Abstract
Current theory holds that macrochimerism is essential to the development of transplant tolerance. Hematopoietic cell transplantation from the solid organ donor is necessary to achieve macrochimerism. Over the last 10 to 20 years, trials of tolerance induction with combined kidney and hematopoietic cell transplantation have moved from the preclinical to the clinical arena. The achievement of macrochimerism in the clinical setting is challenging, and potentially toxic due to the conditioning regimen necessary to hematopoietic cell transplantation and due to the risk of graft-versus-host disease. There are differences in chimerism goals and methods of the three major clinical stage tolerance induction strategies in both HLA-matched and HLA-mismatched living donor kidney transplantation, with consequent differences in efficacy and safety. The Stanford protocol has proven efficacious in the induction of tolerance in HLA-matched kidney transplantation, allowing cessation of immunosuppressive drug therapy in 80% of study participants, with the safety profile of conventional transplantation. In HLA-mismatched transplantation, multi-lineage macrochimerism of over a year’s duration can now be consistently achieved with the Stanford protocol, with complete withdrawal of immunosuppressive drug therapy during the second posttransplant year as the next experimental step and test of tolerance.
Introduction
Tolerance, the specific absence of a destructive immune response to a transplanted tissue in the absence of immunosuppression, is necessary for successful complete immunosuppressive drug withdrawal following solid organ transplantation. Since Owen’s discovery of red blood cell chimerism and immune tolerance in fraternal twin cattle (1), the theory of acquired immune tolerance of Burnet (2), and the experimental work of Billingham, Brent and Medawar in neonatal mice (3, 4) over half a century ago, the creation of hematopoietic cell chimerism has been thought to be the pathway to transplant tolerance (5). In clinical kidney transplantation, the primary method used to achieve tolerance has been transplantation of hematopoietic cells in combination with transplantation of the kidney from the same donor. With this approach, tolerance and successful immunosuppressive drug withdrawal have been accomplished in living donor transplantation, in HLA-matched and in HLA-mismatched donor/recipient pairs.
Chimerism and its Types
Hematopoietic cell chimerism is defined as the presence of foreign (donor) hematopoietic cells in the blood circulation of the recipient (host) (Table 1). Complete chimerism is present when all the hematopoietic cells in the recipient are of donor origin. Mixed chimerism is the presence of a variable number of donor cells of multiple lineages in the recipient. Microchimerism is present when the donor cells in the recipient are below the level of detection by flow cytometry or by DNA-based short tandem repeat (STR) analysis (less than 1%) and can be detected only by more sensitive polymerase chain reaction (PCR) methods. Complete chimerism and mixed chimerism, referred to herein, are macrochimerism.
Table 1.
Chimerism and its types
| Chimerism |
| Presence of foreign (donor) hematopoietic cells |
|
|
| Complete chimerism |
| All hematopoietic (100%) are of donor origin |
|
|
| Mixed chimerism |
| Variable number of donor cells of multiple lineages (>1% donor type cells) |
|
|
| Microchimerism |
| Donor cells are below the level of detection by flow cytometry or STR analysis (<1%), and can be detected only by more sensitive PCR methods |
Requirements and Challenges to Achievement of Chimerism
In present day, hematopoietic cell transplantation (HCT) from the kidney donor is used for the achievement of chimerism. HCT necessitates a conditioning regimen to enable its engraftment. HLA disparity challenges engraftment. HCT carries risk of graft versus host disease (GVHD). A conditioning regimen carries risk of myelosuppression and its consequences.
Chimerism Goals in the Tolerance Induction Trials
Mixed chimerism, either transient or persistent, or complete chimerism is the goal of the current tolerance induction trials (Table 2). Complete chimerism, which is the goal of treatment directed at cancers of hematopoietic origin, replaces the recipient (host) immune cells with the donor (graft) immune cells. Host versus graft tolerance cannot be tested in complete chimerism given the absence of host immune cells. With the loss of host immune cells comes significant risk of graft versus host disease. In mixed chimerism both donor and recipient immune cells are present. Both host versus graft tolerance and the reverse, graft versus host tolerance, can be tested. And with mixed chimerism, there is less risk of graft versus host disease.
Table 2.
