Abstract
White adipose tissue (WAT) and brown adipose tissue (BAT) have sympathetic nervous system (SNS) and sensory innervations. Previous studies from our laboratory revealed central neuroanatomical evidence of WAT sensory and BAT SNS crosstalk with double labeling of inguinal WAT (IWAT) sensory and interscapular BAT (IBAT) SNS neurons. We previously demonstrated that WAT lipolysis increases IBAT temperature, but this effect is absent when IWAT afferents are surgically denervated, which severs both sensory and SNS nerves. It is possible that WAT sensory feedback can regulate SNS drive to itself and other WAT and BAT depots, and thus contribute to the existence of differential SNS outflow to fat during different energy challenges. Here we selectively denervated IWAT sensory nerves in Siberian hamsters using capsaicin and measured norepinephrine turnover (NETO) i.e., SNS drive to WAT and BAT depots, IBAT uncoupling protein 1 (UCP1) expression, body mass, fat mass, blood glucose, and food consumed after a 24-hour cold exposure. IWAT sensory denervation decreased both IWAT and IBAT NETO and IBAT UCP1 expression. IWAT sensory denervation, however, increased mesenteric WAT (MWAT) NETO after the 24-hour cold exposure and did not modify epididymal WAT (EWAT) and retroperitoneal WAT (RWAT) NETO compared with respective controls. Body mass, fat mass, blood glucose, and food consumed were unchanged across groups. RWAT and EWAT mass decreased in capsaicin-injected hamsters, but did not in the vehicle hamsters. These results functionally demonstrate the existence of IWAT sensory and IBAT SNS crosstalk and that a disruption in this sensory-SNS feedback mechanism modifies SNS drive to IWAT, IBAT, and MWAT, but not EWAT and RWAT.
Keywords: norepinephrine turnover, Siberian hamsters, cold exposure, capsaicin
1. INTRODUCTION
Humans and rodent models have two classic types of adipose tissues. White adipose tissue (WAT) stores excess triacylglycerols, whereas brown adipose tissue (BAT) mainly generates heat for thermoregulation during cold exposure via dissipation of the mitochondrial proton gradient by uncoupling protein 1 (UCP1) [for reviews see: [1–4]]. The sympathetic nervous system (SNS) regulates WAT and BAT [for reviews see: [5–7]] by liberating stored triacylglycerols through initiating lipolysis during times of energetic demands [8–10]. Non-esterified free fatty acids (NEFAs) generated from lipolysis stimulate UCP1 synthesis and activation during β oxidation for BAT nonshivering thermogenesis [11].
Studies using NE turnover (NETO), a direct neurochemical measure of SNS activity in fat, revealed differential SNS outflow to WAT and interscapular BAT (IBAT) depots within the same hamsters that were given energy challenges of either cold exposure, food deprivation, or glucoprivation [8, 9, 12, 13]. Subcutaneous, inguinal WAT (IWAT) consistently had increased NETO in all of the previously mentioned challenges [9, 12]. NETO of intra-abdominal WAT depots such as epididymal WAT (EWAT) and retroperitoneal WAT (RWAT), however, were unaffected by glucoprivation and food deprivation, respectively [9, 12]. Visceral, mesenteric WAT (MWAT) NETO remained low and unchanged during food deprivation [13]. IBAT NETO was unaffected in situations of energy deficit i.e., food deprivation and glucoprivation, but increased during cold exposure to trigger nonshivering thermogenesis [9, 12]. Heterogeneous SNS drive has also been demonstrated in rats and mice [14–17].
Viral tract tracers, pseudorabies virus (PRV) and the H129 strain of the herpes simplex virus, have respectively demonstrated the presence of SNS [12, 13, 18, 19] and sensory [20, 21] innervations within IWAT and IBAT depots [22–24]. Doubly labeled SNS and sensory neurons were found across many brain sites when PRV and H129 were both injected into IWAT [22], which implicates a bidirectional communication between WAT and the central nervous system (CNS) as a potential feedback mechanism to maintain energetic homeostasis. Evidence suggests that WAT sensory nerves communicate energy availability via leptin. Leptin intra-IWAT injections increases IWAT sensory nerve activity and cFos labeling in the dorsal root ganglion (DRG) [25]and central or peripheral administration of leptin selectively increases SNS drive to WAT depots [26]. These findings indicate an ability to stimulate the sympatho-excitatory reflex regulation between WAT sensory and SNS pathways [27, 28]. The existence of non-uniform SNS outflow to WAT depots during energetic challenges may be due to WAT afferents providing sensory feedback to central premotor SNS nuclei regulating fat metabolism.
