Abstract
Dendritic cells (DC) are rare, bone marrow (BM)-derived innate immune cells that critically maintain self-tolerance in the healthy steady-state. Regulatory DC (DCreg) with capacity to suppress allograft rejection and promote transplant tolerance in pre-clinical models can readily be generated from BM precursors or circulating blood monocytes. These DCreg enhance allograft survival via various mechanisms, including promotion of regulatory T cells. In nonhuman primates receiving minimal immunosuppressive drug therapy (IS), infusion of DCreg of donor origin, one week before transplant, safely prolongs renal allograft survival and selectively attenuates anti-donor CD8+ memory T cell responses in the early post-transplant period. Based on these observations, and in view of the critical need to reduce patient dependence on nonspecific IS agents that predispose to cardiometabolic side effects and renal insufficiency, we will conduct a first-in-human safety and preliminary efficacy study of donor-derived DCreg infusion to achieve early (18 months post-transplant) complete IS withdrawal in low-risk, living donor liver transplant recipients receiving standard-of-care IS (mycophenolate mofetil, tacrolimus and steroids). We will test the hypothesis that, although donor-derived DCreg are short-lived, they will induce robust donor-specific T cell hyporesponsiveness. We will examine immunological mechanisms by sequential analysis of blood and tissue samples, incorporating cutting-edge technologies.
Keywords: dendritic cells, cell therapy, liver transplantation, tolerance
1. Introduction
1.1. Clinical liver transplant tolerance
Despite its elusive nature, clinical ‘operational’ transplant tolerance (i.e. the maintenance of stable graft function following withdrawal of all immunosuppressive drug therapy (IS), without clinically significant detrimental immune responses and/or immune deficits) can occur “spontaneously,” albeit uncommonly, in liver allograft recipients. The mechanistic basis of liver transplant tolerance is not well-understood, though several mechanisms (including the tolerogenic function of donor-derived or host regulatory immune cells) have been suggested, based on rodent studies [1–4]. In the clinic, complete elective withdrawal of all IS in liver transplantation 6 months to 5 years post-transplant has been documented in numerous reports (adults and children), with an overall approx. 20% drug withdrawal success rate [5–7]. Acute rejection rates after IS withdrawal have ranged from 12–76% and have been generally mild and almost universally reversible. Chronic rejection has been rare (between 0 and 6%), and there has been an extremely low incidence of graft loss due to rejection [5, 6]. Recently, in a multi-center study of early post-transplant IS drug withdrawal (calcineurin inhibitor-based therapy; no induction) in liver transplantation [8], IS minimization starting 12–14 months post transplant was tolerated by the majority of patients, while complete IS withdrawal was achieved in 13% of those qualified for the minimization protocol. This degree of success provides a potential basis for assessment of the impact of innovative strategies aimed at improving the incidence of safe withdrawal of IS therapy and operational tolerance in human liver transplantation.
1.2. The emergence of regulatory immune cell therapy in organ transplantation
Currently, there is considerable interest in the potential of regulatory immune cell therapy, using innate or adaptive immune cells that maintain self-tolerance in the healthy steady-state, to promote the minimization of IS safe withdrawal of all IS in clinical organ transplantation [9]. Thus, for example, the ONE Study (www.onestudy.org) is coordinating efforts at several centers in Europe and the US designed to assess the safety and preliminary efficacy of regulatory immune cell infusion in (living donor) renal transplantation. These studies include the testing of autologous regulatory innate immune (myeloid) cells (regulatory macrophages [Mreg]; and regulatory dendritic cells [DCreg]) and regulatory adaptive immune cells (regulatory T cells [Treg]). In a separate recent pilot study performed in Japan, Todo et al [10] reported that infusion of an ex vivo-generated Treg-enriched autologous cell product early (2 weeks) after living donor liver transplantation in patients treated with mycophenolic acid (MPA), steroids and CNI, together with a single dose of cyclophosphamide on day 5 post-transplant, allowed successful IS drug weaning in 7/10 patients who, at the time of publication, remained IS drug-free 16–33 months post-transplant. Here, we describe the conceptual background and our approach to clinical testing of donor-derived (allogeneic) DCreg and planned accompanying mechanistic studies in adult living donor liver transplant recipients at The University of Pittsburgh Medical Center Starzl Transplantation Institute.
2. Properties of DCreg that make them promising cellular therapeutic agents in transplantation
2.1. Generation and characteristics of DCreg
DC are uniquely well-equipped bone marrow (BM)-derived professional antigen (Ag)-presenting cells (APC) of the innate immune system that promote self-tolerance in the healthy steady-state [11] and regulate both innate and adaptive immunity [12, 13]. Transcriptional determinants of tolerogenic and immunogenic states during DC maturation have been described [14]. Multiple subsets of rodent and human DC [15, 16] with the ability to regulate immune responses, including (in rodents) organ transplant rejection, graft-versus-host disease (GVHD) following hematopoietic (HSC) transplantation [17–19] and various autoimmune disorders [20] have been characterized. The most intensively studied are conventional, myeloid-lineage DC. Myeloid DC with tolerogenic properties can be generated readily from fresh or cryopreserved BM precursor cells or blood monocytes in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) ± IL-4, with the addition of one or more pharmacologic/biologic agents to stably inhibit their maturation and promote their tolerogenicity [21]. These agents include anti-inflammatory cytokines (IL-10; transforming growth factor β [TGFβ]), anti-sense oligonucleotides targeting costimulatory molecules, anti-inflammatory/IS drugs (CNI, rapamycin, mycophenolate mofetil, corticosteroids), vitamin (Vit)D3 and cyclic AMP inducers, in particular prostaglandin E2. At present, there is no consensus as to the optimal agent(s) to use for generation of DCreg for clinical use. Phenotypic characteristics of DCreg include low levels of MHC gene product (MHC class I and II) and T cell costimulatory molecule (CD80; CD86) expression and increased expression of T cell co-inhibitory ligands (e.g. programed death ligand-1 [PD-L1]) and death-inducing ligands (e.g. FasL). Their functional characteristics include low secretion of bioactive Th1 cell-driving IL-12p70 and comparatively high levels of anti-inflammatory cytokine (IL-10; TGFβ) production. In addition, DCreg have potential to expand or induce the de novo generation of Treg [17, 22, 23].