Chimerism goals in the tolerance induction projects
| Nature of replacement of recipient immune and blood-forming cells with donor cells | Complete | Mixed |
|---|---|---|
| Used to treat cancer | ✓ | |
| No recipient immune cells present | ✓ | |
| Both donor and recipient immune cells present | ✓ | |
| Host versus donor tolerance can be tested | ✓ | |
| Donor versus host tolerance can be tested | ✓ | ✓ |
| Significant risk of graft versus host disease | ✓ | |
| Significant risk of infection | ✓ | |
| Goal of Stanford protocol | ✓ |
The Major Tolerance Approaches in Clinical Kidney Transplantation and their Differences
There are presently three major clinical stage tolerance approaches using combined hematopoietic cell and kidney transplantation in the United States. The senior transplant center involved is Massachusetts General Hospital, which began its first clinical trial in 1998 (6,7). Stanford began its first trial in 2000 (8). Northwestern began in 2009 (9). Each of these centers is characterized by investigator leadership with decades of experience in preclinical work in tolerance induction. In an earlier tolerance trial of HLA-mismatched deceased donor kidney transplants, without donor hematopoietic cell transplantation, reported in 1989 by investigators at Stanford and the California Pacific Medical Center, tolerance with complete immunosuppressive drug withdrawal and without subsequent rejection was achieved in three recipients (10). Although the study showed that it was feasible to achieve tolerance in humans without chimerism, only a small minority of patients was completely withdrawn from immunosuppressive drugs. The stability of tolerance over 12 years after transplantation was reported for one of the three recipients who maintained good graft function after the complete withdrawal of immunosuppressive drugs (11).
While all of the more recent trials involve HCT in combination with kidney transplantation, the protocols employed differ in conditioning regimen and its timing, the nature of the HCT and its timing, and goal of type of chimerism (Table 3). Both the MGH and Northwestern protocols employ the more traditional approach to conditioning, a combination of chemotherapy and thymic (MGH) or total body (Northwestern) irradiation. Both also initiate the conditioning days prior to transplantation of the kidney, which restricts these tolerance induction protocols in their current forms to living donor kidney transplantation (living donor kidney transplantation is prescheduled, deceased donor transplantation is not). The Stanford protocol uses a conditioning regimen of rabbit antithymocyte globulin and total lymphoid irradiation that begins following transplantation of the kidney, allowing this approach to be used in deceased as well as living donor organ transplantation. In the MGH protocol, the HCT is unselected marrow, whereas in both the Stanford and Northwestern protocols the HCT is selected subsets of donor cells. In the case of the Northwestern protocol, a number of proprietary “facilitator” cells are added to the HCT. The HCT is given the day of kidney transplantation in the MGH protocol and given one day following kidney transplantation in the Northwestern protocol. In the Stanford protocol, the conditioning regimen ends the eleventh day following kidney transplantation, and the HCT is given that day.
Table 3.
Differences in the major tolerance strategies in clinical kidney transplantation
| Program | Conditioning Regimen | Timing of Conditioning Regimen | Hematopoietic Cell Transplant | Chimerism Goal |
|---|---|---|---|---|
| Massachusetts General Hospital 1998- | Chemotherapy + thymic irradiation | Pre-kidney transplant | Unselected | Transient mixed |
| Stanford 2000- | rATG + total lymphoid irradiation | Post-kidney transplant | Selected | Mixed |
| Northwestern 2009- | Chemotherapy + total body irradiation | Pre-kidney transplant | Selected + “facilitator” cell | Complete |
Transient mixed chimerism was the goal of the MGH protocol. Permanent chimerism was the goal of the Northwestern protocol. Mixed chimerism, ideally persistent, was the goal of the Stanford protocol.
As the MGH and Northwestern protocols and trials are discussed in further detail elsewhere in this special issue, their methods and results will not be further discussed here.