Brain regions comprising the central SNS outflow circuits to WAT and BAT are similar as shown by patterns of overlap in PRV labeled SNS neurons [12, 13, 18, 19]. Therefore, anatomical similarities in SNS pathways along with fat sensory innervations make communication among distinct fat depots probable. The explicit role of IWAT sensory nerves in regulating SNS drive to itself and other fat depots SNS has not been investigated. Injections of H129 into IWAT and PRV into IBAT revealed doubly labeled, IWAT sensory and IBAT SNS central neurons [24]. When CL316,243, a β3-AR agonist, was injected into IWAT to initiate lipolysis, there was an acute increase in IWAT sensory nerve activity, cFos labeling in the DRG, and IBAT temperature, but this was absent when IWAT was surgically denervated [29] implying regulation of IBAT function by IWAT afferents. Because surgical denervation severs both SNS and sensory nerves [30], it is unclear whether the decreased IBAT temperature was due to loss of IWAT SNS or sensory input. Nonetheless, the influence of WAT on IBAT function was also observed when EWAT lipectomy significantly decreased IBAT NETO [31].
It is of interest to test whether the absence of sensory feedback from IWAT can modify SNS outflow to WAT depots and IBAT during a cold challenge. In the present study, we selectively denervated IWAT sensory nerves in Siberian hamsters using capsaicin (CAP). Then, we measured SNS drive in WAT depots and IBAT, IBAT UCP1 protein expression, body mass, fat mass, food consumption, and blood glucose in response to a 24-hour cold exposure.
2. MATERIALS and METHODS
2.1.Animals
Adult male Siberian hamsters (Phodopus sungorus, 3 months old) were singly housed under a long day photoperiod light cycle (16 hour light:8 hour dark) and an ambient temperature of 22 ± 2°C. We used Siberian hamsters, because they become obese, gaining 50% fat mass relative to body mass, in response to a change in photoperiod [32], which decreases potential confounding factors associated with other obesity inducing manipulations. Furthermore, the majority of studies investigating the neural innervation of fat have been done using this species [6, 7, 33]. Hamsters were given ad libitum regular rodent chow (13.4% calories from fat, 29.8% calories from protein, and 56.7% calories from carbohydrates; Purina Rodent Chow, St. Louis, MO) and tap water for the duration of the study. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with Public Health Service and United States Department of Agriculture guidelines.
2.2.Bilateral IWAT sensory denervation
After one week of acclimation to single housing, weight-matched hamsters (n=83) were divided into two groups, vehicle control (VC, n=43) and bilateral CAP (n=40) injected hamsters. Body mass was recorded before and after the surgeries. Hamsters were anesthetized with 2–3% isoflurane in oxygen (Baxter Healthcare, Deerfield, IL), and fur around the left and right inguinal region was shaved. An incision was made to first expose the right IWAT, and hamsters were injected with the VC (1:10, 100% ethanol: olive oil) or 20 µg/µl of CAP dissolved in 1:10 ethanol and olive oil (Cat. No. M2028; Sigma-Aldrich, St. Louis, MO) with 2 µl per locus spread across the fat pad for a total of 20 loci [6, 30, 34, 35]. The Hamilton syringe was held in place for 45 seconds after each injection to prevent efflux when removing the syringe. The right incision site was closed with sterile wound clips and an incision was made on the left IWAT following the same procedures for the right IWAT. Hamsters were transferred to clean cages and given daily, subcutaneous injections of Ketofen (5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), an analgesic, for 3 days post-surgery. Wound clips were removed a week after surgeries.
2.3.NETO with 24-hour cold challenge
Two weeks after surgeries, hamsters were further divided into room temperature (VC RT and CAP RT) and 4°C cold exposed (VC cold and CAP cold) groups for measurement of NETO as previously described [12, 30]. Hamsters were handled daily for one week before NETO to adapt them to handling associated with the procedure and to decrease stress induced NE release. On the day that the 24-hour cold exposure began, body mass and regular rodent chow given were measured, and hamsters were placed in clean cages with food, water, and bedding, but no cotton nestlet to avoid confounding factors of hamsters warming themselves. Body mass and food consumed were recorded at the conclusion of the study. NETO was measured during the last 4 hours of the cold exposure [9, 12]. At the 20th hour, RT and cold exposed hamsters in both treatment groups were injected i.p. with α-methyl-p-tyrosine (250 mg/kg α-MPT; Sigma-Aldrich, St. Louis, MO), an active competitive inhibitor for tyrosine hydroxylase (TH), the rate limiting enzyme for NE production [8, 9, 30]. A supplemental dose of α-MPT (125 mg/kg) was given at the 22nd hour to ensure the inhibition of NE synthesis [8, 9, 30]. Four hours after the first α-MPT injection, hamsters were weighed, and then quickly decapitated. IBAT, IWAT, EWAT, MWAT, and RWAT were quickly harvested, weighed, frozen in liquid nitrogen and stored at −80°C until NE extraction. To obtain baseline values of NE content for NETO calculations, one-half of hamsters from each treatment and temperature groups were rapidly decapitated without receiving α-MPT injections as previously performed [8, 9, 12, 30]. Since α-MPT inhibits NE production, it is likely to alter circulating metabolic fuels, thus blood glucose was measured from non-α-MPT injected hamsters immediately after rapid decapitation using blood glucose test strips (ACCU-CHEK Compact Plus meter, Roche, USA).