2.2. Promotion of graft survival by adoptive transfer of DCreg in rodents and non-human primates
In rodents, the adoptive transfer of ex vivo-generated donor-derived DCreg before transplant (in most studies, systemic infusion of DCreg 7 days before transplant has been maximally effective) prolongs organ or skin allograft survival and can promote Ag-specific tolerance, either in the absence of, or in combination with, short-term IS [17, 24–27]. A literature review specifically of the ability to DCreg to prolong liver allograft survival and promote transplant tolerance has been conducted [28]. The capacity of VitD3- or IL-10-treated mouse DC to regulate alloimmunity and inhibit graft rejection or GVHD has been well-documented [29, 30] and we have shown that VitD3- and IL-10-conditioned DCreg prolong graft survival in nonhuman primates (NHP) [31]. This has provided justification for testing VitD3/IL-10 DCreg in our clinical trial. It is also important to note that the use of conventional ‘standard of care’ (SOC) IS agents (MPA, CNI or steroid) together with DCreg, promotes long-term allograft survival in rodents [32–35]. Therefore, the route of administration, dose regimen and duration of dosing (single i.v. infusion of 2.5–10 × 106 donor DCreg/kg, 7days before transplantation) selected in our liver transplant clinical trial (see 3.1 below), are supported by experiments in pre-clinical models [17, 31]. Infusion of autologous DCreg, either pulsed or unpulsed with donor Ag, has also been shown to induce indefinite organ allograft survival in rodents [36]. Indeed, the efficacy of donor Ag-pulsed autologous DCreg in prolonging rodent allograft survival when administered one week and later post-transplant [34] suggests that this cell-based therapeutic approach could be generalized to deceased donor organ transplantation.
In heart-allografted mice, the therapeutic effect of pre-transplant infusion of donor-derived DCreg does not appear to depend on the in vivo persistence of intact donor DCreg [37] that are likely killed by host natural killer (NK) cells, but on the function of quiescent conventional host DC in secondary lymphoid tissue. Indeed, deletion of host DC prevents the therapeutic effect of donor-derived DCreg in mice [38]. These host DC acquire donor Ag via the direct pathway of allorecognition by cross-dressing [39, 40], or via the indirect pathway by Ag transfer from the donor DCreg (cross-priming). A role for donor-derived microvesicles (exosomes) released by DC may be an advantage, since it amplifies the effect of the infused DCreg. Consequently, T cell activation is reduced, indirect pathway T cell deletion occurs, CD4 T cell-B cell help is impaired, and anti-donor antibody (Ab) production by host B cells suppressed [41]. Independence of the immune regulatory effect of donor-derived DCreg on their persistence following systemic administration offers a potential advantage over other cell therapy approaches (e.g. infusion of Treg) that may be dependent on in vivo persistence/replication/function of the adoptively-transferred regulatory immune cells. Also, DCreg have the important ability to regulate CD4+ and CD8+ memory T cell (Tmem) responses [42–45],- a major barrier to the promotion of long-term allograft survival in larger species (NHP and humans).
In NHP, a single pre-transplant (day-7) infusion of donor-derived DCreg (3.5–10×106/kg) generated from purified CD14+ blood monocytes in the presence of VitD3 and IL-10 [46], safely prolongs the survival of life-sustaining MHC-mismatched renal allografts in recipients given a minimal, short-term IS regimen of costimulation blockade (cytotoxic T lymphocyte Ag 4-immunoglobulin; CTLA4Ig [abatacept]) and rapamycin [31]. There was no evidence of adverse effects or of host sensitization, as determined by lack of circulating anti-donor Ab. Moreover, DCreg infusion was associated with attenuation of donor-specific alloreactive Tmem responses. Thus, concomitant expression of CTLA4 (CD152) and programed death-1 (PD1),- both markers of senescence/exhaustion, on circulating donor-reactive CD4+ and CD8+ Tmem cells, 4 weeks post-transplant was enhanced significantly and selectively in those animals given DCreg [31]. At the same time post-transplant, examination of the allograft revealed that DCreg infusion enhanced CTLA4 expression by graft-infiltrating CD4+ and CD8+ T cells compared with non-infused controls [47], suggesting their attenuation in the former group.
2.3. Testing of human DCreg safety and function in vivo
2.3.1. Vaccination and autoimmunity
Human DCreg inhibit naïve and memory T cell responses in vitro [45, 48]. Moreover, in normal healthy human volunteers, local adoptive transfer (subcutaneous injection) of monocyte-derived autologous immature DC inhibits Ag-specific effector T cell responses to cognate Ags (flu matrix peptide and keyhole limpet hemocyanin) and induces Ag (peptide)-specific CD8+ Treg [49, 50]. Various pharmacologic agents and cytokines have been used to generate good manufacturing practice (GMP) grade human DCreg for prospective clinical use in chronic inflammatory diseases [51–56]. Phase I clinical studies in autoimmune disorders have shown that autologous (including autoAg-pulsed) DCreg administered locally are well-tolerated in patients with rheumatoid arthritis (RA) [55, 57] or type-1 diabetes [54], and when administered intraperitoneally in refractory Crohn’s disease [58], with preliminary evidence of biological activity in a recent RA study [55]. A similar approach is envisaged in multiple sclerosis [59]. The results of these studies are summarized in Table 1 and emphasize the feasibility, safety and potential efficacy of DCreg therapy in patients with immune-mediated disease.