The Stanford Protocol
In the Stanford protocol, total lymphoid irradiation (TLI) is the cornerstone of the HCT conditioning regimen, based on work of Strober and colleagues that demonstrated a fivefold increase in survival of skin allografts without bone marrow transplantation following TLI in adult mice (12). TLI in combination with intravenous infusion of bone marrow cells on the day of the skin grafting resulted in long-term acceptance of the skin allografts. TLI was developed for treatment of Hodgkin’s disease (13), and targets the lymph nodes, spleen and thymus with multiple, small doses of irradiation, with other tissues shielded. It is an outpatient procedure and well tolerated. TLI, as opposed to total body irradiation (TBI), creates a tolerogenic microenvironment in the lymphoid tissues that prevents GVHD after bone marrow transplantation (14,15,16). TLI along with antithymocyte globulin contributes to significant naïve T cell depletion, but the more radio-resistant regulatory immune cells are less affected, resulting in dramatically increased ratio of immunosuppressive T regulatory cells, myeloid-derived suppressor cells, tolerogenic dendritic cells and NK T cells to naïve T cells (17,18,19,20,21). This effect, demonstrated in both animal models and humans, favors the tolerogenic milieu by inducing the suppressive cells to secrete suppressive cytokines, including IL-4, IL-10, arginase-1 and indoleamine 2,3-deoxygenase (IDO) (19,20,21). The total dose TLI used in the HCT conditioning regimen is 20–25% that of the total dose used to treat cancer.
In the Stanford protocol, the kidney donor must be willing to donate hematopoietic cells. This is done approximately six weeks prior to kidney transplantation. To mobilize cells from the marrow to the circulation, the donor is treated with five days of granulocyte-colony-stimulating factor, which is standard practice in HCT donation. This is followed by leukopheresis. The cell product is column-enriched for CD34+ hematopoietic progenitor cells. As they are necessary to engraftment, an add-back of CD3+ cells to the HCT product is made. The product is frozen and stored for transplantation.
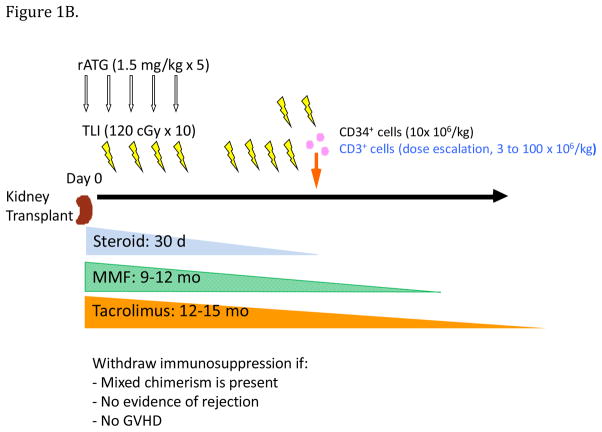
While the Stanford protocol is designed to allow its use in deceased donor transplantation, to date it has been used in only living donor transplantation. There are two trials of the protocol, HLA-matched (Figure 1A) and HLA-mismatched (Figure 1B). The primary difference in the two trials is the target CD34+ cell dose and CD3+ add-back of the HCT cell product. In the HLA-matched trial, the target CD34+ cell dose is ideally 10×106/kg recipient body weight, and the CD3+ add-back is 1×106/kg. In the HLA-mismatched trial, the target CD34+ cell dose is ideally no less than 10×106/kg, and the CD3+ add-back is currently 100×106/kg. The addition of donor T cells to facilitate the engraftment of hematopoietic cells has been demonstrated previously in preclinical models of bone marrow transplantation after TLI and ATG conditioning (15). The donor T cells eliminate host T cells that can reject the donor hematopoietic cells.
Figure 1.
Figure 1A. Stanford tolerance induction protocol; the recipient’s procedures in the HLA-matched trial. rATG, rabbit antithymocyte globin; TLI, total lymphoid irradiation; MMF, mycophenolate mofetil; GVHD, graft versus host disease
Figure 1B. Stanford tolerance induction protocol; the recipient’s procedures in the HLA-mismatched trial. rATG, rabbit antithymocyte globin; TLI, total lymphoid irradiation; MMF, mycophenolate mofetil; GVHD, graft versus host disease
Kidney transplantation occurs on a Monday, followed by initiation of the conditioning regimen of five doses of antithymocyte globulin and ten doses of TLI, and initiation of standard transplant immunosuppression. The recipient is discharged from the hospital Friday and returns the second week to complete the TLI condition regimen as an outpatient. After the final dose of TLI the second Friday (day 11 post-transplant), HCT, which is simply an intravenous infusion, is done.
A modification to standard transplant immunosuppression in the HLA-matched trial is that mycophenolate mofetil is not started until the day of HCT and then discontinued after 30 days.
