Abstract
The proportion of Plasmodium spp. infections carrying gametocytes and gametocyte densities are often reported as surrogate markers for transmission potential. It remains unclear whether parasites under natural conditions adjust commitment to transmission depending on external factors. Population-based surveys comprising mostly asymptomatic low-density infections are always impacted by the sensitivity of the assays used to diagnose infections and detect gametocytes. Asexual parasite density is an important predictor for the probability of detecting gametocytes, and in many cases, it can explain patterns in gametocyte carriage, without the need for an adjustment of the gametocyte conversion rate. When reporting gametocyte data, quantification of blood-stage parasitemia and its inclusion as a confounder in multivariable analyses is essential.
Keywords: Malaria transmission, Molecular gametocyte detection, Population gametocyte prevalence, Gametocyte density, Gametocyte conversion rate, Limit of Detection
Gametocytes as surrogate markers for malaria transmission potential
The prevalence of Plasmodium gametocytes in asymptomatic individuals has garnered increased interest in light of malaria elimination [1]. The proportion of infections carrying gametocytes and gametocyte density are frequently reported, along with estimates for asexual parasite density, in population-based studies as a surrogate marker for human-to-mosquito transmission potential. The identification and treatment of gametocyte reservoirs (see Glossary) is an essential component of malaria elimination strategies.
During each cycle within the red blood cell, a proportion of parasites deviate from asexual replication and develop into gametocytes. The number of blood-stage schizonts that will develop into gametocytes depends on the gametocyte conversion rate. In recent years, great advances were made in our understanding of the processes governing this switch in Plasmodium falciparum (reviewed in [2]), and the AP2-G transcription factor was identified as a key regulator [3]. In parallel, a range of molecular markers for gametocyte detection by reverse-transcription quantitative PCR (RT-qPCR) or nucleic acid sequence-based amplification (NASBA) in field isolates were developed (Box 1).
Box 1. Markers for Gametocyte Detection.
Field studies of gametocyte carriage have long been hampered by the limited sensitivity of light microscopy. While P. falciparum gametocytes can be relatively easily identified by their characteristic shape - the term “falciparum” stems from the curved shape of their gametocytes [66] – P. vivax gametocytes are difficult to distinguish from trophozoites [67]. Sensitive molecular assays for the diagnosis of gametocyte-specific RNA-transcripts allow gametocytes to be examined in low-density infections.
Among the first markers described for gametocyte detection were pfs25 (PF3D7_1031000) [68], pfs16 (PF3D7_0406200) [69] and pfg377 (PF3D7_1250100) [70] for P. falciparum, and pvs25 (PVX_111175) for P. vivax [71]. pfs25 and pvs25 are the most widely applied markers and are predominantly expressed in female gametocytes. pvs25 is expressed at considerably lower levels than pfs25, which results in reduced sensitivity in detecting P. vivax gametocytes [14, 24]. Recently, pfg17 (PF3D7_1319800) was shown to be more sensitive than pfs25 and thus more P. falciparum gametocyte carriers were identified [19]. PfGEXP5 (PF3D7_0936600) is a marker for early (ring-stage) gametocytes [31, 72].
In combination with female-specific markers, male-specific markers, e.g. pfs230p (PF3D7_0209000), pf13 (PF3D7_1311100) [73, 74], and Pf3D7_1469900 [63], enable sex ratios of gametocytes to be determined. This is particularly relevant when testing drugs that might affect male and female gametocytes differentially [55].
As qRT-PCR assays also amplify residual DNA co-extracted with RNA, DNase treatment is required to avoid false-positive results. RT-qPCR assays with primers spanning splice sites of gametocyte-specific transcripts with several exons bypass the need for DNase treatment. Such markers, e.g. Pf11.1 (PF3D7_1038400) [75] and PF3D7_0630000 [76], might result in better detectability, as DNase treatment is expected to lower RNA yields, and simplify laboratory procedures, as a single co-extraction of DNA and RNA becomes feasible. Pf11.1 was found to be 5-fold more sensitive when spanning a splice site, as compared to an assay targeting the same gene but requiring DNase treatment [75]. Yet, due to their lower expression levels, the limit of detection of PF11.1 and PF3D7_0630000 was at least 10-fold higher compared to the pfs25 assay (including DNase treatment). pfs25 and pfg17 are single-exon genes, thus developing assays spanning splice-sites to increase sensitivity of these markers further is not feasible.
Gametocyte-specific, polymorphic transcripts will enable genotyping of individual gametocyte clones that can be followed over time. Several size-polymorphic gametocyte markers have been described, with pfs230 (PF3D7_0209000) and pfg377 (PF3D7_1250100) being the most diverse [75, 77].
Gametocyte conversion rates might vary, depending on factors such as the level of acquired immunity, or as a reaction to treatment. When conditions in the human host deteriorate, or when mosquito densities increase (e.g. in regions of seasonal transmission), a higher investment in transmission would increase survival of the parasite [4]. Indeed, under controlled laboratory conditions, higher proportions of gametocytes were found after subcurative drug treatment [5], in response to host metabolites [6], or in mice co-infected with Schistosoma mansoni [7]. Likewise, adjustments of gametocyte sex ratios to maximize transmission success have been observed in controlled systems [8, 9].