Table 1.
Testing of human DCreg† safety and function in healthy volunteers and autoimmune disease patients
| Subjects | n | DC treatment | Ag pulsing | Route (no. DCreg administered) | Effects observed |
|---|---|---|---|---|---|
| Healthy volunteers | 2 | Immature DC | Influenza matrix peptide (MP); keyhole limpet hemocyanin | s.c. (2.106) | Well-tolerated; no toxicity; specific inhibition of MP-specific CD8+ T effector function; induction of MP-specific CD8+ Treg [49, 50] |
| Type-1 diabetes | 7 | anti-sense oligonucleotides targeting CD40, CD80 and CD86 | None | i.d. (10.106 every 2 wks for 4 administrations) | No adverse effects [54] |
| Rheumatoid arthritis | 18* | NF-κB inhibitor | Four citrullinated peptide Ags | i.d. (7.2.103 to 1.7.104 or 2.7.104 to 6.2.104/kg [0.6.106–4.5.106] | Safe; increased Treg: Teff cells, reduced pro-inflammatory cytokines and chemokines in serum [55] |
| Rheumatoid arthritis | 9 | VitD3 + dexamethasone + monophosphoryl lipid A | Autologous synovial fluid as source of autoAgs | Arthroscopically (1.106, 3.106 or 10.106) | safe; symptoms stabilized in 2 patients given 10.106 DCreg; no systemic clinical or immunoregulatory effects detected [57] |
| Crohn’s Disease | 12 | VitA + dexamethasone then IL-1β, IL-6, TNFα and PGE2 for final 24h of culture | None | i.p. (2.106, 5.106 or 10.106 each either ×1 or biweekly ×3) | Safe; no clear signal of clinical efficacy [58] |
Autologous DCreg
HLA risk genotype-positive patients
2.3.2. Organ (kidney) transplantation
Autologous DCreg infusion, 1 day before transplant, is under examination at the University of Nantes, France, in live donor renal transplantation with SOC triple drug IS (azathroprine, steroid, tacrolimus) as part of the ONE Study. Also in the ONE Study, a related but donor-derived regulatory myeloid cell population (regulatory macrophages; Mreg) given 7 days before transplant, is currently being tested in the same context at the University of Regensburg, Germany. In several publications [60–62], the University of Regensburg group has reported on >20 renal transplant recipients infused once with donor-derived Mreg (referred to in their early publications as “transplant acceptance-inducing cells”), either 7 days before or after transplant. In their most recent (2011) report [63], two patients given donor-derived Mreg (7–8 × 106/kg) i.v. 6 or 7 days before transplant, in combination with azathioprine, tacrolimus and steroid, were minimized to low-dose tacrolimus monotherapy within 24 weeks of transplantation and subsequently maintained excellent graft function. After central venous administration, most of the infused Mreg in these two patients remained viable and trafficked (visualized by radio indium labeling) from the pulmonary vasculature via the blood to the liver, spleen and BM (within 30 hr). This pattern resembled the migration of donor-derived DCreg infused pre-transplant to host secondary lymphoid tissue in a mouse organ transplant model [37]. No adverse effects were observed in these 2 patients, with 3 yr follow-up. Although conducted in a small number of transplant patients, these studies of donor-derived Mreg support the feasibility of our trial, i.e., to administer similar numbers of donor-derived DCreg systemically, 7 days before live donor liver transplant, in combination with MPA, tacrolimus and steroid.
2. Clinical testing of DCreg in live donor liver transplantation
The possibility that DCreg administration as a novel adjunct induction therapy, may promote immunological mechanisms conducive to induction of donor-specific T cell hyporesponsiveness (tolerance) and enable early withdrawal of all IS after liver transplantation carries the potential great advantage of sparing patients the side effects of long-term IS, particularly CNI. Liver transplantation provides a particular opportunity to achieve this goal since liver grafts are better tolerated by the host’s immune system than other solid organ grafts, with IS withdrawal possible in ~20% of patients overall (in the absence of any experimental, immunomodulatory therapy) compared to <5% in renal transplant recipients. Increasing the proportion of liver transplant recipients that can be withdrawn successfully from IS through the administration of donor-derived DCreg could reap significant health benefits for this patient population. Importantly, no similar cellular therapy is currently available to attempt operational tolerance induction in liver transplantation.
3.1. Clinical trial design
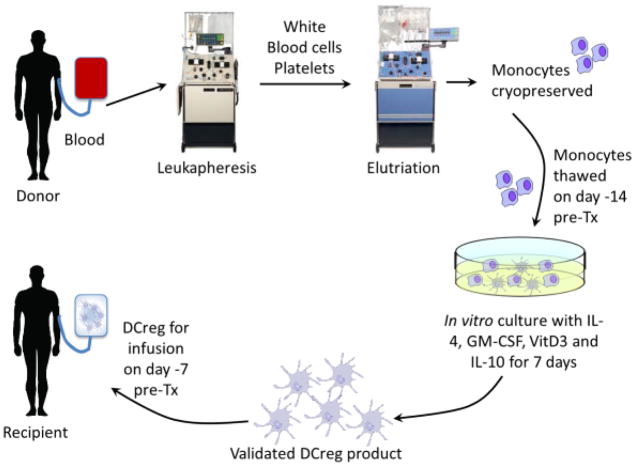
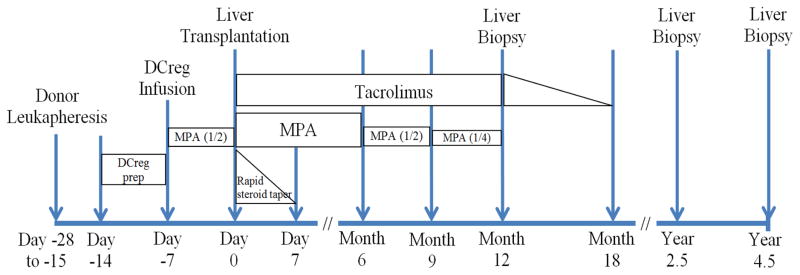
We are initiating an IND- and IRB-approved, first-in-human, single center, open-label, phase I/II study to test the safety and preliminary efficacy of a single infusion of donor-derived DCreg in de novo adult living donor liver transplant recipients. Patients will receive MPA, steroid and tacrolimus, without Ab induction. DCreg will be generated in VitD3 and IL-10 from monocytes obtained by leukapheresis from prospective (non-mobilized) living liver donors and infused as induction therapy into their respective recipients, 7 days before transplant (Fig. 1). The DCreg dose range proposed (2.5–10 × 106/kg) corresponds to the range in which both safety and efficacy were demonstrated in our NHP transplant model [31]. A half dose of MPA will be administered concomitant with DCreg infusion and until the time of transplant. Patients will then receive SOC IS. In eligible patients, determined by permissive liver function tests and (at 12 months) a permissive liver biopsy [64] IS weaning will begin at month 6 and continue through month 18. Follow-up will continue for 3 years following the last dose of IS. Overall study duration for 12 patients will be 6 yr (1.5 yr accrual + 4.5 yr follow-up). During follow-up, clinical data along with peripheral blood (serum and PBMC), host lymph node (at the time of graft implantation) and both SOC protocol and for-cause graft biopsies will be collected and analyzed. The clinical protocol is summarized in Fig. 2.
