Abstract
Proper control of multipotent/stem cell number and fate is essential for ensuing organ formation during development. β1-integrin, a subfamily of cell surface receptors, has a conserved role in maintenance of multipotent/stem cells, including renal progenitor cells, follicle stem cells, epidermal stem cells and neural stem cells. However, it remains unclear whether β1-integrin has a role in cardiac progenitor cell (CPC) development. Here we show that a mesodermal deletion of β1-integrin decreases Isl1+ cell number in the second pharyngeal arch (PA2), where CPCs undergo renewal and expansion. Mesp1 lineage-specific mosaicism revealed that β1-integrin-deleted Isl1+ cells do not proliferate in the PA2. Consistently, β1-integrin-deleted Isl1+ CPCs failed to expand in vitro, independent of PA2 cells. β1-integrin co-localized and physically associated with Numb, a crucial regulator of CPC renewal and expansion. Importantly, Numb/Numbl-deleted CPCs showed dramatic reduction in β1-integrin levels. These findings suggest that β1-integrin is a key mediator of the Numb (Nb) pathway in CPC maintenance.
Keywords: β1-integrin, Cardiac development, Cardiac Progenitor Expansion, ES/iPS cells, Numb/Numbl, Second Heart Field
1. Introduction
Cardiac progenitor cells (CPCs)—identified from embryos or pluripotent stem cell cultures—hold great regenerative potential with their unique ability to expand and differentiate into nearly all cell types and form structures of the heart (Brade et al., 2013; Cho et al., 2014; Garry and Olson, 2006). CPC development initiates as cells expressing mesoderm posterior 1 (Mesp1), a basic helix-loop-helix protein, appear in the nascent mesoderm at the onset of gastrulation (Saga et al., 2000; Saga et al., 1999). Mesp1+ cells migrate anteriorly to form the first heart field (FHF) and the second heart field (SHF). FHF cells contribute to the atria and left ventricle (LV), whereas SHF cells give rise to the right ventricle (RV), outflow tract (OT), and part of the atria (Kelly et al., 2014; Srivastava, 2006). Prior to myocardialization, Mesp1+ cell-derived SHF cells express CPC markers such as Isl1, Tbx1, Sall1(Cai et al., 2003; Huynh et al., 2007; Morita et al., 2016). Recently, a subset of SHF cells, expressing Isl1 but not Nkx2.5, were identified in the second pharyngeal arch (PA2), where they undergo homogeneous renewal and expansion in the presence of PA2 cells (Andersen and Kwon, 2015; Shenje et al., 2014). Intriguingly, the process required the endocytic adopter proteins Numb and Numbl, known to mediate asymmetric cell divisions and self-renewal in other contexts (Petersen et al., 2002; Roegiers and Jan, 2004). However, their downstream mechanisms affecting CPC renewal and expansion remain to be determined.
β1-integrin is expressed ubiquitously during embryonic development and plays an essential role in stem cell maintenance (Campos et al., 2004; Fuentealba et al., 2012; Lu et al., 2012). In neuronal, renal, germline, and epithelial stem/progenitor cells, β1-integrin is a key factor required to maintain stem cell number and renewal and allows interaction with their microenvironment. A loss of β1-intergrin is associated with decreased proliferation and premature differentiation of stem/progenitor cells, causing abnormal organ development. β1-integrin was also shown to mediate endodermal fibronectin signals to induce mesodermal cells (Cheng et al., 2013; Uosaki et al., 2012). However, its role in CPC expansion has not been explored. In this study, we demonstrate that β1-integrin is a key mediator of Numb (Nb) and Numbl (Nbl) for CPC maintenance.
2. Materials and methods
2.1. Mouse Genetics and ES Cells
β1-integrin knockout (KO) or Numb/Numbl double knockout (DKO) mouse embryos were generated by mating Mesp1Cre; β1-integrinflox/+ with β1-integrinflox/flox; RosatdTomato mice or Mesp1Cre; Numbflox/+; Numbl−/+ with Numbflox/flox; Numbl−/+; RosatdTomato (RFP) mice, respectively (Petersen et al., 2002; Raghavan et al., 2000; Shenje et al., 2014). Embryos were harvested from E8.5–9.0. Mesp1Cre; RosaRFP or Mesp1Cre; β1-integrin flox/flox or flox/+; RosaRFP ES cells were derived from corresponding mice. ES cells were maintained and differentiated in culture as done (Uosaki et al., 2012). For generating lineage-specific chimeras, mutant ES cells were injected into wildtype blastocysts (3-5 ES cells/blastocyst) and transferred to E0.5–1.5 pseudopregnant recipient mothers. Chimeric embryos were harvested and analyzed at E9.0.