IWAT, EWAT, RWAT, MWAT, and IBAT were processed and extracted for NE with dihydroxybenzylamine (Sigma-Aldrich, St. Louis, MO), an internal control for extraction efficiency. NETO were measured as described previously [for review see: [30]] and following our methods and the modification of the method of Mefford [36]. Calculations were made according to the following formula: k = (lg[NE]0 − lg[NE]4)/(0.434 × 4) and K = k[NE]0, where k is the constant rate of NE efflux, [NE]0 is the initial NE concentration, [NE]4 is the final NE concentration, and K = NETO.
2.4.Calcitonin gene related peptide (CGRP) enzyme immunoassay
To validate the chemical sensory denervation technique via CAP injections, portions of IWAT from the same tissue samples used for NE extraction were also used to measure levels of CGRP, a marker of sensory nerves, using an enzyme linked immunosorbent assay kit (SPI Bio, Massy, France) according to the manufacturer’s directions as we have previously done [12, 21, 30]. The correlation coefficient was 99.9% for CGRP assays.
2.5.Western blot
IBAT UCP1 protein expression, a molecular indicator of BAT nonshivering thermogenesis, was measured in IBAT as previously described [12, 37]. Primary antibodies used were rabbit anti-UCP1 (1:500; Abcam, Cambridge, MA) and rabbit anti-α tubulin (1:500, Cell Signaling, Danvers, MA) as a loading control. Membranes were blocked with 5% non-fat dry milk in tris buffered saline (TBS), and then incubated with primary antibodies at 4°C overnight. They were washed with TBS with 0.1% tween 20 followed by 2-hour incubation with goat anti-rabbit Alexa Fluor 680 nm antibody (ThermoFisher Scientific, Carlsbad, CA) at room temperature. Protein bands were visualized using Odyssey FC Imaging System (Li Cor Biotechnology, Lincoln, NE). UCP1 protein expression was normalized to α tubulin.
2.6.Statistical analyses
Results are expressed as means ± SE. Statistical analyses were carried out using Systat Software (version 11.0, San Jose, CA). CGRP levels in VC and CAP hamsters were compared by t-test. All other data were statistically analyzed by two-way ANOVA (treatment × temperature). The Holm-Sidak post hoc test was used when differences within or between groups were obtained. Differences were considered statistically significant if p<0.05. Exact probabilities and test values were omitted from the results section for simplicity and clarity of the presentation of results.
3. RESULTS
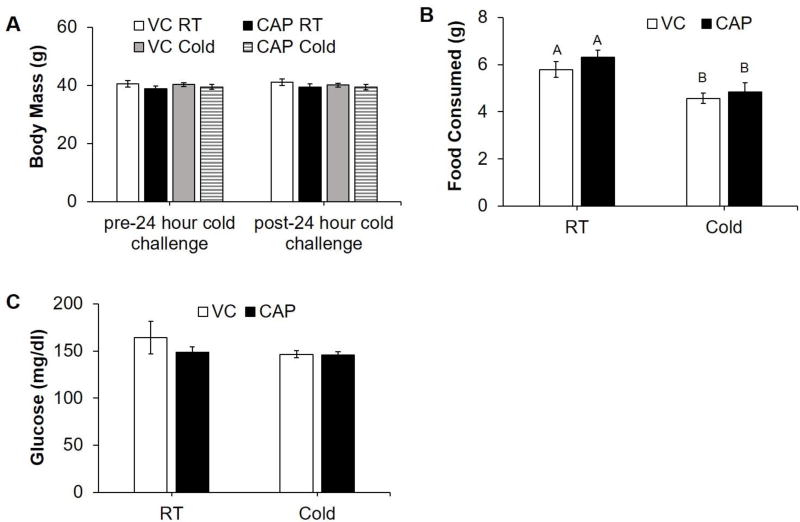
There were no differences in body mass of the different treatment groups before or after the cold challenge (Fig. 1, A). Cold exposed hamsters consumed significantly less chow during the challenge compared with hamsters housed at RT regardless of CAP injections (Fig. 1, B). There was no effect of CAP treatment or cold exposure on blood glucose (Fig. 1, C).
Figure 1.