Fig. 1.
Generation of donor-derived DCreg for infusion into liver allograft recipients.
Fig. 2.
Protocol for clinical trial of donor-derived DCreg with immunosuppressive drug withdrawal in adult, live donor liver transplantation.
We will observe each of the first 3 patients 3 months apart after DCreg infusion before administering DCreg to the next patient. This initial slow pacing of recruitment builds in time for a thorough evaluation of safety on a patient-by-patient basis. Assuming no safety concerns, the remaining patients will be recruited on a rolling basis with ~1 month lag between patients for logistical reasons (e.g. scheduling leukapheresis and DCreg preparation).
3.2. Primary and secondary endpoints
The primary and secondary endpoints of the trial are listed in Tables 2 and 3 respectively.
Table 2.
Primary endpoints
A. Safety: will be determined by assessing the proportion of subjects who experience the following events:
|
| B. Preliminary Efficacy: will be determined by the proportion of patients able to achieve staged IS withdrawal and operational tolerance 1 yr after complete IS cessation based on liver biopsy criteria of the 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology [63] |
Common Terminology Criteria for Adverse Events
Table 3.
Secondary endpoints
| A. DSA levels will be measured early (<6 weeks) and late (>6 weeks) after transplant |
| B. In vitro immunological studies will address: Impact of DCreg infusion on recipients’ immune response to donor alloAgs as measured by:
|
| C. We will assess the fate and longevity of the infused donor DCreg in the circulation and lymphoid tissue* of the recipient. |
obtained at the time of graft implantation
4. Mechanistic studies of DCreg-infused liver allograft recipients
The mechanistic studies that we will undertake will serve two important purposes: (i) to detect early efficacy signals (e.g., specific suppression of anti-donor immune responses by the infused DCreg) and (ii) to guide design of future phase II trials in terms of (a) the need for higher or additional DCreg cell dosing (e.g., transient induction of donor-specific hyporesponsiveness would suggest that repeated DCreg dosing may be advantageous), and (b) which mechanistic studies are best suited for immune monitoring of the graft recipient. Samples for analysis will be collected before DCreg infusion and prospectively at subsequent time-points. Blood and tissue samples from age-matched healthy subjects and from SOC liver transplant patients (receiving MPA, tacrolimus and steroid, without Ab induction) that do not receive DCreg will be collected and used as comparator groups for the mechanistic assays.
4.1. Mechanistic hypothesis
Our trial will test the hypothesis that, although infused donor-derived DCreg may be short-lived [37, 65], they will induce donor Ag-specific T cell hyporesponsiveness, while preserving T cell recall responses to nominal Ags. This could be mediated by decreased donor-specific CD8+ Tmem frequencies due to a possible combination of mechanisms of clonal regulation/exhaustion/anergy or deletion (Table 4), resulting in residual low proliferation, interferon (IFN)-γ and granzyme B (GrB)/Perforin production, but with preserved responses to Epstein Barr virus (EBV) and tetanus toxoid (TT). While DCreg are effective in blunting responses of Tmem [31, 43–45, 48] and de novo-primed naive T cells [17, 41], it remains unclear how long after infusion donor-derived DCreg can be detected and which mechanism(s) may contribute to inducing donor-specific T cell hyporesponsiveness (Table 4). To address these mechanistic questions, blood and tissue samples will be analyzed in order to: i) track the survival of the infused donor-derived DCreg in blood and/or host lymph node tissue (at the time of graft implantation); ii) characterize the phenotype, memory differentiation, and function of CD8 T cells; both CD4 T conventional (Tconv) and T follicular helper (Tfh) cells, - Th subsets that may play distinct roles in promoting cellular and humoral (donor-specific Ab; DSA) alloimmunity respectively after transplantation, and Treg (including Foxp3 demethylation status); iii) assess donor-reactive T cell clonality and function; iv) characterize cellular infiltrates and effector and regulatory molecules in protocol and for-cause biopsy samples; v) perform mass cytometry (CyTOF; [66]) that greatly increases the number of immune cell phenotypic and functional markers that can be probed simultaneously, to enhance mechanistic insights; vi) perform serology to assess the presence and nature of DSA before and after transplant. Use of state-of-the-art techniques will allow valuable insights into mechanistic pathways triggered by DCreg infusion that may be responsible for promoting operational tolerance.
Table 4.