2.2. EdU labeling, Immunohistochemistry, Microscopy, and Western blotting
We used the click-it® chemical reaction protocol for EdU detection followed by immunostaining with primary and secondary antibodies and before DAPI staining. For confocal microscopy, embryos were fixed in 4% paraformaldehyde overnight and then 30% sucrose, and then embedded in OCT, sectioned and stained using standard protocols. Antibodies used were: goat and rabbit anti-β1-integrin (1:400; R&D or 1:1000; Abcam), mouse anti-Isl1 (1:200, Iowa Hybridoma Bank), rabbit anti-RFP (1:400, Clontech), rabbit anti-Numb (pre-absorbed, 1: 500, Abcam or from Dr. Zhong), and mouse anti-PH3 (1:500, Abcam). Alexa Fluor secondary antibodies (Invitrogen) were used for all secondary detection and confocal images acquired with a Zeiss LSM 510 Meta confocal microscope using Zen™ acquisition software. For Western blotting, cell lysate was resolved on SDS-PAGE and electroblotted on nitrocellulose membranes and incubated with primary antibodies in 5% nonfat milk overnight at 4 degrees Celsius. Secondary antibodies were incubated for 1 hour at room temperature. The blots were washed 3×10 mins in TTBS, and detection was by chemiluminescence (Amersham ECL, GE Healthcare Life Sciences).
3. Results
3.1 β1-integrin is required for early heart development
To examine the role of β1-integrin in CPC development, we deleted β1-integrin in Mesp1+ progenitors by crossing Mesp1cre mice with β1-integrinflox/flox mice. The mutant embryos appeared grossly normal at E8.5 (Fig. 1A, E), but became abnormal from E9.0, predominantly affecting formation of the PA2 and the OT/RV of mutant embryos (Fig. 1F, G) compared to controls (Fig. 1B, C). Sectional analysis showed marked depletion of Isl1+ cells and neighboring cells in the PA2 of mutant embryos (Fig. 1D, H). In order to analyze Mesp1 progeny in detail, RosaRFP allele was included in the embryo, and the progeny was traced by RFP expression. We found that RFP+ cells in the PA2 (Isl1+) are seen continuous with the OT in control embryos (Fig. 2A, A′), but are nearly depleted in β1-integrin KO embryos (Fig. 2C, C′). Mutant embryos exhibited a hypoplastic PA2, OT, and RV (Fig. 2C, C″) compared to controls (Fig. 2A, A″). The LV appeared less affected in the mutants. Histological analysis showed a severe depletion of RFP+ Isl1+ cells in the PA2 (Fig. 2B, D). Moreover, phosphohistone H3 (PH3) staining was not detected in the RFP+ Isl1+ cells in the mutant PA2 (Fig. 2B, D). This suggests that β1-integrin is required in Mesp1 progeny for OT/RV development.
Fig. 1. β1–integrin deletion causes an atrophic PA2 and heart at E9.0.
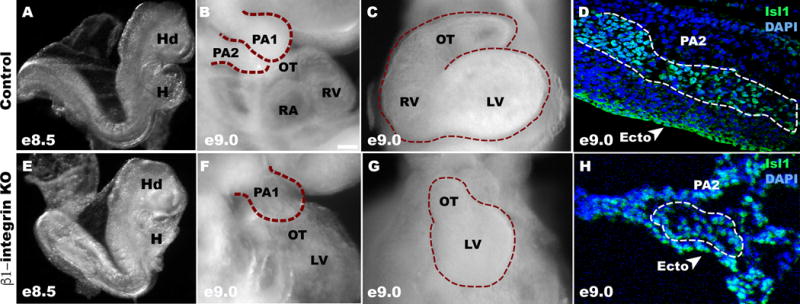
(A, E) Representative control and β1-integrin KO embryos. They are morphologically indistinguishable at E8.5. (B, C, F, G). Lateral (B, F) or frontal (C, G) views of representative control and β1-integrin KO embryos (n=3), showing hypoplastic PA2 and OT/RV at E9.0. (D, H) PA2 sections showing a significant reduction (white dotted trace) of Is1+ cells in β1-integrin KO embryo at E9.0.
Fig. 2. β1-integrin is necessary for OT/RV development and CPC expansion.