With successful HCT engraftment, multi-lineage macrochimerism is evident within two weeks, but monthly monitoring of macrochimerism is begun four weeks after HCT. If multi-lineage, mixed macrochimerism persists for a minimum of six to nine months, without evidence of rejection or GVHD, immunosuppressive drug taper over several months is begun. Monitoring of macrochimerism continues during drug taper. Just prior to complete drug cessation, a surveillance biopsy of the transplant kidney is performed to ascertain there is no subclinical rejection.
In the HLA-matched trial, taper of the calcineurin inhibitor, which at that point is monotherapy, is begun between months six and nine post-transplant, with complete drug cessation at about 12 months. Loss of macrochimerism during the taper does not preclude drug cessation. In the HLA-mismatched trial, taper and withdrawal of mycophenolate mofetil occurs between months nine and 12, followed by taper of the calcineurin inhibitor between months 12 and 15. Loss of macrochimerism during drug taper in the HLA-mismatched trial now ceases the taper.
Following complete immunosuppressive drug withdrawal, weekly measurement of serum creatinine is done for a period of six to nine months. Thereafter the frequency of measurement is reduced to every two weeks, then to every three weeks and eventually to every four weeks, which continues indefinitely. At one year following drug cessation a second surveillance biopsy of the transplant kidney is done to ascertain absence of subclinical acute and chronic rejection.
Infection prophylaxis and monitoring are similar to that in conventional kidney transplantation. Antimicrobial prophylaxis consists of nystatin for one month, trimethoprim-sulfamethoxazole for one year, and valganciclovir for three months - six months if the recipient is at high risk for CMV infection. (If a recipient is at low risk for CMV infection, acyclovir is given in place of valganciclovir.) At the end of the course of CMV prophylaxis with valganciclovir, acyclovir is begun for herpes zoster prophylaxis and continued until 18 months post-transplant. Monitoring of blood for BK virus is done at months 1, 2, 3, 4, 5, 6, 9 and 12.
In the HLA-mismatched trial, monitoring for donor-specific antibody is done at months 6, 8, 12 and 18.
Exclusion criteria for the trials include primary kidney disease at high risk for recurrence (primary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, atypical hemolytic uremic syndrome), increased risk of post-transplant lymphoproliferative disease (EBV antibody-positive donor/EBV antibody-negative recipient), and age greater than 65 years in the HLA-match trial or greater than 60 years in the HLA-mismatched trial. Additional exclusion criteria for the HLA-mismatched trial include panel reactive antibody greater than 20%, the presence of donor-specific antibody, and donor/recipient ABO blood type mismatch.
The HLA-matched Trial
The HLA-matched trial began in 2005, as a proof-of-principle study following unsuccessful immunosuppressive drug withdrawal in the first version of the HLA-mismatched trial (see below) (17,18,22). To date there have been 29 transplantations in the HLA-matched trial.
Figure 2 shows four examples of multi-lineage, mixed macrochimerism achieved in the HLA-matched trial. Macrochimerism has been durable (patient #1), lost early - before six months (patient #3), lost late - after one year (patient #4), and lost after drug withdrawal (patient #5). Immunosuppressive drug cessation was successful in the setting of durable macrochimerism and in the setting of late loss of macrochimerism, either before or after complete drug withdrawal.
Figure 2.
Stanford tolerance induction protocol; examples of macrochimerism patterns in the HLA-matched trial.
Of the 29 recipients, 23 achieved multi-lineage, mixed macrochimerism of sufficient duration to allow immunosuppressive drug withdrawal. Twenty-two of the 23 enjoy rejection-free transplant kidney survival, up to nine years off drug. Of the 23, nine have not lost macrochimerism, and 14 lost macrochimerism after the first year. Of the 14 who lost macrochimerism, one sustained an acute rejection episode after being off drug for four years. The rejection responded to treatment with pulse corticosteroid, and the patient continues to do well, with excellent transplant kidney function on resumed maintenance immunosuppression.
Five of the recipients did not achieve macrochimerism sufficient to allow drug withdrawal and are doing well on maintenance immunosuppression. Four of the five were in the first nine transplantations. The most recently transplanted recipient is in drug taper.
There have been two deaths in the HLA-matched trial. Both recipients had been withdrawn from immunosuppression. The first was sudden death in a recipient with coronary artery disease, at three years off drugs. The second was sudden death in a recipient with type 1 diabetes mellitus, a 40-kg weight gain following transplantation, and sleep apnea, at one year off drug. There has been one recent loss of a transplant kidney, to recurrent lupus nephritis, 9 years following transplantation.