Proposed mechanisms of T cell hyporesponsiveness induced by DCreg infusion
| T cell | Exhaustion | Anergy | Regulation | Deletion |
|---|---|---|---|---|
| Progressive state of functional hyporesponsiveness in response to chronic Ag challenge | Quick state of functional hyporesponsiveness in response to TCR signaling in the absence of costimulatory signals | Modulation of immune responses inducing tolerance Treg: thymic-derived Tr1: memory Treg cells induced in periphery |
Terminally differentiated state post-exhaustion or anergy due to apoptosis | |
| Phenotype | PD-1hi ; TIM3 hi; CTLA4hi | Treg:CD25hiCD127lo HELIOS, CD39, CTLA4 Tr1: CD49b, LAG-3, CTLA4, PD-1;TIGIT |
||
| Function | IL-2lo TNF-α lo IFN-γlo Proliferationlo | IL-2lo Proliferationlo | Treg: TGFβ; IL-10 Tr1: IL-10; IFN-γ |
|
| Transcription factors | Tbet hi/Eomeshi GATA3, BATF | Treg: Foxp3+; TSRD demethylation Tr1: Foxp3−; EOMES+/T-bet+ |
||
| CyTOF | Phenotypic and functional analyses enhanced by identification of impaired signaling pathways (e.g. AKTlo; protein tyrosine phosphatases (PTP)hi; STAT5lo; STAT3hi;) | |||
| TCR cloning | Pre-Tx alloreactive TCR clones maintained or expanded | Pre-Tx alloreactive TCR clones maintained | Pre-Tx alloreactive TCR clones maintained or expanded | Pre-Tx alloreactive TCR clones decreased/ lost post-Tx |
4.2. Proposed mechanistic assays
4.2.1. Flow cytometry
(a) Persistence of donor DCreg after infusion
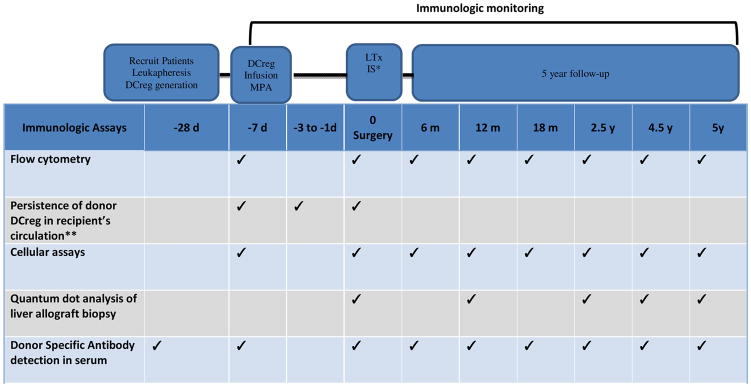
Previously [67] we have detected donor T cells in blood of transplant recipients by flow cytometry, with threshold sensitivity of one donor cell in 1000 recipient cells (0.1%). The conditions under which donor-derived DCreg can be tracked are as follows: a) detection of donor-specific HLA that can be detected and b) Y chromosome detection in male to female transplants. We will identify donor DCreg in whole blood (and host lymphoid tissue, obtained 7 days post-infusion, i.e. immediately before graft implantation) by flow staining for Lin− DR+BDCA1(CD1c)+CD11c+ DC, in conjunction with staining for a mis-matched donor MHC allele at the time-points indicated in Table 5.
Table 5.
Schedule of immunologic monitoring events in liver transplant recipients receiving DCreg
The plan for weaning immunosuppression (IS) is depicted in Figure 2. Standard of care IS will be restored if rejection occurs after IS weaning.
Before implantation (0 d), a lymph node will be harvested to determine if donor DCreg migration to secondary lymphoid tissue of the recipient can be detected.
(b) Frequency, phenotype and memory differentiation profiles of host T cells
We will assess the proportions and absolute numbers of CD3+CD8+ T cells, CD3+CD4+CXCR5+ Tfh cells and CD3+CD4+CXCR5− Tconv helper cells, as well as of Treg (CD4+CD25hiFoxp3+CD127− CD39+ Helios+ TIGIT [T cell receptor with Ig and ITIM domains]+) in peripheral blood before and after DCreg infusion, as indicated in Table 5 and determine: (i) their memory status based on CD45RO/CD62L/CCR7 expression; (ii) their polarization based on CCR6+ (Th17), CXCR3+ (Th1) and CXCR3−/CCR6− (Th2) expression; (iii) their expression of co-stimulatory (CD28, ICOS [inducible T cell co-stimulator] and CD40L) and co-inhibitory (PD-1, TIM3 [T cell Ig and mucin domain 3] and CTLA-4) receptors that are up-regulated on recently-activated or regulatory/exhausted T cells and are critical for cross-talk with DC, in conjunction with expression of Annexin V/7-AAD to track apoptosis. We will also track Epstein Barr virus (EBV)-specific CD8+ T cells and tetanus toxoid (TT)-specific CD4+ T cells as controls for recall responses.
(c) Functional polarization of circulating T cells and their donor specificity
Short (24 hr) stimulation of whole blood with (i) SEB (Staphylococcal Enterotoxin B; non-specific stimulation), (ii) donor-derived cell lysate (indirect pathway) and CD3-depleted donor cells (direct pathway) (alloAg-specific) or (iii) third-party Ags will allow us to measure T cell cytokine release by flow cytometry. Specifically, we will gate on memory CD8+ T cells, CD4+ Tfh and CD4+ Tconv subsets and further on CXCR3+CCR6− (Tc1/Th1), CXCR3−CCR6− (Tc2/Th2) and CXCR3−CCR6+ (Tc17/Th17 cells), and determine intracellular (i/c) expression of IFN-γ, IL-2, IL-4, IL-17, IL-21, IL-10, GrB/Perforin, as well as co-expression of the T box transcription factors T-bet/Eomes, GATA-3, Bcl-6, BATF (basic leucine zipper transcription factor) and ROR-γt to confirm their lineage identity. Stimulation with EBV peptide and TT-peptide mixtures (that span multiple HLA alleles) will also be performed, followed by similar screening, as above. These data, generated ex vivo, will allow us assess the potential mechanism(s) of hyporesponsiveness, since it is recognized that no single biomarker can be used to define the state or anergy, regulation or exhaustion.