Body mass, food consumption, and glucose of control (VC) and IWAT sensory denervated (CAP) hamsters kept at room temperature (RT) or given a 24-hour cold (4°C) challenge. (A) Body mass before and after cold challenge. (B) Food consumed during cold challenge. (C) Blood glucose after cold exposure. Within each panel, bars with different letters are significantly different from each other (p<0.05).
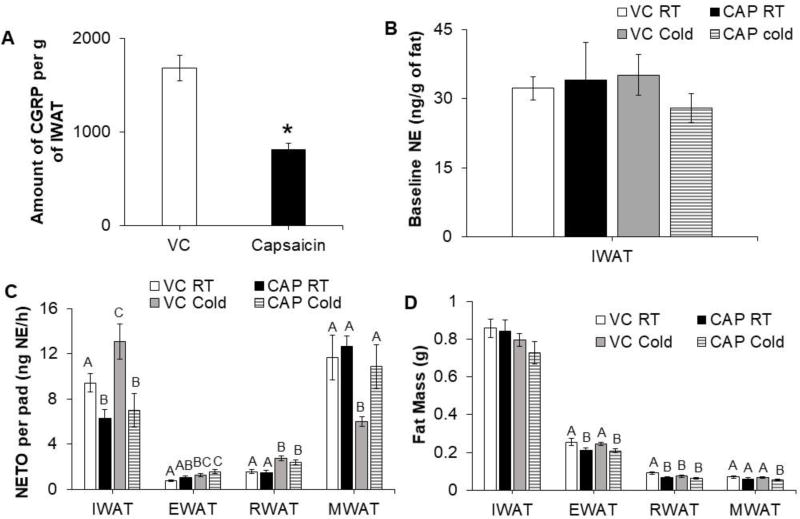
CGRP, a sensory nerve associated peptide, was measured in IWAT to validate the efficacy of sensory denervation. CGRP levels were significantly lower in IWAT depots of CAP injected hamsters compared with VC hamsters (Fig. 2, A); whereas baseline NE level was not different in hamsters injected with either vehicle or CAP (Fig. 2, B). This indicated that CAP injection specifically destroyed sensory but not sympathetic nerves. SNS outflow varied among different fat depots across treatment groups (Fig. 2, C). VC cold exposed hamsters had a significant increase in IWAT, EWAT, and RWAT NETO compared with VC hamsters at RT housing (Fig. 2, C). CAP injected hamsters had significantly decreased IWAT NETO compared with VC hamsters at RT and cold exposure failed to increase IWAT NETO in CAP injected hamsters (Fig. 2, C). By contrast, IWAT sensory denervation did not affect EWAT and RWAT NETO at RT or during cold exposure (Fig. 2, C). Unexpectedly, cold exposure caused a significant decrease in MWAT NETO of VC hamsters, whereas MWAT NETO of CAP injected hamsters was unchanged by cold exposure compared to RT hamsters (Fig. 2, C). IWAT mass was not significantly different across groups (Fig. 2, D) whereas EWAT and RWAT mass were significantly decreased in CAP injected hamsters (Fig. 2, D). Cold exposure caused a significant decrease in RWAT mass of VC hamsters, but not CAP injected hamsters. By contrast, cold exposure decreased MWAT mass in CAP injected hamsters, but not the VC controls (Fig. 2, D).
Figure 2.
IWAT sensory denervation differentially changes SNS drive to WAT depots in hamsters given 24-hour cold (4°C) exposure. (A) Levels of calcitonin gene related peptide (CGRP) in IWAT of controls (VC) and capsaicin injected (CAP) hamsters. (B) Baseline NE levels in IWAT of controls (VC) and CAP injected hamsters at either room temperature (RT) or 4°C. (C) NE turnover (NETO) and (D) fat mass of WAT depots from VC and CAP injected hamsters kept at RT or 4°C. *p<0.05, vs. VC. Within each panel, bars with different letters are significantly different from each other (p<0.05).
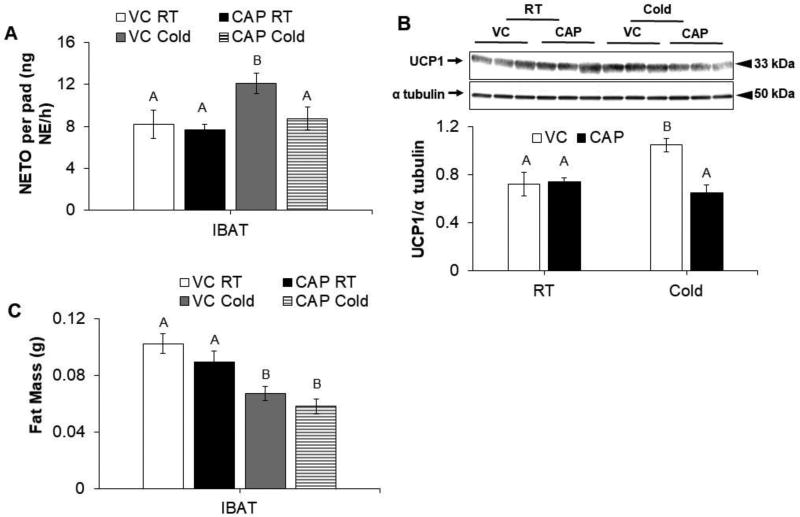
There was a significant increase in IBAT NETO and IBAT UCP1 expression of cold exposed VC hamsters, but these responses were absent in CAP injected hamsters (Fig. 3, A–B). IBAT mass was significantly reduced by cold exposure in both VC and CAP injected hamsters (Fig. 3, C).