It remains unclear whether parasites in the field adapt the gametocyte conversion rate in response to external factors to an extent that it is measurable in population-wide studies. A better understanding of commitment to transmission could help control programs to target gametocytes specifically through drugs and transmission-blocking vaccines [10].
Here, we propose that many observations on gametocyte carriage at the population level do not require an adjustment of the conversion rate by way of explanation. Rather, they can be explained by the correlation between asexual parasite and gametocyte densities, the limit of detection of parasites and gametocytes at low densities, and a delay of P. falciparum peak gametocytemia after parasitemia. In addition, measurement errors by commonly used qPCR assays [11, 12], and often small volumes of blood examined, confound results.
Assay sensitivity as a key determinant of the proportion of gametocyte-positive infections
Several studies have shown that each 10-fold increase in Plasmodium vivax genome density, as measured by qPCR, results in 2-3-fold higher odds in detecting gametocytes [13-15]. For P. falciparum, this association was weaker, yet parasite density remained a significant predictor for gametocyte carriage [14, 16].
Given that parasite density is a strong predictor for the detection of gametocytes, the proportion of gametocyte-positive infections, and thus population gametocyte prevalence, depends heavily on the sensitivity of both the detection of asexual parasites and gametocytes. Clearly, assays that are more sensitive in detecting gametocytes will result in higher numbers of gametocyte carriers. This is evident when microscopy is compared to RT-qPCR [14, 17, 18], or when RT-qPCR markers of different sensitivity are compared [19].
In most studies, in order to limit the number of RNA extractions and RT-qPCRs to be done, only infections that test positive for parasites by DNA-based PCR are screened for gametocytes [13, 20-22]. Therefore, in such cases, the population gametocyte prevalence will depend on the sensitivity of the initial screening for parasites, as will the proportion of gametocyte-positive infections detected. If the sensitivity of blood-stage detection decreases (e.g. when a less sensitive PCR is used), very low-density infections are no longer detected, and therefore the average density of the infections detected will be higher. These infections are more likely to carry detectable gametocytes compared to very low-density infections. Thus, if gametocyte detection has a constant sensitivity, the proportion of gametocyte-positive infections will increase as the sensitivity of blood-stage detection decreases.
Figure 1 shows data from a cross-sectional survey in Papua New Guinea, where 2,083 individuals of the general population were surveyed [14]. All samples were screened by microscopy, nested PCR, or qPCR. Among the positive samples, P. falciparum and P. vivax gametocytes were subsequently detected by RT-qPCR. Based on qPCR, which was the most sensitive diagnostic assay used in this study, gametocytes were detected in 60% of P. falciparum and 49% of P. vivax infections. Using nested PCR (nPCR), which is less sensitive, fewer infections were detected. The mean density of nPCR-positive infections was higher than the density of infections positive by qPCR only, and thus the chance of detecting gametocytes by RT-qPCR was higher. As a result, gametocytes were detected in 66% and 52% of P. falciparum and P. vivax nPCR-positive infections, respectively. Microscopy was the least sensitive diagnostics used in this study and detected only infections with medium or high densities. The chance of detecting gametocytes (by RT-qPCR) among these infections was high. Indeed, 88% of P. falciparum and 79% of P. vivax microscopy-positive infections carried gametocytes. Depending on the method for blood-stage parasite diagnosis, the population gametocyte prevalence ranged from 6.1% to 11.2% for P. falciparum and from 5.3% to 6.3% for P. vivax (Figure 1).
Figure 1.
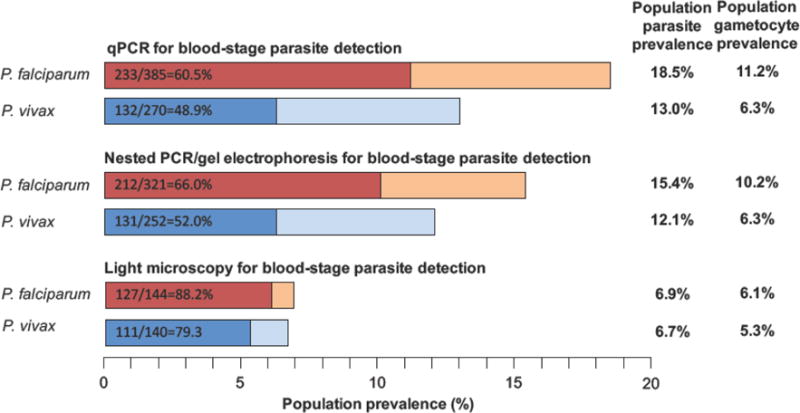
Population parasite and gametocyte prevalence in relation to the diagnostic method used to detect blood-stage infections (qPCR, nested PCR, or microscopy). In each bar, the number of asexual infections, the number of infections with gametocytes detected, and the proportion of gametocyte-positive infections are given. Gametocytes were detected by RT-qPCR of marker transcripts pfs25 and pvs25 among samples that tested positive for P. falciparum or P. vivax by qPCR, nested PCR, or microscopy. Data are from a cross-sectional survey in Papua New Guinea, involving 2083 individuals [14].