(d) Deep profiling of immune cell subsets
Mass cytometry using CyTOF enables comprehensive profiling of the phenotypic and functional details of patient immune systems and offers to enhance discovery of predictive biomarkers [66, 68, 69]. In consultation with The Human Immune Monitoring Center at Stanford University, we will use a custom-made CyTOF immunophenotyping panel with heavy metal ion-tagged Abs, standardized at Stanford, to ascertain the additive value of using CyTOF as a complementary approach to immune cell subset (PBMC and potentially graft-infiltrating cell) characterization (immunophenotyping, i/c cytokines, signaling pathways) in the liver transplant patients receiving DCreg infusion and controls.
4.2.2. Cellular assays
(a) Donor-specific clonality of circulating CD4+ and CD8+ T cells
To track donor-reactive T cells before and after DCreg infusion, and also in control patients without DCreg infusion, we will use a deep sequencing approach that identifies the CD8+ and CD4+ Tconv and Tfh cell repertoire by amplifying the T cell receptor (TCR) CDR3 regions with primers designed for all 54 known Vβ-regions and all known 13 Jβ sequences, using ImmunoSEQ in a solid phase PCR and high throughput sequencing (Adaptive Biotechnologies, Seattle WA). To accomplish this, we will sort (by flow cytometry) naïve (N=CD45RO−CD62L+) and memory (central memory CM=CD45RO+CD62L+; effector memory EM=CD45RO+CD62L−; terminally-differentiated effector memory TemRA=CD45RO−CD62L−) CD8+, Tconv and Tfh CD4+ T cells and Treg from recipients before DCreg infusion (day-7) and label them with the proliferation dye carboxyfluorescein succinimidyl ester (CFSE). We will then incubate each cell subset with PKH-16-labeled, CD3-depleted donor PBMC in a CFSE-MLR for 5 days. Proliferating donor-reactive T cells identified in each memory/naïve subset will be used for genomic DNA isolation and subsequently for high-throughput TCR deep sequencing. We will then search for TCR sequences detected before DCreg infusion in the recipient’s post-transplant blood CD8+ and CD4+ T cell subsets. This will allow us assess the potential mechanism of hyporesponsiveness induced by DCreg infusion and distinguish deletion from regulation/anergy/exhaustion.
(b) Gene expression profiles of DCreg-induced CD4 and CD8 T cells
To further characterize T cell subsets induced by DCreg infusion, microarrays will be performed on purified RNA from sorted CD4 and CD8 T cells hybridized on Affymetrix H133 2.0 Plus Array platforms. Results as data sets will be curated and genes that are significantly differentially expressed plotted as hierarchical cluster heat-maps. Differentially-expressed gene lists will be imported into Ingenuity Pathway Analysis (Ingenuity, Redwood City, CA) for pathway analysis. Distinct transcriptional profiles will be compared within each patient pre- and at different times post-transplant (3 month, 1 and 2 yr), as well as among DCreg-infused patients and to those from control patients (no DCreg infusion) acquired at the same time-points. Specifically, inflammatory/anti-inflammatory pathways, Treg-associated markers and transcriptional regulator markers will be assessed. In addition, identification of unexpected novel genes expressed differentially in T cells as a result of DCreg infusion will be assessed. Gene expression array results will be confirmed by RT-PCR measurements of cytokines/chemokines found to be up-regulated or down-regulated in the array. The data may help enhance identification of the relationship between anti-inflammatory pathways and mechanisms that may control donor-reactive T responses leading to induction of hyporesponsiveness (regulation/anergy or exhaustion).
4.2.3. Histological analysis of graft and lymphoid tissue
Changes that occur within the liver graft, both in terms of structure and cellular content, will provide insight into the interactions between the recipient’s immune system and the transplant and will inform as to whether these interactions are beneficial or detrimental to the graft. To investigate this, fresh frozen or formalin-fixed, paraffin-embedded liver allograft tissue will be immunostained using multiplex quantum dot staining, followed by whole slide automated image analysis [70–72]. Staining panels with anti-CD45RO, anti-CCR7, anti-CD8 and anti-CD3 Abs in conjunction with CXCR3, CXCR6 or CXCR5, Foxp3, Tbet/Eomes and GrB/Perforin staining will be designed. We will specifically enumerate T cell subset infiltrates. Results will be expressed as numbers of positive cells/mm2, or as T cell-APC clusters, and compared to cellular infiltrate results from biopsies obtained from control patients that did not received DCreg infusion. C4d deposition will also be investigated.
A one-time excision of an abdominal lymph node as well as a fragment of the excised native liver will be obtained from the recipient at the time of graft implantation (i.e. 7 days after DCreg infusion). This will allow us characterize secondary lymphoid organ cellular infiltrates,-specifically the presence and location of infused DCreg and their possible interaction with host T cells. We will perform the same quantum dot staining and analyses on lymph node tissue, as described above by in situ immunostaining. We will add to the mAb T cell panel detailed above anti-CD11c and anti-HLA-DR mAbs, paired when possible, with staining that distinguishes the HLA mismatch between the recipient and donor, to distinguish recipient DC from infiltrating donor-derived infused DCreg, and their interaction with recipient T cells.