This trial has proven the protocol to be safe in HLA-matched transplantation. There has been no serious myelosuppression (neutrophil count less than 100/mm3) with the conditioning regimen, no engraftment syndrome, and no GVHD. The nature and frequency of infection has been no different than that encountered in conventional transplantation, aside from herpes zoster. The incidence of zoster has decreased since the adoption of prolonged prophylaxis with acyclovir. There has been no BK nephropathy. There has been one case of cancer, stage IA (T1b N0 M0) invasive ductal breast cancer diagnosed at month 23 post-transplant.
The success of this trial has caused us to now think of the protocol as our standard of care in HLA-matched, living donor, kidney transplantation. The promise of freedom from immunosuppressive drugs, and the efficacy and safety of the protocol, holds remarkable appeal to patients.
The HLA-mismatched Trial
The first version of the HLA-mismatched trial began in year 2000 (8,23). There were six transplantations done, ending in 2003. Two of the six recipients developed transient, low-level, multi-lineage, mixed chimerism, which at the time was considered sufficient to attempt immunosuppressive drug cessation. Drugs were stopped at approximately 12 months in the two, one a recipient of an unrelated transplant and the other a recipient of a one-haplotype match transplant. They remained off drugs for 3.5 and 5.5 months, respectively, before developing acute rejection manifest as a mild increase in serum creatinine, verified by transplant kidney biopsy. Treatment for acute rejection was given, and maintenance immunosuppression was resumed. The two patients continue to do well, with good transplant kidney function, 17 years and 16 years following transplantation.
The current version of the HLA-mismatched trial began in 2010 (8). Enrollment is restricted to one-haplotype match donor-recipient pairs. To date there have been 21 transplantations involving kidney and hematopoietic cells.
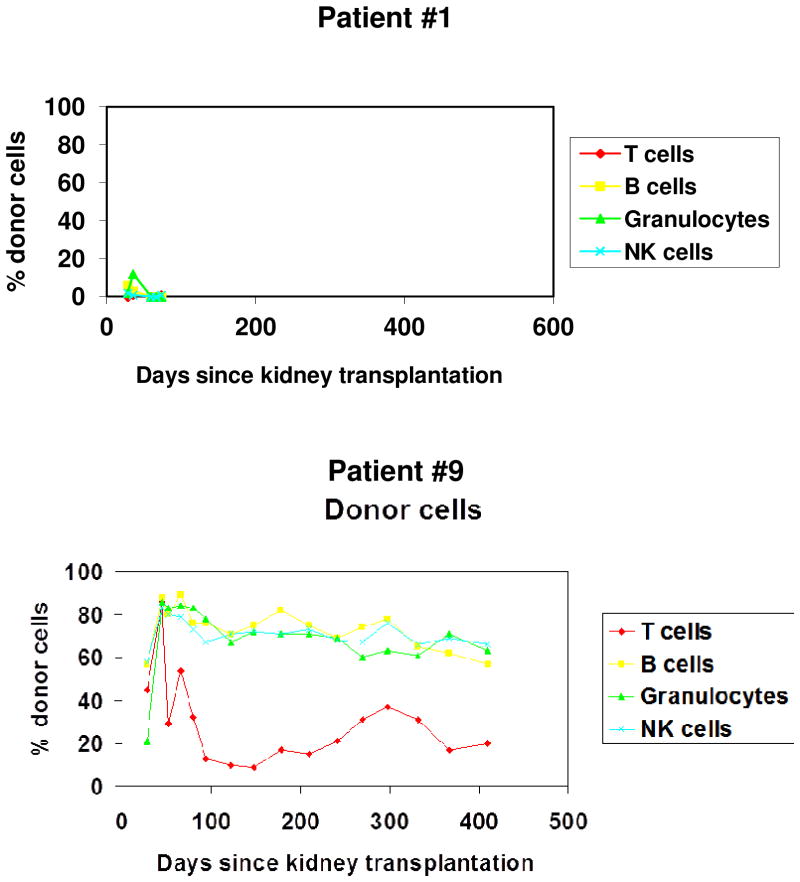
Figure 3 shows two examples of multi-lineage, mixed macrochimerism achieved in the current HLA-mismatched trial. The pattern seen in Subject 1 was transient and low level, reminiscent of that seen in the two patients in the first version of the trial in years 2000–2001. The CD3+ cell dose in this patient was 3×106/kg. The pattern seen in Subject 9 was achieved with a CD3+ cell dose of 50×106/kg.