The same principle applies when applying more sensitive parasite diagnostics: ultra-sensitive assays targeting multicopy genes [23], or diagnosis based on rRNA which is present in thousands of copies per parasite [24], resulted in higher parasite prevalence. Yet, the proportion of infections with detectable gametocytes was lower in infections detected by ultra-sensitive assays only [23, 24]. On the other hand, in studies that extracted DNA from a lower volume of blood (e.g. from dried blood spots on filter paper), and thus had limited sensitivity to detect low-density infections, nearly all infections carried gametocytes [25, 26].
Gametocyte densities in cross-sectional surveys
Across studies, P. vivax gametocyte densities, as measured by RT-qPCR using marker pvs25, correlated to parasite densities, as measured by qPCR. In particular, no individuals with high asexual parasite density but absence of gametocytes were observed (Figure 2A) [13, 14, 21, 22, 26]. Substantial variation in qPCR and RT-qPCR assays was observed between technical replicates [12], which might largely explain the variation observed in Figure 2A. At low parasite densities, gametocytes are likely to be below the limit of detection. This is expected to be frequent, in particular in the case of P. vivax, as parasite densities are often lower than for P. falciparum, and as pvs25 expression is lower than pfs25 expression [14, 24]. In addition, during the development of schizonts, the number of genomes increases approximately 10-fold within a few hours, while gametocyte densities are not expected to fluctuate within such short periods of time. It appears that no adjustment to the gametocyte conversion rate between strains is required to explain the P. vivax gametocyte densities observed.
Figure 2.
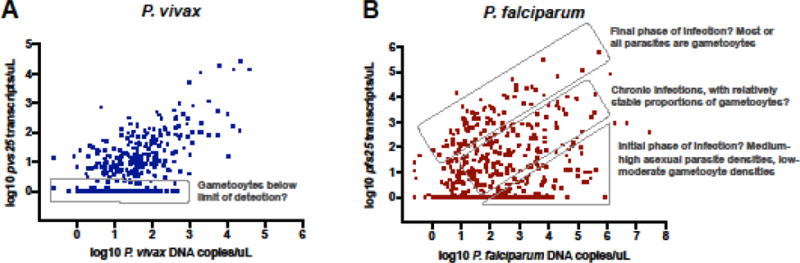
P. vivax pvs25 (A) and P. falciparum pfs25 (B) transcript density vs. genome density in cross-sectional surveys. Possible scenarios resulting in infections with above or below proportions of gametocytes among all parasites are indicated. As each gametocyte contains one genome, high pvs25 or pfs25 densities when genome copies are very low (top-left region in graphs) are not observed. Data are from two cross-sectional surveys conducted in Papua New Guinea (4,600 individuals surveyed, 766 positive for P. vivax, 611 positive for P. falciparum by qPCR) [20].
The pattern for P. falciparum is more complex (Figure 2B) [14, 21, 27]. Several factors complicate the study of the association between P. falciparum asexual parasite density and gametocyte density: (i) Developing gametocytes are sequestered for approximately two weeks [28]. Only mature (termed ‘stage V’) gametocytes are observed in peripheral blood. Gametocytes then circulate for approximately one week, although this period can reach up to a month [29]. (ii) Late P. falciparum stages are sequestered, thus parasite densities in peripheral blood do not reflect overall parasitemia. (iii) Drug treatment can result in the release of sequestered P. falciparum gametocytes.
Can the pattern in Figure 2B be explained by taking into account these aspects of the biology of P. falciparum gametocytes, or does it imply differences in conversion rate among infections? Even though conclusions on temporal trends remain speculative in cross-sectional surveys, it appears that the 2-week sequestration of developing gametocytes and the long circulation of mature gametocytes could result in the patterns observed, without the need for adjustments of the conversion rate. In the first phase of the infections, asexual parasite density peaks, but as developing gametocytes are sequestered, their density in peripheral blood remains low. After treatment, or in the final phase of the infection, all remaining parasites in the blood might be gametocytes [30]. In between these groups, ‘chronic’ infections with an intermediate proportion of gametocytes are observed.
Cohort studies with frequent sampling will be required to assess whether these processes indeed explain P. falciparum gametocyte densities in the general population. In addition to marker pfs25, which is specific for mature female gametocytes, markers for early gametocytes [31] will help to elucidate whether P. falciparum infections in the field differ with respect to gametocyte conversion.
Gametocyte carriage in response to external factors
In order to maximize transmission success, parasites might adjust gametocyte conversion according to external factors. Such processes were indeed observed in in-vitro and animal studies [5, 6, 8, 9], yet it remains unknown whether similar processes occur in the field. In particular, gametocyte densities in most of these experiments were several orders of magnitude higher than commonly observed in field samples, and well above the threshold for successful mosquito infection [32, 33]. Thus, they might not represent common natural situations, and patterns observed in field studies might be primarily shaped by detectability as discussed above.
Seasonality
In a number of malaria endemic countries, transmission is limited to a brief wet season, and absent during the dry season. P. falciparum infections persist at low densities during the dry season and initiate transmission once mosquitos become abundant in the wet season. It has been speculated that parasites might increase gametocyte conversion at the beginning of the transmission season [34]. From an evolutionary point of view, a parasite could benefit from not producing gametocytes during the dry season. The resources invested in gametocyte development will be lost if no mosquitos are present and thus no chance for onward transmission exists. These gametocytes could also induce transmission blocking immunity [35] and thus reduce infectivity in the transmission season.