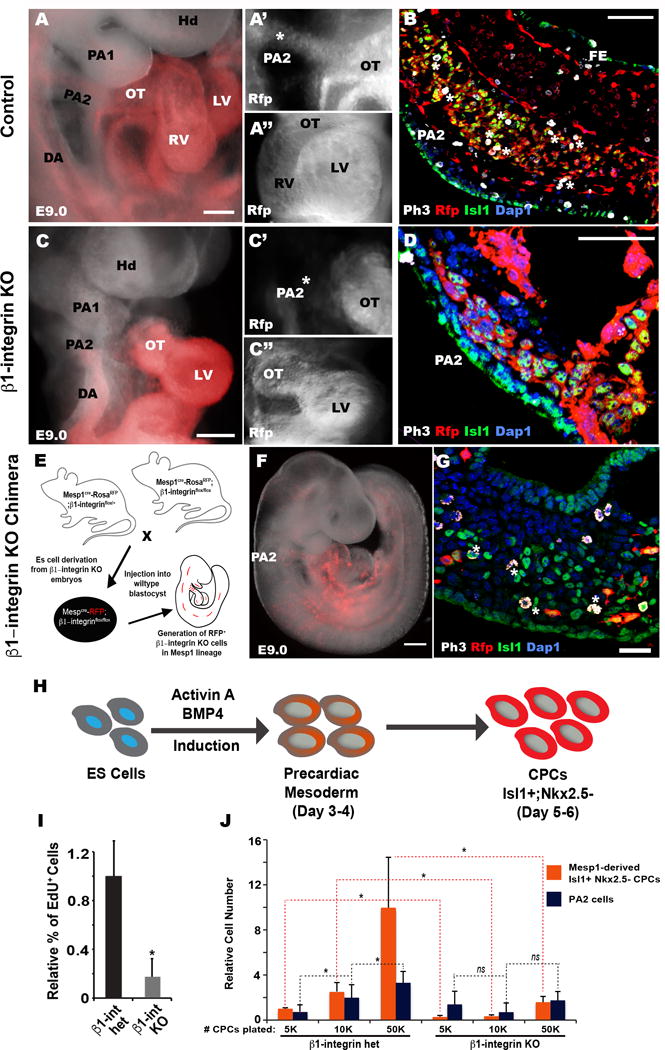
(A–B, C–D) Mesp1-RosaRFP cell-traced control (A–B) or β1-integrin KO (C–D) embryos analyzed at E9.0. RFP marks Mesp1 progeny (n=4). Control embryos show continuous RFP expression from PA2 to heart (asterisk, A’), but the arch is severely underdeveloped in β1-integrin KO embryos without noticeable RFP expression (C’). (A”, C”) Frontal views of control or mutant embryos showing normal heart morphology (A”) or an atrophic heart (C”). (B, D) Confocal images of transverse sections through the PA2 (12-microns) of control (B) and β1-integrin KO (D) embryos, immunostained with PH3, RFP, Isl1 antibodies (n=4). Internal boundaries of PA2 are outlines in dashes, showing a cluster of Isl1+ CPC cells that undergo proliferation (asterisks indicate PH3+ and Isl1+ cells) in the control PA2 (B). β1-integrin KO Isl1+ cells are markedly reduced in the PA2 and do not express PH3 (D). (E) Schematic diagram of β1-integrin KO chimera generation. (F) Mesp1 lineage-specific β1-integrin KO chimera at E9.0 (n=5). (G) Confocal image of chimeric PA2 section, immunostained with PH3, Isl1, RFP antibodies (n=5). (H) Schematic of cardiac differentiation in the ES cell system. (I) Relative percentage of EdU+ cells in Isl1+ CPCs derived from control (Mesp1Cre; β1-integrinflox/+; RosaRFP) and β1-integrin KO (Mesp1Cre; β1-integrinflox/flox; RosaRFP) ES cells (n=3). (J) Relative number of control or β1-integrin KO Isl1+ CPCs (orange) or PA2 cells (blue) after 48 hours of co-culture at a density of 10K cells/cm2 of PA2 cells with indicated numbers of CPCs (n=3). Dapi (blue) was used to counterstain the nuclei. *P < 0.05. ns, not significant. Scale bars, 50μm (B, D, G), 150μm (A, C, F). hd, head; da, dorsal aorta; pa, pharyngeal arch; ot, outflow tract; rv, right ventricle; lv, left ventricle. fe, foregut endoderm.
