et al., 2003, Braak et al., 2004). Of these nuclei, the LC is the main noradrenergic producer and is compromised early in PD (Marien and Briley, 1994, Marien et al., 2004, Vermeiren and De Deyn, 2017). The LC has numerous and widespread projection targets, including the substantia nigra (Benarroch, 2009, Delaville et al., 2011). Multiple studies in animal models of PD indicate that LC noradrenergic activity alters neurotransmission in the nigrostriatal system (Antelman and Caggiula, 1977, Marien et al., 2004). Our ELISA data show increased norepinephrine concentrations in the SN of Pink1 −/− rats compared to WT. Norepinephrine-dopamine containing systems are interlinked and it is hypothesized that dopamine is counterbalanced by noradrenergic activity (Antelman and Caggiula, 1977); an increase in norepinephrine in the SN of Pink1 −/− rats may regulate dopamine activity through inhibition, although this hypothesis has yet to be experimentally confirmed in this model. In the 6-OHDA model of PD, selective denervation of LC norepinephrine using N-ethyl-2-bromobenzylamine aggravates dopamine deficiency (Srinivasan and Schmidt, 2003). Because we did not see a decrease in dopamine concentration in the SN or SR, we speculate that the norepinephrine deficiencies are not sufficient to induce or potentiate dopamine loss at this early stage.
A pertinent finding in this study is the significant reduction in LC norepinephrine concentration. This is consistent with previous data showing a reduction in the number of TH-labeled cells in the LC (Grant et al., 2015). The data are compatible with the RT qPCR data that show a significant increase in Comt expression; Comt mRNA codes for a protein involved in the inactivation of catecholamines. Previous work has found Comt mRNA present at high levels in neurons of the rat brain (Matsumoto et al., 2003). While we did not directly test for protein levels of COMT, in the 6-ODHA model of PD, COMT protein in dopaminergic terminals is unchanged suggesting that Comt is not located in the nigrostriatal projection neurons (Kaakkola et al., 1987). Additionally, COMT protein is not altered in the LC (via DSP-4 injection) noradrenergic terminals of the rat brain (Schendzielorz et al., 2013). These data suggest that the modulation of the LC noradrenergic neurons is a factor for early-onset behavior deficits in rodent PD models. Elevated levels of Comt in the Pink1 −/− rat may also alter the metabolism of levodopa, as there is evidence that COMT shortens the biological half-life. Translationally, COMT inhibitors block action of the enzyme involved in noradrenergic degradation and are often prescribed in conjunction with levodopa in the later stages of PD in order to make catecholamines more available to neurons. There was no significant difference in the relative expression of other catecholamine-related genes including Th, Mao-a, and Net. There was a upregulation trend for Dat in the SR in Pink1 −/− animals; as nigrostriatal deficits progress, compensatory mechanisms in the SR may be intensified (Robinson and Whishaw, 1988). The dysregulation of norepinephrine presented in this paper highlights the complexity within the catecholamine circuitry in this rodent model and suggests an important role for rescuing norepinephrine in early PD.
These data also suggest a relationship between noradrenergic dysfunction and ultrasonic vocalization production. Here, norepinephrine concentrations in the LC are positively correlated to percent of complex calls produced by Pink1 −/− rats, and previous data link norepinephrine loss to decreases in vocal intensity (Grant et al., 2015). These data stress the importance of the LC in vocalization production in rats. This is important because vocalization deficits emerge early in the progression of PD and standard pharmacological and surgical interventions aimed at treating nigrostriatal dopamine do not ameliorate voice deficits (Stewart et al., 1995, Harel et al., 2004b, Rusz et al., 2013). Thus, a rat model of norepinephrine dysfunction is useful for studying potential treatments.
The Pink1 −/− rat is a useful tool to study early-stage disease because rats with this genotype have observable sensorimotor deficits as well as brainstem pathology consistent with early-stage human PD (Dave et al., 2014, Grant et al., 2015, Kelm-Nelson et al., 2016b). In addition to classic motor deficits, compromised brainstem catecholaminergic systems can have significant impacts on sensorimotor behavior. Norepinephrine loss has been implicated in the early pathology of PD (Braak et al., 2003). We found a link to the degradation of vocalizations that are non-responsive to levodopa treatment and brainstem norepinephrine pathology. This study has important implications for using the Pink1 −/− rat to model early-stage PD, especially with regard to loss of norepinephrine and increases in Comt in the LC.
Research Highlights.
Levodopa has variable effects on limb and vocalization behavior in Pink1 −/− rats.
Pink1 −/− rats have reduced levels of norepinephrine in the locus coeruleus.
Pink1 −/− rats have increased norepinephrine concentrations in the substantia nigra.
Catecholamine protein concentrations are positively correlated with motor behavior and complex calls in Pink1 −/− rats.
Relative expression of Comt in the locus coeruleus is increased in the Pink1 −/− rat.