Figure 3.
IWAT sensory denervation decreases SNS drive to IBAT in hamsters given 24-hour cold (4°C) exposure. (A) NE turnover (NETO), (B) relative IBAT UCP1 protein expression normalized to α tubulin, and (C) IBAT mass of control (VC) and capsaicin injected (CAP) hamsters kept at room temperature (RT) or 4°C. Within each panel, bars with different letters are significantly different from each other (p<0.05).
4. DISCUSSION
The neuroanatomy of WAT sensory innervation has been documented since the mid-1980s [27, 28, 38–41], but its function in energetic homeostasis has only been studied in depth within the last decade [20, 22, 24, 25, 29, 42, 43]. Here, we have functionally investigated the contribution of WAT sensory feedback to the SNS regulation of discrete fat depots. We measured NETO in WAT depots and IBAT of hamsters that had IWAT sensory denervation using CAP, the pungent component of red chili peppers. CAP chemically destroys sensory nerves and is more specific than surgical denervation where both SNS or sensory nerves are likely to be severed due to their close proximity to each other [30, 34, 35, 44, 45]. Unchanged TH staining in CAP injected WAT verified CAP’s specificity for destroying afferents [35, 46]. In the present study, we also found that CAP injection in IWAT significantly lowered CGRP levels without changing baseline NE levels, further confirming the specificity of CAP for destroying sensory nerves without affecting the sympathetic branch. In addition, it was previously demonstrated that capsaicin remains localized within the site of injection [46]. Using a within animal control, capsaicin was injected into the left IWAT depot of Siberian hamsters while the right IWAT was injected with the vehicle. Left IWAT depots had a significant decrease in CGRP levels compared with right IWAT depots. This supports that, in addition to chemical specificity of capsaicin targeting only the sensory branch, capsaicin does not diffuse out to affect nearby tissues.
IWAT sensory denervation significantly reduced both IWAT and IBAT NETO and decreased IBAT UCP1 protein expression after a 24-hour cold challenge, which supports the necessity of IWAT sensory signaling to not only maintain function regulating its own SNS drive, but also the SNS drive to IBAT. On the other hand, with the exception of MWAT, SNS drive to other WAT depots were unaffected by IWAT sensory denervation. With the application of transneuroanl viral tract tracers, we are just beginning to understand the complex neuronal networks of SNS and sensory nerves innervating different fat pads. For example, retrograde transneuronal viral tract tracer studies revealed both discrete and shared sympathetic neuronal networks that innervate IBAT and IWAT, as well as IWAT and MWAT [12, 13]. In addition, bidirectional crosstalk between sensory and sympathetic neuronal networks in IBAT and IWAT was also observed [24]. It is possible that the IWAT sensory afferents may be wired more closely to brain centers that lead to sympathetic innervation of IWAT and IBAT than those of EWAT and RWAT. However, more studies will be needed to delineate sensory and sympathetic neural circuits that innervate different fat pads in order to thoroughly understand the neuronal crosstalk and communications among these fat pads. Alternatively, NETO appears to be much lower in EWAT and RWAT compared to other fat pads. Thus, it is possible that lower NETO in these fat pads might make it difficult to observe further reductions in NETO triggered by IWAT sensory denervation.
As previously observed, the SNS outflow to IWAT was increased in VC cold exposed hamsters compared with RT housed hamsters [9, 12]. SNS drive to IWAT in CAP injected hamsters at RT and cold exposed conditions, however, was significantly decreased likely because of the absence of IWAT sensory inflow i.e., its neural signaling of energy availability to the CNS. Thus, without this sensory information central nuclei involved in the control of fat metabolism did not increase SNS drive to IWAT for lipid mobilization. Furthermore, IWAT sensory denervation significantly decreased SNS outflow to IBAT and reduced its UCP1 expression, which may also have translated into reduced IBAT temperature [12]. These data provide new evidence of WAT sensory and BAT SNS functional crosstalk and complement a study from our laboratory that used surgical denervation to show that IWAT sensory nerves influence IBAT temperature in addition to IWAT lipolysis [29]. Our data from IWAT sensory chemical denervation suggest that the decrease in IBAT temperature that was observed in animals with surgical denervation of IWAT was indeed due to sensory and not SNS denervation.