3.2. β1-integrin-deleted CPCs have proliferative defects, independent of PA2 cells
Deletion of β1-integrin in Mesp1 progeny severely affects PA2 and heart morphogenesis, making it difficult to assess its cell-autonomous role in vivo. To determine if the β1-integrin KO phenotype reflects the intrinsic role of β1-integrin for CPCs in the PA2, we generated chimeric embryos lacking β1-integrin specifically in the Mesp1 lineages (Fig. 2E, 2F). We initially established Mesp1cre; RosaRFP; β1-integrinflox/+ (β1-integrin het) and Mespcre; RosaRFP; β1-integrinflox/flox (β1-integrin KO) ES cell lines. The ES cells were injected into host blastocysts of wildtype mice to generate chimeras. The resulting chimeras developed normally and showed mosaic RFP+ cells lacking β1-integrin exclusively in Mesp1 progeny. In the PA2, a cluster of Isl1+ cells formed normally and expressed the proliferation marker PH3 (Fig. 2G). However, none of the donor RFP+ Isl1+ cells were positive for PH3 in the PA2s (Fig. 2G), suggesting that Isl1+ cells in the PA2 lost their ability to expand in the absence of β1-integrin.
To determine the CPC-autonomous role of β1-integrin in vitro, we turned to a ES cell system that can be used to recapitulate early cardiac development (Kattman et al., 2011). To do this, the control and mutant ES cells were differentiated into Isl1+ Nkx2.5− CPCs as described previously (Shenje et al., 2014) (Fig. 2H). When compared to control CPCs (β1-integrin het), β1-integrin-deleted CPCs showed a drastic reduction in proliferation (Fig. 2I). The proliferation defect was also observed in the presence of PA2 cells (Fig. 1J), indicating β1-integrin acts cell-autonomously for CPC expansion. Intriguingly, increasing control CPC number resulted in a corresponding increase in PA2 cell number, but the effect was not observed with β1-integrin-deleted CPCs (Fig. 2J). This may explain the hypoplastic PA2 in β1-integrin KO embryos (Fig. 1F, H and Fig. 2C, D) and suggest that β1-integrin regulates the number of PA2 cells in a non-cell-autonomous manner. Together, these data suggest that β1-integrin is an intrinsic factor required for CPC renewal and expansion in the PA2.
3.3. β1-integrin levels are maintained in CPCs by Numb/Numbl
We previously showed that Nb/Nbl deletion results in the atrophic PA2 and OT/RV and that Isl1+ cells require Numb/Numbl for their renewal and expansion in the PA2 (Shenje et al., 2014). Given the phenotypic similarity of β1-integrin KO and Numb/Numbl DKO embryos, we examined their expression patterns in precardiac mesoderm. We found that β1-integrin co-localized with Numb in the PA2 (Fig 3. A–A″). Moreover, co-immunoprecipitation assay revealed that β1-integrin physically associates with Numb in ES cell-derived CPCs (Fig. 3B, 3C). Strikingly, β1-integrin was not detected in Numb/Numbl-deleted CPCs (Fig. 3D), suggesting that Numb/Numbl regulate β1-integrin levels. Deletion of Numb/Numbl in Mesp1 progeny severely affects PA2 and heart morphogenesis, making it difficult to assess its role on β1-integrin levels in vivo. Thus, we generated chimeric embryos lacking Numb/Numbl specifically in the Mesp1 lineages (Shenje et al., 2014) (Fig. 4A). β1-integrin was normally expressed in host (RFP−) cells in the PA2 and heart (Fig. 4B, C). However, its levels were markedly compromised in Numb/Numbl DKO (RFP+) cells (Fig. 4B′, B″, C′, C″). This suggests that Numb/Numbl are required to maintain normal levels of β1-integrin in vivo.
Fig. 3. β1–integrin physically interacts with Numb and its levels are regulated by Numb/Numbl in CPCs.
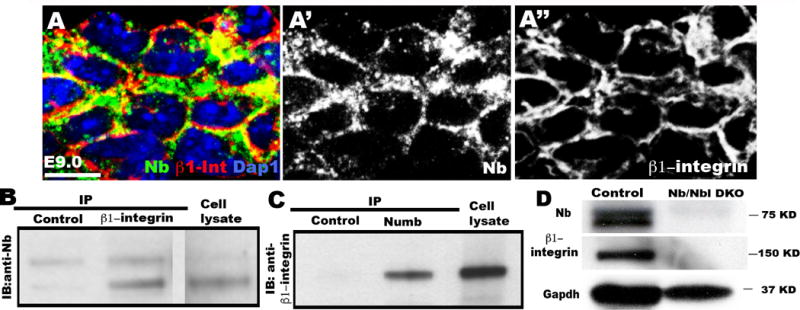
(A-A”) Confocal images of PA2 section, showing co-localization of β1-integrin and Numb. Scale bar, 50μm. (B, C) Immunoprecipitation (IP) assay shows physical interaction of β1–integrin and Numb interaction. (D) Western blot analysis shows β1-integrin protein is depleted in Numb/Numb1 DKO CPCs. IB, immunoblot.