Acknowledgments
We gratefully acknowledge John C. Szot, Katie M. Yang, Jacob M. Lake, Forrest J. Stehula, Cagla Muslu, and Mackenzie L. K. Sinnen for their assistance with data acquisition and Drs. Tiffany Glass and Miranda Cullins for their assistance with manuscript editing.
Funding: F32 D014399 (Kelm-Nelson), R01 DC014358 (Ciucci), UW Department of Surgery Pilot Funds (Kelm-Nelson), UW Department of Surgery (Ciucci); IBS-SRP (Trevino).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure/Conflict of Interest: None
References
- Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195:646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- Baptista MAS, Dave KD, Sheth NP, De Silva SN, Carlson KM, Aziz YN, Fiske BK, Sherer TB, Frasier MA. A strategy for the generation, characterization and distribution of animal models by The Michael J. Fox Foundation for Parkinson’s Research. Dis Model Mech. 2013;6:1316–1324. doi: 10.1242/dmm.011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The locus ceruleus norepinephrine system: Functional organization and potential clinical significance. Neurology. 2009;73:1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched Environment Confers Resistance to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine and Cocaine: Involvement of Dopamine Transporter and Trophic Factors. J Neurosci. 2003;23:10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18(Supplement 1):S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Dekker MCJ, Vanacore N, Fabbrini G, Squitieri F, Marconi R, Antonini A, Brustenghi P, Dalla Libera A, De Mari M, Stocchi F, Montagna P, Gallai V, Rizzu P, van Swieten JC, Oostra B, van Duijn CM, Meco G, Heutink P. Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7. Neurol Sci. 2002;23:s59–s60. doi: 10.1007/s100720200069. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bredberg E, Lennernas H, Paalzow L. Pharmacokinetics of levodopa and carbidopa in rats following different routes of administration. Pharm Res. 1994;11:549–555. doi: 10.1023/a:1018970617104. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-khz ultrasonic vocalization in rats. Behav Neurosci. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study. Behav Brain Res. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of Bilateral Subthalamic Nucleus Stimulation and Medication on Parkinsonian Speech Impairment. J Voice. 2008;22:365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC, III, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MAS, Fiske BK, Sherer TB, Frasier MA. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70:190–203. doi: 10.1016/j.nbd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- de Lau LML, Giesbergen PCLM, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Boon P, Van Borsel J. Levodopa-induced alterations in speech rate in advanced Parkinson’s disease. Acta Neurol Belg. 2006;106:19–22. [PubMed] [Google Scholar]
- Delaville C, Deurwaerdère PD, Benazzouz A. Noradrenaline and Parkinson’s Disease. Front Syst Neurosci. 2011;5:31. doi: 10.3389/fnsys.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and Progressive Sensorimotor Anomalies in Mice Overexpressing Wild-Type Human α-Synuclein. J Neurosci. 2004a;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut P-O, Mehta A, DiCarlo CD, Seaman RL, Chesselet M-Fo. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004b;187:418–429. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The Science and Practice of LSVT/LOUD: Neural Plasticity-Principled Approach to Treating Individuals with Parkinson Disease and Other Neurological Disorders. Semin Speech Lang. 2006;27:283–299. doi: 10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- Grant LM, Kelm-Nelson CK, Hilby BL, Blue KV, Rajamanickam ESP, Pultorak J, Fleming SM, Ciucci MR. Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 knockout rat model of Parkinson disease. J Neurosci Res. 2015;93:1713–1727. doi: 10.1002/jnr.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Wang L, He D, Yang Q, Duan Z, Zhang X, Nie L, Yan X, Tang B. Clinical features and 11C-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurol Sci. 2011;32:35–40. doi: 10.1007/s10072-010-0360-z. [DOI] [PubMed] [Google Scholar]
- GÜVen M, Elalmis DD, Binokay S, Tan Ü. Population-level right paw preference in rats assessed by a new computerized forod-reaching test. Int J Neurosci. 2003;113:1675–1689. doi: 10.1080/00207450390249258. [DOI] [PubMed] [Google Scholar]
- Harel B, Cannizzaro M, Snyder PJ. Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: A longitudinal case study. Brain Cogn. 2004a;56:24–29. doi: 10.1016/j.bandc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ. Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment. J Neurolinguist. 2004b;17:439–453. [Google Scholar]
- Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted Training of Ultrasonic Vocalizations in Aged and Parkinsonian Rats. JOVE. 2011:e2835. doi: 10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakkola S, Mannisto PT, Nissinen E. Striatal membrane-bound and soluble catechol-O-methyl-transferase after selective neuronal lesions in the rat. J Neural Transm. 1987;69:221–228. doi: 10.1007/BF01244343. [DOI] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Brauer AFL, Barth KJ, Lake JM, Sinnen MLK, Stehula FJ, Muslu C, Marongiu R, Kaplitt MG, Ciucci MR. Characterization of early-onset motor deficits in the Pink1 −/− mouse model of Parkinson disease. Brain Res. 2017 doi: 10.1016/j.brainres.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Brauer AFL, Ciucci MR. Vocal training, levodopa, and environment effects on ultrasonic vocalizations in a rat neurotoxin model of Parkinson disease. Behav Brain Res. 2016a;307:54–64. doi: 10.1016/j.bbr.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Stevenson SA, Ciucci MR. Atp13a2 expression in the periaqueductal gray is decreased in the Pink1 −/− rat model of Parkinson disease. Neurosci Lett. 2016b;621:75–82. doi: 10.1016/j.neulet.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Stevenson SA, Ciucci MR. Data in support of qPCR primer design and verification in a Pink1 −/− rat model of Parkinson disease. Data in Brief. 2016c;8:360–363. doi: 10.1016/j.dib.2016.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Yang KM, Ciucci MR. Exercise Effects on Early Vocal Ultrasonic Communication Dysfunction in a PINK1 Knockout Model of Parkinson’s Disease. J Parkinsons Dis. 2015;5:749–763. doi: 10.3233/JPD-150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent AP, Stern GM, Webster RA. The effect of benserazide on the peripheral and central distribution and metabolism of levodopa after acute and chronic administration in the rat. Br J Pharmacol. 1990;100:743–748. doi: 10.1111/j.1476-5381.1990.tb14085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien MM, Briley M. Noradrenergic mechanisms in Parkinson’s disease. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol Behav. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JD, Said CM, Dillon LC, Hoffman M, Hughes AJ. Gait analysis in patients with Parkinson’s disease and motor fluctuations: Influence of levodopa and comparison with other measures of motor function. Mov Disord. 1998;13:900–906. doi: 10.1002/mds.870130607. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson G. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier; 2005. [Google Scholar]
- Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, Ellis C, Rodriguez AD, Burkhead LM, Rosenbek JC. Perceptual characteristics of Parkinsonian speech: A comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation. 2009;24:131–144. doi: 10.3233/NRE-2009-0462. [DOI] [PubMed] [Google Scholar]
- Ringel LE, Basken JN, Grant LM, Ciucci MR. Dopamine D1 and D2 receptor antagonism effects on rat ultrasonic vocalizations. Behav Brain Res. 2013;252:252–259. doi: 10.1016/j.bbr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. In: Parametric Measures of Effect Size In: The Handbook of Research Synthesis. Cooper H, V HL, editors. New York: Russel Sage Foundation; 1994. [Google Scholar]
- Rusz J, Čmejla R, Růžičková H, Klempíř J, Majerová V, Picmausová J, Roth J, Růžička E. Evaluation of speech impairment in early stages of Parkinson’s disease: a prospective study with the role of pharmacotherapy. J Neural Transm. 2013;120:319–329. doi: 10.1007/s00702-012-0853-4. [DOI] [PubMed] [Google Scholar]
- Ryczko D, Dubuc R. Dopamine and the Brainstem Locomotor Networks: From Lamprey to Human. Front Neurosci. 2017;11:295. doi: 10.3389/fnins.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir S, Spielman JL, Ramig LO, Story BH, Fox C. Effects of Intensive Voice Treatment (The Lee Silverman Voice Treatment [LSVT]) on Vowel Articulation in Dysarthric Individuals With Idiopathic Parkinson Disease: Acoustic and Perceptual Findings. J Speech Lang Hear Res. 2007;50:899–912. doi: 10.1044/1092-4388(2007/064). [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol. 2003a;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003b;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. Compulsive, abnormal walking caused by anticholinergics in akinetic, 6-hydroxydopamine-treated rats. Science. 1978;199:1461–1463. doi: 10.1126/science.564552. [DOI] [PubMed] [Google Scholar]
- Schendzielorz N, Oinas J-P, Myöhänen TT, Reenilä I, Raasmaja A, Männistö PT. Catechol-O-Methyltransferase (COMT) Protein Expression and Activity after Dopaminergic and Noradrenergic Lesions of the Rat Brain. PLoS One. 2013;8:e61392. doi: 10.1371/journal.pone.0061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord. 2000;33:59–88. doi: 10.1016/s0021-9924(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci. 2003;17:2586–2592. doi: 10.1046/j.1460-9568.2003.02684.x. [DOI] [PubMed] [Google Scholar]
- Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, Brin MF. Speech dysfunction in early Parkinson’s disease. Mov Disord. 1995;10:562–565. doi: 10.1002/mds.870100506. [DOI] [PubMed] [Google Scholar]
- Sung HY, Kim J-S, Lee K-S, Kim Y-I, Song I-U, Chung S-W, Yang D-W, Cho YK, Park JM, Lee IS, Kim SW, Chung I-S, Choi M-G. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord. 2010;25:2361–2368. doi: 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Takahashi S. The effects of L-threo-3,4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect. 1993;5:27–34. doi: 10.1007/BF02260912. [DOI] [PubMed] [Google Scholar]
- Vermeiren Y, De Deyn PP. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem Int. 2017;102:22–32. doi: 10.1016/j.neuint.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Yoshioka JG. Handedness in Rats. The Pedagogical Seminary and Journal of Genetic Psychology. 1930;38:471–474. [Google Scholar]