4.2.4. Donor-specific Ab
The tissue type of the donor and recipient pairs will be ascertained to enable interpretation of DSA results and to promote understanding of Treg responses. To identify and characterize circulating DSA IgG subclass distribution and C1q binding ability in the case of post-DCreg infusion sensitization, we will use single Ag bead assay and Luminex platforms as described [73] to determine DSA specificity, mean fluorescence intensity and titer and their C1q-fixing ability. DSA subclass will be assessed by a modified single Ag assay using mAbs specific for IgG1–4 subclasses [74]. These techniques will allow in-depth understanding of the potential pathogenicity versus non-inflammatory nature of DSA if sensitization occurs post DCreg infusion or post-liver transplantation.
5. Conclusions
Based on extensive preclinical data and their safety profile in preliminary human studies, DCreg show considerable promise for control of T cell responses and the therapy or organ allograft rejection. In liver transplantation, operational tolerance is more readily achieved that with other types of organ allograft, making this a suitable clinical scenario in which to first test the safety and preliminary efficacy of donor-derived DCreg and their capacity to enhance the regulation of host immune responses to donor. Our mechanistic studies are designed to identify immunological mechanisms responsible for promotion of operational tolerance and biomarkers that may be predictive of this state.
Acknowledgments
Our studies have been supported by National Institutes of Health grants R34AI123033 (AWT), R21AI116746 (DMM) and U01TR000005 (AWT), the Starzl Transplantation Institute and University of Pittsburgh Medical Center Enterprises.
Abbreviations
- CNI
calcineurin inhibitor(s)
- DC
dendritic cell(s)
- DCreg
regulatory DC(s)
- IS
immunosuppressive(ion)
- SOC
standard of care
- Treg
regulatory T cell(s)
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 2.Knechtle SJ, Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin Liver Dis. 2009;29:91. doi: 10.1055/s-0029-1192058. [DOI] [PubMed] [Google Scholar]
- 3.Yokota S, Yoshida O, Ono Y, Geller DA, Thomson AW. Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl. 2016;22:536. doi: 10.1002/lt.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate anti-donor T cell responses in mouse liver transplant tolerance. Hepatology. 2017 doi: 10.1002/hep.29529. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 6.Londono MC, Rimola A, O’Grady J, Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J Hepatol. 2013;59:872. doi: 10.1016/j.jhep.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Feng S. Spontaneous and induced tolerance for liver transplant recipients. Curr Opin Organ Transplant. 2016;21:53. doi: 10.1097/MOT.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 8.Shaked A, Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, et al. Early post-transplant immunosuppression (IS) withdrawal - final outcomes of the ITN030ST AWISH Study. Am J Transplant. 2016;16:269. [Google Scholar]
- 9.Geissler EK, Hutchinson JA. Cell therapy as a strategy to minimize maintenance immunosuppression in solid organ transplant recipients. Curr Opin Organ Transplant. 2013;18:408. doi: 10.1097/MOT.0b013e328363319d. [DOI] [PubMed] [Google Scholar]
- 10.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 11.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 14.Vander Lugt B, Riddell J, Khan AA, Hackney JA, Lesch J, DeVoss J, et al. Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J Cell Biol. 2017;216:779. doi: 10.1083/jcb.201512012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 16.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 17.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 18.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers NM, Isenberg JS, Thomson AW. Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am J Transplant. 2013;13:1125. doi: 10.1111/ajt.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 2010;29:156. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 21.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25−/loFoxp3− effector T cells. J Immunol. 2010;185:5003. doi: 10.4049/jimmunol.0903446. [DOI] [PubMed] [Google Scholar]
- 23.Raker VK, Domogalla MP, Steinbrink K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Seminars in Immunology. 2011;23:252. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill M, Cuturi MC. Negative vaccination by tolerogenic dendritic cells in organ transplantation. Curr Opin Organ Transplant. 2010;15:738. doi: 10.1097/MOT.0b013e32833f7114. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Shan J, Guo Y, Li S, Long D, Li Y, et al. Effects of adoptive transfer of tolerogenic dendritic cells on allograft survival in organ transplantation models: an overview of systematic reviews. J Immunol Res. 2016;2016:5730674. doi: 10.1155/2016/5730674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia MJ, Shan J, Li YP, Zhou YN, Guo YJ, Sun GX, et al. Adoptive transfusion of tolerogenic dendritic cells prolongs the survival of liver allograft: a systematic review. J Evid Based Med. 2014;7:135. doi: 10.1111/jebm.12094. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 30.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 31.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 33.Eun SC, Baek RM, Park CG. Prolongation of the rat composite tissue allograft survival by the combination of tolerogenic immature dendritic cells and short-term treatment with FK506. Transplant Proc. 2013;45:1792. doi: 10.1016/j.transproceed.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Ikeguchi R, Sacks JM, Unadkat JV, Solari M, Horibe EK, Thomson AW, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85:237. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 35.Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, et al. Modified dendritic cells coexpressing self and allogeneic major histocompatibility complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15:987. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 36.Beriou G, Moreau A, Cuturi MC. Tolerogenic dendritic cells: applications for solid organ transplantation. Curr Opin Organ Transplant. 2012;17:42. doi: 10.1097/MOT.0b013e32834ee662. [DOI] [PubMed] [Google Scholar]