Indirect evidence for this WAT and BAT sensorimotor interaction has also been documented when leptin injections into EWAT caused a steady increase in IBAT SNS nerve activity in rats [28]. Furthermore, whole body CAP densensitization resulted in IBAT atrophy and decreased mitochondrial protein [47]. The caveat to this study is that it is unclear whether IBAT atrophy was due to IWAT or IBAT sensory denervation or a non-specific side effect of systemically administered CAP. Nevertheless, tract tracing studies provides neuroanatomical evidence for bidirectional WAT sensory and BAT SNS feedback [24], where 50% of IWAT sensory and IBAT SNS neurons were found to colocalize across the brain [24] in nuclei that have been previously shown to be involved in SNS mediated thermogenesis and lipolysis [48–50]. Thus, our functional demonstration of IWAT sensory input influencing IBAT SNS signaling is likely mediated by centrally distributed SNS nuclei involved in the regulation of fat metabolism.
Though the current study described here did not attempt to determine the molecular mechanism of WAT sensory and BAT SNS crosstalk, WAT sensory denervation may result in the absence of certain factors or molecules that might otherwise be sensed by the brain through WAT afferents as indications of WAT energy and/or metabolic status. Possible candidate molecules include fatty acids such as eicosopentanoic acid (EPA) and arachidonic acid (AA) [29] or leptin [25, 28]. Independent injections of EPA or AA into IWAT and a mixture of EPA and AA significantly increased IWAT sensory nerve activity [29]. In addition, leptin receptors located on DRG neurons are activated by leptin injections into IWAT [25]. Further studies are needed in order to identify potential WAT-derived molecules that may act as WAT sensory signals that regulate the IWAT sensory and IBAT SNS crosstalk.
On the other hand, SNS brain sites may sense the deficit in NEFA available for IBAT β-oxidation due to decreased IWAT SNS drive in CAP injected hamsters. WAT lipolysis typically produces a large supply of NEFA that are predominantly delivered to BAT through the circulation [51], while BAT lipolysis produces a smaller NEFA supply [52]. Though NEFA released from intact WAT depots can still influence IBAT SNS outflow and its residual function, the reduction in IWAT substrates for β-oxidation likely has a role in attenuating IBAT activity. This has been shown when loss of NEFA source i.e., removal of WAT by lipectomy significantly decreased SNS drive to IBAT [31].
Because only the sensory nerves in IWAT were destroyed and the SNS outflow to IBAT and IBAT UCP1 protein expression were significantly reduced, but not completely eliminated compared with respective controls, the contribution made by sensory nerves from IBAT and intact WAT depots to regulate IBAT thermogenic activity also need to be taken into consideration [20, 21, 23]. The activation of sensory signaling from one fat tissue can plausibly increase efferent activity in other distant fat depots presumably through central sensory and SNS crosstalk. For example, leptin injections into EWAT increase EWAT afferent activity and SNS activity to IWAT and IBAT [28]. Thus, remaining intact fat depots (IBAT, EWAT, RWAT, and MWAT) likely contributed sensory input to premotor SNS brain regions to support IBAT function in CAP injected hamsters in this experiment.
This is the first time the SNS outflow to MWAT, the true visceral fat in rodent models, was measured during a cold challenge. RT housed hamsters had significantly higher MWAT NETO compared with cold exposed VC hamsters. Since MWAT is the most highly innervated fat depot compared with EWAT, RWAT, and IWAT [53], its high NETO values in RT housed hamsters likely reflect this innervation by the SNS. In vitro studies have shown that primary adipocytes from MWAT have increased basal levels of free fatty acids and glycerol compared with adipocytes from EWAT [54]. Thus, the high levels of NETO in MWAT of RT housed hamsters may be accounted for by high levels of basal lipolysis. Interestingly, while cold exposure significantly reduced SNS drive to MWAT in VC injected hamsters, SNS drive to MWAT remained the same in CAP injected hamsters at RT or 4°C. This preserved SNS drive to MWAT, combined with unchanged SNS drives to EWAT and RWAT in CAP injected hamsters, may compensate for the decreased IWAT and IBAT NETO in CAP injected hamsters, and thus, may provide necessary fuels for these CAP injected hamsters to survive the cold challenge. In addition, our data point to a possibility that MWAT sympathetic innervation may be wired to respond reciprocally to that of other WAT depots, regardless of whether the afferent innervation is intact. This might suggest that visceral fat coordinates with other fat depots to maintain homeostasis during changes in temperature and energy availability (in this case, sensory denervation). However, this may not always be the case. MWAT NETO is unaffected by IWAT or EWAT SNS denervation using 6-hydroxydopamine, but MWAT NETO is increased as result of central leptin administration [26, 55]. Thus, it is possible that MWAT responds reciprocally to other fat depots, but to what specific stimuli or physiological status it responds is unknown. Further studies will have to be performed to definitively understand visceral fat function in maintenance of homeostasis in accordance with other fat tissues. Alternatively, despite our best efforts to dissect MWAT from the viscera for NE extraction, it is possible that some blood vessels remained in the tissue since MWAT is highly vascularized resulting in overestimation of MWAT NETO in RT housed and cold exposed CAP injected hamsters.