Figure 3.
Stanford tolerance induction protocol; examples of macrochimerism patterns in the HLA-mismatched trial.
Of the 18 mismatched recipients followed for at least one year, nine thus far have achieved multi-lineage, mixed macrochimerism of sufficient duration to allow immunosuppressive drug withdrawal. The range of donor cell chimerism among whole blood cells at day 270 post-transplant was from 13 to 60% in the latter recipients. Macrochimerism has persisted during and following withdrawal of mycophenolate mofetil. However, chimerism was lost in six patients following withdrawal of tacrolimus to subtherapeutic blood levels or discontinuation. Similar to the experience of the first version of the trial in 2000–2001, one of these six patients did well for three months off immunosuppressive drugs before sustaining an acute rejection and returning to maintenance immunosuppression. Two others did well for shorter periods of time off immunosuppressive drugs before loss of macrochimerism prompted resumption of tacrolimus monotherapy. The remaining three never had evidence of rejection during tacrolimus tapering and are maintained on immunosuppressive drugs. Two patients with evidence of rejection developed donor-specific antibody that subsequently resolved on immunosuppressive drugs. The loss of chimerism after immunosuppressive drug withdrawal in the mismatched patients indicates that these patients had immune reactivity to donor alloantigens that eliminated the donor hematopoietic cells. The results suggest that permanent clonal deletion was not induced by prolonged chimerism during the first year, in contrast to the preclinical studies in cattle and mice (1–5).
Two recently transplanted patients are now off mycophenolate mofetil and are beginning taper of tacrolimus during their second transplant year.
There has been one death in the HLA-mismatched trial. The recipient had been withdrawn from mycophenolate mofetil and was in taper of tacrolimus. The death was due to pulmonary embolism that occurred after the development of autoimmune hemolytic anemia. There have been no other losses of a transplant kidney.
This trial has proven the protocol to be safe in HLA-mismatched transplantation. There has been no significant myelosuppression with the conditioning regimen. There have been two cases of engraftment syndrome (probably more aptly named dis-engraftment syndrome), manifest as acute transplant kidney dysfunction at the time of early loss of the donor cell chimerism (24). Both episodes resolved with corticosteroid treatment. There has been no GVHD. The nature and frequency of infection have been no different than that encountered in conventional transplantation, aside from herpes zoster. There has been no BK nephropathy. There has been one case of cancer, chronic myelogenous leukemia diagnosed at 38 months post-transplant, in a patient with a history of pre-transplant treatment of lupus nephritis with cyclophosphamide and antimetabolite drugs. The disease is in remission.
Stepwise escalation of the CD3+ dose, now at 100×106/kg, coupled with a minimum CD34+ dose of 10×106/kg, has yielded persistent multi-lineage, mixed macrochimerism of a year’s duration. However, thus far this macrochimerism has proven to be immunosuppression-dependent. In this trial we will soon learn if the protocol will yield immunosuppression-independent macrochimerism and tolerance in HLA-mismatched transplantation.
Conclusion
The Stanford protocol successfully achieves persistent macrochimerism, specifically multi-lineage, mixed chimerism, and transplant tolerance in HLA-matched, living 17 donor, kidney transplantation, allowing complete immunosuppressive drug withdrawal. Macrochimerism in this setting was not immunosuppression-dependent in one-third of the patients following cessation of transplant immunosuppression. Transplant tolerance in this HLA-matched setting is not macrochimerism-dependent; tolerance has almost uniformly persisted following loss of macrochimerism following immunosuppressive drug withdrawal.
The protocol now also successfully achieves persistent macrochimerism in HLA-mismatched, living donor, kidney transplantation. Successful immunosuppressive drug withdrawal has not yet been achieved. To date, macrochimerism in this setting has been immunosuppression-dependent; it has not persisted following cessation of transplant immunosuppression. In contrast to the experience in the HLA-matched trial, transplant tolerance in this setting may be macrochimerism-dependent. With this protocol in the HLA-mismatched setting, persistent macrochimerism may prevent acute and chronic rejection regardless of whether it is immunosuppression-dependent or –independent. Work continues to determine if these early observations are reproducible in larger numbers of patients.