- 37.Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant. 2012;12:1398. doi: 10.1111/j.1600-6143.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805. doi: 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. 2016:1. doi: 10.1126/sciimmunol.aaf8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morelli AE, Thomson AW. Orchestration of transplantation tolerance by regulatory dendritic cell therapy or in-situ targeting of dendritic cells. Curr Opin Organ Transplant. 2014;19:348. doi: 10.1097/MOT.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111:2091. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- 43.Kenna TJ, Waldie T, McNally A, Thomson M, Yagita H, Thomas R, et al. Targeting antigen to diverse APCs inactivates memory CD8+ T cells without eliciting tissue-destructive effector function. J Immunol. 2010;184:598. doi: 10.4049/jimmunol.0900032. [DOI] [PubMed] [Google Scholar]
- 44.Nasreen M, Waldie TM, Dixon CM, Steptoe RJ. Steady-state antigen-expressing dendritic cells terminate CD4+ memory T-cell responses. Eur J Immunol. 2010;40:2016. doi: 10.1002/eji.200940085. [DOI] [PubMed] [Google Scholar]
- 45.Kleijwegt FS, Jansen DT, Teeler J, Joosten AM, Laban S, Nikolic T, et al. Tolerogenic dendritic cells impede priming of naive CD8(+) T cells and deplete memory CD8(+) T cells. Eur J Immunol. 2013;43:85. doi: 10.1002/eji.201242879. [DOI] [PubMed] [Google Scholar]
- 46.Zahorchak AF, Kean LS, Tokita D, Turnquist HR, Abe M, Finke J, et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation. 2007;84:196. doi: 10.1097/01.tp.0000268582.21168.f6. [DOI] [PubMed] [Google Scholar]
- 47.Ezzelarab MB, Lu L, Guo H, Zahorchak AF, Shufesky WF, Cooper DK, et al. Eomesoderminlo CTLA4hi alloreactive CD8+ memory T cells are associated with prolonged renal transplant survival induced by regulatory dendritic cell infusion in CTLA4 immunoglobulin-treated nonhuman primates. Transplantation. 2016;100:91. doi: 10.1097/tp.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang XN, et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84:124. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 51.Harry RA, Anderson AE, Isaacs JD, Hilkens CM. Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis. Ann Rheum Dis. 2010;69:2042. doi: 10.1136/ard.2009.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naranjo-Gomez M, Raich-Regue D, Onate C, Grau-Lopez L, Ramo-Tello C, Pujol-Borrell R, et al. Comparative study of clinical grade human tolerogenic dendritic cells. J Transl Med. 2011;9:89. doi: 10.1186/1479-5876-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142:332. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7:290ra87. doi: 10.1126/scitranslmed.aaa9301. [DOI] [PubMed] [Google Scholar]
- 56.Thomas R, Street S, Ramnoruth N. Safety and preliminary evidence of efficacy in a phase I clinical trial of autologous tolerizing dendritic cells exposed to citrullinated peptides (Rheumavax) in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:169. [Google Scholar]
- 57.Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA, et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017;76:227. doi: 10.1136/annrheumdis-2015-208456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jauregui-Amezaga A, Cabezon R, Ramirez-Morros A, Espana C, Rimola J, Bru C, et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s Disease: A Phase I study. J Crohns Colitis. 2015;9:1071. doi: 10.1093/ecco-jcc/jjv144. [DOI] [PubMed] [Google Scholar]
- 59.Raich-Regue D, Grau-Lopez L, Naranjo-Gomez M, Ramo-Tello C, Pujol-Borrell R, Martinez-Caceres E, et al. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur J Immunol. 2012;42:771. doi: 10.1002/eji.201141835. [DOI] [PubMed] [Google Scholar]
- 60.Hutchinson JA, Riquelme P, Brem-Exner BG, Schulze M, Matthai M, Renders L, et al. Transplant acceptance-inducing cells as an immune-conditioning therapy in renal transplantation. Transpl Int. 2008;21:728. doi: 10.1111/j.1432-2277.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 61.Hutchinson JA, Brem-Exner BG, Riquelme P, Roelen D, Schulze M, Ivens K, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008;21:742. doi: 10.1111/j.1432-2277.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 62.Hutchinson JA, Roelen D, Riquelme P, Brem-Exner BG, Witzke O, Philipp T, et al. Preoperative treatment of a presensitized kidney transplant recipient with donor-derived transplant acceptance-inducing cells. Transpl Int. 2008;21:808. doi: 10.1111/j.1432-2277.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 63.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 64.Demetris AJ, Bellamy C, Hubscher SG, O’Leary J, Randhawa PS, Feng S, et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816. doi: 10.1111/ajt.13909. [DOI] [PubMed] [Google Scholar]
- 65.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leipold MD, Newell EW, Maecker HT. Multiparameter Phenotyping of Human PBMCs Using Mass Cytometry. Methods Mol Biol. 2015;1343:81. doi: 10.1007/978-1-4939-2963-4_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metes D, Logar A, Rudert WA, Zeevi A, Woodward J, Demetris AJ, et al. Four-color flow cytometric analysis of peripheral blood donor cell chimerism. Hum Immunol. 2003;64:787. doi: 10.1016/s0198-8859(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33:323. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair N, Mei HE, Chen SY, Hale M, Nolan GP, Maecker HT, et al. Mass cytometry as a platform for the discovery of cellular biomarkers to guide effective rheumatic disease therapy. Arthritis Res Ther. 2015;17:127. doi: 10.1186/s13075-015-0644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macedo C, Walters JT, Orkis EA, Isse K, Elinoff BD, Fedorek SP, et al. Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation. Transplantation. 2012;93:813. doi: 10.1097/TP.0b013e318247a717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Demetris AJ, Lunz JG, 3rd, Randhawa P, Wu T, Nalesnik M, Thomson AW. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transpl Int. 2009;22:120. doi: 10.1111/j.1432-2277.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 72.Isse K, Grama K, Abbott IM, Lesniak A, Lunz JG, Lee WM, et al. Adding value to liver (and allograft) biopsy evaluation using a combination of multiplex quantum dot immunostaining, high-resolution whole-slide digital imaging, and automated image analysis. Clin Liver Dis. 2010;14:669. doi: 10.1016/j.cld.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 74.Jordan SC. Donor-specific HLA antibody IgG subclasses are associated with phenotypes of antibody-mediated rejection in sensitized renal allograft recipients. J Am Soc Nephrol. 2016;27:6. doi: 10.1681/ASN.2015060608. [DOI] [PMC free article] [PubMed] [Google Scholar]