After the 24-hour cold challenge, hamsters with denervated IWAT sensory nerves had similar body mass, food consumption, and blood glucose compared to intact hamsters. As reported previously [9], cold exposed hamsters most likely maintained body mass as a result of having ad lib access to chow and the acute duration of the cold exposure. Cold exposed hamsters regardless of CAP or VC injections ate relatively less than RT hamsters possibly because of short bouts of torpor, which is known to occur during cold exposure in an attempt to conserve energy during thermoregulatory demands [56, 57]. Food intake and bouts of torpor are negatively correlated, but the causal relationship is still unclear [56]. Nevertheless, IWAT sensory denervated hamsters were still able to mobilize stored fat for energy and had intact glucose control as shown by differential increases in SNS outflow to WAT depots and homogeneous blood glucose levels across groups, respectively.
Interestingly, we found that IWAT CAP injected hamsters failed to increase NETO during cold exposure; whereas they still displayed reduced IBAT mass comparable to VC injected cold-exposed hamsters. We hypothesize that sympathetic nervous system independent mechanisms may be responsible for IBAT lipolysis in cold exposed CAP injected hamsters resulting in reduced IBAT mass despite reduced sympathetic drive. Factors such as FGF21 from the liver directly acting on IBAT and natriuretic peptides mainly from the heart acting through natriuretic peptide receptors are known to activate BAT function and thermogenesis [58]. It is possible that these factors promotes BAT activation and lipolysis independent of reduced sympathetic drive.
Here, we have functionally demonstrated the existence of IWAT sensory and IBAT SNS crosstalk and that a disruption in this sensory-SNS feedback mechanism via IWAT sensory denervation decreases IWAT and IBAT SNS drive and attenuates IBAT UCP1 expression. In addition, IWAT sensory denervation modified SNS drive to MWAT, but not EWAT and RWAT.
Highlights.
White fat sensory and brown fat sympathetic crosstalk exists.
Disruption of white and brown fat neural feedback changes sympathetic drive.
Sympathetic drive to brown fat decreases when white fat afferents are ablated.
Acknowledgments
We thank Candace L. Barr (undergraduate research assistant) and Dr. J. Christopher Ehlen for technical assistance with tissue collection and chromatograph, respectively. We also thank Drs. Ruth Harris, Aaron Roseberry, Cheryl Vaughan, and Vitaly Ryu for insightful comments on the manuscript. Lastly, we thank GSU Department of Animal Resources for husbandry care. This research was supported by NIH R37DK035254 to T.J.B. and NIH R01DK035254, R01DK078358, R01DK107544, and R01HL107500, American Heart Association 17GRNT33670492 and American Diabetes Association 1-18-IBS-260 to B.X.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose, financial or otherwise.