Fig. 4. β1-integrin levels are maintained by Numb/Numbl in vivo.
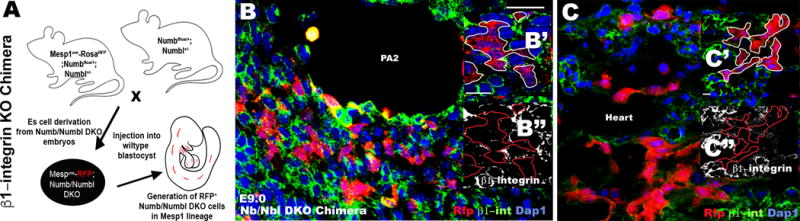
(A) Schematic diagram of β1-integrin KO chimera generation. (B, C) PA2 (B) or heart (C) section of Numb/Numbl KO Chimera, immunostained with RFP and β1–integrin antibody (n=4). β1–integrin levels are markedly compromised in RFP+ cells. RFP+ cells are outlined in white or red (B′, B″, C′, C″).
4. Conclusion
Through the use of mouse genetics, embryonic stem cell culture, and lineage-specific mosaicism, we demonstrate an unrecognized role of β1-integrin in CPC development, acting as a cell-autonomous factor for the proliferation of undifferentiated CPCs. Surprisingly, β1-integrin mutants phenocopied Numb/Numbl DKO embryos, and β1-integrin levels were markedly decreased in the absence of Numb/Numbl. These findings suggest that β1-integrin is a key mediator in the pathway involving Numb/Numbl in CPC maintenance. Similar to Numb/Numbl DKO embryos, LV formation appeared unaffected in the β1-integrin mutant embryos. This suggests that β1-integrin may be dispensable for FHF development. It will be of importance to elucidate the mechanisms by which Numb proteins regulate β1-integrin levels. It is worth noting that β1-integrin deficiency in CPCs negatively affected PA2 cell number, suggesting a non-cell-autonomous role in niche cell development. It will be interesting to investigate how CPCs influence PA2 cells via β1-integrin.
Highlights.
β1-integrin is required for PA2 and OT/RV development.
β1-integrin regulates SHF CPC expansion in a cell-autonomous manner.
β1-integrin forms a physical complex with Numb.
β1-integrin levels are maintained by Numb/Numbl in CPCs
Acknowledgments
This study was supported by awards from the National Institutes of Health (R01HD086026), the American Heart Association and the Children’s Heart Foundation, and the Maryland Stem Cell Research Fund.
Footnotes
Disclosures
None.
References
- Andersen P, Kwon C. Ex Vivo Culture of Pharyngeal Arches to Study Heart and Muscle Progenitors and Their Niche. J Vis Exp. 2015:e52876. doi: 10.3791/52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade T, Pane LS, Moretti A, Chien KR, Laugwitz KL. Embryonic heart progenitors and cardiogenesis. Cold Spring Harb Perspect Med. 2013;3:a013847. doi: 10.1101/cshperspect.a013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Cheng P, Andersen P, Hassel D, Kaynak BL, Limphong P, Juergensen L, Kwon C, Srivastava D. Fibronectin mediates mesendodermal cell fate decisions. Development. 2013;140:2587–2596. doi: 10.1242/dev.089052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho GS, Fernandez L, Kwon C. Regenerative medicine for the heart: perspectives on stem-cell therapy. Antioxid Redox Signal. 2014;21:2018–2031. doi: 10.1089/ars.2014.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Huynh T, Chen L, Terrell P, Baldini A. A fate map of Tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis. 2007;45:470–475. doi: 10.1002/dvg.20317. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Andersen P, Hotta A, Tsukahara Y, Sasagawa N, Hayashida N, Koga C, Nishikawa M, Saga Y, Evans SM, et al. Sall1 transiently marks undifferentiated heart precursors and regulates their fate. J Mol Cell Cardiol. 2016;92:158–162. doi: 10.1016/j.yjmcc.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Shenje LT, Andersen P, Uosaki H, Fernandez L, Rainer PP, Cho GS, Lee DI, Zhong W, Harvey RP, Kass DA, et al. Precardiac deletion of Numb and Numblike reveals renewal of cardiac progenitors. Elife. 2014;3:e02164. doi: 10.7554/eLife.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Uosaki H, Andersen P, Shenje LT, Fernandez L, Christiansen SL, Kwon C. Direct Contact with Endoderm-like Cells Efficiently Induces Cardiac Progenitors from Mouse and Human Pluripotent Stem Cells. PLoS One. 2012 doi: 10.1371/journal.pone.0046413. [DOI] [PMC free article] [PubMed] [Google Scholar]