Indeed, early studies noted fewer gametocyte carriers in the transmission-free season [36-38]. Yet, already in these studies, based on microscopy, the correlation between parasite density and gametocytemia was apparent [36, 37, 39]. When sensitive molecular diagnostics were applied, gametocytes were repeatedly detected in the low-transmission season [18, 40-42]. In several studies, the proportion of gametocyte-positive infections was lower in the dry season, but also mean parasite densities were much lower. Few studies have quantified parasites by qPCR and thus were able to correct for this factor. A study in Peru did so for P. vivax, and after correcting, no longer found any significant differences in gametocyte carriage between seasons [13]. The evidence gathered based on the current state of research indicates that parasites do not adjust gametocyte conversion to seasonality, and the apparent absence of gametocytes during the transmission-free season can be explained by the very low density of these infections.
Age of human host
Across studies, lower proportions of infections with gametocytes have been found with increasing age, both by microscopy [38, 43, 44] and PCR [14, 18, 43]. In parallel, some studies reported that in those adults carrying gametocytes, the proportion of gametocytes among all parasites was up to 20-fold higher than in children [43, 44].
As the result of naturally acquired immunity, in regions of high transmission intensity, parasite densities decrease strongly with age; 10-fold or higher differences in mean density between young children and adults are often observed [20]. To investigate whether parasites adapt their gametocyte conversion rate to host age, differences in the proportion of gametocyte-positive infections between age groups needs to be disentangled from the effect of differences in parasite density. The speed of acquisition and longevity of gametocyte-specific immunity differs from immunity against asexual parasites [45, 46]. Differences in the rate of clearance of asexual parasites and gametocytes in different age groups thus complicate patterns further.
Based on the difficulty in detecting gametocytes in low-density infections, it seems likely that fewer individuals with gametocytes among adults might largely be the result of limited detectability and stochastic variation in the proportion of gametocytes, in particular in the case of studies based on microscopy. If parasite density is low, gametocytes are only detected if they constitute a high proportion of all parasites within an infection. As a result, among infections with detectable gametocytes in adults, a higher proportion of all parasites are gametocytes, as compared to children. Even when applying sensitive RT-qPCR, gametocytes are often detected in as few as 20% of infections in adults, compared to much higher proportions in children [14, 18, 43]. Likely, many adults carry gametocytes below the limit of detection even by RT-qPCR. More sensitive markers for gametocytes [19], or concentration of RNA from larger blood volumes during extraction, will be needed to understand gametocyte carriage in these very low-density infections.
Mixed-species infections
Two studies in Papua New Guinea found lower gametocyte densities in individuals co-infected with P. falciparum and P. vivax, after correcting for parasite densities [14, 15]. Parasite densities were similar in single-species and mixed-species infections [14]. It is not known whether this can be explained by cross-reactivity of gametocyte-specific antibodies resulting in fast clearance of gametocytes [47], or whether the conversion rate is downregulated in mixed-species infections. A limited number of studies have addressed gametocyte carriage in co-infections of other species; by microscopy, slightly higher P. falciparum gametocytes densities were observed in Kenyan individuals co-infected with P. malariae or P. ovale [39]. As this result was not corrected for parasite density, it might have been prone to confounding: high density facilitates both the detection of mixed-species infections, and of gametocytes. In Vietnam, gametocytes of P. falciparum, P. vivax and Plasmodium knowlesi were frequently detected in individuals co-infected with several species, but interactions were not assessed [48].
Drug treatment and anemia
At the recommended safe level, commonly used drugs, with the exception of primaquine, have moderate effects on gametocytes [49, 50]. P. falciparum gametocytemia up to 2 weeks following treatment is thus frequently observed [29], and treated individuals might be an important source of transmission during convalescence [51-54]. But does drug treatment result in a change of gametocyte conversion rates? This was indeed observed following chloroquine treatment at subcurative doses under carefully controlled laboratory conditions, whereby there was an increase in P. falciparum gametocytogenesis [5]. It remains very challenging to show whether certain drugs result in increased gametocyte conversion rates in the field.
In drug trials combined with studies on gametocyte carriage and infectivity, higher gametocyte numbers and higher infection success in patients with treatment failure were observed for up to a month following treatment [51-53]. To assess whether this is the result of a change of the gametocyte conversion rate, several confounders need to be considered: (i) Taking into account the 10-day development of P. falciparum gametocytes, effects of treatment on the conversion rate would not yet be apparent at day 7 after treatment. (ii) Gametocyte density at day 7 reflects parasite density approx. 3 days before diagnosis and treatment. In most cases, this density is unknown. (iii) While some drugs increase clearance of gametocytes [55], treatment can also result in the release of sequestered gametocytes, thus increasing their density in peripheral blood [56]. (iv) When infectivity is evaluated in addition to gametocyte density, a drug might not clear gametocytes, but still affect their infectivity [52, 57, 58]. In addition, drugs might act differently on male and female gametocytes [55], and transcripts might be detected even if gametocytes are rendered infertile.