References
- 1.Cannon B, Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays in biochemistry. 1985;20:110–64. [PubMed] [Google Scholar]
- 2.Griggio MA. Thermogenic mechanisms in cold-acclimated animals. Braz J Med Biol Res. 1988;21:171–6. [PubMed] [Google Scholar]
- 3.Heldmaier G, Steinlechner S, Ruf T, Wiesinger H, Klingenspor M. Pho toperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. Journal of biological rhythms. 1989;4:251–65. [PubMed] [Google Scholar]
- 4.Sell H, Deshaies Y, Richard D. The brown adipocyte: update on its metabolic role. Int J Biochem Cell Biol. 2004;36:2098–104. doi: 10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–93. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–47. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 9.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1445–52. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–9. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem. 2000;275:25073–81. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol. 2017;312:R132–R45. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2014;306:R375–86. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garofalo MA, Kettelhut IC, Roselino JE, Migliorini RH. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. Journal of the autonomic nervous system. 1996;60:206–8. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- 15.Nautiyal KM, Dailey M, Brito N, Brito MN, Harris RB, Bartness TJ, et al. Energetic responses to cold temperatures in rats lacking forebrain-caudal brain stem connections. Am J Physiol Regul Integr Comp Physiol. 2008;295:R789–98. doi: 10.1152/ajpregu.90394.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, Kemnitz JW, Bray GA. Lateral hypothalamic lesions and norepinephrine turnover in rats. J Clin Invest. 1983;72:919–27. doi: 10.1172/JCI111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka K, Yoshida T, Kondo M. Effect of acute cold-exposure on norepinephrine turnover and thermogenesis in brown adipose tissue and metabolic rate in MSG-induced obese mice. The Japanese journal of physiology. 1989;39:957–62. doi: 10.2170/jjphysiol.39.957. [DOI] [PubMed] [Google Scholar]
- 18.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–9. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 19.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–78. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 20.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;96:R501–11. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1049–58. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R886–900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci. 2015;35:2181–90. doi: 10.1523/JNEUROSCI.3306-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu V, Watts AG, Xue B, Bartness TJ. Bidirectional Crosstalk between the Sensory and Sympathetic Motor Systems Innervating Brown and White Adipose Tissue in Male Siberian Hamsters. Am J Physiol Regul Integr Comp Physiol. 2017 doi: 10.1152/ajpregu.00456.2015. ajpregu 00456 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–47. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–21. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 27.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. Journal of the autonomic nervous system. 1998;73:19–25. doi: 10.1016/s0165-1838(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 28.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neuroscience letters. 1999;262:125–8. doi: 10.1016/s0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 29.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab. 2016;5:626–34. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods in enzymology. 2014;537:199–225. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiology & behavior. 2004;81:535–42. doi: 10.1016/j.physbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1499–505. doi: 10.1152/ajpregu.2001.281.5.R1499. [DOI] [PubMed] [Google Scholar]
- 33.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. Int J Obes Suppl. 2015;5:S35–9. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R514–R20. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 35.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–37. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 36.Mefford IN. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods. 1981;3:207–24. doi: 10.1016/0165-0270(81)90056-x. [DOI] [PubMed] [Google Scholar]
- 37.Cui X, Nguyen NL, Zarebidaki E, Cao Q, Li F, Zha L, et al. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiological reports. 2016;4 doi: 10.14814/phy2.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253:R942–4. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 39.Fredholm bb. Nervous control of circulation of metabolism in white adipose tissue. Boston: Butterworth; 1985. [Google Scholar]
- 40.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. Journal of neurocytology. 1996;25:125–36. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 41.Hill B, Ralevic V, Crowe R, Burnstock G. Innervation and nitric oxide modulation of mesenteric arteries of the golden hamster. Eur J Pharmacol. 1996;317:275–83. doi: 10.1016/s0014-2999(96)00727-3. [DOI] [PubMed] [Google Scholar]
- 42.Kosacka J, Nowicki M, Kacza J, Borlak J, Engele J, Spanel-Borowski K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J Neurosci Res. 2006;83:1160–9. doi: 10.1002/jnr.20811. [DOI] [PubMed] [Google Scholar]
- 43.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. Journal of applied physiology. 2012;112:1008–14. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 44.Jancso G, Kiraly E, Jancso-Gabor A. Direct evidence for an axonal site of action of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1980;313:91–4. doi: 10.1007/BF00505809. [DOI] [PubMed] [Google Scholar]
- 45.Jancso G, Kiraly E, Joo F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neuroscience letters. 1985;59:209–14. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- 46.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1630–7. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 47.Cui J, Himms-Hagen J. Rapid but transient atrophy of brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. 1992;262:R562–7. doi: 10.1152/ajpregu.1992.262.4.R562. [DOI] [PubMed] [Google Scholar]
- 48.Foster MT, Song CK, Bartness TJ. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18:682–9. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–76. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 50.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295:R417–28. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in lipid research. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calderon-Dominguez M, Mir JF, Fucho R, Weber M, Serra D, Herrero L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5:98–118. doi: 10.1080/21623945.2015.1122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 54.Wueest S, Yang X, Liu J, Schoenle EJ, Konrad D. Inverse regulation of basal lipolysis in perigonadal and mesenteric fat depots in mice. Am J Physiol Endocrinol Metab. 2012;302:E153–60. doi: 10.1152/ajpendo.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 2012;20:1355–64. doi: 10.1038/oby.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruf T, Klingenspor M, Preis H, Heldmaier G. Daily torpor in the Djungarian hamster (Phodopus sungorus): interactions with food intake, activity, and social behaviour. Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology. 1991;160:609–15. [Google Scholar]
- 57.Ruf T, Heldmaier G. Reduced locomotor activity following daily torpor in the Djungarian hamster: recovery from hypothermia? Die Naturwissenschaften. 1992;79:574–5. doi: 10.1007/BF01131417. [DOI] [PubMed] [Google Scholar]
- 58.Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–43. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]