Acknowledgments
We thank Dr. Maria Millan for the initial design of figure 1A. The research was supported by grants from the National Institutes of Health (P01HL075462 and R01 AI 1085024), a grant from the California Institute of Regenerative Medicine (CLIN2-09439), the Stanford Institute for Immunity, Transplantation and Infection, the John and Marsha Goldman Foundation, the John M. Sobrato Fund, the Robert and Patricia Moore Family Foundation, Sarah E. Stein and Michael A. Cohn, MD, and Stanford Health Care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 2.Burnet FM, Fenner F. The production of antibodies. Macmillan; Melbourne, Australia: 1949. [Google Scholar]
- 3.Billingham RE, Brent L, Medawar PB. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance. Phil Trans R Soc Lond B. 1956;239:357–414. [Google Scholar]
- 5.Simpson E. Medawar’s legacy to cellular immunology and clinical transplantation: a commentary on Billingham, Brent and Medawar (1956) ‘Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance’. Phil Trans R Soc B. 2015;370:20140382. doi: 10.1098/rstb.2014.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, Ballen K, Delmonico F, Saidman S, Sachs D, Cosimi AB. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–676. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao B, Wong W, Morris H, Lo Cascio SA, Sayre P, Shonts B, Williams WW, Jr, Smith R-N, Colvin RB, Sykes M, Cosimi AB. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scandling JD, Busque S, Shizuru JA, Lowsky R, Hoppe R, Dejbakhsh-Jones S, Jensen K, Shori A, Strober JA, Lavori P, Turnbull BB, Engleman EG, Strober S. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA-matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant. 2015;15:695–704. doi: 10.1111/ajt.13091. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal JR, Eliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, Mathew JM, Konieczna I, Ison MG, Galvin J, Mehta J, Badder MD, Abecassis MMI, Miller J, Gallon L, Ildstad S. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99:288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 10.Strober S, Dhillon M, Schubert M, Holm B, Engleman E, Benike C, Hoppe R, Sibley R, Myburgh JA, Collins G, Levin B. Acquired immune tolerance to cadaveric renal allografts. N Engl J Med. 1989;321:28–33. doi: 10.1056/NEJM198907063210106. [DOI] [PubMed] [Google Scholar]
- 11.Strober S, Benike C, Krishnaswamy S, Engleman EG, Grumet FC. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti-donor reactivity. Transplantation. 2000;69:1549–1554. doi: 10.1097/00007890-200004270-00005. [DOI] [PubMed] [Google Scholar]
- 12.Slavin S, Strober S, Fuks Z, Kaplan HS. Long-term survival of skin allografts in mice treated with fractionated total lymphoid irradiation. Science. 1976;19:1252–1254. doi: 10.1126/science.785599. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan HS. Hodgkin’s Disease. Harvard University Press; Cambridge, MA: 1972. pp. 216–279. [Google Scholar]
- 14.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, Laport GG, Stockerl-Goldstein KE, Johnston LJ, Hoppe RT, Bloch DA, Blume KG, Negrin RS, Strober S. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 15.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protect against graft-versus-host disease. Blood. 2009;113:4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1. 1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 17.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 18.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, Shizuru JA, Lowsky R, Engleman EG, Strober S. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12:1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581–1589. doi: 10.1182/blood-2011-08-371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hongo D, Tang X, Baker J, Engleman EG, Strober S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am J Transplant. 2014;14:2467–2477. doi: 10.1111/ajt.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hongo D, Tang X, Zhang X, Engleman EG, Strober S. Tolerogenic interactions between CD8+ dendritic cells and NKT cells prevent rejection of bone marrow and organ grafts. Blood. 2017;129:1717–1728. doi: 10.1182/blood-2016-07-723015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation (letter to the editor) N Engl J Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, Tan JC, Salvatierra O, Hoppe RT, Strober S. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Farris AB, Taheri D, Kawai T, Fazlollahi L, Wong W, Tolkoff-Rubin N, Spitzer TR, Iafrate AJ, Preffer FI, Lo Cascio SA, Sprangers B, Saidman S, Smith RN, Cosimi AB, Sykes M, Sachs DH, Colvin RB. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am J Transplant. 2011;11:1464–1477. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]