To show an increase in the conversion rate, or even a terminal investment, that is, parasites invest their remaining resources in transmission [4], the input from sequestered gametocytes needs to be disentangled from gametocyte clearance and de-novo gametocyte development. Given the confounders listed above, this remains challenging in field studies. Controlled human infections in malaria-naïve individuals could help to address these questions [30, 31]. Such studies have detected P. falciparum gametocyte rings immediately following detection of asexual infections, without the presence of triggers such as high-density infections [31], and waves of gametocytes after drug treatment were observed together with recrudescent asexual parasitemia [30, 31]. This primarily confirms that developing gametocytes are not cleared by the drugs used in these studies, with no evidence that drug treatment triggers gametocyte conversion [30, 31]. It remains difficult to assess whether treatment results in an increase in gametocyte conversion. Assays that can measure gametocyte commitment, i.e. targeting genes that are responsible for initiating the switch from asexual to sexual development, might be required to resolve this issue.
In several drug trials [59, 60] and cross-sectional surveys [14, 61] anemia was identified as a risk factor for gametocyte carriage. However, as anemia is often the result of prolonged or repeated infections, or high parasite density, this does not necessarily show that anemia results in a change of gametocyte conversion. Rather, a prolonged duration of infection might give P. falciparum more time to develop gametocytes, and will also affect levels of acquired immunity.
Concluding remarks and future perspectives
Despite the observation of parasites adjusting the gametocyte conversion rate in in-vitro studies [5, 6], it remains very challenging to find evidence for such processes in the field (see Outstanding Questions). Given the importance of parasite density as a predictor to detect gametocytes, it is essential that all studies assessing the proportion of gametocyte-positive infections, or gametocyte density, quantify parasite densities and include them as confounders in multivariable analyses. Likewise, a good understanding of the limit of detection and technical variation of laboratory assays applied is crucial for the correct interpretation of gametocyte data in low-density infections.
Outstanding Questions.
Do Plasmodium spp. alter their commitment to transmission, i.e. the gametocyte conversion rate, in response to external factors in population-based studies?
How can we measure gametocyte commitment in field studies? I.e. can we develop markers that detect parasites committed to gametocyte development, before committed schizonts sequester?
Can we increase comparability of reporting of gametocyte densities assessed by different protocols? How do different RNA collection (e.g. filter paper vs. blood collected into RNA preservation reagent) and extraction methods (e.g. spin column vs. magnetic bead-based protocols) affect the number of transcripts per gametocyte detected?
Does P. falciparum gametocyte density follow asexual density with a lag of 10-14 days?
How will the inclusion of male-specific markers in addition to female markers pfs25 and pvs25 in population-based surveys affect results?
Due to differences in protocols used, comparisons of studies conducted by different laboratories are often difficult. This hampers the ability to assess potential key factors of transmission epidemiology, such as the relationship between gametocyte conversion rate and transmission intensity, and to conduct formal meta-analyses. The volume of blood screened for parasites and gametocytes, and the limit of detection of qPCR and RT-qPCR assays must be reported in all studies. In addition, more accurate methods for genome [12] and transcripts quantification, protocols to quantify sequestered parasites [62], and markers for gametocyte commitment and for early gametocytes [31] are expected to allow for a better understanding of gametocyte commitment. To fully evaluate the effect of the 2-week sequestration of developing P. falciparum gametocytes in field studies, cohorts with frequent sampling (e.g. daily) will be needed.
It appears that parasite densities can explain many of the patterns of gametocyte carriage observed at the population level. Changes across age groups, i.e. overall fewer gametocyte carriers with increasing age [14], but possibly including some individuals with very high proportions of gametocytes [43], warrant further investigation. Likewise, future studies might reveal why fewer gametocytes are found in mixed-species infections. To understand the possible effect of drug treatment on gametocyte conversion, a marker for sexually committed ring stages, as recently published [31], will be of great help. This marker allows the detection of committed P. falciparum gametocytes up to 10 days earlier than markers for mature gametocytes, and the same study showed that committed ring stages circulate in peripheral blood and can be quantified before being sequestered [31]. Adjustments to gametocyte sex ratios as a response to the environment might be more pronounced than adjustments of overall gametocyte densities. The use of both male and female gametocyte markers will enable the study of such processes in the field [63].
Given the strong dependence of gametocyte density on asexual parasite density, measures of asexual parasite density might often provide sufficiently reliable estimates of transmission potential, in particular for P. vivax. Importantly, while the presence of male and female gametocytes is a prerequisite for transmission, many additional factors need to be taken into consideration when assessing the contribution to transmission of specific groups within the population [1, 64]. This includes the assessment of the infectivity of gametocytes through mosquito feeding assays [32, 33]. While several of the factors discussed here might have limited effects on gametocyte density, they might affect infectivity, either because gametocytes are infertile, or because of transmission blocking immunity elicited by the human host [65].
Highlights.
Understanding who carries gametocytes and therefore is likely to be infective to mosquitos, including in the case of asymptomatic infection, is key to malaria elimination.
When the proportion of gametocyte-positive infections and gametocyte densities are measured in population-based surveys, the results are substantially impacted by imperfect detectability.
Blood-stage parasite density is a key variable for the probability of detecting gametocytes.
Many factors associated with gametocyte carriage in univariate analyses are no longer significant if blood-stage parasite density is included in multivariable analyses as a confounder.
Acknowledgments
Funding statement
This work was supported by grants from the National Institutes of Health (U19 AI129326, R01 AI050243 and D43 TW001505). The funders had no role in the decision to publish, or preparation of the manuscript.
Glossary
- Blood-stage schizont
Matured malaria parasite, representing the final stage of the asexual cycle of the parasite in human blood. A schizont contains approximately a dozen daughter parasites (merozoites), which upon release invade red blood cells. All merozoites of a schizont develop either asexually, or, in the case of a sexually committed schizont, develop into gametocytes. P. falciparum schizonts are sequestered in inner organs and not observed in peripheral blood
- Conversion rate
Proportion of parasites that deviate from asexual replication and develop into gametocytes
- Gametocyte reservoir
All gametocytes in a population, i.e. a combination of the number of gametocyte carriers and gametocyte densities. Gametocyte reservoirs do not have a unit of measurement
- Gametocytemia
Gametocyte density (gametocytes/μL blood)
- Infectivity
Ability of a parasite to infect a mosquito upon feeding blood. Two main parameters are assessed – the number of mosquitos infected, and the number of oocysts (developing parasites in the mosquito midgut) per mosquito
- Nucleic acid sequence-based amplification (NASBA)
Method for the quantification of RNA, and thus for measuring gene expression. In contrast to RT-qPCR, the amplification step occurs at a constant temperature
- Parasitemia
Parasite density (parasites/μL blood)
- Population gametocyte prevalence
number of gametocyte carriers divided by the general population (including parasite-free individuals)
- Proportion of gametocyte-positive infections
Number of gametocyte carriers divided by those infected
- Reverse-transcription quantitative PCR (RT-qPCR)
Method for the quantification of RNA, and thus for measuring gene expression. RNA is first transcribed to complementary DNA (cDNA), and then the cDNA is amplified and quantified
- Terminal investment
According to this theory, individuals invest increasingly in reproduction (gametocyte production in the case of malaria parasites) if conditions deteriorate and survival of the parent is at risk
- Transmission-blocking immunity
Immunity specifically against gametocytes or mosquito stages, which can result in clearance of gametocytes from the blood
- Transmission-blocking vaccines (TBV)
A vaccine that inhibits parasite development in the mosquito. Antibodies generated by the human immune system upon vaccination are taken up by the mosquito during a blood-meal, and interfere with proteins of the parasite expressed in the mosquito. TBVs do not offer a direct benefit for the individual vaccinated but aim to reduce transmission at the community level. Several TBVs are being developed, but to date none have been approved
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.malERA Refresh Consultative Panel on Characterising the Reservoir Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002452. doi: 10.1371/journal.pmed.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson SK, et al. Targeting Human Transmission Biology for Malaria Elimination. PLoS Pathog. 2015;11(6):e1004871. doi: 10.1371/journal.ppat.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancucci NMB, et al. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014;16(2):165–176. doi: 10.1016/j.chom.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Carter LM, et al. Stress and sex in malaria parasites: Why does commitment vary? Evol Med Public Health. 2013;2013(1):135–47. doi: 10.1093/emph/eot011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckling A, et al. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118(Pt 4):339–46. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 6.Brancucci NMB, et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell. 2017;171(7):1532–44. doi: 10.1016/j.cell.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriyasu T, et al. Schistosoma mansoni infection suppresses the growth of Plasmodium yoelii parasites in the liver and reduces gametocyte infectivity to mosquitoes. PLoS Negl Trop Dis. 2018;12(1):e0006197. doi: 10.1371/journal.pntd.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reece SE, et al. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453(7195):609–14. doi: 10.1038/nature06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitri C, et al. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc Biol Sci. 2009;276(1673):3721–6. doi: 10.1098/rspb.2009.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theisen M, et al. Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert Rev Vaccines. 2017;16(4):329–336. doi: 10.1080/14760584.2017.1276833. [DOI] [PubMed] [Google Scholar]
- 11.Walker M, et al. Improving statistical inference on pathogen densities estimated by quantitative molecular methods: malaria gametocytaemia as a case study. BMC Bioinformatics. 2015;16:5. doi: 10.1186/s12859-014-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koepfli C, et al. Sensitive and accurate quantification of human malaria parasites using droplet digital PCR (ddPCR) Sci Rep. 2016;6:39183. doi: 10.1038/srep39183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovira-Vallbona E, et al. Predominance of asymptomatic and sub-microscopic infections characterizes the Plasmodium gametocyte reservoir in the Peruvian Amazon. PLoS Negl Trop Dis. 2017;11(7):e0005674. doi: 10.1371/journal.pntd.0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koepfli C, et al. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. PLoS One. 2015;10(5):e0126747. doi: 10.1371/journal.pone.0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wampfler R, et al. Effects of liver-stage clearance by Primaquine on gametocyte carriage of Plasmodium vivax and P. falciparum. PLoS Negl Trop Dis. 2017;11(7):e0005753. doi: 10.1371/journal.pntd.0005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandeu MM, et al. Do the venous blood samples replicate malaria parasite densities found in capillary blood? A field study performed in naturally-infected asymptomatic children in Cameroon. Malar J. 2017;16(1):345. doi: 10.1186/s12936-017-1978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwingira F, et al. Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar J. 2014;13:433. doi: 10.1186/1475-2875-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coalson JE, et al. High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J. 2016;15(1):527. doi: 10.1186/s12936-016-1587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essuman E, et al. A Novel Gametocyte Biomarker for Superior Molecular Detection of the Plasmodium falciparum Infectious Reservoirs. J Infect Dis. 2017;216(10):1264–1272. doi: 10.1093/infdis/jix442. [DOI] [PubMed] [Google Scholar]
- 20.Koepfli C, et al. Sustained Malaria Control Over an 8-Year Period in Papua New Guinea: The Challenge of Low-Density Asymptomatic Plasmodium Infections. J Infect Dis. 2017;216(11):1434–1443. doi: 10.1093/infdis/jix507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguitragool W, et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasit Vectors. 2017;10(1):512. doi: 10.1186/s13071-017-2407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadesse FG, et al. The shape of the iceberg: quantification of submicroscopic Plasmodium falciparum and Plasmodium vivax parasitaemia and gametocytaemia in five low endemic settings in Ethiopia. Malar J. 2017;16(1):99. doi: 10.1186/s12936-017-1749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann N, et al. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12(3):e1001788. doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wampfler R, et al. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8(9):e76316. doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbosa S, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8(8):e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadesse FG, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018 doi: 10.1093/cid/cix1123. [DOI] [PubMed] [Google Scholar]
- 27.Gadalla AAH, et al. Associations between Season and Gametocyte Dynamics in Chronic Plasmodium falciparum Infections. Plos One. 2016;11(11) doi: 10.1371/journal.pone.0166699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilar R, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123(7):959–66. doi: 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousema T, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasay CJ, et al. Piperaquine Monotherapy of Drug-Susceptible Plasmodium falciparum Infection Results in Rapid Clearance of Parasitemia but Is Followed by the Appearance of Gametocytemia. Journal of Infectious Diseases. 2016;214(1):105–113. doi: 10.1093/infdis/jiw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farid R, et al. Initiation of gametocytogenesis at very low parasite density in Plasmodium falciparum infection. Journal of Infectious Diseases. 2017;215(7):1167–1174. doi: 10.1093/infdis/jix035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiattibutr K, et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int J Parasitol. 2017;47(2–3):163–170. doi: 10.1016/j.ijpara.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goncalves BP, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun. 2017;8(1):1133. doi: 10.1038/s41467-017-01270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babiker HA, et al. Gametocytes: insights gained during a decade of molecular monitoring. Trends Parasitol. 2008;24(11):525–30. doi: 10.1016/j.pt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone WJ, et al. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. 2016;143(2):187–98. doi: 10.1017/S0031182015001341. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg R, et al. Seasonal fluctuation of Plasmodium falciparum gametocytaemia. Trans R Soc Trop Med Hyg. 1990;84(1):29–33. doi: 10.1016/0035-9203(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 37.Boudin C, et al. Epidemiology of Plasmodium falciparum in a rice field and a savanna area in Burkina Faso: seasonal fluctuations of gametocytaemia and malarial infectivity. Ann Trop Med Parasitol. 1991;85(4):377–85. doi: 10.1080/00034983.1991.11812580. [DOI] [PubMed] [Google Scholar]
- 38.Nacher M, et al. Seasonal fluctuations in the carriage of Plasmodium vivax gametocytes in Thailand. Ann Trop Med Parasitol. 2004;98(2):115–20. doi: 10.1179/000349804225003145. [DOI] [PubMed] [Google Scholar]
- 39.Andagalu B, et al. Longitudinal study on Plasmodium falciparum gametocyte carriage following artemether-lumefantrine administration in a cohort of children aged 12-47 months living in Western Kenya, a high transmission area. Malar J. 2014;13:265. doi: 10.1186/1475-2875-13-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdel-Wahab A, et al. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J Infect Dis. 2002;185(12):1838–42. doi: 10.1086/340638. [DOI] [PubMed] [Google Scholar]
- 41.Nassir E, et al. Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol. 2005;35(1):49–55. doi: 10.1016/j.ijpara.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Adomako-Ankomah Y, et al. Host age and Plasmodium falciparum multiclonality are associated with gametocyte prevalence: a 1-year prospective cohort study. Malar J. 2017;16(1):473. doi: 10.1186/s12936-017-2123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouedraogo AL, et al. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar J. 2010;9:281. doi: 10.1186/1475-2875-9-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drakeley C, et al. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22(9):424–30. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Drakeley CJ, et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol. 2006;28(5):185–90. doi: 10.1111/j.1365-3024.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 46.Ouedraogo AL, et al. Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect Immun. 2011;79(12):4957–64. doi: 10.1128/IAI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal GP, et al. Antibodies elicited during natural infection in a predominantly Plasmodium falciparum transmission area cross-react with sexual stage-specific antigen in P. vivax. Acta Trop. 2017;170:105–111. doi: 10.1016/j.actatropica.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeno Y, et al. Plasmodium knowlesi and human malaria parasites in Khan Phu, Vietnam: Gametocyte production in humans and frequent co-infection of mosquitoes. Parasitology. 2017;144(4):527–535. doi: 10.1017/S0031182016002110. [DOI] [PubMed] [Google Scholar]
- 49.Delves M, et al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9(2):e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Group, W.G.S. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14:79. doi: 10.1186/s12916-016-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beshir KB, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208(12):2017–24. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallett RL, et al. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob Agents Chemother. 2004;48(10):3940–3. doi: 10.1128/AAC.48.10.3940-3943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djimde AA, et al. Gametocyte clearance dynamics following oral artesunate treatment of uncomplicated falciparum malaria in Malian children. Parasite. 2016;23:3. doi: 10.1051/parasite/2016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin JT, et al. Single dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission in Cambodia: An open-label randomized trial. PLoS One. 2017;12(6):e0168702. doi: 10.1371/journal.pone.0168702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White NJ, et al. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J. 2014;13:483. doi: 10.1186/1475-2875-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karl S, et al. Gametocyte Clearance Kinetics Determined by Quantitative Magnetic Fractionation in Melanesian Children with Uncomplicated Malaria Treated with Artemisinin Combination Therapy. Antimicrob Agents Chemother. 2015;59(8):4489–96. doi: 10.1128/AAC.00136-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goncalves BP, et al. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med. 2016;14:40. doi: 10.1186/s12916-016-0581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beavogui AH, et al. Low infectivity of Plasmodium falciparum gametocytes to Anopheles gambiae following treatment with sulfadoxine-pyrimethamine in Mali. Int J Parasitol. 2010;40(10):1213–20. doi: 10.1016/j.ijpara.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karl S, et al. Risk factors for Plasmodium falciparum and Plasmodium vivax gametocyte carriage in Papua New Guinean children with uncomplicated malaria. Acta Trop. 2016;160:1–8. doi: 10.1016/j.actatropica.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 60.von Seidlein L, et al. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65(5):523–7. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Z, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J. 2016;15(1):421. doi: 10.1186/s12936-016-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desakorn V, et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2005;99(7):517–24. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Stone W, et al. A Molecular Assay to Quantify Male and Female Plasmodium falciparum Gametocytes: Results From 2 Randomized Controlled Trials Using Primaquine for Gametocyte Clearance. J Infect Dis. 2017;216(4):457–467. doi: 10.1093/infdis/jix237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bousema T, Drakeley C. Determinants of Malaria Transmission at the Population Level. Cold Spring Harb Perspect Med. 2017;7(12) doi: 10.1101/cshperspect.a025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bousema T, et al. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int J Parasitol. 2011;41(3–4):293–300. doi: 10.1016/j.ijpara.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welch WH, Thayer WS. Malaria 1897 [Google Scholar]
- 67.World Health Organisation. Basic Malaria Microscopy 2010 [Google Scholar]
- 68.Niederwieser I, et al. Plasmodium falciparum: expression of gametocyte-specific genes in monolayer cultures and malaria-positive blood samples. Exp Parasitol. 2000;95(3):163–9. doi: 10.1006/expr.2000.4536. [DOI] [PubMed] [Google Scholar]
- 69.Schneider P, et al. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol. 2004;137(1):35–41. doi: 10.1016/j.molbiopara.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 70.Maeno Y, et al. A dried blood sample on filter paper is suitable for detecting Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Acta Tropica. 2008;107(2):121–127. doi: 10.1016/j.actatropica.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Lima NF, et al. Plasmodium vivax: reverse transcriptase real-time PCR for gametocyte detection and quantitation in clinical samples. Exp Parasitol. 2012;132(3):348–54. doi: 10.1016/j.exppara.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiburcio M, et al. Specific expression and export of the Plasmodium falciparum Gametocyte EXported Protein-5 marks the gametocyte ring stage. Malar J. 2015;14:334. doi: 10.1186/s12936-015-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santolamazza F, et al. Detection of Plasmodium falciparum male and female gametocytes and determination of parasite sex ratio in human endemic populations by novel, cheap and robust RTqPCR assays. Malar J. 2017;16(1):468. doi: 10.1186/s12936-017-2118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider P, et al. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol. 2015;199(1–2):29–33. doi: 10.1016/j.molbiopara.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Wampfler R, et al. Novel genotyping tools for investigating transmission dynamics of Plasmodium falciparum. J Infect Dis. 2014;210(8):1188–97. doi: 10.1093/infdis/jiu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanron AE, et al. Multiplex, DNase-free one-step reverse transcription PCR for Plasmodium 18S rRNA and spliced gametocyte-specific mRNAs. Malar J. 2017;16(1):208. doi: 10.1186/s12936-017-1863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menegon M, et al. Genotyping of Plasmodium falciparum gametocytes by reverse transcriptase polymerase chain reaction. Mol Biochem Parasitol. 2000;111(1):153–61. doi: 10.1016/s0166-6851(00)00314-5. [DOI] [PubMed] [Google Scholar]


